Abstract
Aim
To evaluate the efficacy of a non-thermal plasma (NTP) at atmospheric pressure on ex vivo biofilm in root canals of extracted teeth.
Methodology
Intra-canal contents from three teeth with root canal infections were collected, pooled, and grown in thirty-five microCT-mapped root canals of extracted and instrumented human teeth. One group of teeth was treated with NTP, another with 6% NaOCl, and one set was left untreated. The intra-canal contents from twenty-seven teeth (nine teeth in each group) were plated on agar and colony forming units were determined. Parametric test of one-way Analysis of Variance (ANOVA) was used to analyze statistical significance. The remaining teeth were cut open, stained with LIVE/DEAD® and examined with confocal laser scanning microscopy.
Results
The untreated root canals were covered with biofilm of varying thickness. The treatment with the non-thermal plasma decreased the number of viable bacteria in these biofilms by one order of magnitude, while the NaOCl control achieved a reduction of more than four magnitudes. Both the NTP and the NaOCl treatment results were significantly different from the negative control (P< 0.05).
Conclusion
The non-thermal plasma displayed antimicrobial activity against endodontic biofilms in root canals, but was not as effective as the use of 6 % NaOCl.
Keywords: Endodontics, non-thermal plasma, biofilm, micro-CT
Introduction
Several microscopic studies that examined endodontic infections in situ have described polymicrobial biofilms (Nair 2004, Nair et al. 2005, Carr et al. 2009), whose elimination is commonly achieved by a combination of antimicrobial irrigants along with mechanical instrumentation (Haapasalo et al. 2005) (). Despite advancements in root canal treatment, the complete removal or inactivation of biofilms within the root canal system remains a demanding procedure with success rates ranging from 68 % to 85 % (Ng et al. 2007). For this reason, alternative treatment protocols and devices have been tested, one of which is the non-thermal plasma-based technology (Yu et al. 2006, Jiang et al. 2009a, Jiang et al. 2012). In addition to solid, liquid and gas, plasma represents the fourth state of matter with temperatures usually exceeding thousands of Kelvin. Recently developed atmospheric-pressure non-thermal plasma (NTP) jets, typically in the shape of fine plumes of partially ionized gases, were generated by a proprietary device powered with ultra-short (<200 ns pulse duration) kilovolt electric pulses (Jiang et al. 2009a). These plasma jets are highly non-equilibrium and generate efficiently reactive plasma species including ions, ozone, and oxygen radicals by energetic collisions of electrons, while the gas temperature of the plasma remains virtually at room temperature. The interaction of plasma species with the bacterial membrane causes their disruption and consequently the death of bacterial cells (Laroussi et al. 2002, Jiang & Schaudinn 2011, Jiang et al. 2012). The possibility to gently sterilize surfaces at ambient temperature has made the NTP an attractive tool for a wide range of applications including the sterilization of clinical instruments (Lee et al. 2006) and food (Vleugels et al. 2005). So far, the efficacy of NTP to kill and remove bacteria or yeasts has been shown on a number of species. In these studies, the targeted bacteria were predominantly grown as single species biofilms in diverse models, for instance on agar in petri-dishes (Sladek et al. 2004, Jiang et al. 2009b, Rupf et al. 2010), membrane filters (Lee et al. 2006, Yu et al. 2006), hydroxyapatite discs (Jiang & Schaudinn 2011) or dentine slices (Rupf et al. 2010) and were therefore directly and easily accessible to the plasma plume. Only a few attempts have been made to tackle biofilms in root canals (Jiang et al. 2009a). In a previous study, the poly-microbial biofilm was visibly disrupted, but the effects were limited to the first millimetre of the root canal where the plasma directly reached the biofilm so that the overall reduction was minimal. In this proof-of-concept study, a dental plasma probe was engineered with a needle-fine plasma plume, which made it possible to penetrate the entire length of root canals. The hypothesis was that the NTP “needle” had antimicrobial effect against ex vivo multispecies biofilms grown inside root canals of extracted human teeth.
