Abstract
A 65-year-old man with back pain had plain radiographs that showed multiple osteolytic bone lesions of the pelvis, femur and L-spine; an magnetic resonance imaging scan of the L-spine showed extensive bony resorption with a posterior epidural mass involving the L1 spinous process; these findings suggested multiple myeloma or bony metastasis. However, all serology testing was negative. The parathyroid hormone and serum calcium levels were found to be abnormally elevated. A fine needle aspiration biopsy suggested that the L-spine lesion was consistent with the diagnosis of osteitis fibrosa cystica. A pathological fracture of the spine compressed the spinal cord, and surgical intervention was required. The neck computed tomography and Tc-99m sestamibi scan showed a solitary parathyroid mass. A minimally invasive parathyroidectomy using intraoperative parathyroid hormone monitoring was performed and two enlarged parathyroid glands identified. This case illustrates the importance of the consideration of a rare brown tumor associated with primary hyperparathyroidism in patients with the bone lesions suggestive of a malignancy.
Keywords: Hyperparathyroidism, Osteitis fibrosa cystica, Brown tumor
INTRODUCTION
Primary hyperparathyroidism results from excessive secretion of parathyroid hormone (PTH). It can be caused by a solitary adenoma in 80% patients, parathyroid hyperplasia in 15%, multiple adenomas in 5%, and parathyroid carcinoma in less than 5% of patients [1]. Osteitis fibrosa cystica is a skeletal disease related with long lasting, end-stage hyperparathyroidism and is sometimes referred to as a "brown tumor" because of its reddish color [2]. However, it is not a true neoplasm but rather a reactive osteolytic lesion of bone and may mimic other diseases such as giant cell tumors, multiple bone metastasis or multiple myeloma [3,4]. Because it is rare, it may not be initially considered in the differential diagnosis of bony tumors. Here we report a patient with hyperparathyroism caused by parathyroid hyperplasia who had osteitis fibrosa cystica mistaken for a malignancy.
CASE REPORT
A 65-year-old man who had severe back pain for one year was referred from a local orthopedic clinic to the department of oncology in our hospital, because plain radiographs showed multiple osteolytic lesions of the pelvis, femur and vertebrae (Fig. 1). Three years previously, he had a general physical examination showing no specific problems. However, one year ago, he was treated for a left femur fracture. The lumbar spine MRI showed that the vertebral bodies had low signal intensity due to osteoporosis, a compression fracture of the L2 spine and the L1 spinous process with a mass lesion; these findings were suggestive of bone metastasis or multiple myeloma (Fig. 2). The blood chemistry findings showed that the serum beta-2 microglobulin was 7,860 ng/mL (700-1,800) but the serum IgG, IgA, IgM and tumor markers were all negative and the serum protein electrophoresis and urine protein electrophoresis were negative for a monoclonal gammopathy. However, the alkaline phophatase was 6,788 IU/L (40-250), total calcium was 12.8 mg/dL (8.2-10.5), ionized calcium was 6.5 pg/mL (4.3-5.0) and the PTH was 1,889 pg/dL (0-65). The chest X-ray showed diffuse osteoblastic and some osteolytic lesions of the bony thorax. The bone mineral density studies of the spine and femur were consistent with osteoporosis. The computed tomography (CT) of the abdomen and pelvis demonstrated parenchymal calcinosis of the liver and medullary calcinosis of both kidneys. The entire skeleton had diffuse osteoblastic and osteolytic lesions. The fludeoxyglucose-positron emission tomography (FDG-PET) showed multiple areas of FDG uptake in the bones consistent with the pattern of bony metastasis from a malignancy; however, we could not identify a primary lesion (Fig. 3).
