Abstract
A principal role of tight junctions is to seal the apical intercellular space and limit paracellular flux of ions and molecules. Despite the fact that tight junctions form heavily cross-linked structures, functional studies have fostered the hypothesis that the tight junction barrier is dynamic and defined by opening and closing events. However, it has been impossible to directly measure tight junction barrier function with sufficient resolution to detect such events. Nevertheless, recent electrophysiological and sieving studies have provided tremendous insight into the presence of at least two pathways of trans-tight junction flux: a high-capacity ion-selective “pore” pathway and a low-capacity “leak” pathway that allows the passage of macromolecules. Furthermore, it is now known that the tight junction molecular structure is highly dynamic and that dynamics are correlated with barrier function. Taken together, these data support a dynamic model of tight junction conductance and suggest that regulation of tight junction openings and closings may provide sensitive means of barrier regulation.
Keywords: tight junction, FRAP, claudin, occluding, ZO-1
Fluid-filled structures throughout the body are lined by sheets of specialized epithelial or endothelial cells. One of the principal roles for these cells is to regulate luminal fluid composition through absorption and secretion of ions, organic molecules, and water. While transporters in the apical and basolateral cell membranes of these cells are critical to the active transport of ions against concentration gradients, solute would easily diffuse back if it were not for the paracellular barrier established by tight junctions. Now, almost 50 years after tight junctions were first identified at the apical intercellular space, we understand that the tight junction barrier is more than a simple seal of the paracellular space. Rather, tight junctions are dynamic structures with a capacity to finely regulate the paracellular passage of ions and molecules via different size- and charge-selective pathways.
Tight junction ultrastructure provides theoretical basis of tight junction “pores”
Tight junction ultrastructure, demonstrated by freeze-fracture scanning electron microscopy, provides insight into the organization of the tight junction barrier. Lipid bilayers fracture along hydrophobic planes and lateral views of the intercellular space reveal multiple anastomosing stands at the tight junction. The number of strands varies between cell type and organ, and strand number correlates roughly with barrier function.1 For example, leaky epithelia, like the proximal convoluted tubule and gallbladder, have, on average, one or two strands, but tight epithelia, such as urinary bladder, have five or more tight junction strands. A simple model of the tight junction barrier would be to consider each strand as a resistor. Since resistors sum in series, overall transepithelial electrical resistance (TER) should be directly proportional (or conductance inversely proportional) to the number of strands. However, this turns out not to be the case. Rather, TER is a logarithmic function of the number of tight junction strands.2 One possible explanation to account for such nonlinear behavior is that strands are populated by “pores” that can open and close to regulate tight junction ion conductance.2 To date, it has not been possible to measure tight junction function with high enough resolution to detect such openings and closings, primarily due to limitations in the ability to electrically isolate a single tight junction area. However, despite the lack of local tight junction recordings, multiple studies have demonstrated that alterations in the molecular composition of the tight junction may have profound effects on pore-like tight junction barrier, even though differences at the ultrastructural level are not detected. One large family of tight junction proteins, the claudins, has been hypothesized to provide the structural basis of the paracellular pore.
Members of the claudin family are a major component of tight junction strands, and, when expressed in fibroblasts lacking tight junctions, claudins can direct formation of tight junction strand-like structures between adjacent cells.3 These interactions have been proposed to occur through homotypical and heterotypical interactions of claudin extracellular domains, and are believed to form the basis of small ion permeability. For example, overexpression of claudin-2 in high-resistance MDCK I cells, which express little claudin-2, induces an 85% decrease in overall electrical resistance, and an 8.5-fold increase in Na+ permeability.4 Similarly, there is an 82% decrease in Na+ permeability and a fourfold increase in TER in Caco-2 cells after knockdown of claudin-2.5 Furthermore, expression of other claudins, such as claudin-4 or -5, has the capacity to decrease Na+ permeability,6,7 or in the case of claudin-10a, induce anion selectivity.8 Multiple studies have demonstrated that the first extracellular loop of claudins is critical in defining the ion selectivity. For example, expressing chimeric claudins where one loop one of claudin-2 is swapped with that of claudin-4 makes claudin-2 behave like claudin-4, while swapping the same extracellular loop of claudin-4 with that of claudin-2 makes claudin-4 behave more like claudin-2. These changes occur without any alterations in tight junction strand ultrastructure.9 Since claudins from one cell interact with claudins on adjacent cells,10–12 and more than one claudin is expressed in a single cell, it is possible that different claudins can cooperate to form a broad range of pores with unique sieving properties. For example, claudin-16 and claudin-19 can interact with one another and cooperatively form sodium-selective paracellular pores in the thick ascending loop of Henle that play an important role in generating transepithelial ion gradients. These gradients are in turn essential to driving reabsorption of divalent cations Mg2+ and Ca2+ (see Ref. 13). Indeed, inherited mutations of either claudin-16 or claudin-19 result in a syndrome associated with hypomagnesemia and hypercalciuria.14 Thus, regulation of claudin expression provides a possible mechanism to finely regulate pore-like tight junction behavior. However, in addition to ions, it is well recognized that noncharged molecules with varying sizes, such as serum albumin and various sized dextrans, can pass through the tight junction. Since these molecules would be too large to pass through ion-selective pores, an additional pathway to allow larger molecules to pass must exist.