Material and Methods
Tooth preparation
Appropriate Institutional Review Board approval (USC UPIRB #UP-10-00182) was obtained. Thirty-five single-rooted human teeth were collected after surgical extraction, and stored in 10 % formalin. The extracted teeth were non-restorable and non-salvageable and removed as part of necessary and routine clinical care of patients at the Ostrow School of Dentistry of USC. All patients gave informed consent for the procedure. The root canals of all teeth were enlarged employing the ProTaper rotary system (Dentsply Tulsa Dental, Tulsa, OK, USA) to a F2 size 25, 08taper with 1 mL NaOCl (6 %) used between each instrumentation step. All teeth were finally rinsed with 5 mL EDTA (17 %) (Roth Int. Chicago, Il, USA) for smear layer removal.
Micro- Computed Tomography (micro-CT) analysis
To investigate tooth morphology and endodontic surface area, virtual endoscopy was performed on each single tooth before the actual experiment started using high-resolution micro-computed tomography (Inveon™, Siemens Medical Solutions, Knoxville, TN, USA). The specimens were placed in a sample holder in the posterior-anterior direction and scanned using a high-resolution micro-CT system at a spatial resolution of 18.676 μm (voxel dimension) and 1,536×1,536 pixel matrices. After scanning, the 2D image data were stored in the Digital Imaging and Communications in Medicine (DICOM) format, transferred to a computer and a 3D reconstruction and analysis was performed (Freire et al. 2011). Microtomographic slice images were reoriented as volume of interest (VOI) using Amira™ software. Threshold equal 480 HU was used to investigate the teeth. The volume and surface area of interior the root canal was determined using OnDemand3D (Cybermed Inc. Irvine, CA, USA).
Biofilm growth conditions
After sterilization of all specimens by autoclaving, the root canals were coated with (10 mg/mL) bovine dermal type I collagen (Gibco®, Invitrogen, Carlsbad, CA, USA) by injecting the collagen into the canals and incubated overnight at 4 °C (Shen et al. 2009). Three teeth were extracted from three patients with acute pain and the diagnosis of irreversible pulpitis. Intra-canal contents were carefully sampled canals with sterile paper points in a laminar hood directly after the extraction. At first, each sample was cultivated separately at 37 °C under anaerobic conditions (BD GasPak™ 100 System, Franklin Lakes, NJ, USA) in brain heart infusion broth (BHI) (HiVeg™ Media, Mumbai, India), which was supplemented with 1 μg/mL vitamin K, 10 μg/mL haemin and 1 % l-cysteine. These ex vivo cultures were then combined and grown for 24 h, as described above. The culture was concentrated to 1010 cells/mL, filled with a 25-5/8 gauge needle in the root canals until the liquid started to drip out of foramen of the tooth, and pre-incubated for 4 h at 37 °C under anaerobic conditions to allow the bacteria to adhere. Subsequently, the root canals were rinsed with 1 mL media for one minute to remove unbound bacteria and placed in six-well plates. The wells were filled with 8 mL supplemented BHI, and cultivated at 37 °C under anaerobic conditions for 14 days. Fifty percent of the media was changed every third day.
Treatment of endodontic biofilms
Prior to treatment, the liquid in the root canals was largely removed by tapping the root canal orifices on sterile filter paper. In order to minimize the strong, foul odour, the biofilm on the outer surfaces of all teeth was carefully removed with 70 % ethanol-soaked swabs, while the openings to the root canals were both plugged with sterile parafilm during this procedure. Twelve teeth were randomly chosen and treated with the NTP for 30 min (3 × 10 min with 2 min pauses in between). The NTP was powered by 6.5 kV, 150 ns voltage pulses at 1.5 kHz. The average power of the plasma device was maintained below 0.5 W throughout the treatment. A laminar gas flow (1 l/min) of a He/O2 (99:1) mixture (Airgas, Lakewood, CA, USA) was used to support the 1 mm-in-width plasma plume. Eleven teeth were randomly chosen and filled for 30 min with 500 μL 6 % NaOCl. The remaining twelve teeth were filled with 500 μL sterile 0.9 % saline (pH 7.2) by injecting the liquid in the root canals with 25-5/8 gauge needles. In some cases, excess of liquid dripped out of the foramen of the tooth. Although the needles were inserted completely into the canals (up to a length of 14–15 mm from the canal orifices), they did not reach the end of the canal. After treatment, all NaOCl-treated root canals were rinsed with 1 mL 5 % sodium thiosulfate to inactivate NaOCl, while the plasma-treated and untreated root canals were rinsed with 1 mL sterile 0.9 % saline.
