Abstract
Introduction
Human immunodeficiency virus (HIV) infection is a primary cause of acquired heart disease, particularly of accelerated atherosclerosis, symptomatic heart failure, and pulmonary arterial hypertension. Cardiac complications often occur in late-stage HIV infections as prolonged viral infection is becoming more relevant as longevity improves. Thus, multi-agent HIV therapies that help sustain life may also increase the risk of cardiovascular events and accelerated atherosclerosis.
Discussion
Before highly active antiretroviral therapy (HAART), the two-to-five-year incidence of symptomatic heart failure ranged from 4 to 28% in HIV patients. Patients both before and after HAART also frequently have asymptomatic abnormalities in cardiovascular structure. Echocardiographic measurements indicate left ventricular (LV) systolic dysfunction in 18%, LV hypertrophy in 6.5%, and left atrial dilation in 40% of patients followed on HAART therapy. Diastolic dysfunction is also common in long-term survivors of HIV infection. Accelerated atherosclerosis has been found in HIV-infected young adults and children without traditional coronary risk factors. Infective endocarditis, although rare in children, has high mortality in late-stage AIDS patients with poor nutritional status and severely compromised immune systems. Although lymphomas have been found in HIV-infected children, the incidence is low and cardiac malignancy is rare. Rates of congenital cardiovascular malformations range from 5.6 to 8.9% in cohorts of HIV-uninfected and HIV-infected children with HIV-infected mothers. In non-HIV-infected infants born to HIV-infected mothers, foetal exposure to ART is associated with reduced LV dimension, LV mass, and septal wall thickness and with higher LV fractional shortening and contractility during the first two years of life.
Conclusions
Routine, systematic, and comprehensive cardiac evaluation, including a thorough history and directed laboratory assays, is essential for the care of HIV-infected adults and children as cardiovascular illness has become a part of care for long-term survivors of HIV infection. The history should include traditional risk factors for atherosclerosis, prior opportunistic infections, environmental exposures, and therapeutic and illicit drug use. Laboratory tests should include a lipid profile, fasting glucose, and HIV viral load. Asymptomatic cardiac disease related to HIV can be fatal, and secondary effects of HIV infection often disguise cardiac symptoms, so systematic echocardiographic monitoring is warranted.
Keywords: HIV, AIDS, child, cardiac outcomes, antiretroviral therapies, therapeutic complications, cardiovascular risk
Introduction
(HIV) infection is a primary cause of acquired heart disease, particularly of accelerated atherosclerosis, symptomatic heart failure, and pulmonary arterial hypertension [1–17]. Cardiac complications often occur in the later stages of HIV infection with prolonged viral infection and are therefore becoming more relevant as longevity improves [1–17]. Multi-agent HIV therapies that help sustain life may also directly increase the risk of cardiovascular events and accelerated atherosclerosis [1,18–22].
By 2011, between 31 and 36 million people were living with HIV [23], an estimated 0.8% of all people aged 15–49 years. Globally, treatment and burden of this epidemic varies greatly from one region to the next. One of the most severely afflicted regions is sub-Saharan Africa, where 69% of all people living with HIV reside and nearly 1 in every 20 adults is infected [23]. In 2011, 330,000 children acquired HIV infection (90% of whom are in sub-Saharan Africa), 43% less than in 2003 [23].
HIV-infected children did not usually receive antiretroviral therapy (ART) or only received monotherapy with zidovudine in the early 1990s. These children often experienced abnormal left ventricular (LV) structure and function, a predictor of mortality [24]. Although the cardiovascular effects of HIV and ART are not fully understood, HIV-infected children are routinely exposed to ART or highly active antiretroviral therapy (HAART) while the cardiovascular system is still developing. Sub-clinical cardiac abnormalities may develop into symptomatic cardiomyopathy in adulthood.
Cardiac abnormalities (Table 1) associated with HIV infection include premature myocardial infarction (MI) or stroke, pericardial effusion, lymphocytic interstitial myocarditis, LV diastolic dysfunction, dilated cardiomyopathy (frequently with myocarditis) infective endocarditis, and malignancy (myocardial Kaposi's sarcoma and B-cell immunoblastic lymphoma; Table 1) [3,4,25,26]. Treatment-related drug effects and interactions are considerably more prevalent and directly challenge the cardiovascular system through lipid abnormalities with protease inhibitors (PIs) and an increased statin serum concentration with PIs [18]. Therapies may also change repolarization or prolong the QT interval, increasing the risk of sudden cardiac death [18].
Table 1.
