Abstract
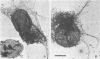
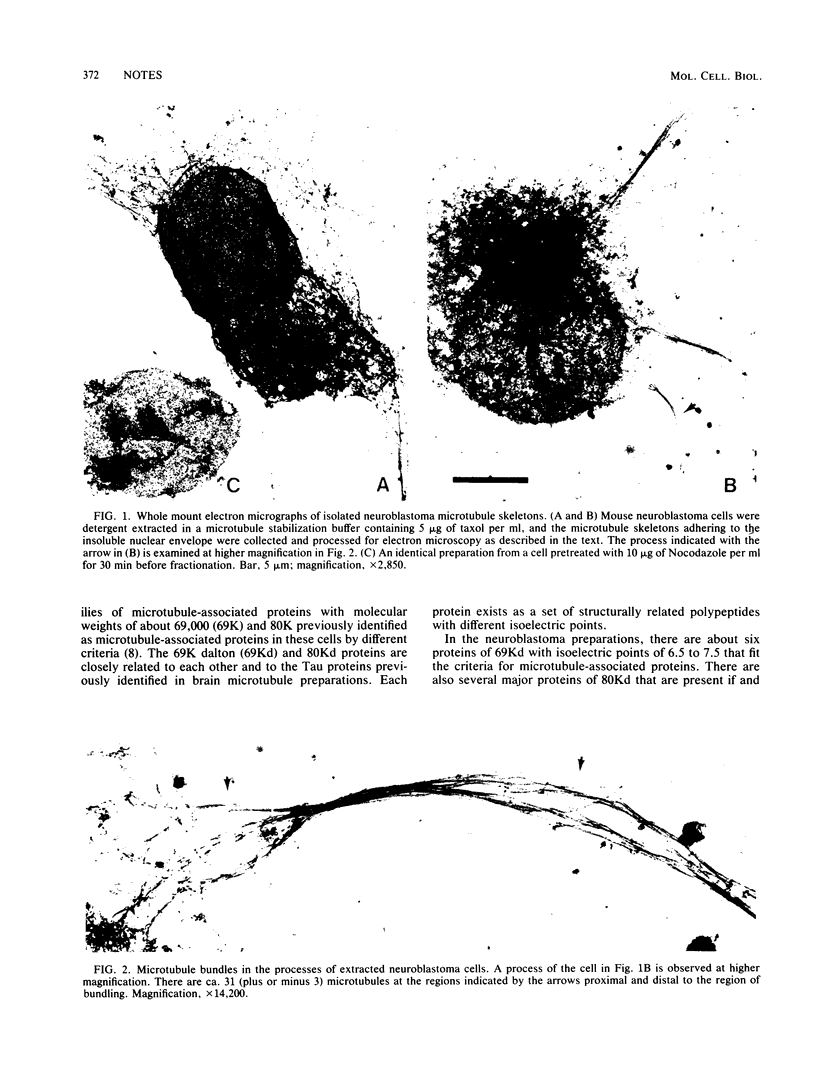
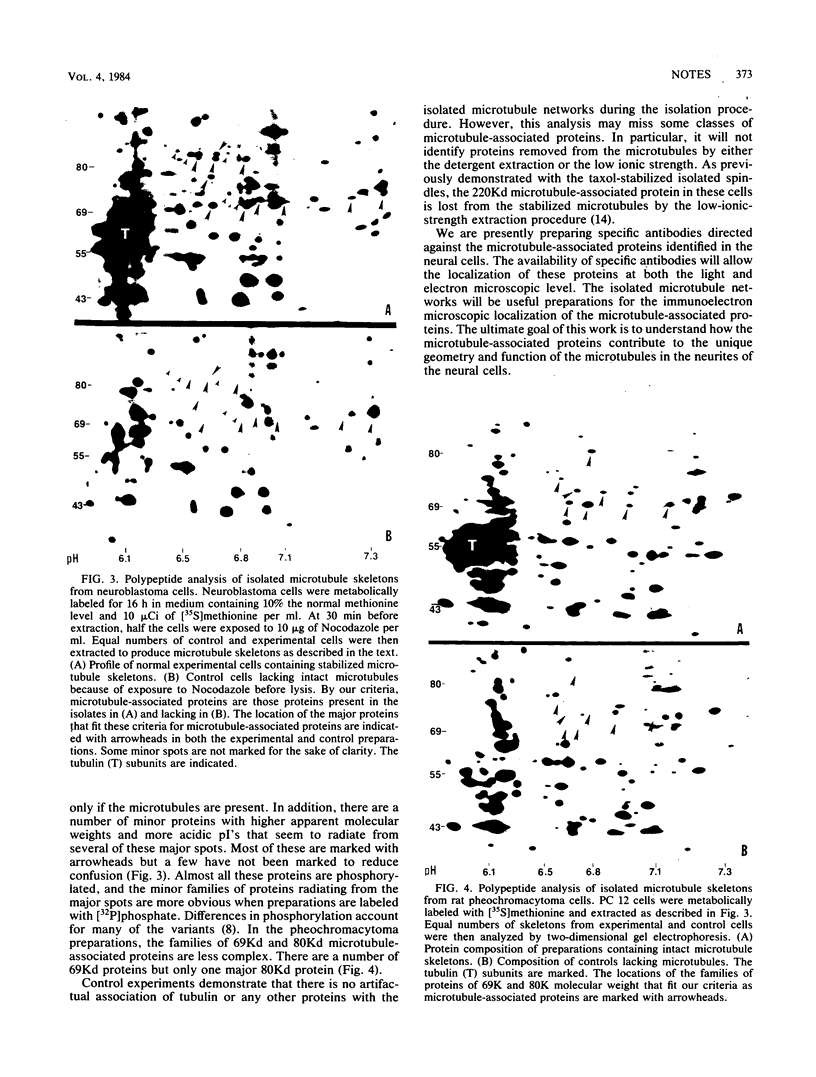
The microtubule skeletons of cultured neuronal cells have been isolated in their original form utilizing taxol and rigorous extraction conditions. Continuous microtubules are visible throughout the cell body and into the processes. These preparations include the complex set of microtubule-associated proteins of 69,000 and 80,000 daltons previously identified in these cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burton P. R., Paige J. L. Polarity of axoplasmic microtubules in the olfactory nerve of the frog. Proc Natl Acad Sci U S A. 1981 May;78(5):3269–3273. doi: 10.1073/pnas.78.5.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M., Thomson J. N. Organization of neuronal microtubules in the nematode Caenorhabditis elegans. J Cell Biol. 1979 Jul;82(1):278–289. doi: 10.1083/jcb.82.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerr A., Pallas D., Solomon F. Molecular analysis of cytoplasmic microtubules in situ: identification of both widespread and specific proteins. Cell. 1981 Apr;24(1):203–211. doi: 10.1016/0092-8674(81)90516-x. [DOI] [PubMed] [Google Scholar]
- Euteneuer U., McIntosh J. R. Polarity of midbody and phragmoplast microtubules. J Cell Biol. 1980 Nov;87(2 Pt 1):509–515. doi: 10.1083/jcb.87.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidemann S. R., Landers J. M., Hamborg M. A. Polarity orientation of axonal microtubules. J Cell Biol. 1981 Dec;91(3 Pt 1):661–665. doi: 10.1083/jcb.91.