Colony forming units (CFU) and imaging
Nine randomly selected teeth of each group were subsequently flushed thoroughly with 1 mL of supplemented BHI into an Eppendorf tube, by inserting 25-5/8 gauge needles into the root canals. The samples were then diluted to 10−8, all dilution steps were plated on supplemented BHI agar, incubated for 24 hours and the colony forming units were counted. At least one randomly selected tooth from each group was cut transversally using a diamond disc (911H Hyperflex disc, Brasseler, Savannah, GA, USA), while another tooth from each group was cut longitudinally. All tooth fragments were stained with Live/Dead® BacLight™ according to the manufacturer’s instructions (Molecular Probes®, Invitrogen™, Carlsbad, CA, USA) and imaged with the cLSM (LSM710, Carl Zeiss Microimaging, LLC, Thornwood, NY, USA) in chamber slides (Lab-Tek®, Electron Microscopy Sciences, Hatfield, PA, USA).
Statistical analysis
The samples were grouped as listed in Table 1. Each treatment group contained 9 specimens for the microbiology analysis. The area density of CFU counts (CFUs/mm2) was calculated using the data obtained with micro-CT and transformed by log10 in order to make the treatment results comparable and reduce the error in variance of specimens.
Table 1.
Overview on sample processing pathways
| Control group (12 teeth) | NTP group (12 teeth) | NaOCl group (11 teeth) |
| CFU (9 teeth) | CFU (9 teeth) | CFU (9 teeth) |
|
cLSM: (3 teeth) transversally cut (2 teeth) longitudinally cut (1 tooth) |
cLSM: (3 teeth) transversally cut (2 teeth) longitudinally cut (1 tooth) |
cLSM: (2 teeth) transversally cut (1 tooth) longitudinally cut (1 tooth) |
The CFU area density values were presented as mean and standard deviation. The parametric test of one-way Analysis of Variance (ANOVA) was used to analyze statistical significance, and the results were considered statistically significant when P< 0.05.
Results
The three-dimensional micro-anatomy of root canals was characterized by micro-CT (Figs. 1a–d). Root canal morphology was characteristic for each respective tooth used in the study, making them representative samples of teeth that would be encountered clinically. A small subset of teeth demonstrated secondary or accessory canals, which can also be encountered clinically in some instances. Canal shape for all teeth was predominantly linear to curvilinear since molar teeth were not used. The majority of teeth had a single apical foramen, and apical foramina were patent in all teeth. There were no incidences of root fusion, internal resorption or periapical cysts or lesions associated with the teeth.
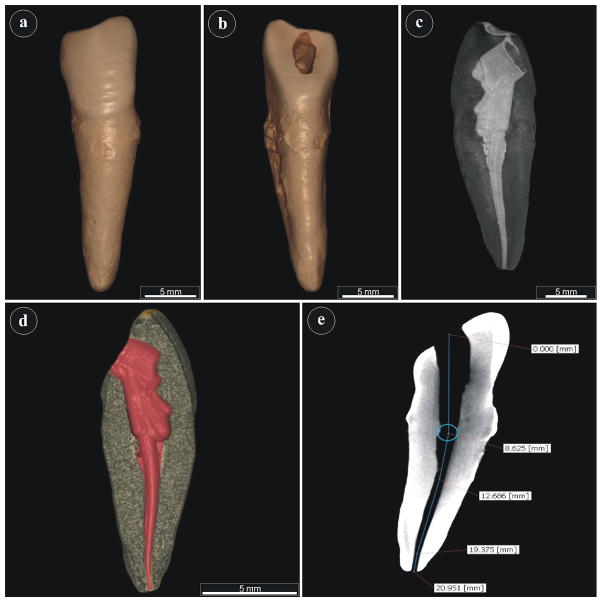
Figure 1.
Three-dimensional reconstruction of morphological features of single rooted teeth by micro-CT scan. Different planes are illustrated, including (a) facial, (b) lingual, (c) longitudinal endodontic canal cross-section, (d) endodontic canal volume in red, e) distance measurements.
Quantitative evaluation allowed measurement of the root canal lengths, surface area, and volume with accuracy (Fig. 1e). The average root canal length was calculated to be 19.7 mm ± 2.7 mm, the radius 0.75 mm ± 0.09 mm, the average root canal surface area 93.03 mm2 ± 19.95 mm2, and the average root canal volume 18 mm3 ± 3.9 mm3. Individual measurements have also been presented (Table 2, supporting information). In order to visualize the complex anatomical structures, such as secondary canals, virtual endoscopy was performed on all root canals (representative example video, supporting information).