Summary of HIV-associated cardiovascular diseases
| Disease | Possible causes | Incidence/prevalence | Diagnosis | Treatment |
|---|---|---|---|---|
| Accelerated atherosclerosis | Protease inhibitors, atherogenesis with virus-infected macrophages, chronic inflammation, glucose intolerance, dyslipidemia, endothelial dysfunction | Up to 8% prevalence | ECG, Stress testing, echocardiography, lipid profile, CT angiography, and calcium scoring | Smoking cessation, low fat diet, aerobic exercise, blood pressure control, guideline based statin use, percutaneous coronary intervention, coronary artery bypass surgery |
| Dilated cardiomyopathy systolic dysfunction |
Coronary Artery Disease Drug related:
cocaine, AZT, IL-2, doxorubicin, interferon Infectious: HIV, toxo-plasma, coxsackievirus group B, EBV, CMV, adenovirus Metabolic or endocrine: Selenium or carnitine deficiency, anaemia, hypocalcemia, hypophosphatemia, hyponatremia, hypokalemia, hypoalbuminemia, hypothyroidism, growth hormone deficiency, adrenal insufficiency, hyperinsulinemia Cytokines: TNF-α, nitric oxide, TGF-β, endothelin-I, interleukins Immunodeficiency: CD4 <100 Autoimmune |
Up to 8% of asymptomatic patients Up to 25% of autopsy cases |
Chest radiograph findings
ECG: Nonspecific conduction abnormalities, PVCs, PACs Echocardiogram findings: low-normal LV wall thickness, increased LV mass, dilated LV, systolic LV dysfunction. Possible laboratory studies: Troponin T, brain natriuretic peptide level, CD4 count, viral load, viral PCR, toxoplasma serology, thyroid-stimulating hormone, cortisol, carnitine, selenium, serum ACE, stress testing, myocardial biopsy, cardiac catheterization |
Diuretics, digoxin, ACE inhibitors,
β-blockers Adjunctive treatment in HIV patients Treatment of infection nutritional replacement IVIg Intensify antiretroviral therapy Follow-up serial echocardiograms |
| LV diastolic dysfunction |
TNF, Interleukin (6) Hypertension Chronic viral infection |
Up to 37% asymptomatic |
Echocardiography Tissue doppler imaging |
Treat hypertension Intensify antiretroviral therapy |
| Pulmonary hypertension | Plexogenic pulmonary arteriopathy | 0.5% | ECG, echocardiography, right heart catheterization | Anticoagulation, vasodilators, prostacyclin analogs Endothelin antagonists, PDE-5 Inhibitors |
| Pericardial disease |
Bacteria: Staphylococcus, Streptococcus, Proteus, Klebsiella, Pericardiocentesis Enterococcus, Listeria, Nocardia, Mycobacterium Viral pathogens: HIV, HSV, CMV, adenovirus, echovirus Other pathogens: Cryptococcus, Toxoplasma, Histoplasma Malignancy: Kaposi’s sarcoma, lymphoma, capillary leak/wasting/malnutrition Hypothyroidism Immunodeficiency Uremia |
11%/year- markedly reduced in post HAART studies. Spontaneous resolution in 42% of affected patients Approximately 30% increase in six-month mortality |
Pericardial rub on examination Echocardiogram Fluid analysis for gram stain, and culture, cytology ECG-low voltage/PR depression Associated pleural and peritoneal fluid analysis Pericardial biopsy |
Treat the cause Followup: Serial echocardiograms Intensify antiretroviral therapy Pericardiocentesis or window Histoplasma |
| Infective endocarditis |
Autoimmune Bacteria: Staphylococcus aureus or Staphylococcus epidermidis, Salmonella, Streptococcus, Hemophilus parainfluenzae, Pseudallescheria boydii, HASEK organisms Fungal: Aspergillus fumigatus, Candida, Cryptococcus neoformans |
Increased incidence in IVDA, regardless of HIV status | Blood cultures; Echocardiogram | IV antibiotics, valve replacements |
| Nonbacterial thrombotic endocarditis | Valvular damage, vitamin C deficiency, malnutrition, wasting, DIC, hypercoagulable state, prolonged acquired immunodeficiency | Rare condition, but clinically relevant emboli in 42% of cases | Echocardiogram | Anticoagulation, treat vasculitis or underlying illness |
| Malignancy | Kaposi’s sarcoma, non-Hodgkin lymphoma, leiomyosarcoma Low CD4 count, prolonged immunodeficiency HHV-8, EBV | Approximately 1% incidence Usually metastatic in HIV+ patients |
Echocardiogram, biopsy | Chemotherapy possible |
| Right ventricle disease | Recurrent pulmonary infections, pulmonary arteritis, microvascular pulmonary emboli, COPD | ECG, echocardiography, right heart catheterization | Diuretics, treat underlying lung infection or disease, anticoagulation as clinically indicated | |
| Vasculitis | Drug therapy with antibiotics and antivirals | Increasing incidence | Clinical diagnosis | Systemic corticosteroids, withdrawal of drug |
| Autonomic dysfunction | CNS disease, drug therapy, prolonged immunodeficiency, malnutrition, sedentary lifestyle | Increased in patients, with CNS disease | Tilt-table test, Holter or Event monitoring | Procedural precautions |
| Arrhythmias | Drug therapy, pentamidine, autonomic dysfunction, acidosis electrolyte abnormalities | ECG—long QT, Holter monitoring, exercise stress testing | Discontinue drug, procedural precautions Electrolyte replacement |
|
| Lipodystrophy | Drug therapy: protease inhibitors | Echocardiography, lipid profile, cardiac catheterization, coronary calcium score | Lipid therapy (beware of drug interactions), aerobic exercise, altered antiretroviral, therapy, cosmetic surgery/fat implantation |
ACE=angiotensin-converting enzyme; AZT=azidothymidine; CMV=cytomegalovirus; CNS=central nervous system; DIC=disseminated intravascular coagulation; EBV=Epstein-Barr virus; ECG=electrocardiogram; HHV=human herpes virus; HIV=human immunodeficiency virus; HSV=herpes simplex virus; HTN=hypertension; IL-2=interleukin-2; IVDA=intravenous drug abuse; IVIg=intravenous immunoglobulin; LV=left ventricular; PAC=premature atrial complex; PCR=polymerase chain reaction; PVC=premature ventricular complex; TGF=transforming growth factor; TNF=tumour necrosis factor.
Modified with permission from Fisher SD, Lipshultz SE. Chapter 72: Cardiovascular abnormalities in HIV-infected individuals. In: Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine, Ninth Edition. Editors: Bonow RO, Mann DL, Zipes DP, Libby P. Philadelphia: Elsevier Saunders. 1618–27. 2011 ISBN: 978-1-4377-0398-6.