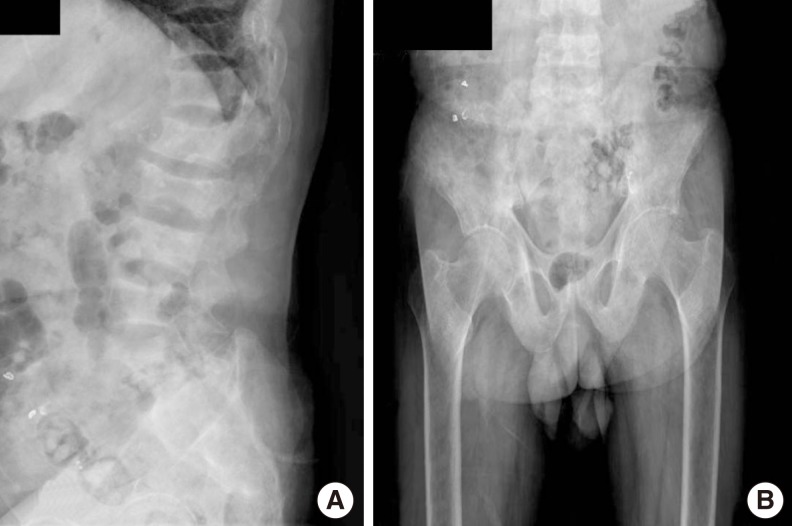
Fig. 1.
Plain radiographs of lumbar spine (A), pelvis and femur (B) showing subperiosteal erosion and generalized osteopenia.
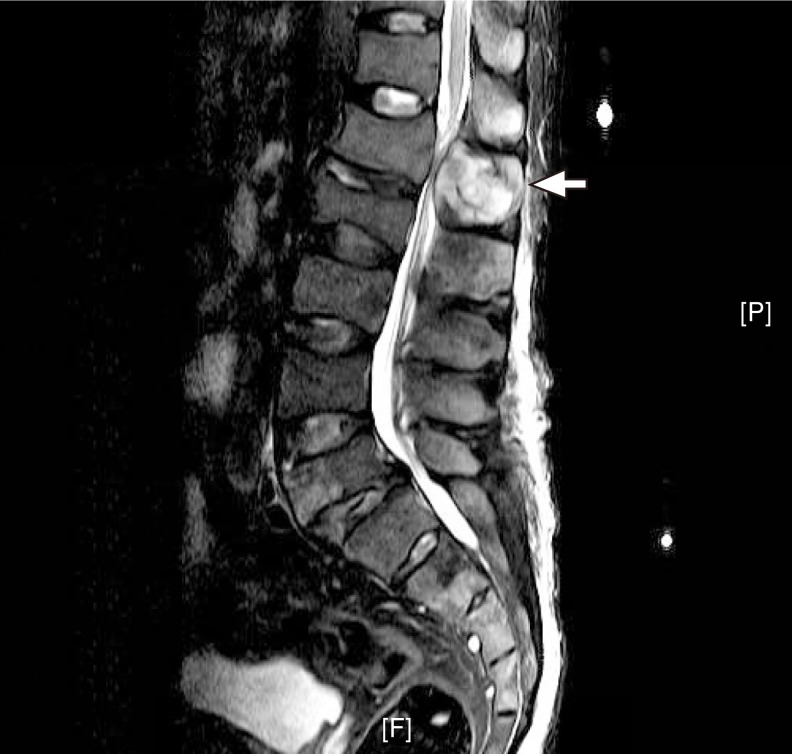
Fig. 2.
T2 weighted sagittal magnetic resonance imaging scan of the lumbar spine showing a compression fracture in L2 and an enhancing expansile mass (arrow) in the L1 spinous process.
Fig. 3.
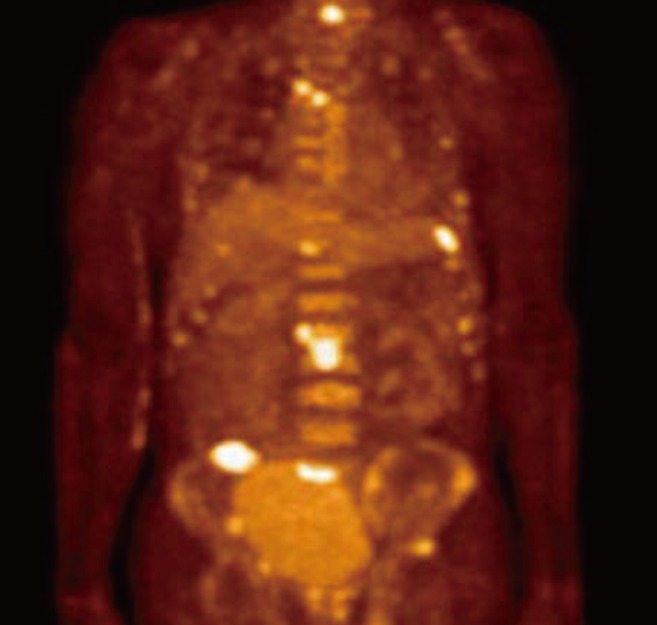
Fludeoxyglucose-positron emission tomography (FDG-PET) image showing multiple intense FDG uptake in the bones: spine, ribs, both iliac bones and acetabulums, and diffuse bone marrow activation.