For the actual NTP treatment, the randomly chosen teeth were placed one by one in a plastic holder directly beneath the NTP needle, as shown in Fig. 2.
Figure 2.
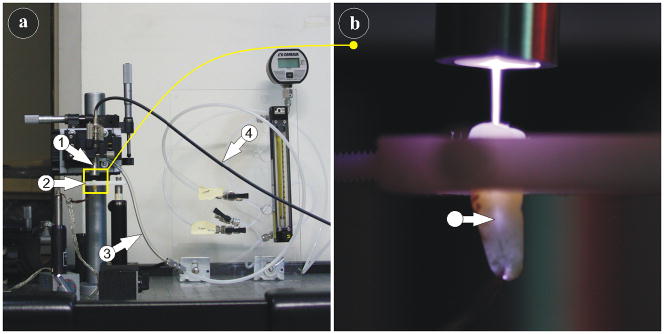
Setup of the dental plasma probe: consisting of the plasma dental probe (arrow 1), the tooth holder (arrow 2), the gas-flow tubing (arrow 3) and the power supply cable (arrow 4). (b) The needle-like NTP enters and illuminates (arrow) the root canal of an extracted tooth.
The mean log10 (CFU counts/mm2) data in the different treatment groups (Fig. 3) revealed that both the NTP and the 6 % NaOCl treatment groups are significantly different from the negative control group (P< 0.001). While the results of the NTP treatment group was only one order of magnitude lower than the untreated group, the NaOCl treatment group achieved a reduction of four orders of magnitude. This indicated that NaOCl was more effective (P< 0.05) at biofilm removal compared to the NTP for the same exposure time.
Figure 3.
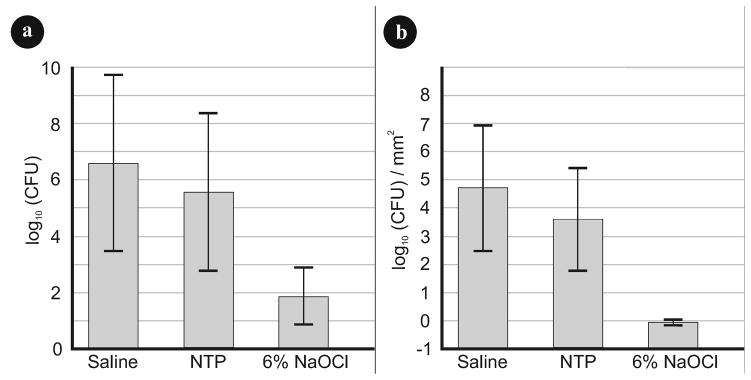
Microbiology analysis: (a) Viable bacteria counts (CFU) and (b) standardized viable bacteria counts (CFU/mm2) of the endodontic biofilms for all treatment protocols after log transformation (ANOVA, P < 0.05).
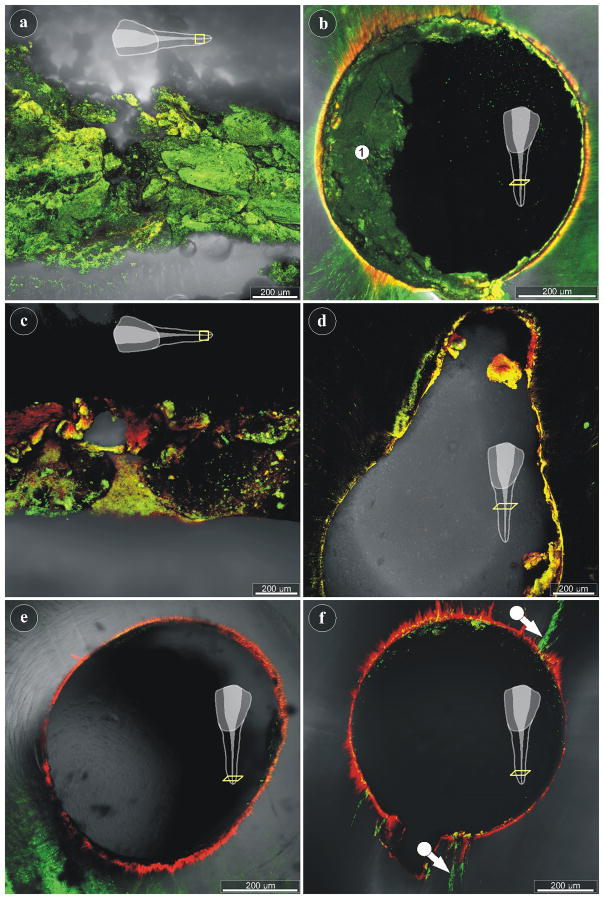
The longitudinally-sectioned as well as the transversely-sectioned tooth fragments, which were left untreated, revealed dense, unevenly distributed biofilm in the root canal with a strong green signal, indicating the viability of the bacteria (Fig. 4a, 4b). Depending on the focal plane, the dentine in all samples showed green, yellow and orange autofluorescence, in particular at the edge of the canal (Fig. 4b). The NTP-treated samples displayed areas with dead bacteria (red signal), damaged cells (yellow signal), and also living bacteria (green signal) (Figs. 4c, 4d), when imaged with the cLSM. Large parts of the NTP-treated root canals (also in the apical region) did not reveal traces of a signal, suggesting that the biofilms of these areas were removed. The NaOCl-treated root canals revealed only scattered, dead bacteria throughout the entire length of the root canals (Figs. 4e, 4f). Nonetheless, despite the NaOCl treatment, Live/Dead® staining also exposed vital biofilm in a number of small side canals in at least one of the examined teeth (Fig. 4f).
Figure 4.
cLSM imaging of untreated and treated root canals: (a) Longitudinal-section through the apical part of a root canal, as indicated in the scheme. Live/Dead® staining of an untreated specimen revealing vital biofilm (green signal). (b) The cross-section through a root canal of an untreated tooth also shows vital biofilm (area 1). (c) The longitudinal-section through the apical part of a plasma-treated root canal reveals areas with live (green) and dead (red) bacteria, while large regions have no signal and remain black. (d) Cross-section through the middle part of a plasma-treated root canal, showing little biofilm with mostly compromised bacteria (yellow signal). (e, f) The apical regions of two NaOCl-treated root canals demonstrate the almost complete removal of biofilm from the root canals walls. Only few small side-canals show persisting biofilm with viable bacteria (f).