Discussion
Accelerated atherosclerosis
Since the advent of ART, patients with HIV infection have longer life expectancies, but chronic conditions including atherosclerotic and metabolic disease are becoming more prevalent in this population [27]. Highly active ART (HAART) causes a metabolic syndrome well-characterized in adults as unfavourable body composition (reduction in subcutaneous and increase in visceral fat), insulin resistance and abnormal glucose metabolism, and dyslipidemia [28,29]. The physiologic effect of the metabolic syndrome places patients at risk for atherosclerotic cardiovascular disorders. In fact, in adults with HAART-related fat redistribution, several studies have suggested an increase in the risk of MI relating to the level of viral control (increased inflammation) or to ART exposures (including PIs and certain nucleoside reverse transcriptase inhibitors) [30–32]. Acute MI can be the primary presentation of atherosclerotic disease [33]. However, there is controversy over whether the metabolic syndrome in HIV-infected patients is exclusively related to ART exposure or HIV infection itself. Synergistic causes may include traditional risk factors such as family history, high LDL cholesterol, low HDL cholesterol, diabetes, hypertension, age >55, HIV viral load, and medication specific ART exposure. Studies in children show similar although not identical findings, including abnormal body composition, insulin resistance, and dyslipidemia with the use of ART, with increased risk at older age and longer duration of HAART [34–40]. The onset of puberty has been proposed as another factor that is associated with accelerating these changes [41].
Early studies in children showed that PI therapy improved weight, weight-for-height and mid-arm muscle circumference of HIV-infected children, independent of the concurrent decrease in HIV viral load and improved CD4 T-lymphocyte counts [42]. The immediate treatment effects were most apparent with an improvement in weight and mid-arm muscle circumference and there was a trend towards increased height and lean body mass. In addition to the positive improvements in growth and lean body mass, however, HAART is also associated with abnormalities in fat distribution in children though some studies report similar lean mass in HIV-infected and uninfected children [43]. Arpadi et al. observed similar total fat, trunk fat, and percentage of total fat between HIV-infected and uninfected children, but lower leg and higher arm fat in infected children [44]. Jacobson et al. showed there were decreased limb/trunk fat ratios in HIV-infected children when compared with HIV-exposed uninfected (HIVEU) [35]. These findings suggest that both peripheral lipoatrophy, as well as central obesity occur in these children. Further studies have shown that a majority of children develop fat redistribution within three years of initiating a protease inhibitor (PI) - containing regimen, and that these changes progress over time [45]. Other studies have identified metabolic abnormalities induced by other specific classes of drugs. Stavudine use has been associated with lipoatrophy [46], potentially by altering mitochondrial number and function [47].
Following exposure to ART, there are increases in total, LDL, and HDL cholesterol in both adults and children. Children newly exposed to ART experienced a rapid rise in LDL cholesterol over the first six months that continued through 12 months [48]. A total of 10% of a cohort of 449 children in the United Kingdom had LDL cholesterols over the 95% for age and PIs caused greater rises in total cholesterol than non-nucleoside reverse transcriptase inhibitors. The authors concluded that dietary and exercise interventions and a change in ART might help address these metabolic abnormalities [49]. In children with incident hypercholesterolemia, Jacobson et al. found that a switch in the ART regimen was associated with cholesterol levels that returned to normal [50]. There was limited power to detect the effects of switching to specific ARTs; however, a higher viral load at baseline was associated with the normalization of cholesterol. According to the Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents, switching from one PI to another PI or to the same PI at a lower dosing frequency may reduce dyslipidemia [51]. Evaluating metabolic changes in children as they start or change ART can be helpful to determine specific effects of ART because children have fewer confounding psychosocial factors (such as smoking, alcohol, obesity) that can independently impact metabolic outcomes.
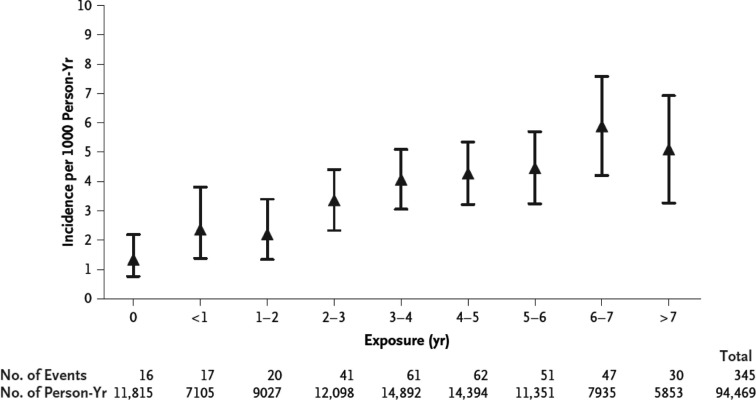
Atherosclerotic cardiovascular disease (CVD) often results from an environment that is hostile to the endothelium, which may occur from a complex interaction of HIV, the adverse effects of ART, traditional risk factors for CVD, inflammation and co-infections [52]. Autopsies in HIV-infected patients aged 23–32 years who died unexpectedly revealed atherosclerotic plaque with features common to both coronary atherosclerosis and transplant vasculopathy, histologic characteristics more frequently seen with single-vessel disease in which the cause of MI is plaque rupture [53,54]. Imaging data suggest inflammation as the cause of such premature cardiovascular events (Table 2). Endothelial dysfunction is one possible causative link between HIV infection and atherosclerosis. HIV-infected patients have increased expression of vascular adhesion molecules (E-selectin, ICAM, VCAM) and inflammatory cytokines such as interleukin (IL-6) and tumour necrosis factor (TNF)-α [55,56]. The presence of an endothelial response to injury is supported by the correlation of viral load with higher plasma TNF-α, IL-6, and von Willebrand factor concentrations [37,55,57]. The risk of myocardial infarction has been found to increase with the exposure to combination ART (Figure 1) [57].