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas D., Solomon F. Cytoplasmic microtubule-associated proteins: phosphorylation at novel sites is correlated with their incorporation into assembled microtubules. Cell. 1982 Sep;30(2):407–414. doi: 10.1016/0092-8674(82)90238-0. [DOI] [PubMed] [Google Scholar]
- Schiff P. B., Fant J., Horwitz S. B. Promotion of microtubule assembly in vitro by taxol. Nature. 1979 Feb 22;277(5698):665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- Seeds N. W., Gilman A. G., Amano T., Nirenberg M. W. Regulation of axon formation by clonal lines of a neural tumor. Proc Natl Acad Sci U S A. 1970 May;66(1):160–167. doi: 10.1073/pnas.66.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon F., Magendantz M., Salzman A. Identification with cellular microtubules of one of the co-assemlbing microtubule-associated proteins. Cell. 1979 Oct;18(2):431–438. doi: 10.1016/0092-8674(79)90062-x. [DOI] [PubMed] [Google Scholar]
- Solomon F. Neuroblastoma cells recapitulate their detailed neurite morphologies after reversible microtubule disassembly. Cell. 1980 Sep;21(2):333–338. doi: 10.1016/0092-8674(80)90469-9. [DOI] [PubMed] [Google Scholar]
- Vallee R. B. A taxol-dependent procedure for the isolation of microtubules and microtubule-associated proteins (MAPs). J Cell Biol. 1982 Feb;92(2):435–442. doi: 10.1083/jcb.92.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieve G., Solomon F. Proteins specifically associated with the microtubules of the mammalian mitotic spindle. Cell. 1982 Feb;28(2):233–242. doi: 10.1016/0092-8674(82)90341-5. [DOI] [PubMed] [Google Scholar]