Discussion
The ex vivo biofilm model
This study sought to assess the potential of a recently engineered NTP needle to kill and remove microbial biofilms in root canals. Laboratory methods for testing the efficacy of root canal treatment is challenging because of the lack of a commonly accepted model. Previous work has been based on extracted teeth to evaluate different treatment protocols against E. faecalis in dentinal tubules (Berber et al. 2006, Estrela et al. 2009) or involved the collection of subgingival plaque or intra-canal content grown ex vivo on either collagen coated hydroxyapatite discs (Shen et al. 2009) or hemi-sections of root canal apices (Clegg et al. 2006). The benefits of both models, the use of ex vivo biofilms as well as the employment of extracted teeth, were combined to test the NTP under more challenging conditions than are posed by flat, horizontal surfaces and mono-species biofilms. Concentrations of 6 % NaOCl were shown to efficiently kill and remove biofilm in in vitro experiments (Clegg et al. 2006, Dunavant et al. 2006).
The advantages of using micro-CT
The use of micro-CT enabled the depiction of the topographic challenges to be confronted by the NTP needle. This approach would have allowed a possible explanation, if aberrant results had occurred. Additionally, micro-CT imaging permitted the root canal surface area of each tooth to be determined. This enabled the viable bacterial load before and after the treatment - counted as CFUs – to be normalized to a standard area.
Limitation of the ex vivo model
The ex vivo model had a number of limitations. It is well known that only about 50 % of the oral bacterial species are culturable (Kroes et al. 1999). A shift from the natural habitat to an ex vivo system would inevitably never represent the original microbial community. Furthermore, certain species of the oral microbial communities show only slow growth, at least in in vitro systems such as agar plates. Hence, a twenty-four hour cultivation, as performed for the CFU counts, is necessarily biased by these fast-growing microorganisms.
Comparison with other studies
Due to the limited use of NTP in endodontic research, the specific experimental approach used allows only limited comparisons to be made. However, some interesting parallels do appear. Vianna et al. (2006) examined endodontic biofilms in infected root canals in situ. The median amount of microorganisms that were recovered from the infected root canals before the treatment ranged between 2.8 × 106 and 7.6 × 106 depending on the testing assay. In the present ex vivo test model, the median arrived at 4.7 × 106 bacteria per root canal (in absolute numbers), which is well within the range reported. Furthermore, the in situ NaOCl treatment of root canals (2.5 % NaOCl, 20 min) by Vianna et al. achieved bacterial reduction rates of 99.93 % (mean) and 100 % for the median. The 30 min 6 % NaOCl treatment in the present study reached similar reduction rates of 99.998 % for the mean and 99.999 % in case of the median.