Multiple pathways of trans-tight conductance
In order to assess the differential permeability of the tight junction to small and large molecules (i.e., tight junction size selectivity), there are two commonly used techniques. The first technique assesses the transepithelial flux of series polyethylene glycol oligomers ranging in radius from 2.8 Å to 7.0 Å,15,16 and a second approach relies on electrical reversal potential measurements after apical or basolateral substitution of Na+, with different sized cations ranging in size from 1.0 Å to 3.6 Å.17 Using either method, plotting tracer permeability as a function of size, reveals a bimodal relationship between size and permeability in a variety of epithelial cells. A high-capacity size-restrictive component has a sharp radius cut-off to molecules with radii larger than about 3.5 Å and a low-capacity pathway has no apparent upper cutoff, up to at least 7.5 Å (Fig. 1A). These two pathways have been termed pore and leak pathways, respectively, and data support that the structural basis of these two pathways is distinct. Specifically, expression of claudin-2 increases only pore flux, whereas occludin and tricellulin appear to be important to leak pathway regulation (Fig. 1B).15,17–19 Furthermore, multiple studies have demonstrated that these two pathways are differentially regulated as stimuli affecting tight junction permeability to small ions and larger macromolecules do not always correlate.
Figure 1.
Size dependence of paracellular flux (solid black line) indicates the presence of two pathways. (A) A high-capacity pore pathway (dashed green line) is responsible for trans-tight junction flux of small ions and molecules, whereas a leaky pathway (dashed red line) allows paracellular flux of larger molecules (after Refs. 14, 16, and 17). (B) Claudins primarily regulate the pore pathway, while occludin appears to be important to leak pathway regulation (after Ref. 29).15,17,28
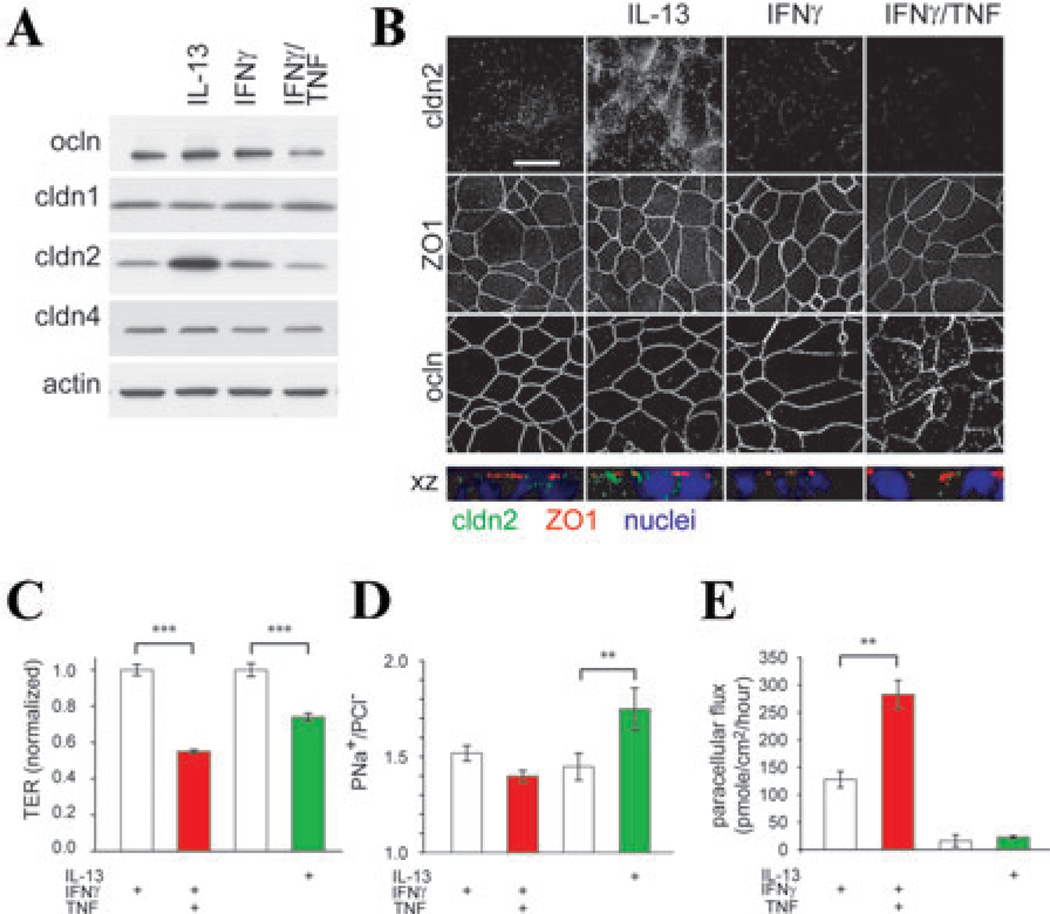
One good example of selective pore regulation is that which occurs following activation of apical sodium-glucose cotransport (SGLT1). In the presence of high apical glucose, there is reduction in TER along with an increase in the flux of the small molecule mannitol (radius = 3.6 Å), yet there is no change in the flux of larger inulin (r = 11.5Å).20 These pore and leak pathways may also be differentially regulated via pathological stimuli such as inflammatory cytokines IL-13 and TNF-α. IL-13 