The fine needle aspiration biopsy from the L1 spinous mass revealed reactive fibroblastic tissue with scattered multinucleated giant cells, and hemorrhage suggestive of osteitis fibrosa cystica. The neck CT scan showed a well enhanced mass at the posteroinferior site of the right thyroid gland (Fig. 4A), and the parathyroid Tc-99m sestamibi scan also showed increased uptake at the same site (Fig. 4B). Because the serology and imaging tests were only suggestive of hypercalcemia due to hyperparathyroidism, the patient was referred to the ENT department where the diagnosis of primary hyperparathyroidism with osteitis fibrosa cystica due to a parathyroid tumor was considered. We planned to perform parathyroidectomy. Prior to the surgery, the radiating pain to the right leg, due to the compression fracture of the L-spine, worsened and emergency surgery was performed to remove the mass lesion in addition to a L2-T12 fusion.
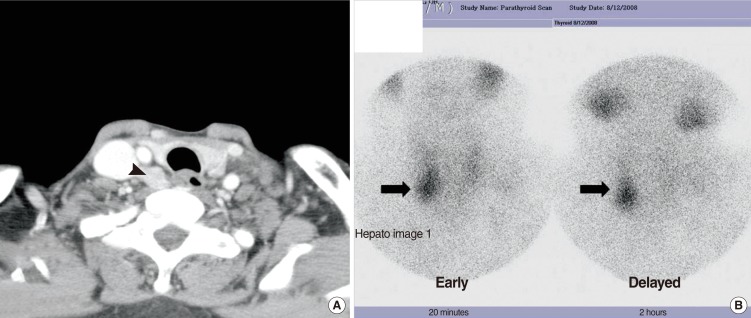
Fig. 4.
(A) Neck computed tomography, axial scan showing mass lesion (arrow head) in the posterior part of the right thyroid gland suggestive of a parathyroid tumor. (B) A sestamibi scan was performed and nodular uptake of radiotracer in the inferior pole of the right thyroid area (arrow) was observed on the early and delayed images; the delayed image shows washout of the normal thyroid tissue activity and focal uptake is seen as discrete.
The histopathological findings reported from the mass lesion were consistent with osteitis fibrosa cystica (Fig. 5). Next, a minimally invasive parathyroidectomy was performed using intraoperative PTH monitoring techniques. We found an enlarged parathyroid in the posteroinferior portion of the right thyroid and one additionally enlarged gland that was not detected on the preoperative imaging studies. The serology showed that the total calcium, ionized calcium, and PTH recovered to within normal range on postoperative day 1. However, on postoperative day 2, the patient developed the hungry bone syndrome (total calcium was 6.5, ionized calcium was 3.9 and serum phosphorus was 2.1). We conlsuted endocrinology department for this problem, and it resolved with vitamin D and calcium replacement within 2 weeks. The histopathological diagnosis was parathyroid hyperplasia affecting two glands.
Fig. 5.

This section shows diffuse proliferation of osteoclast-type giant cells intermixed osteoblastic proliferation, and neovascularizaion, suggestive of the characteristic findings of osteitis fibrosa cystica (H&E, ×200).
DISCUSSION
Osteitis fibrosa cystica was first reported by von Recklinghausen in 1891; it presents as the end stage findings of hyperparathyroidism [3]. However recently, with the technical development of imaging and laboratory screening methods, hypercalcemia due to primary or secondary hyperparathyroidism can often be detected early; as a result the frequency of osteitis fibrosa cystica has declined [3,5,6].
The clinical symptoms associated with hyperparathyroidism are general weakness, fatigue, urinary stones, fractures [7] and osteitis fibrosa cystica, which causes pain and a mass-like lesion [3,4,6]. Because it appears that hyperparathyroidism is associated with bone loss largely at cortical sites, plain radiographic findings include subperiosteal resorption as well as osteopenia [8]. Osteitis fibrosa cystica appears as a well-defined expansile osteolytic lesion with osteopenia on plain radiographs, and as a well enhanced mass on CT and MRI T2-weighted imaging, and is isodense on T1-weighted images [2]. Excessive secretion of PTH causes bone resorption accompanied by fibrovascular marrow replacement and increased osteoblastic activity; the imbalance of osteoclastic and osteoblastic activity manifest as a slow enlarging painful bony mass, osteitis fibrosa cystica, which can lead to pathological fractures [8-10].
