Abstract
Orexigenic and anorexigenic pathways mediate food intake and may be affected by meal composition. Our objective was to determine whether changes in levels of active ghrelin and peptide YY (PYY) differ in obese vs. normal-weight adolescent girls following specific macronutrient intake and predict hunger and subsequent food intake. We enrolled 26 subjects: 13 obese and 13 normal-weight girls, 12–18 years old, matched for maturity (as assessed by bone age) and race. Subjects were assigned a high-carbohydrate, high-protein, and high-fat breakfast in random order. Active ghrelin and PYY were assessed for 4 h after breakfast and 1 h after intake of a standardized lunch. Hunger was assessed using a standardized visual analog scale (VAS). No suppression in active ghrelin levels was noted following macronutrient intake in obese or normal-weight girls. Contrary to expectations, active ghrelin increased in obese girls following the high-carbohydrate breakfast, and the percent increase was higher than in controls (P = 0.046). Subsequent food intake at lunch was also higher (P = 0.03). Following the high-fat breakfast, but not other breakfasts, percent increase in PYY was lower (P = 0.01) and subsequent lunch intake higher (P = 0.005) in obese compared with normal-weight girls. In obese adolescents, specific intake of high-carbohydrate and high-fat breakfasts is associated with greater increases in ghrelin, lesser increases in PYY, and higher intake at a subsequent meal than in controls. Changes in anorexigenic and orexigenic hormones in obese vs. normal-weight adolescents following high-carbohydrate and high-fat meals may influence hunger and satiety signals and subsequent food intake.
Introduction
Obesity is a global problem, and 17% of US children are obese (BMI > 95th percentile) and 16.5% overweight (BMI between 85th and 95th percentiles) (1,2). It is increasingly recognized that many neuroendocrine peptides including ghrelin, an orexigenic hormone, and peptide YY (PYY), an anorexigenic hormone, regulate appetite, satiety, and food intake (3–6), and secretion of these hormones is decreased in obesity (7–9). However, the role of these peptides in physiological regulation of food intake remains to be determined. Of note, recent data in adults suggest that specific nutrient intake may profoundly affect neuroendocrine pathways activated during feeding (10–12) and impact hunger, satiety, and subsequent food intake.
Ghrelin, secreted by the stomach (13–15), is present in acylated and deacylated forms, with acylated ghrelin having orexigenic effects. Ghrelin peaks before a meal, and its administration increases food intake (5), suggesting that ghrelin is important for meal initiation (16). An important observation has been that postprandial ghrelin secretion may depend on specific macronutrient intake. In adults, total ghrelin increases after protein ingestion and decreases after carbohydrate and fat intake (10–12), and ghrelin levels are inversely related to insulin secretion in adults and in children (17–19). Although studies have examined ghrelin responses to different macronutrients in adults, data are limited in children. Adolescence is a period of tremendous fux in the hormonal milieu, and total ghrelin levels decrease with progression through puberty (20). Given these pubertal alterations in ghrelin, it may not be possible to extrapolate data from adults to an adolescent population. In fact, children do not seem to have the same inhibition of total ghrelin seen in adults after a standardized light breakfast (21) or a mixed meal (22).
Data in children are also limited regarding PYY. PYY is secreted by the colonic mucosa (23–25) and increases postprandially (26,27). PYY inhibits hypothalamic neurons activated by ghrelin (28), and PYY administration decreases food intake in humans (8). It has been proposed that PYY deficiency may be involved in the pathogenesis of obesity through decreased anorexigenic signals (3,8). PYY is present as PYY1–36 and PYY3–36 and the latter is biologically active. One study in obese adolescents demonstrated higher total PYY following a high-fat diet compared with a high-carbohydrate diet (29). However, active PYY was not examined, and the study population was not compared to controls. It is therefore unknown whether effects of specific intake of carbohydrates, fat, or protein on anorexigenic or orexigenic hormones differ in obese vs. normal-weight children and whether such differences relate to satiety and food intake.
We investigated postprandial responses of active ghrelin and PYY3–36 to high-fat, high-protein, and high-carbohydrate intake at breakfast in obese and normal-weight adolescents. We hypothesized that changes in active ghrelin and PYY3–36 following specific macronutrient intake at breakfast would predict hunger scores and subsequent food intake at a standardized lunch.
Methods and Procedures
Subject selection
Twenty-six adolescent girls (13 obese and 13 normal weight), 12–18 years old, matched for bone age and race were enrolled. All obese girls had BMIs > 95th percentile for age, and normal-weight girls had BMIs between 15th and 85th percentiles. Exclusion criteria were pregnancy, smoking, use of medications affecting appetite, present or past history of eating disorders, bariatric surgery, and significant weight changes (>2 kg) within three months of the study. Among obese and normal-weight girls, 10 each were white, two African American, and one of mixed racial background. The study was restricted to girls because girls are at higher risk for obesity than boys and have greater fat accumulation during adolescence (2). Subjects were recruited through mass mailings to providers and advertisements within the Partners HealthCare network. Applicable institutional and governmental regulations concerning ethical use of human volunteers were followed. Our institutional review board approved the study, and informed assent and consent were obtained from all.