Table 2.
Imaging and support that atherosclerosis is inflammatory in HIV-infected people
| Modality | HIV vs Matched Controls | Associations |
|---|---|---|
| Carotid ultrasound Carotid intimal-medial thickness | First to show higher rates of atherosclerosis 0.04 mm thicker in HIV (metanalysis) |
Smoking, dyslipidemia, low nadir CD4 T-cell count, and increased lymphocyte activation correlated with higher IMT and progression |
| Computed tomography calcium scores | HIV-infected have higher mean Agatston scores and proportion of scores >0 | Framingham risk, metabolic syndrome, higher levels of asymmetric dimethylarginine, and fatty liver |
| CT angiography | Higher prevalence of noncalcified plaque | CD4/CD8 ratio and HIV duration independently predict plaque burden |
| Magnetic resonance angiography | Association of HIV viremia and atherosclerotic plaque burden in the aorta Extensively used in cerebral and peripheral vascular beds |
|
| Flow-mediated brachial artery dilation | Impaired in HIV-infected | Degree of HIV viremia, injection drug use, periodontal disease, and vitamin D deficiency Statins, niacin, and pentoxifylline have been beneficial in improving flow-mediated dilation |
| Future potential imaging Intravascular ultrasound Intracoronary optical coherence tomography Future PET imaging of 18 FDG uptake Molecular targeted magnetic resonance imaging |
Modified with permission from Fisher SD, Lipshultz SE. Chapter 72: Cardiovascular abnormalities in HIV-infected individuals. In: Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine, Ninth Edition. Editors: Bonow RO, Mann DL, Zipes DP, Libby P. Philadelphia: Elsevier Saunders. 1618–27. 2011 ISBN: 978-1-4377-0398-6.
Figure 1.
Risk of myocardial infarction according to exposure to combination antiretroviral therapy. The adjusted relative rate of myocardial infarction according to cumulative exposure to combination antiretroviral therapy was 1.16 per year of exposure (95% CI, 1.09–1.23). The I bars denote the 95% CIs. Reproduced with permission from ref. 57.
Premature cerebrovascular disease is also prevalent in HIV-infected adults and providers should be aware of its risks in young adults. A review of autopsies from 1983 to 1987 found that AIDS patients had an estimated 8% prevalence of stroke. Evidence of cerebral emboli was found in four of the 13 patients with stroke and the embolus had a clear cardiac source in three of these four patients. Considering these patients were in the pre-HAART era, the aetiology is possibly different. As HIV-infected children age, the common origins of stroke should be sought and atherosclerotic disease should be suspected. Premature atherosclerosis is generally found in children treated with ART, although it is not clearly ART related. Acute stroke investigation in HIV-infected individuals should be somewhat different than in the general population as a result of infectious and immune-mediated vasculopathy, tumours, opportunistic infections, and cardioembolism [58].
Prevention of premature CVD should be directed at identifying and decreasing known risk factors. Low cholesterol diets reduce the incidence of dyslipidemia [59]. In addition, patients should be encouraged to follow heart-healthy diets with increased aerobic activities and avoid smoking as it has been found that exercise and smoking cessation also markedly lower lipid levels and help prevent lipodystrophy [60]. It is also recommended that the patient's glucose and lipid concentrations be monitored regularly [59,60]. Current guidelines should be followed for patients with dyslipidemia for primary and secondary risk prevention. Known drug interactions should be avoided, such as that with simvastatin and ritonavir, which can lead to a 400-fold increase in simvastatin concentrations [61,62].
Left ventricular systolic dysfunction
Incidence
Before HAART therapy, the two-to-five-year incidence of symptomatic heart failure ranged from 4 to 28% in HIV patients, suggesting a prevalence of symptomatic HIV-related heart failure of between 4 and 5 million cases worldwide [5,6]. The incidence of clinically important cardiac disease in HIV-infected patients has been markedly reduced by HAART. However, HAART is only available to a minority of those in need [6,63–65].
Patients receiving HAART also frequently have asymptomatic abnormalities in cardiac structure [66]. Echocardiographic measurements indicate 18% have LV systolic dysfunction, 6.5% have LV hypertrophy, and 40% have left atrial dilation [64]. A history of MI, current tobacco smoking, and elevated highly sensitive C-reactive protein were associated with LV systolic dysfunction [64].
During pre-HAART era HIV-infected children aged ten or younger, 25% died from chronic cardiac disease [5,6], and 28% experienced serious cardiac events after an AIDS-defining illness [1,5,6,65]. Increased mortality is only associated with a mild decrease in LV systolic function or an increase in LV mass in children [24]. The NHLBI-funded Highly Active Antiretroviral Therapy-Associated Cardiotoxicity (CHAART-II) study collected longitudinal echocardiographic measurements in HIV-infected children and adolescents exposed to HAART or multi-drug ART. When compared to HIV-infected but relatively less ART-exposed children from the HIV-infected cohort from the Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted HIV Infection (P2C2 HIV) study, the CHAART-II patients had persistently decreased LV mass. Although in infancy, the CHAART-II patients had significantly better LV contractility compared to the P2C2 HIV group, at ten years of follow-up, LV contractility significantly decreased in the CHAART-II group to a level equivalent to decreased LV systolic function in infancy in the P2C2 HIV group. These findings suggest that long-term HAART exposure may be cardioprotective for a finite period early in life, but decreases as this HIV-infected population ages into adolescence and early adulthood. Further longitudinal follow-up studies are needed in adolescents and young adults who were perinatally infected with HIV to better characterize their future cardiac risk. After 11 years of HAART exposure, in CHAART-II patients, LV function was equivalent to that of the HAART-unexposed P2C2 HIV-infected cohort. The conclusion was that the protective effects of HAART exposure on cardiac function appeared to diminish 11 years after exposure. A larger, but otherwise similar HIV-infected paediatric cohort from the NIH-funded Pediatric HIV/AIDS Cohort Study's Adolescent Master protocol required only a single echocardiogram. Generally, measures of LV structure and function were better in this long-term HAART-exposed group than in the relatively HAART-unexposed P2C2 HIV cohort, but were not as normal as those in an HIVEU control group [67]. Although the general conclusion was that HAART exposure in HIV-infected children appeared to be cardioprotective, the cross-sectional study could not support conclusions regarding the long-term trajectories of cardiac health or dysfunction. Serial echocardiographic and other cardiovascular risk screening in this cohort as they age could inform the long-term cardiovascular risk in perinatally HIV-infected children in the HAART era.