NTP results and future development
Although the endodontic test model seemed to produce fairly reliable results, the key question regarding the efficacy of the NTP needle remains. In a number of pre-experiments it was ensured that the NTP plume would penetrate the entire length of any root canal shorter than three centimetres. However, it is clear from the results that the visible presence of the plasma plume in the root canal is not sufficient to effectively eliminate bacterial biofilm. Previous experiments have shown that direct, short-distance exposure of bacteria to NTP killed, destroyed and removed the bacteria cells within minutes (Yu et al. 2006, Jiang et al. 2009b, Jiang et al. 2012). The observed limitation of the NTP plume in the root canal is obviously due to its inability to act on bacteria over a longer distance. As a consequence, the next generation of NTP needle needs to be flexible and capable of insertion into the root canal so that the plasma jet directly impinges on the biofilm bacteria. The construction of such a micro-NTP, based on a flexible 30-gauge irrigating needle, is currently under way. Nonetheless, this proof-of-concept approach represents a necessary step in the successful disinfection of hard-to-access biofilms in endodontic root canals.
Conclusion
The antimicrobial effect of the needle-shaped NTP against endodontic biofilms in human root canals was demonstrated. However, the efficacy of biofilm removal by the NTP was less than achieved in the treatment with 6% NaOCl. The effectiveness of the NTP-based treatment needs to be improved to be comparable to conventional irrigation methods.
Supplementary Material
Individual endodontic canal measurements.
Virtual endoscopy through the endodontic canal by micro-CT.
Acknowledgments
The work is supported by a research grant (R21-DE020167-01A1) from the National Institute of Dental and Craniofacial Research (NIDCR), National Institutes of Health.
Footnotes
We affirm that there is no potential conflict of interest and no financial affiliation or involvement with any commercial organization with direct interest in the subject or materials of the presented work.
References
- Berber VB, Gomes BP, Sena NT, et al. Efficacy of various concentrations of NaOCl and instrumentation techniques in reducing Enterococcus faecalis within root canals and dentinal tubules. International Endodontic Journal. 2006;39:10–7. doi: 10.1111/j.1365-2591.2005.01038.x. [DOI] [PubMed] [Google Scholar]
- Buchanan LS. Cleaning and shaping the root canal system: negotiating canals to the termini. Dentistry Today. 1994;13:78–81. [PubMed] [Google Scholar]
- Carr GB, Schwartz RS, Schaudinn C, Gorur A, Costerton JW. Ultrastructural examination of failed molar retreatment with secondary apical periodontitis: an examination of endodontic biofilms in an endodontic retreatment failure. Journal of Endodontics. 2009;35:1303–9. doi: 10.1016/j.joen.2009.05.035. [DOI] [PubMed] [Google Scholar]
- Clegg MS, Vertucci FJ, Walker C, Belanger M, Britto LR. The effect of exposure to irrigant solutions on apical dentin biofilms in vitro. Journal of Endodontics. 2006;32:434–7. doi: 10.1016/j.joen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Dunavant TR, Regan JD, Glickman GN, Solomon ES, Honeyman AL. Comparative evaluation of endodontic irrigants against Enterococcus faecalis biofilms. Journal of Endodontics. 2006;32:527–31. doi: 10.1016/j.joen.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Estrela C, Sydney GB, Figueiredo JA, Estrela CR. Antibacterial efficacy of intracanal medicaments on bacterial biofilm: a critical review. Journal of Applied Oral Sciences. 2009;17:1–7. doi: 10.1590/S1678-77572009000100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire MO, Sedghizadeh PP, Schaudinn C, et al. Development of an animal model for Aggregatibacter actinomycetemcomitans biofilm-mediated oral osteolytic infection: a preliminary study. Journal of Periodontology. 2011;82:778–89. doi: 10.1902/jop.2010.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapasalo M, Endal U, Zandi H, Coil JM. Eradication of endodontic infection by instrumentation and irrigation solutions. Endodontic Topics. 2005;10:77–102. [Google Scholar]