specifically upregulates expression of claudin-2 (Fig. 2A and B), results in a decrease in TER (Fig. 2C), and increases the relative permeability of Na+ to Cl− (Fig. 2D).21–23 However, IL-13 does not induce increased flux of 4 kDa FITC-dextran (Fig. 2E).23 In contrast, TNF-α, induces TER loss with a corresponding increase in macromolecular flux (Fig. 2C–D), representative of a leak pathway.23,24 Consistent with what is known about the molecular basis of the leak pathway, occludin internalization is associated with TNF-induced barrier dysfunction (Fig. 2A and B). Since TNF-α does not induce a change in PNa+/PCl− (Fig. 2D). TER loss corresponds with equivalent increases in tight junction permeability to both Na+ and Cl−.23 Since such noncharge-selective increases in Na+ and Cl− permeability would be expected to bring PNa+/PCl− closer to 1.0, the fact that this ratio is only minimally reduced supports that tight junction “leak” is a low-capacity pathway, contributing at most ~20% to overall ion conductance, even in the presence of TNF-α.
Figure 2.
IL-13 and TNF-α differentially regulate pore and leak pathways of tight junction flux. (A) Claudin-2 protein was increased by 308%, and occludin protein was reduced by 60% after respective treatments with IL-13 or TNF-α. (B) IL-13 specifically increased claudin-2 localization at the tight junction without affecting occludin or ZO-1. In contrast, TNF-α caused occludin internalization without affecting ZO-1 or claudin-2. Bar, 10 µm. (C) TNF-α (red; 2.5 ng/mL) and IL-13 (green; 0.1 ng/mL) reduced TER of T84 monolayers cells at 4 and 12 h, respectively. (D) IL-13 (green), but not TNF-α (red), increased PNa+/PCl− of T84 monolayers. (**P ≤ 0.01; ***P ≤ 0.001, SEM). (E) TNF-α (red), but not IL-13 (green), increased the paracellular permeability of 4 kDa dextran (from Ref. 23).
Thus, these data support the presence of distinct and differentially regulated high-capacity pore and low-capacity leak pathways across the tight junction. However, despite our knowledge of multiple distinct pathways, current assessments of barrier function reflect average measurements of epithelial barrier and do not provide insight on a local, molecular level. Nonetheless, recent studies of fluorescently tagged tight junction protein dynamics after local photobleaching (i.e., FRAP (fluorescence recovery after photobleaching) studies) reveal that global tight junction function does in fact correlate with tight junction dynamics, at a molecular level.