The CHAART-I study collected serial echocardiograms in a cohort of HIVEU children exposed perinatally to either multi-drug ART or HAART [68]. At age two, these children had below-normal LV mass, LV dimension, and septal wall thickness, indicating smaller hearts. In contrast, LV function was increased. These differences were more pronounced in girls [68]. In a larger cohort of HIVEU, perinatally HAART-exposed and slightly older (aged 3–5 years) children from the PHACS SMARTT protocol, preliminary results from a single echocardiogram showed that 16% of children had at least one abnormal echocardiographic measure. First trimester exposure to various ART agents was associated with specific echocardiographic abnormalities. For instance, first trimester exposure to abacavir was associated with decreased LV wall thickness. In a separate study of the PHACS SMARTT HIVEU cohort, serum cardiac biomarker measurements suggested that HIVEU children perinatally exposed to multiple ART agents might have subclinical myocardial inflammation. Specifically, abacavir exposure was potentially associated with deleterious cardiac effects [69].
The results of cardiac biomarkers in the PHACS AMP HIV-infected cohort are still being analyzed and could provide further insights into both the long-term pathophysiologic effects of HAART exposure as well as how best to evaluate long-term cardiovascular risk. Currently, additional analyses are on-going comparing the cross-sectional echocardiographic measures in this PHACS SMARTT cohort to the relatively ART-unexposed P2C2 HIV cohort and the smaller but longitudinally followed CHAART-I perinatally HAART-exposed cohort. The results of these on-going analyses may better elucidate the effects of prenatal HIV and ART exposures on cardiac measures of structure and function in HIVEU children.
Recent data show a marked decline in the incidence of both clinical cardiomyopathy and structural abnormalities and an apparent cardioprotective effect of HAART in children and adolescents [58,63–65,68].
Clinical presentation
Concurrent pulmonary infections, anaemia, pulmonary hypertension, malnutrition, portal hypertension, and malignancy can modify or confuse the distinctive signs in HIV-infected patients that define heart failure in other populations. Patients can present with LV systolic dysfunction that is anywhere from asymptomatic to New York Heart Association Class III (marked functional limitations) or IV heart failure (severe functional limitations) [62].
Echocardiography, including strain measurements, and cardiac magnetic resonance imaging is useful for assessing LV function, in addition to diagnosing LV dysfunction. Images often reveal LV hypertrophy, dilation, or low-to-normal wall thickness, as well as left atrial dilation [5,24,33,62,64]. Echocardiographic assessment is recommended at baseline and every 1–2 years thereafter, or as indicated, in any patient at elevated cardiovascular risk who has unexplained or persistent pulmonary symptoms or viral co-infections or with any clinical manifestations of CVD [5,54,62,64].
Electrocardiography (ECG) often reveals nonspecific conduction defects or repolarization changes in ART naïve patients. Chest radiography has low sensitivity and specificity for diagnosing heart failure in HIV-infected patients treated or untreated with ART. Several small studies of HIV-infected individuals revealed that blood brain natriuretic peptide concentrations were inversely correlated with LV ejection fraction. This inverse correlation can be useful in the differential diagnosis of congestive cardiomyopathy in HIV-infected patients [33,69,70].
Progressive LV dilation is common in children infected with HIV. LV dilation may precede heart failure (five-year cumulative incidence, 12.3%) and is associated with elevated LV afterload, LV hypertrophy, and reduced LV function [65]. Early and continuous treatment with HAART for at least five years in HIV-infected children prevented clinically important heart failure better than in earlier groups and preserved cardiac structure and function, indicating that HAART may be cardioprotective [65].
Both the PHACS and CHAART studies suggest that any cardiac changes in the HAART era are generally subclinical in children. Further, in addition to characterizing lifetime ART exposure, traditional non-HIV cardiovascular risk factors, will be needed to best determine differences in global cardiovascular risk between perinatally HIV-infected and HIVEU children, and that in the general population.
Pathogenesis in children
Two mechanisms of pathogenesis have been described in children treated with ART in the pre-HAART with perinatally-transmitted HIV infection: dilation of the LV with a reduced ratio of the LV wall thickness to end-systolic dimension and concentric hypertrophy of the muscle and dilation, in which the ratio of LV thickness to end-systolic dimension remains normal or is increased [5].
Pathogenesis in young adults
As these children enter young adulthood, adult pathogenesis of the disease becomes more relevant. Several causative agents have been postulated for HIV-related cardiomyopathy in children and adults receiving treatment who are from the pre-HAART era (Table 1) [62,65,68].