Anthropometric measurements
Subjects were weighed to the nearest 0.1 kg wearing a hospital gown on an electronic scale. Waist–hip ratio was determined (waist measurements at umbilicus; maximum hip circumference). Subcutaneous adipose tissue (SAT) and visceral adipose tissue were assessed using magnetic resonance imaging at the lumbar 4–5 level. We used a single stadiometer at the General Clinical Research Center to measure height to the nearest 0.1 cm using an average of three measurements. Bone age was assessed using methods of Greulich and Pyle by a single pediatric endocrinologist.
Experimental protocol
The screening visit included a history, physical examination, and screening labs. A normal TSH, fasting glucose <126 mg/dl, 2-h post glucose value of <200 mg/dl (following an oral glucose-tolerance test), and hematocrit >30% were required for study participation. Indirect calorimetry was performed prior to macronutrient specific study visits to determine resting energy expenditure. We calculated insulin area under the curve (AUC) and homeostasis model assessment of insulin resistance from the 2-h oral glucose-tolerance test.
Eligible subjects were randomized to the order in which they would receive the high-fat, high-carbohydrate, or high-protein breakfast. Subjects returned for three separate visits when they received these meals, with 1–4 weeks between visits. On the day of the visit, subjects arrived at the General Clinical Research Center having fasted since midnight. Frequent sampling was started after the breakfast tray was brought to the subject. Subjects were required to finish the test breakfast within 30 min.
If subjects could not finish the breakfast, they were asked to consume equal amounts of different components of the breakfast as far as possible (see “Test meals”). They were also provided a standard lunch of macaroni and cheese 4 h after breakfast. Subjects were given the following instructions for lunch: “Please eat as much or as little as you want. If you would like more, please ask.” Plates were weighed before and after subjects completed breakfast and lunch. Frequent sampling for active ghrelin and PYY3–36 was performed 15 min before the breakfast test meal, at the start of the test meal, and every 20 min subsequently ending 1 h after lunch (total of 5 h, 15 min). With each blood sample, subjects completed a 10-cm validated visual analog scale (VAS) questionnaire (30,31).
Test meals
The quantity of test breakfast was based on metabolic needs, calculated as 130% of calories of the resting energy expenditure determined during the baseline visit using indirect calorimetry (32,33). Caloric content of the breakfast test meal was based on 25–30% of metabolic needs, estimated to be the typical caloric content for breakfast. The composition of the test meal was 60–65% fat, protein, or carbohydrate with the remaining 35–40% split between the other two macronutrients. The high-fat test meal consisted of orange juice, turkey sausage, and pancakes (made with four, baking soda, ProMod protein supplement powder, salt, eggs, heavy cream, butter, and water) served with sugar-free pancake syrup. The high-protein test meal consisted of milk mixed with Carnation Instant Breakfast and ProMod protein supplement powder, scrambled eggs (whole egg, egg white, ProMod protein supplement powder, and mozzarella cheese), and vegetarian soy sausage. The high-carbohydrate meal consisted of orange juice, Honey Nut Cheerios and milk, and whole-wheat toast served with peanut butter. We chose to administer such meals rather than a beverage to approximate real-life situations more closely, and food items were selected to reflect foods acceptable to an adolescent group.
Biochemical assessment
Samples were assayed in duplicate using the same assay. We used radio-immune assay to measure active ghrelin (Linco, St Louis, MO; intraassay coefficient of variation 7.4%, sensitivity 7.8 pg/ml), PYY3–36 (Phoenix Pharmaceuticals, Belmont, CA; intraassay coefficient of variation 4.5%, sensitivity 5 pg/ml), and insulin (Diagnostic Products, Los Angeles, CA; coefficient of variation 3.1–9.3%, sensitivity 1.2 µIU/ml).
Statistical methods
Data are described as mean ± s.d. A P value of <0.05 was used to denote significance. AUC was calculated using the trapezoidal rule using NCSS sofware (NCSS, Kaysville, UT). We used the Student's t-test to compare differences in means for two groups. The Wilcoxon rank-sum test was used when data were not normally distributed. Repeated measures analyses were used to compare changes in active ghrelin and PYY3–36 from baseline levels over time in the two groups following different macronutrient breakfasts. Our premise was that percent change (%Δ) from baseline would reflect acute changes in active ghrelin and PYY3–36 with specific macronutrient administration and better indicate macronutrient-induced effects, whereas absolute levels would reflect a chronic adaptive state to the underlying obesity or normal weight. We therefore primarily report AUC for %Δ ghrelin and PYY3–36 in this study. Pearson's correlations were conducted to determine associations between variables (Spearman's correlation was used when data were not normally distributed). We used regression modeling to control for caloric intake and entered diagnostic group (obese or normal weight) and caloric intake into the model, with %Δ ghrelin and PYY3–36 as the dependent variable. We used a P value of 0.11 to enter and 0.10 to leave the model.
Results
Clinical characteristics
The groups did not differ for maturity as assessed by bone age and Tanner stage (Table 1). As a consequence, and given known effects of obesity on maturity, obese girls were somewhat younger than normal-weight girls. BMI ranged from 25.4 to 49.0 kg/m2 in obese girls and 18.2 to 25.3 kg/m2 in controls. No significant differences in BMI occurred during the course of the study.