Osteitis fibrosa cystica can be locally aggressive but it is not a true malignancy. However, because of the characteristics of this disease, it can be difficult to distinguish it from primary bony tumors, multiple myeloma and metastatic disease. Therefore, fine needle aspiration biopsy (FNAB) of the mass-like lesion can be helpful as well as imaging studies, high serum calcium and PTH level. The histopathology shows diffuse proliferation of osteoclastic multinucleated giant cells intermixed with fibrocellular proliferation and hemorrhagic foci, changes that may be related to a microfracture undergoing organization with the release of hemosiderin and appears as a reddish brown mass [2,3,11]. However, the histopathological feature alone cannot confirm the diagnosis because other giant cell lesions such as giant cell granuloma and aneurismal bone cysts have similar histological features. In addition, the diagnosis by FNA cytology of the bone lesions is controversial. However, it allows for the exclusion of a malignant process and prevents unnecessary studies [12].
In our case, the plain radiographic findings and MRI showed multiple bony osteolytic lesions and an enhanced mass-like change in the lumbar spine. In a review of the medical literature there are several reports of osteitis cystica of the facial bones, long bones, ribs and pelvis, but not the spine [5,13]. Our initial impression in the case was of a malignant process. The serology and imaging studies including serum and urine monoclonal immunoglobulins, abdominal ultrasonography and abdominal and pelvic CT focused on multiple myeloma and determination of an unknown primary tumor. However, the pathology of the FANB excluded malignant disease, and suggested osteitis fibrosa cystica; the parathyroid gland related serology was consistent with hyperparathyroidism. Then, we could focus our investigations on the PTH level, neck CT and Tc-99m sestamibi scan, which confirmed a parathyroid lesion. Treatment of osteitis fibrosa cystica caused by primary hyperparathyroidism is surgical removal of the parathyroid lesions. The bone lesions tend to recover spontaneously after correction of the PTH level, and surgical removal of the osteitis fibrosa cystica is usually unnecessary [13,14]. In our case, the mass-like lesion caused a compression fracture of the lumbar spine, surgical removal should be performed.
The surgical techniques used for parathyroid lesions have changed from exploration of all parathyroid glands to minimally invasive surgery for targeted parathyroid glands [15-17]. The major cause of primary hyperparathyroidism is a solitary adenoma (80%); localization is available with current imaging techniques and intraoperative PTH assay. In our case, the neck CT and Tc-99m sestamibi scan both showed a solitary parathyroid mass. We removed the targeted lesion and performed intraoperative PTH assay and found that the levels were still high; therefore, we proceeded to explore the remaining parathyroid glands and found one additional parathyroid mass.