Myocarditis
Dilated cardiomyopathy can be related to the direct action of HIV on myocardial tissue or to proteolytic enzymes or cytokine mediators induced by HIV alone or with co-infecting viruses [71]. Endomyocardial biopsy specimens have revealed Toxoplasma gondii, coxsackievirus group B, Epstein-Barr virus, cytomegalovirus, adenovirus, and HIV in myocytes. Further research is required to determine if these co-infecting agents also apply in the post-HAART era.
Only scant and patchy inflammatory cell infiltrates in the myocardium have been identified in autopsy and biopsy findings [5,62,71,72], indicating that HIV can infect myocardial interstitial cells and are rarely found in cardiomyocytes. Patients with confirmed myocarditis have an increased number of infected interstitial cells where proteolytic enzymes or increased concentrations of TNF-α or interleukin may injure the myocytes. Studies have revealed that these affected patients have increased concentrations of TNF-α, inducible nitric oxide synthase, and IL-6 [5,62,71,73]. About 40% of patients with HIV-related cardiomyopathy have no opportunistic infection before the onset of cardiac symptoms [5,6], although this cardiomyopathy is commonly not associated with specific opportunistic infections.
Cytokine alterations
Increased TNF-α production induced by HIV infection can elevate nitric oxide production and alter intracellular calcium homeostasis, transforming growth factor-β and endothelin-1 activity [74]. When nitric oxide concentrations were elevated experimentally, myocytes were killed or injured, causing negative inotropic effects [74]. Clinical trials are needed in order to determine the effect of cytokine alterations in the current post-HAART era.
Nutritional deficiencies
Nutritional deficiencies are common in HIV-infected individuals, particularly in the late stages and in young infants. Electrolyte imbalances and deficiencies in elemental nutrients are often a result of diarrhoea and poor absorption. Deficiencies of trace elements have been associated with cardiomyopathy. For example, coxsackievirus is more virulent in selenium-deficient cardiac tissue [54]. Left ventricular function is restored and cardiomyopathy is reversed with selenium replacement. Concentrations of vitamin B12, carnitine, growth hormone, and thyroid hormone can be altered in HIV disease; all have been associated with LV dysfunction [69,75].
Course of disease
Patients with asymptomatic LV dysfunction, defined as a LV fractional shortening <28% with global LV hypokinesis, may have echocardiographically defined transient disease. One serial echocardiographic study reported that three out of six patients with abnormal LV fractional shortening had normal readings after a mean of nine months. The three patients with persistently depressed LV function all died within one year of diagnosis of LV systolic dysfunction [5].
Prognosis
Mortality has increased in HIV-infected patients with cardiomyopathy, independently of CD4 count, sex, age, or HIV risk group. In the pre-HAART era, median survival from diagnosis to AIDS-related death was 101 days in patients with LV dysfunction. Patients with normal hearts had a median survival of 472 days at a similar stage of infection [1,5]. Neither isolated right ventricular dysfunction nor borderline LV dysfunction increased the risk of AIDS-related death.
In the P2C2 HIV study, the median age was 2.1 years and five-year cumulative survival was 64% [5]. Children with baseline measurements showing depressed LV fractional shortening or increased LV dimension, mass, thickness, heart rate, blood pressure, or wall stress had a higher mortality. Increased LV wall thickness and decreased LV fractional shortening also predicted adjusted survival (Figure 2) [5]. Although increased LV wall thickness identified a population at risk only 18–24 months before death, LV fractional shortening was abnormal for three years before death. Although most patients received zidovudine at some point during the P2C2 study, a separate report found that zidovudine was not associated with cardiac complications [76]. Thus, LV fractional shortening may be a useful long-term predictor of mortality, and LV wall thickness, a useful short-term predictor in children receiving ART from the pre-HAART era [5,24,65,77].
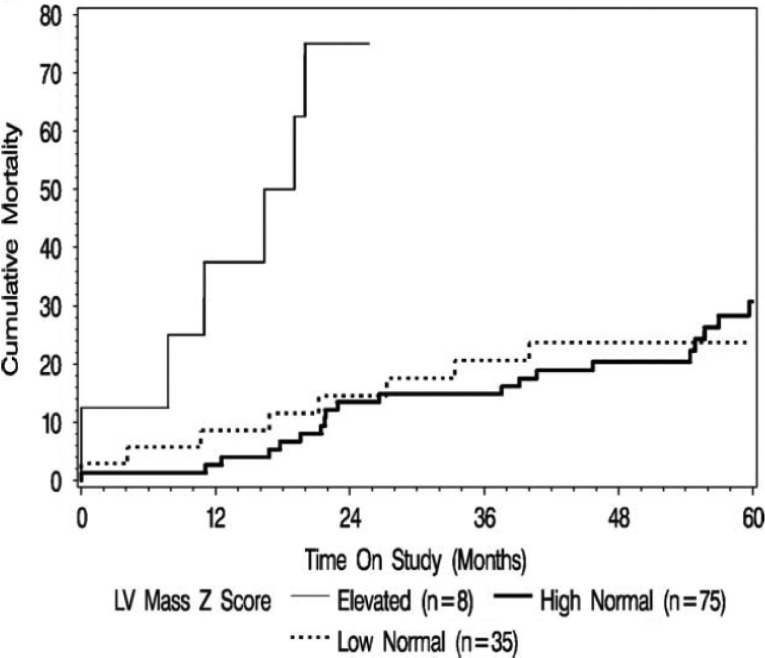
Figure 2.
Mildly increased LV mass is a risk marker for early HIV mortality even though it is still inadequate for LV dimension. Reproduced with permission from ref. 24.