Table 1. Clinical characteristics of study groups.
| Normal-weight girls (n = 13) | Obese girls (n = 13) | P | |
|---|---|---|---|
| Age (years) | 15.9 ± 1.9 | 14.3 ± 1.9 | 0.03 |
| Bone age (years) | 15.8 ± 2.0 | 15.4 ± 1.7 | ns |
| Tanner stage (breasts) | 4.5 ± 1.1 | 4.5 ± 0.9 | ns |
| Tanner stage (pubic hair) | 4.2 ± 1.1 | 4.1 ± 1.0 | ns |
| Weight (kg) | 57.1 ± 7.8 | 87.0 ± 22.6 | 0.0001 |
| BMI (kg/m2) | 21.8 ± 2.0 | 34.4 ± 7.7 | <0.001 |
| BMI-SDS | 0.1 ± 0.4 | 3.5 ± 1.5 | <0.0001 |
| Waist–hip ratio | 0.80 ± 0.05 | 0.87 ± 0.08 | 0.005 |
| Subcutaneous adipose tissue (cm2) | 146 ± 53 | 439 ± 189 | <0.0001 |
| Visceral adipose tissue (cm2) | 19.7 ± 7.7 | 43.6 ± 17.7 | 0.0002 |
| nsulin AUC (OGTT) (µIU/ml·120 min) | 4,453 ± 1,323 | 9,682 ± 5,079 | 0.003 |
| HOMA-IR | 1.1 ± 0.8 | 2.1 ± 1.2 | 0.02 |
| Fasting ghrelin (pg/ml) | 55.5 ± 18.1 | 39.7 ± 17.7 | 0.04* |
| Fasting PYY3–36 (pg/ml) | 89.4 ± 43.3 | 79.2 ± 29.8 | ns |
| Resting energy expenditure (kcal) | 1,374 ± 178 | 1,744 ± 164 | 0.002 |
Mean ± s.d.
AUC, area under the curve; HOMA-IR, homeostasis model assessment of insulin resistance; OGTT, oral glucose-tolerance test; SDS, standard deviation score.
Wilcoxon rank-sum test.
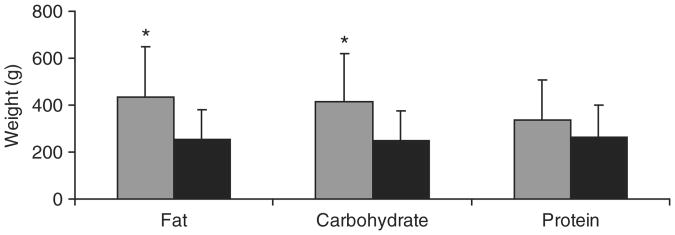
Table 2 indicates the actual proportion of macronutrients consumed at the different breakfasts by subjects and demonstrates that we were able to achieve the desired macronutrient composition in our subjects. Absolute caloric intake at breakfast (estimated to be 25–30% of daily caloric needs (130% of resting energy expenditure)) was higher in obese vs. normal-weight subjects for each macronutrient breakfast, per study design. Within each group, caloric intake at the high- carbohydrate and high-fat breakfasts did not differ; however, caloric intake at the high- protein breakfast was lower than expected, particularly within the normal-weight group due to lower food consumption. Because the caloric intake for the high-protein breakfast was lower than expected, we controlled for caloric intake while comparing ghrelin, PYY, and subsequent food intake between the groups.
Table 2. Actual macronutrient intake for the different kinds of breakfast.
| Breakfast | Normal-weight girls (n = 13) | Obese girls (n = 13) | P |
|---|---|---|---|
| High-carbohydrate breakfast | |||
| Total caloric intake (cal) | 440 ± 89 | 543 ± 129 | 0.03 |
| Difference between administered and consumed calories | 78.6 ± 85.8 | 67.5 ± 95.9 | ns |
| Carbohydrates (% total calories) | 65.8 ± 2.3 | 66.2 ± 2.5 | ns |
| Proteins(% total calories) | 16.9 ± 1.5 | 15.6 ± 1.7 | ns |
| Fats (% total calories) | 17.4 ± 1.8 | 18.2 ± 3.3 | ns |
| High-protein breakfast | |||
| Total caloric intake (cal) | 269 ± 123 | 403 ± 155 | 0.03 |
| Difference between administered and consumed calories | 302.1 ± 137.0 | 260.9 ± 174.8 | ns |
| Carbohydrates (% total calories) | 15.4 ± 2.5 | 16.1 ± 1.5 | ns |
| Proteins (% total calories) | 64.4 ± 6.9 | 64.7 ± 6.9 | ns |
| Fats (% total calories) | 20.2 ± 6.9 | 19.2 ± 7.5 | ns |
| High-fat breakfast | |||
| Total caloric intake (cal) | 399 ± 88 | 581 ± 141 | 0.0009 |
| Difference between administered and consumed calories | 32.9 ± 119.4 | 123.9 ± 162.2 | ns |
| Carbohydrates (% total calories) | 18.8 ± 2.6 | 17.0 ± 1.3 | ns |
| Proteins (% total calories) | 17.3 ± 1.2 | 17.0 ± 1.5 | ns |
| Fats (% total calories) | 64.0 ± 2.4 | 66.1 ± 2.4 | ns |
Mean ± s.d.