Tight junction dynamics correlate with tight junction function
The principle behind FRAP experiments is that the time course of fluorescence recovery, after transient photobleaching of a fluorescently tagged tight junction protein, provides insight into the stability of molecular interactions. By comparing the mobilities of different tight junction proteins, it is possible to infer interactions at a molecular level. For example, the FRAP kinetics of ZO-1, occludin, and claudin-1 are each unique, and recovery occurs from different pools of unbleached proteins. Experimental data, recapitulated in simulations (Fig. 3A–E), show that occludin exchanges within the tight junction and with non-tight junction lateral membrane pools. In contrast, claudin-1 is distinguished by a much larger immobile fraction, and ZO-1 does not diffuse as an intact tight junction complex along the plasma membrane, but rather exchanges with intracellular pools.
Figure 3.
Tight junction proteins are dynamic and undergo continuous remodeling in steady state. (A) Computer models were established based on fluorescence recovery after photobleaching of fluorescently labeled claudin-1, occludin, or ZO-1. The model reflects distinct dynamic behavior and exchange of these proteins within three cellular compartments: tight junction, lateral membrane, and cytosol. Claudin-1 is largely anchored at the tight junction, while occludin exchanges with a small lateral membrane pool (20% of total). ZO-1 is immobile at the tight junction and exchanges with intracellular pools (from Ref. 30). (B) A laser is used to transiently photobleach a region of tight junction–expressing EGFP-labeled tight junction proteins (here demonstrated for ZO-1 before and after a laser pulse at t = 0). (C–E) Kymographs demonstrate time course of fluorescence recovery in the tight junction region indicated by the dashed line in Figure 3C (simulation parameters from Ref. 31). (C) ZO-1 recovery occurs entirely from intracellular pools. (D) Occludin recovery is fast and occurs from the tight junction and lateral membrane. (E) Claudin-1 recovery is slow and occurs from a mobile pool of claudin in the adjacent area of the tight junction.31
Furthermore, the dynamics of ZO-1 exchange between the intracellular pools and the tight junction are highly dependent on interactions with the actin cytoskeleton.25 Thus, these data support that tight junctions undergo continuous remodeling at steady state, and it will be important to understand how these dynamics correlate with barrier regulation. One hypothesis is that stimuli that influence tight junction mobility will tend to stabilize the tight junction within particular conformations, that is, either in open or closed states.
An example of such dynamic tight junction regulation involves CK2 phosphorylation of occludin in its C-terminal tail, which causes the mobilities of occludin, claudin-1, claudin-2, and ZO-1 converge.5 These data, combined with complementary binding assays, support the formation of a protein complex and favors reductions in small ion permeability, consistent with pore pathway regulation. Furthermore, inhibition of phosphorylation prevents IL-13–induced barrier dysfunction, consistent with the known role of IL-13 in increasing claudin-2 pores. Thus, in addition to the role for occludin in pathologic leak pathway regulation, there exists a possible role for occludin in the regulation of the pore pathway, via modulation of claudin-2 pore formation. These data suggest that dynamic pore-like behavior relies both on claudin expression and also the stability of claudin interactions with other tight junction proteins. It is presently unclear if claudin-2 dynamics may provide the basis of tight junction pore openings and closings, as proposed by Claude,2 since it is presently not possible to measure the tight junction barrier with similar molecular resolution. Understanding tight junction pore behavior requires understanding of pore number, pore size, charge selectivity, as well as the frequency and duration of pore openings. Nevertheless, some predictions, based on macroscopic tight junction behavior, have been possible about how altering the open and closing probabilities of claudin pores would be expected to influence barrier function.
Modeling the tight junction function
In order to better understand how dynamic pore properties could potentially play an important role in barrier regulation, it will be important to first consider a simple static model of pore function. A reductionist model of pore function ignores stand-to-strand and regional variations in tight junction composition as well as potential unique properties of different claudins, and rather will focus on claudin-2. Claudin-2 is one of the best defined pore-forming claudins, and is known to regulate pore behavior in multiple cell lines in vitro and in vivo.5,17,23,26 Charge-altering amino acid substitutions and cysteine scanning mutagenesis approaches have helped to identify critical amino acids within the first extracellular loop of claudin-2 that are responsible for pore-like behavior when expressed in MDCK cells.11 Such experiments have enabled Yu et al. to model the claudin-2 selectivity filter as a cylinder with 70pS conductance.17 The simplest possible static model of the tight junction barrier is therefore one that considers each tight junction strand as a resistor populated by a fixed number of such claudin-2 pores.