In the P2C2 HIV-infected cohort, echocardiographic evidence of increased LV mass was associated with post-mortem cardiomegaly and documented chronically increased heart rate before death but not with anaemia, HIV viral load, or encephalopathy [5]. Mild persistent depression of LV function and elevated LV mass were associated with higher all-cause mortality in children infected with HIV [24,65,68]. A reduction in LV fractional shortening from 34 to 30% in a ten-year old, equivalent to a reduction of 2 Z-scores, is associated with an increase from 15 to 55% in five-year mortality [65,68]. Fractional shortening was higher in HIV-uninfected children of HIV-infected mothers with in utero exposure to ART than in HIV-uninfected children of HIV-infected mothers unexposed to ART. However, exposure to ART was associated with decreased LV mass, LV dimension, and septal thickness [68]. Any exposure to HAART in perinatally-infected children with HIV markedly affects LV mass, LV contractility, and LV afterload [78]. Rapid-onset heart failure has a grim prognosis in both HIV-infected children and adults. More than half of patients die from primary cardiac failure within a year of presentation [1,5,62].
Therapy
Similar to non-ischemic cardiomyopathy, therapy for dilated cardiomyopathy associated with HIV infection includes diuretics, digoxin, aldosterone antagonists, β-blockers, and angiotensin-converting enzyme inhibitors, as tolerated. The efficacy of specific cardiac therapeutic regimens other than intravenous immunoglobulin is unknown [2]. Due to low systemic vascular resistance, patients may be very sensitive to angiotensin-converting enzyme inhibitors. Preventing heart failure using HAART remains the best treatment [59,60,62].
Infections should be treated to improve or resolve related cardiomyopathy. Right ventricular biopsy may assist in target therapy in addition to identifying infectious causes of failure [62]. Right ventricular biopsy may be underused [6,62,64,71].
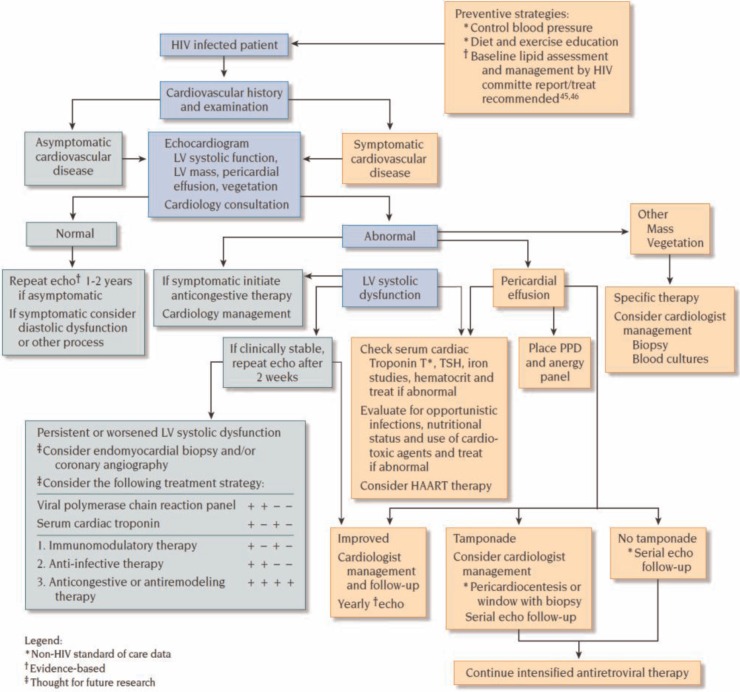
Serial echocardiographic measurements should be performed at clinically relevant intervals, such as four months, after medical therapy is begun. Monitoring recommendations for testing and timing of follow-up are based on studies relating impaired LV fractional shortening to a worse prognosis. A biopsy should be considered if cardiac function continues to deteriorate or if the clinical course worsens. Patients with heart failure who have not responded to two weeks of medical therapy may benefit from cardiac catheterization and endomyocardial biopsy, which may reveal lymphocytic infiltrates suggesting myocarditis or treatable opportunistic infections (by special stains), permitting aggressive therapy of an underlying pathogen [5,52,62,68,71,74]. Angiography should be performed selectively if there are risk factors for atherosclerotic disease or suggestive clinical symptoms (Figure 3) [33,44].
Figure 3.
Cardiac dysfunction in HIV-infected patients. HAART=highly active antiretroviral therapy; LV=left ventricular; PPD=purified protein derivative; TSH=thyroid-stimulating hormone. Reproduced with permission from “Fisher SD, Lipshultz SE. Chapter 72: Cardiovascular abnormalities in HIV-infected individuals. In: Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine, Ninth Edition. Editors: Bonow RO, Mann DL, Zipes DP, Libby P. Philadelphia: Elsevier Saunders. 1618–27. 2011 ISBN: 978-1-4377-0398-6.”
In HIV-uninfected children, intravenous immunoglobulins help treat acute congestive cardiomyopathy and nonspecific myocarditis. Monthly immunoglobulin infusions have minimized LV dysfunction, increased LV wall thickness, and reduced peak LV wall stress in HIV-infected children, suggesting that both impaired myocardial growth and LV dysfunction can be immunologically mediated [2].
Although transplantation therapy is not widely available, it remains an area of active research and has been successfully performed [70].
Animal models
Exposure to a ubiquitous environmental agent, heat-killed Mycobacterium avium complex, results in exaggerated myocardial pathology in Rhesus macaques infected with simian immunodeficiency virus. In this model, enternacept (a TNF antagonist) prevented LV dysfunction, suggesting a TNF-α-dependent pathway in the development of cardiomyopathy in HIV infection [74].