Active ghrelin
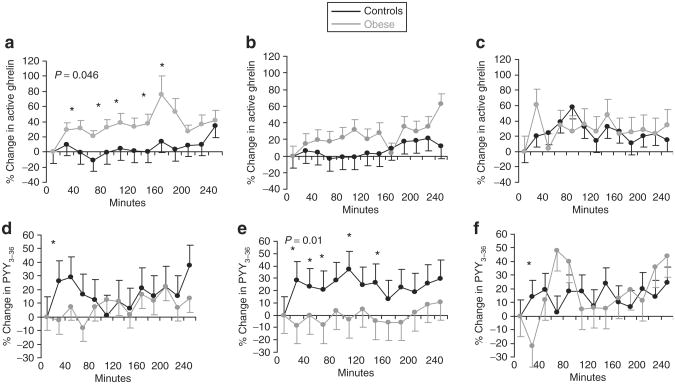
Interestingly, ghrelin levels did not suppress following food intake in our subjects. Instead, following the high- carbohydrate breakfast (but not the high-fat or high-protein breakfast), there was an increase in ghrelin from baseline in obese girls, and the percent change (%Δ) was higher in obese compared with normal-weight girls (Figure 1). Similarly, on repeated measures analysis, %Δ ghrelin was overall higher over 4 h after the high-carbohydrate breakfast in obese compared with normal-weight girls (P = 0.046), as was AUC for %Δ ghrelin from baseline (Table 3 ). This difference remained significant after controlling for caloric intake (13.4% of the variability). For the group as a whole, AUC for %Δ ghrelin correlated inversely with basal ghrelin following the high-protein (r = −0.44, P = 0.03), high-carbohydrate (r = −0.66, P = 0.005), and high-fat breakfasts (r = −0.41, P = 0.04).
Figure 1.
Percent change in active ghrelin and PYY3–36 from baseline in obese (gray line) and normal-weight (black line) girls following the various breakfasts. (a–c) Percent change from baseline in active ghrelin in obese (gray line) vs. normal-weight girls (black line) after (a) high-carbohydrate, (b) high-fat, and (c) high-protein breakfasts. Compared to normal-weight girls, obese girls had greater increases in ghrelin at various time points after the high-carbohydrate breakfast (P = 0.046 for the sampling period using repeated measures analysis), but not other breakfasts. (d–f) Percent change from baseline in PYY3–36 in obese (gray line) vs. normal-weight girls (black line) after (d) high-carbohydrate, (e) high-fat, and (f) high-protein breakfasts. Compared to normal-weight girls, obese girls had lesser increases in PYY3–36 at various time points after the high-fat breakfast (P = 0.02 for the sampling period using repeated measures analysis), but not other breakfasts. *P < 0.05 for differences between obese and normal-weight girls. PYY, peptide YY.
Table 3. AUC for percent change in active ghrelin and PYY following high-carbohydrate and high-fat breakfasts.
| Normal-weight girls (n = 13) | Obese girls (n = 13) | P | |
|---|---|---|---|
| AUC for % change in ghrelin | |||
| High-carbohydrate breakfast | 1,104 ± 5,958 | 8,716 ± 10,513 | 0.04 |
| High-fat breakfast | 1,609 ± 6,703 | 5,708 ± 13,178 | ns |
| AUC for % change in PYY3–36 | |||
| High-carbohydrate breakfast | 3,894 ± 9,250 | 1,853 ± 7,040 | ns |
| High-fat breakfast | 5,624 ± 7,523 | -273 ± 35,347 | 0.02 |
Mean ± s.d.
AUC, area under the curve; PYY, peptide YY.
Fasting ghrelin was lower in obese girls compared with controls (Table 1). Similarly, absolute ghrelin AUC was lower for obese than normal-weight girls for the high-carbohydrate (14,035 ± 3,883 vs. 19,501 ± 6,368 pg/ml·240 min, P = 0.03) and high-fat breakfasts (15,028 ± 5,222 vs. 20,761 ± 7,793 pg/ml·240 min, P = 0.04), but not for the high-protein breakfast (23,093 ± 7,949 vs. 20,126 ± 6,763 pg/ml·240 min, P = 0.36). Absolute ghrelin AUC following the high-carbohydrate breakfast correlated inversely with BMI and SAT (r ≤ −0.43, P ≤ 0.04) for the entire group and with SAT and visceral adipose tissue (r ≤ −0.61, P < 0.05) for obese girls. For the entire group, there was no association of insulin AUC or homeostasis model assessment of insulin resistance with absolute ghrelin AUC or %Δ ghrelin AUC following any breakfast. Within obese girls, there was an inverse association of insulin AUC with %Δ ghrelin AUC following the high-carbohydrate breakfast (r = −0.60, P = 0.04) only.
PYY3–36
Percent change (%Δ) in PYY3–36 was lower in obese than normal-weight girls following the high-fat breakfast, but not after the high-carbohydrate or high-protein breakfast (Figure 1). On repeated measures analysis, %Δ PYY3–36 was overall lower over 4 h after the high-fat breakfast in obese compared with normal-weight girls (P = 0.01), as was AUC for %Δ PYY3–36 from baseline (Table 3). This difference remained significant after controlling for caloric intake (20.3% of the variability). %Δ PYY3–36 AUC correlated inversely with basal PYY3–36 following the high-protein (r = −0.55, P = 0.005) and high-carbohydrate (r = −0.60, P = 0.001) breakfasts.