- Jiang C, Chen MT, Schaudinn C, et al. Nanosecond pulsed plasma dental probe. Plasma Processes and Polymers. 2009a;6:479–83. [Google Scholar]
- Jiang C, Chen MT, Schaudinn C, et al. Plused Atmospheric-Pressure Cold Plasma for Endodontic Disinfeciton. IEEE Transactions on Plasma Science. 2009b;37:1190–5. [Google Scholar]
- Jiang C, Schaudinn C. A Curving Bactericidal Plasma Needle. IEEE Transactions on Plasma Science. 2011;39:2966–7. [Google Scholar]
- Jiang C, Schaudinn C, Jaramillo DE, Webster P, Costerton JW. In Vitro Antimicrobial Effect of a Cold Plasma Jet against Enterococcus faecalis Biofilms. ISRN Dentistry. 2012;2012:295736. doi: 10.5402/2012/295736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroes I, Lepp PW, Relman DA. Bacterial diversity within the human subgingival crevice. Proceedings of the National Academy of Sciences USA. 1999;96:14547–52. doi: 10.1073/pnas.96.25.14547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroussi M, Alexeff I, Richardson JP, Dyer FF. The resistive barrier discharge. IEEE Transactions on Plasma Science. 2002;30:158–9. [Google Scholar]
- Lee K, Paek KH, Ju WT, Lee Y. Sterilization of bacteria, yeast, and bacterial endospores by atmospheric-pressure cold plasma using helium and oxygen. Journal of Microbiology. 2006;44:269–75. [PubMed] [Google Scholar]
- Nair PN. Pathogenesis of apical periodontitis and the causes of endodontic failures. Critical Reviews in Oral Biology and Medicine. 2004;15:348–81. doi: 10.1177/154411130401500604. [DOI] [PubMed] [Google Scholar]
- Nair PN, Henry S, Cano V, Vera J. Microbial status of apical root canal system of human mandibular first molars with primary apical periodontitis after “one-visit” endodontic treatment. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics. 2005;99:231–52. doi: 10.1016/j.tripleo.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Ng YL, Mann V, Rahbaran S, Lewsey J, Gulabivala K. Outcome of primary root canal treatment: systematic review of the literature - part 1. Effects of study characteristics on probability of success. International Endodontic Journal. 2007;40:921–39. doi: 10.1111/j.1365-2591.2007.01322.x. [DOI] [PubMed] [Google Scholar]
- Rupf S, Lehmann A, Hannig M, et al. Killing of adherent oral microbes by a nonthermal atmospheric plasma jet. Journal of Medical Microbiology. 2010;59:206–12. doi: 10.1099/jmm.0.013714-0. [DOI] [PubMed] [Google Scholar]
- Shen Y, Qian W, Chung C, Olsen I, Haapasalo M. Evaluation of the effect of two chlorhexidine preparations on biofilm bacteria in vitro: a three-dimensional quantitative analysis. Journal of Endodontics. 2009;35:981–5. doi: 10.1016/j.joen.2009.04.030. [DOI] [PubMed] [Google Scholar]
- Sladek RE, Stoffels E, Walraven R, Tielbeek PJ, Koolhoven RA. Plasma Treatment of Dental Cavities: A Feasibility Study. IEEE Transactions on Plasma Science. 2004;32:1540–3. [Google Scholar]
- Vianna ME, Horz HP, Gomes BP, Conrads G. In vivo evaluation of microbial reduction after chemo-mechanical preparation of human root canals containing necrotic pulp tissue. International Endodontic Journal. 2006;39:484–92. doi: 10.1111/j.1365-2591.2006.01121.x. [DOI] [PubMed] [Google Scholar]
- Vleugels M, Shama G, Deng XT, Greenacre E, Brocklehurst T, Kong MG. Atmospheric plasma inactivation of biofilm-forming bacteria for food safety control. IEEE Transactions on Plasma Science. 2005;22:824–8. [Google Scholar]
- Yu H, Perni S, Shi JJ, Wang DZ, Kong MG, Shama G. Effects of cell surface loading and phase of growth in cold atmospheric gas plasma inactivation of Escherichia coli K12. Journal of Applied Microbiology. 2006;101:1323–30. doi: 10.1111/j.1365-2672.2006.03033.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Individual endodontic canal measurements.
Virtual endoscopy through the endodontic canal by micro-CT.