In epithelia-expressing claudin-2, there would need to be approximately four 70 pS claudin pores per 100 nm of tight junction strands in order to account for measured TER values.17 Also, in order to obtain a reasonable conductance in the absence of claudin-2, a relatively small claudin-2–independent tight junction conductance of 2 pS per 100 nm will be assumed here. In a simple static model, claudin-2 conductance is inversely proportional to the number of tight junction strands, ranging from 70 pS/100 nm for four strands and increasing to 282 pS/100 nm for one strand (Fig. 4A). Furthermore, in this static model, conductance is directly proportional to the number of claudin-2 pores. However, as described earlier, when conductance is assessed across a range of epithelial with different numbers of tight junction strands, conductance is not inversely correlated with strand number, which has been an argument for the presence of tight junction pores.2 In order to assess how opening and closing of pores could potentially provide a means to regulate the tight junction barrier, the static model will be compared to a dynamic model.
Figure 4.
Static and dynamic simulations are used to predict tight junction pore barrier. (A) In a static model, each tight junction strand has a low conductance (2 pS per 100 nm) and is populated by four 70 pS claudin pores per 100 nm of tight junction.17 (B) In a dynamic model, only a percentage of claudins form conductive pores, and pores randomly flicker between open and closed states with defined probabilities. The percentage of open pores is defined by the probability of a closed claudin moving to an open state and an open claudin moving to a closed state. (C) In a dynamic model (red), conductance is higher than would be predicted for a simple inverse relationship with strand number (as is true for the static model, green), and conductance may be quite sensitive to the percentage of open claudin-2 pores.
Since the ultrastructural appearance of a tight junction strand is continuous, it is conceivable that there may be many more claudins in a strand than was predicted in the above static model. Therefore, a starting point for a dynamic model will be to assume that only 10% of claudins form conductive pores, which requires the presence of 53 claudin-2 proteins per 100 nm of tight junction strand to match the known global epithelial conductance. Also, in order to maintain this relationship in steady state, the probability of an open pore moving to a closed state is necessarily 10-fold higher than the probability of a closed pore moving to an open state. If one looks at the average conductance of a population of pores in steady-state using time as a spatially averaged simulation, the frequency and rate of transition between pore states averages out, and it is possible to determine the relationship between conductance and strand number. As suggested by Claude, this relationship no longer follows a simple inverse relationship. For a low number of strands, conductance is higher than would be predicted from an analogous static model (Fig. 4C). Intuitively, this can be explained by the fact that synchronized openings on multiple strands are more likely to occur as strand number decreases. It also follows that barrier function may be exquisitely sensitive to the number of claudin pores per strand, which is in contrast to what one would expect for a static model, where conductance is directly proportional to the number of pores per strand. For example, in the dynamic model, decreasing all claudins (open and closed) by 75% results in an 89% decrease in conductance, while increasing claudins by 75% results in a 92% increase in overall conductance. In contrast, for a static model, changing the number of pores by 75% in either direction would result in a corresponding 75% change in barrier function. Thus, a dynamic model may be more sensitive to changes in expression level, and may partially explain the apparent nonlinear relationship between claudin-2 expression and measured barrier function (unpublished personal observation).
In addition to the above differences between a static and dynamic model, an important feature of the dynamic model is that it provides two additional parameters, pore open and closed probability, which may be sensitive to small perturbations. This is best illustrated by the profound effects on barrier function, which occur when the percentage of open pores is varied, while keeping the total number of claudins constant (Fig. 4C). For example, starting with 10% of claudins in the open pore state, a decrease in the pore-forming probability by 50% decreases overall conductance by 60%, and increasing pore openings by 50% increases conductance by 84%. Thus, a dynamic model can better describe known tight junction properties and may provide a potential sensitive means of barrier regulation, even though it is presently not possible to experimentally detect pore openings and closing with molecular resolution.