Fasting PYY3–36 and AUC for absolute PYY3–36 did not differ between obese and control girls following the different macronutrient breakfasts and did not correlate with insulin AUC (oral glucose-tolerance test) or homeostasis model assessment of insulin resistance (data not shown). However, insulin AUC correlated inversely with %Δ PYY3–36 following the high-carbohydrate and high-fat breakfasts for the group as a whole (r = −0.43 and −0.49, P = 0.04 and 0.02), following the high- carbohydrate breakfast within obese girls (r = −0.70, P = 0.008), and following the high-fat breakfast for controls (r = −0.66, P = 0.01). For the group as a whole, %Δ PYY3–36 following the high-carbohydrate breakfast correlated inversely with BMI, SAT, and visceral adipose tissue (r ≤ −0.42, P ≤ 0.03). In obese girls, %Δ PYY following the high-fat breakfast correlated inversely with BMI and SAT (r ≤ −0.71, P ≤ 0.01).
VAS
The VAS asked the following questions: “How much can you eat?” “How full are you?” “How hungry do you feel?” “How satisfied are you?” “Would you like to eat something sweet?” “Would you like to eat something salty?” “Would you like to eat something fatty?” and “Would you like to eat something savory?” Overall, obese girls expressed that they could eat more, were less satisfied and less full following the three kinds of breakfast; however, differences were not statistically significant. We developed a composite VAS score for the question “How hungry do you feel?” by adding values from all time points in response to this question following the specific breakfasts. There were strong positive associations between composite VAS hunger scores following the different kinds of breakfast within obese girls (r = 0.91, P = 0.0002 for high-carbohydrate vs. high-fat breakfasts, r = 0.77, P = 0.04 following the high-carbohydrate vs. high-protein breakfasts, and r = 0.97, P = 0.0003 following the high-protein vs. high-fat breakfasts). In normal-weight controls, associations were similar but less robust. Therefore, girls who felt hungrier following a particular kind of meal, felt similarly hungry regardless of the macronutrient content of the meal.
Food intake at lunch
Compared with normal-weight girls, obese girls consumed more food at lunch, and this difference was significant after the high-fat (70% greater intake) and high-carbohydrate (67% greater intake) breakfasts, but not high-protein breakfast (Figure 2). Of note, these changes were no longer significant after controlling for resting energy expenditure. Similar to the composite VAS score, there were positive associations between lunch intake of food following the different kinds of breakfast within obese girls (r = 0.62, P = 0.03 following high-fat vs. high-carbohydrate breakfasts; r = 0.81, P = 0.003 following high-protein vs. high-carbohydrate breakfasts; and r = 0.58, P = 0.06 following high-protein vs. high-fat breakfasts). Similar, but less strong, associations were observed within normal-weight girls. Therefore, girls likely to eat more at lunch did so regardless of macronutrient content of the preceding meal.
Figure 2.
Food intake at lunch following the high-fat, high-carbohydrate, and high-protein breakfasts in obese (gray bars) and normal-weight (black bars) girls. Lunch intake was higher in obese compared with normal-weight girls following the high-fat and high-carbohydrate breakfasts but not the high-protein breakfast. *P < 0.05 for differences between obese and normal-weight girls.
Discussion
This is the first study to comprehensively examine active ghrelin and PYY3–36 responses to all three macronutrient dense meals in a randomized controlled study in adolescent obese and normal-weight girls. In contrast to adults, ghrelin did not suppress following meal intake in our subjects. We demonstrate that ghrelin and PYY3–36 responses differ in obese vs. normal-weight adolescents and importantly that carbohydrate and fat, but not protein intake, are key regulators of these responses. Specifically, obese girls had a greater percent increase in active ghrelin following the high-carbohydrate breakfast than normal-weight girls and a lower percent increase in PYY3–36 following the high-fat breakfast. Consistent with these findings, obese girls consumed more food at lunch than normal-weight girls following the high-fat and high-carbohydrate breakfasts, but not the high-protein breakfast.
Studies have reported lower total (34,35) and acylated ghrelin (36) in the fasting state in obese compared with lean children, and lower total ghrelin AUC in obese adults after a mixed meal buffet (37) and after oral glucose in obese children (38,39). Consistent with this, fasting active ghrelin and absolute ghrelin AUC were lower in obese girls compared with controls following the high-carbohydrate and high-fat breakfasts, likely an adaptive response to a state of overnutrition. In contrast to the suppression of ghrelin following food intake reported in adults (37), but consistent with data following a mixed meal in prepubertal children (21,22), we found no attenuation of ghrelin following macronutrient specific meals.
Instead, we observed increases in active ghrelin following the high-carbohydrate breakfast in obese girls (significantly different from normal-weight girls), and commensurate with this potentially orexigenic stimulus, food intake at lunch following the high-carbohydrate breakfast was higher in obese compared with normal-weight girls. These data are in contrast to those from a study in 16 healthy adults that reported marked suppression of active ghrelin following a high-protein meal, followed by high-carbohydrate and high-fat meals (40). However, 80% of the calories (administered as a beverage) in this study in adults were derived from the macronutrient of interest, with only 10% derived from the other two classes. Similar data were reported with total ghrelin from another study in 14 healthy adults, in which 77% and 75% of total calories were derived from carbohydrates or fat respectively (12). In our study in adolescents, only 65% of calories administered at breakfast (as regular food items rather than a beverage) were from the macronutrient of interest, therefore, a more physiological approximation of real-life situations than other studies. This more physiological administration of specific macronutrients in adolescents may partly account for differences in postprandial ghrelin secretion compared with adults.