In conclusion, osteitis fibrosa cystica has become a rare disease. However, awareness of this disease entity is important in the differential diagnosis of bony lesions which suggest malignant disease.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Khan A, Samtani S, Varma VM, Frost A, Cohen J. Preoperative parathyroid localization: prospective evaluation of technetium 99m sestamibi. Otolaryngol Head Neck Surg. 1994 Oct;111(4):467–472. doi: 10.1177/019459989411100413. [DOI] [PubMed] [Google Scholar]
- 2.Takeshita T, Tanaka H, Harasawa A, Kaminaga T, Imamura T, Furui S. Brown tumor of the sphenoid sinus in a patient with secondary hyperparathyroidism: CT and MR imaging findings. Radiat Med. 2004 Jul-Aug;22(4):265–268. [PubMed] [Google Scholar]
- 3.Hsieh MC, Ko JY, Eng HL. Pathologic fracture of the distal femur in osteitis fibrosa cystica simulating metastatic disease. Arch Orthop Trauma Surg. 2004 Sep;124(7):498–501. doi: 10.1007/s00402-004-0697-y. [DOI] [PubMed] [Google Scholar]
- 4.Atabek ME, Pirgon O, Sert A, Esen HH. Extensive brown tumors caused by parathyroid adenoma in an adolescent patient. Eur J Pediatr. 2008 Jan;167(1):117–119. doi: 10.1007/s00431-007-0414-2. [DOI] [PubMed] [Google Scholar]
- 5.Guney E, Yigitbasi OG, Bayram F, Ozer V, Canoz O. Brown tumor of the maxilla associated with primary hyperparathyroidism. Auris Nasus Larynx. 2001 Nov;28(4):369–372. doi: 10.1016/s0385-8146(01)00099-2. [DOI] [PubMed] [Google Scholar]
- 6.Dinkar AD, Sahai S, Sharma M. Primary hyperparathyroidism presenting as an exophytic mandibular mass. Dentomaxillofac Radiol. 2007 Sep;36(6):360–363. doi: 10.1259/dmfr/19204128. [DOI] [PubMed] [Google Scholar]
- 7.Shanmugham MS, Alhady SF. Hyperparathyroidism with osteitis fibrosa cystica in the maxilla. J Laryngol Otol. 1984 Apr;98(4):417–420. doi: 10.1017/s0022215100146821. [DOI] [PubMed] [Google Scholar]
- 8.Khan A, Bilezikian J. Primary hyperparathyroidism: pathophysiology and impact on bone. CMAJ. 2000 Jul;163(2):184–187. [PMC free article] [PubMed] [Google Scholar]
- 9.Kalambokis G, Economou G, Kamina S, Papachristou DJ, Bai M, Tsianos EV. Multiple brown tumors of the ribs simulating malignancy. J Endocrinol Invest. 2005 Sep;28(8):738–740. doi: 10.1007/BF03347558. [DOI] [PubMed] [Google Scholar]
- 10.Parfitt AM. Parathyroid hormone and periosteal bone expansion. J Bone Miner Res. 2002 Oct;17(10):1741–1743. doi: 10.1359/jbmr.2002.17.10.1741. [DOI] [PubMed] [Google Scholar]
- 11.Demay MB, Rosenthal DI, Deshpande V. Case records of the Massachusetts General Hospital: Case 16-2008 - a 46-year-old woman with bone pain. N Engl J Med. 2008 May;358(21):2266–2274. doi: 10.1056/NEJMcpc0802020. [DOI] [PubMed] [Google Scholar]
- 12.Kemp AM, Bukvic M, Sturgis CD. Fine needle aspiration diagnosis of osteitis fibrosa cystica (brown tumor of bone): a case report. Acta Cytol. 2008 Jul-Aug;52(4):471–474. doi: 10.1159/000325556. [DOI] [PubMed] [Google Scholar]
- 13.Triantafillidou K, Zouloumis L, Karakinaris G, Kalimeras E, Iordanidis F. Brown tumors of the jaws associated with primary or secondary hyperparathyroidism: a clinical study and review of the literature. Am J Otolaryngol. 2006 Jul-Aug;27(4):281–286. doi: 10.1016/j.amjoto.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Knee TS, Drake AJ, 3rd, Turton D, Shakir KM. Effect of parathyroid adenoma resection on bone density in primary hyperparathyroidism and osteitis fibrosa cystica. Orthopedics. 2001 Oct;24(10):1000–1002. doi: 10.3928/0147-7447-20011001-25. [DOI] [PubMed] [Google Scholar]
- 15.Vignali E, Picone A, Materazzi G, Steffe S, Berti P, Cianferotti L, et al. A quick intraoperative parathyroid hormone assay in the surgical management of patients with primary hyperparathyroidism: a study of 206 consecutive cases. Eur J Endocrinol. 2002 Jun;146(6):783–788. doi: 10.1530/eje.0.1460783. [DOI] [PubMed] [Google Scholar]
- 16.Halevy A, Stepansky A, Halpern Z, Wassermann I, Chen-Levy Z, Pytlovich S, et al. Quick parathormone assay in the surgical management of hyperparathyroidism. Isr Med Assoc J. 2003 Nov;5(11):775–777. [PubMed] [Google Scholar]
- 17.Chen H, Pruhs Z, Starling JR, Mack E. Intraoperative parathyroid hormone testing improves cure rates in patients undergoing minimally invasive parathyroidectomy. Surgery. 2005 Oct;138(4):583–587. doi: 10.1016/j.surg.2005.06.046. [DOI] [PubMed] [Google Scholar]