Tight junction leak is explained by a rare high conductance pathway
Much less is known about the biophysical basis of tight junction leak flux. One possible explanation for tight junction leak is that it is spatially restricted to only a small portion of total linear tight junction. For example, tricellulin has been shown to form a barrier to macromolecules in tricellular tight junctions without contributing increased ion permeability.19 However, a second possibility is that the leak pathway is dynamic like the pore pathway. Leak may be explained by infrequent high conductance tight junction openings that are large enough to allow macromolecules to pass but rare enough to maintain overall tight junction ion selectivity when studied on a global scale. Alternatively, dynamic leak openings may be associated with transient breaks or separations of a tight junction strands. Such breaks would allow diffusion of molecules across a strand and thus diffusion across the entire tight junction may occur in a stepwise fashion followed by resealing of the first strand and breakage of a new strand. This may occur pathophysiologically during injury, but may also occur normally during cell extrusion, during cell proliferation, or even as a result of normal tight junction strand rearrangements.27 A leak event may be rare, relative to the probability of pore openings, and the overall effect on tight junction conductance would depend on the original number of tight junction strands. The impact of such an event may be equivalent to moving to the left along one of the conductance curves in Figure 4C, while keeping the pore open probability constant. It is intriguing to consider that heavily branched tight junction structures may provide a functional advantage by limiting lateral diffusion between tight junction strands. Functionally, this means that a high conductance leak event would only be expected to affect ~500 nm of linear tight junction.
Summary and future directions
Tight junctions were once thought to be static structures with the ability to seal off the intercellular space. This hypothesis was bolstered by the fact that tight junctions are heavily cross-linked structures. However, predictions based on early ultra structural studies have suggested that this cannot be correct, and many have proposed the existence of a dynamic pore pathway. Recent electrophysiological sieving studies support the existence of such an ion-selective pore pathway, and are also consistent with an additional low-capacity leak pathway, but it has been impossible to study these tight junction functions at a molecular level. Additional molecular insight into tight junction function can be obtained from high-resolution FRAP studies, and there is a strong correlation between the mobility of different tight junction proteins and epithelial barrier function. Taken together, these data support a dynamic model of the tight junction barrier and also suggest that regulation of tight junction openings and closings may provide a sensitive means to modulate barrier function without changing protein expression. However, single tight junction opening and closing events have never been measured, and our ability to detect single openings and closings will require novel high resolution techniques. Improved insight into these pathways will likely facilitate the development of more specific pharmacologic modulators designed to target and modulate specific aspects of barrier function in the future.
Footnotes
Conflicts of interest
The author declares no conflicts of interest.
References
- 1.Claude P, Goodenough DA. Fracture faces of zonulae occludentes from “tight” and “leaky” epithelia. J. Cell. Biol. 1973;58:390–400. doi: 10.1083/jcb.58.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Claude P. Morphological factors influencing transepithelial permeability: a model for the resistance of the zonula occludens. J. Membr. Biol. 1978;39:219–232. doi: 10.1007/BF01870332. [DOI] [PubMed] [Google Scholar]
- 3.Furuse M, Sasaki H, Fujimoto K, Tsukita S. Asingle gene product, claudin-1 or-2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J. Cell. Biol. 1998;143:391–401. doi: 10.1083/jcb.143.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amasheh S, Meiri N, Gitter AH, et al. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J. Cell. Sci. 2002;115:4969–4976. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- 5.Raleigh DR, Boe DM, Yu D, et al. Occludin S408 phosphorylation regulates tight junction protein interactions and barrier function. J. Cell. Biol. 2011;193:565–582. doi: 10.1083/jcb.201010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J. Clin. Invest. 2001;107:1319–1327. doi: 10.1172/JCI12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wen H, Watry DD, Marcondes MC, Fox HS. Selective decrease in paracellular conductance of tight junctions: role of the first extracellular domain of claudin-5. Mol. Cell. Biol. 2004;24:8408–8417. doi: 10.1128/MCB.24.19.8408-8417.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Itallie CM, Rogan S, Yu A, Vidal LS, Holmes J, Anderson JM. Two splice variants of claudin-10 in the kidney create paracellular pores with different ion selectivities. Am. J. Physiol. Renal Physiol. 2006;291:F1288–F1299. doi: 10.1152/ajprenal.00138.2006. [DOI] [PubMed] [Google Scholar]