In addition, studies in adults suggest that an adequate increment of insulin following food intake and sensitivity to insulin are necessary for a commensurate decrease in ghrelin (18,19). Erdmann et al. (41) reported greater increases in insulin in adults following a carbohydrate-dense meal compared to a protein-dense meal, and suppression of ghrelin following the former, but not the latter. It is possible that a threshold level of insulin secretion necessary for postprandial ghrelin suppression is attained with carbohydrate-dense but not protein-dense meals. An inverse association between insulin resistance and fasting ghrelin (17) has been reported in children, although data regarding postprandial ghrelin are lacking. Importantly, pubertal children are relatively insulin resistant, which may explain why ghrelin did not suppress following food intake in our normal-weight adolescents, and why obese adolescents, who are even more insulin resistant, actually had an increase in postprandial ghrelin. Although we did not assess insulin levels postprandially, insulin AUC during an oral glucose-tolerance test correlated inversely with %Δ ghrelin AUC following the high-carbohydrate breakfast, but not with absolute ghrelin AUC. Despite this association, ghrelin did not suppress following the high-carbohydrate breakfast, and %Δ ghrelin was actually higher in obese girls than controls. It is possible that children are overall refractory to inhibitory effects of insulin on postprandial ghrelin secretion while effects on fasting ghrelin (17) are maintained. Studies are necessary with insulin measured concurrently with ghrelin to better understand the mechanism underlying lack of ghrelin suppression with food intake in children.
Studies have reported lower total PYY AUC in obese adults compared to controls after a buffet meal (8) and following a high-carbohydrate vs. high-fat diet (29), and a blunted total PYY response with a mixed meal in obese adolescents (27). However, the three macronutrients have not been compared against each other in children, and our findings are particularly of interest in that the change in PYY3–36 levels was lower in obese than normal-weight girls following the high-fat breakfast, and consistent with the reduced anorexigenic stimulus, subsequent food intake at lunch was higher in obese girls. Consistent with other reports in children (27), fasting PYY3–36 levels did not differ between the groups. In addition, absolute values of PYY3–36 AUC did not differ between groups for the various breakfasts, and we found no associations of insulin AUC with absolute PYY3–36 AUC. Interestingly, however, insulin AUC correlated inversely with %Δ PYY3–36 following the high-carbohydrate and high-fat breakfasts, and this association merits further investigation.
These data suggest that the change in active ghrelin and PYY3–36 after a meal may better predict subsequent food intake than absolute levels of these hormones, whereas absolute levels indicate a set point that is body weight dependent. This is further supported in our subjects by (i) inverse correlations of BMI and fat measures with ghrelin AUC, similar associations reported by other investigators (39,42–44), and (ii) inverse associations of basal ghrelin and PYY3–36 with subsequent changes in these hormones over time. Of note, within subjects, there was a strong correlation between the composite VAS hunger score for the different kinds of breakfasts, and a similar strong correlation between food intake at lunch following the differ-ent breakfasts. These data suggest that cues regulating hunger and food intake are consistent within individuals, regardless of macronutrient content of the preceding meal. Interestingly, VA S scores did not correlate with ghrelin or PYY3–36, or with changes in these peptides over time.
A study limitation is that visits occurred within 1–4 weeks of each other and girls were at different stages of their menstrual cycles for the visits, which may have impacted results. In addition, subjects consumed less than the expected amount of calories for the high-protein breakfast, particularly in the normal-weight group, which may explain the lack of difference between groups for ghrelin, PYY3–36, and subsequent food intake following this breakfast. However, even after controlling for caloric intake, no differences were observed between groups for the high-protein breakfast. Calories consumed following the high-carbohydrate and high-fat breakfasts did not differ within groups. Further studies are necessary to better elucidate associations of food intake with these hormones. In addition, regulation of food intake is a complex mechanism, and other neuroendocrine and gastrointestinal hormones are likely also involved in this process, which will be important to investigate in future studies.
In summary, our study demonstrates (i) increased food intake at lunch following a high-carbohydrate or high-fat breakfast in obese girls compared with controls and (ii) differences in the patterns of active ghrelin and PYY3–36 secretion in obese and normal-weight adolescents following specific macronutrient intake. Unlike adults, specific macronutrient intake is not associated with the suppression of ghrelin levels. It remains to be determined whether these alterations are causative for increased food intake in obese children, whether these are adaptive, or whether there are innate hitherto unknown factors that govern appetite and lead to fairly consistent food intake regardless of these hormonal alterations. Greater increases in ghrelin and lesser decreases in PYY3–36 following a high-carbohydrate or a high-fat breakfast respectively in obese adolescents suggest the need for further studies to determine whether meals that are protein enriched should be recommended for obese adolescents.
Acknowledgments
We thank the Bionutrition Core of the Clinical Research Center of Massachusetts General Hospital for its help with the protocol and for preparing the test meals. This work was supported in part by NIH grants M01-RR-01066, F32-DK072816, K23 RR018851, DK46200, and 5P30DK4620-15.
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Hedley AA, Ogden CL, Johnson CL, et al. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 2.Dietz WH. Overweight in childhood and adolescence. N Engl J Med. 2004;350:855–857. doi: 10.1056/NEJMp048008. [DOI] [PubMed] [Google Scholar]
- 3.Batterham RL, Cowley MA, Small CJ, et al. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 4.Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 5.Wren AM, Seal LJ, Cohen MA, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 6.Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 7.Tschöp M, Weyer C, Tataranni PA, et al. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50:707–709. doi: 10.2337/diabetes.50.4.707. [DOI] [PubMed] [Google Scholar]
- 8.Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3–36. N Engl J Med. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 9.Tschöp M, Wawarta R, Riepl RL, et al. Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest. 2001;24:RC19–RC21. doi: 10.1007/BF03351037. [DOI] [PubMed] [Google Scholar]
- 10.Erdmann J, Lippl F, Schusdziarra V. Differential effect of protein and fat on plasma ghrelin levels in man. Regul Pept. 2003;116:101–107. doi: 10.1016/s0167-0115(03)00195-2. [DOI] [PubMed] [Google Scholar]
- 11.Erdmann J, Töpsch R, Lippl F, Gussmann P, Schusdziarra V. Postprandial response of plasma ghrelin levels to various test meals in relation to food intake, plasma insulin, and glucose. J Clin Endocrinol Metab. 2004;89:3048–3054. doi: 10.1210/jc.2003-031610. [DOI] [PubMed] [Google Scholar]
- 12.Monteleone P, Bencivenga R, Longobardi N, Serritella C, Maj M. Differential responses of circulating ghrelin to high-fat or high-carbohydrate meal in healthy women. J Clin Endocrinol Metab. 2003;88:5510–5514. doi: 10.1210/jc.2003-030797. [DOI] [PubMed] [Google Scholar]
- 13.Date Y, Kojima M, Hosoda H, et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- 14.Dornonville de la Cour C, Björkqvist M, Sandvik AK, et al. A-like cells in the rat stomach contain ghrelin and do not operate under gastrin control. Regul Pept. 2001;99:141–150. doi: 10.1016/s0167-0115(01)00243-9. [DOI] [PubMed] [Google Scholar]
- 15.Kojima M, Hosoda H, Date Y, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 16.Cummings DE, Purnell JQ, Frayo RS, et al. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 17.Bacha F, Arslanian SA. Ghrelin suppression in overweight children: a manifestation of insulin resistance? J Clin Endocrinol Metab. 2005;90:2725–2730. doi: 10.1210/jc.2004-1582. [DOI] [PubMed] [Google Scholar]
- 18.Flanagan DE, Evans ML, Monsod TP, et al. The influence of insulin on circulating ghrelin. Am J Physiol Endocrinol Metab. 2003;284:E313–E316. doi: 10.1152/ajpendo.00569.2001. [DOI] [PubMed] [Google Scholar]
- 19.Saad MF, Bernaba B, Hwu CM, et al. Insulin regulates plasma ghrelin concentration. J Clin Endocrinol Metab. 2002;87:3997–4000. doi: 10.1210/jcem.87.8.8879. [DOI] [PubMed] [Google Scholar]
- 20.Whatmore AJ, Hall CM, Jones J, Westwood M, Clayton PE. Ghrelin concentrations in healthy children and adolescents. Clin Endocrinol (Oxf) 2003;59:649–654. doi: 10.1046/j.1365-2265.2003.01903.x. [DOI] [PubMed] [Google Scholar]
- 21.Bellone S, Castellino N, Broglio F, et al. Ghrelin secretion in childhood is refractory to the inhibitory effect of feeding. J Clin Endocrinol Metab. 2004;89:1662–1665. doi: 10.1210/jc.2003-031207. [DOI] [PubMed] [Google Scholar]
- 22.Lomenick JP, Clasey JL, Anderson JW. Meal-related changes in ghrelin, peptide YY, and appetite in normal weight and overweight children. Obesity (Silver Spring) 2008;16:547–552. doi: 10.1038/oby.2007.129. [DOI] [PubMed] [Google Scholar]
- 23.Böttcher G, Sjölund K, Ekblad E, et al. Coexistence of peptide YY and glicentin immunoreactivity in endocrine cells of the gut. Regul Pept. 1984;8:261–266. doi: 10.1016/0167-0115(84)90034-x. [DOI] [PubMed] [Google Scholar]
- 24.Lundberg JM, Tatemoto K, Terenius L, et al. Localization of peptide YY (PYY) in gastrointestinal endocrine cells and effects on intestinal blood flow and motility. Proc Natl Acad Sci U S A. 1982;79:4471–4475. doi: 10.1073/pnas.79.14.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nilsson O, Bilchik AJ, Goldenring JR, et al. Distribution and immunocytochemical colocalization of peptide YY and enteroglucagon in endocrine cells of the rabbit colon. Endocrinology. 1991;129:139–148. doi: 10.1210/endo-129-1-139. [DOI] [PubMed] [Google Scholar]