- 9.Colegio OR, Itallie CV, Rahner C, Anderson JM. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am. J. Physiol. Cell. Physiol. 2003;284:C1346–C1354. doi: 10.1152/ajpcell.00547.2002. [DOI] [PubMed] [Google Scholar]
- 10.Mitic LL, Unger VM, Anderson JM. Expression, solubilization, and biochemical characterization of the tight junction transmembrane protein claudin-4. Protein Science : a publication of the Protein Society. 2003;12:218–227. doi: 10.1110/ps.0233903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angelow S, Yu AS. Structure-function studies of claudin extracellular domains by cysteine-scanning mutagenesis. J. Biol. Chem. 2009;284:29205–29217. doi: 10.1074/jbc.M109.043752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Itallie CM, Mitic LL, Anderson JM. Claudin-2 forms homodimers and is a component of a highmolecular weight protein complex. J. Biol. Chem. 2011;286:3442–3450. doi: 10.1074/jbc.M110.195578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou J, Renigunta A, Konrad M, et al. Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J. Clin. Invest. 2008;118:619–628. doi: 10.1172/JCI33970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou J, Renigunta A, Gomes AS, et al. Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc. Natl. Acad. Sci. USA. 2009;106:15350–15355. doi: 10.1073/pnas.0907724106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Itallie CM, Holmes J, Bridges A, et al. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J. Cell. Sci. 2008;121:298–305. doi: 10.1242/jcs.021485. [DOI] [PubMed] [Google Scholar]
- 16.Watson CJ, Rowland M, Warhurst G. Functional modeling of tight junctions in intestinal cell monolayers using polyethylene glycol oligomers. Am. J. Physiol. Cell. Physiol. 2001;281:C388–C397. doi: 10.1152/ajpcell.2001.281.2.C388. [DOI] [PubMed] [Google Scholar]
- 17.Yu AS, Cheng MH, Angelow S, et al. Molecular basis for cation selectivity in claudin-2-based paracellular pores: identification of an electrostatic interaction site. J. Gen. Physiol. 2009;133:111–127. doi: 10.1085/jgp.200810154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu AS, McCarthy KM, Francis SA, et al. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am. J. Physiol. Cell. Physiol. 2005;288:C1231–C1241. doi: 10.1152/ajpcell.00581.2004. [DOI] [PubMed] [Google Scholar]
- 19.Krug SM, Amasheh S, Richter JF, et al. Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol. Biol. Cell. 2009;20:3713–3724. doi: 10.1091/mbc.E09-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turner JR, Rill BK, Carlson SL, et al. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am. J. Physiol. 1997;273:C1378–C1385. doi: 10.1152/ajpcell.1997.273.4.C1378. [DOI] [PubMed] [Google Scholar]
- 21.Prasad S, Mingrino R, Kaukinen K, et al. Inflammatory processes have differential effects on claudins 2, 3, and 4 in colonic epithelial cells. Lab. Invest. 2005;85:1139–1162. doi: 10.1038/labinvest.3700316. [DOI] [PubMed] [Google Scholar]
- 22.Heller F, Florian P, Bojarski C, et al. Interleukin-13 Is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Weber CR, Raleigh DR, Su L, et al. Epithelialmyosin light chain kinase activation inducesmucosal interleukin-13 expression to alter tight junction ion selectivity. J. Biol. Chem. 2010;285:12037–12046. doi: 10.1074/jbc.M109.064808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang F, Graham WV, Wang Y, et al. Interferongamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by upregulating myosin light chain kinase expression. Am. J. Pathol. 2005;166:409–419. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu D, Marchiando AM, Weber CR, et al. MLCK-dependent exchange and actin binding region-dependent anchoring of ZO-1 regulate tight junction barrier function. Proc. Natl. Acad. Sci. USA. 2010;107:8237–8241. doi: 10.1073/pnas.0908869107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 intoMadin-Darby canine kidney I cells. J. Cell. Biol. 2001;153:263–272. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcial MA, Madara JL. Analysis of absorptive cell occluding junction structure-function relationships in a state of enhanced junctional permeability. Lab. Invest. 1987;56:424–434. [PubMed] [Google Scholar]
- 28.Van Itallie CM, Fanning AS, Bridges A, Anderson JM. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol. Biol. Cell. 2009;20:3930–3940. doi: 10.1091/mbc.E09-04-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am. J. Pathol. 2006;169:1901–1909. doi: 10.2353/ajpath.2006.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu. Rev. Physiol. 2011;73:283–309. doi: 10.1146/annurev-physiol-012110-142150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen L, Weber CR, Turner JR. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J. Cell. Biol. 2008;181:683–695. doi: 10.1083/jcb.200711165. [DOI] [PMC free article] [PubMed] [Google Scholar]