- 26.Adrian TE, Ferri GL, Bacarese-Hamilton AJ, et al. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. 1985;89:1070–1077. doi: 10.1016/0016-5085(85)90211-2. [DOI] [PubMed] [Google Scholar]
- 27.Stock S, Leichner P, Wong AC, et al. Ghrelin, peptide YY, glucose-dependent insulinotropic polypeptide, and hunger responses to a mixed meal in anorexic, obese, and control female adolescents. J Clin Endocrinol Metab. 2005;90:2161–2168. doi: 10.1210/jc.2004-1251. [DOI] [PubMed] [Google Scholar]
- 28.Riediger T, Bothe C, Becskei C, Lutz TA. Peptide YY directly inhibits ghrelin-activated neurons of the arcuate nucleus and reverses fasting-induced c-Fos expression. Neuroendocrinology. 2004;79:317–326. doi: 10.1159/000079842. [DOI] [PubMed] [Google Scholar]
- 29.Essah PA, Levy JR, Sistrun SN, Kelly SM, Nestler JE. Effect of macronutrient composition on postprandial peptide YY levels. J Clin Endocrinol Metab. 2007;92:4052–4055. doi: 10.1210/jc.2006-2273. [DOI] [PubMed] [Google Scholar]
- 30.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 31.Parker BA, Sturm K, MacIntosh CG, et al. Relation between food intake and visual analogue scale ratings of appetite and other sensations in healthy older and young subjects. Eur J Clin Nutr. 2004;58:212–218. doi: 10.1038/sj.ejcn.1601768. [DOI] [PubMed] [Google Scholar]
- 32.Ritz R, Cunningham J. Indirect calorimetry. In: Kacmarek RM, Hess D, Stoller JK, editors. Monitoring in Respiratory Care. Mosby-Yearbook; St. Louis, MO: 1993. pp. 407–441. [Google Scholar]
- 33.Segal KR. Comparison of indirect calorimetric measurements of resting energy expenditure with a ventilated hood, face mask, and mouthpiece. Am J Clin Nutr. 1987;45:1420–1423. doi: 10.1093/ajcn/45.6.1420. [DOI] [PubMed] [Google Scholar]
- 34.Reinehr T, de Sousa G, Roth CL. Obestatin and ghrelin levels in obese children and adolescents before and after reduction of overweight. Clin Endocrinol (Oxf) 2008;68:304–310. doi: 10.1111/j.1365-2265.2007.03042.x. [DOI] [PubMed] [Google Scholar]
- 35.Zou CC, Liang L, Wang CL, Fu JF, Zhao ZY. The change in ghrelin and obestatin levels in obese children after weight reduction. Acta Paediatr. 2009;98:159–165. doi: 10.1111/j.1651-2227.2008.00997.x. [DOI] [PubMed] [Google Scholar]
- 36.Lányi E, Csernus K, Erhardt E, et al. Plasma levels of acylated ghrelin during an oral glucose tolerance test in obese children. J Endocrinol Invest. 2007;30:133–137. doi: 10.1007/BF03347411. [DOI] [PubMed] [Google Scholar]
- 37.le Roux CW, Patterson M, Vincent RP, et al. Postprandial plasma ghrelin is suppressed proportional to meal calorie content in normal-weight but not obese subjects. J Clin Endocrinol Metab. 2005;90:1068–1071. doi: 10.1210/jc.2004-1216. [DOI] [PubMed] [Google Scholar]
- 38.Baldelli R, Bellone S, Castellino N, et al. Oral glucose load inhibits circulating ghrelin levels to the same extent in normal and obese children. Clin Endocrinol (Oxf) 2006;64:255–259. doi: 10.1111/j.1365-2265.2006.02441.x. [DOI] [PubMed] [Google Scholar]
- 39.Soriano-Guillén L, Barrios V, Martos G, et al. Effect of oral glucose administration on ghrelin levels in obese children. Eur J Endocrinol. 2004;151:119–121. doi: 10.1530/eje.0.1510119. [DOI] [PubMed] [Google Scholar]
- 40.Foster-Schubert KE, Overduin J, Prudom CE, et al. Acyl and total ghrelin are suppressed strongly by ingested proteins, weakly by lipids, and biphasically by carbohydrates. J Clin Endocrinol Metab. 2008;93:1971–1979. doi: 10.1210/jc.2007-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erdmann J, Leibl M, Wagenpfeil S, Lippl F, Schusdziarra V. Ghrelin response to protein and carbohydrate meals in relation to food intake and glycerol levels in obese subjects. Regul Pept. 2006;135:23–29. doi: 10.1016/j.regpep.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 42.Misra M, Miller KK, Kuo K, et al. Secretory dynamics of ghrelin in adolescent girls with anorexia nervosa and healthy adolescents. Am J Physiol Endocrinol Metab. 2005;289:E347–E356. doi: 10.1152/ajpendo.00615.2004. [DOI] [PubMed] [Google Scholar]
- 43.Soriano-Guillén L, Barrios V, Campos-Barros A, Argente J. Ghrelin levels in obesity and anorexia nervosa: effect of weight reduction or recuperation. J Pediatr. 2004;144:36–42. doi: 10.1016/j.jpeds.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 44.Misra M, Miller KK, Tsai P, et al. Elevated peptide YY levels in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2006;91:1027–1033. doi: 10.1210/jc.2005-1878. [DOI] [PubMed] [Google Scholar]