Left ventricular diastolic dysfunction
Diastolic dysfunction is relatively common in long-term survivors of HIV infection, as suggested by clinical and echocardiographic data. Such LV dysfunction may precede LV systolic dysfunction and mark an early manifestation of HIV-associated cardiac disease [18,75,79–81]. However, LV diastolic function has not been characterized in HIV-uninfected children exposed in utero to ART. Slower LV relaxation during diastole leads to a decrease in early diastolic filling. Left ventricular compliance decreases as LV diastolic dysfunction worsens and left atrial pressure increases. Moderate-to-severe LV diastolic dysfunction, an independently predicts mortality, regardless of normal LV systolic function [82]. The clinical impact of LV diastolic function has been studied in children with cardiomyopathy along with other co-morbidities, such as obesity, generalized autoimmune disease, and diabetes [83–88].
In one cross-sectional study, early diastolic mitral valve annular velocity was lower in HIVEU children born to HIV-infected mothers who were exposed in utero to ART compared to a group of HIV-uninfected children born to HIV-uninfected mothers with no perinatal ART exposure. In addition, lower early diastolic mitral valve annular velocity was associated with lower maternal CD4 counts in the final trimester [89]. The longitudinal CHAART-I study found subclinical LV diastolic abnormalities in both LV compliance and relaxation among HIVEU children exposed perinatally to multi-drug ART [83]. In a study of 656 asymptomatic HIV-infected adults, 26% had screening echocardiographic evidence of LV diastolic dysfunction [64]. Adults with LV diastolic dysfunction, compared to those without, were older, tended to have higher body mass indexes, more likely to have hypertension, and had been infected longer [81]. Whether LV diastolic dysfunction is associated with an increased risk of early coronary disease is unknown [75,81]. In children, also unknown is the clinical importance of LV systolic versus diastolic dysfunction in HIV-infected and HIVEU children perinatally exposed to multi-drug ART. The temporal occurrence of these LV systolic versus diastolic echocardiographic changes is important in determining the effects of HIV exposure and ART exposure.
Uncontrolled HIV replication and ART increase IL-6 concentrations [90]. Viral proteins or replication in the myocardial macrophages in animal models may cause LV diastolic dysfunction. Longitudinal mitral inflow and tissue Doppler echocardiographic studies of Rhesus macaques infected with simian immunodeficiency virus found that LV diastolic dysfunction was common and strongly correlated with the extent of viral replication in the myocardium [90].
Pulmonary hypertension
Pulmonary arterial hypertension (PAH) occurs in about 0.5% of HIV-infected patients. This does not include cases of elevated pulmonary pressure secondary to interstitial lung disease or chronic obstructive pulmonary disease where the pathophysiology and response to therapies differ. The introduction of HAART has not changed the prevalence of pulmonary arterial hypertension [3,91–94]. In HIV-infected patients, normal endothelial structure is replaced by plexogenic pulmonary arteriopathy, which is characterized by remodelling of the pulmonary vasculature with intimal fibrosis [92,93]. Perfusion scans are normal and lung fields may be clear on examination and chest radiographs [92]. Pulmonary arterial hypertension has been reported in HIV-infected patients without a history of thromboembolic disease, intravenous drug use, or pulmonary infections associated with HIV [3,93,94].
Primary pulmonary hypertension has been found in patients with haemophilia receiving lyophilized factor VIII, intravenous drug users, and patients with LV dysfunction, obscuring any relationship with HIV [3,93]. Whether PAH is associated with human herpesvirus 8 is unclear. HIV or a co-infection might cause endothelial damage and mediator-related vasoconstriction of the pulmonary arteries.
Two recent studies found that CD4 count was independently associated with survival in 154 patients with HIV and PAH, with pulmonary hypertension as the direct cause of death in 72% of those affected. Survival rates at one, two, and three years were 73, 60, and 47%, respectively. Survival rates in New York Heart Association functional Class III–IV patients at the time of diagnosis were 60, 45, and 28% at one, two, and three years [93,94], respectively. In one year, 52% of 549 patients with HIV and PAH died with 51% from right heart failure [94].
Standard treatments for PAH, such as PDE-5 inhibitors, endothelin antagonists, and prostacyclin analogues, have been effective in HIV-infected patients. Therapy also includes anticoagulation (on the basis of individual risk-benefit analysis) [92]. Affected patients have continued HAART. In patients with HIV and PAH, PAH should be aggressively treated because it is life-threatening as set forward in the American College of Cardiology Foundation's treatment guidelines for PAH [92]. Morbidity and mortality seem to be caused by PAH more than by HIV infection and, therefore, should be clinically managed based on current recommendations from the American College of Cardiology expert consensus document on pulmonary hypertension [92].
Pericardial effusion
Incidence
Pericardial effusions were found in up to 11% of patients with AIDS before the HAART era. The prevalence of effusion in asymptomatic AIDS patients reaches a mean of about 22% after 25 months, rising over time [95]. In a recent study, only 2 out of 802 HAART-treated patients had clinically important effusions, indicating the greatly reduced incidence with treatment of HIV [95].
Clinical presentation
HIV-infected patients with pericardial effusions generally have lower CD4 counts than those without effusions [92]. Effusions are generally small and asymptomatic. HIV infection should be suspected whenever a patient presents with unexplained pericardial effusion or tamponade. In a retrospective series from a city hospital, 13 out of 37 (35%) patients with cardiac tamponade had HIV infection [95]. Although rare, tuberculosis has been found as a presenting infection for pericardial effusions in underdeveloped areas where tuberculosis is prevalent [95,96]. These cases have therapeutic implications and deserve special attention [97].
Pathogenesis
Pericardial effusion is often part of a generalized serous effusive process also involving pleural and peritoneal surfaces. Enhanced cytokine production in AIDS may be associated with this “capillary leak”. Other well-described associations (Table 1) include uremia from HIV-associated nephropathy or drug nephrotoxicity. Effusion nearly triples the risk of death among AIDS patients [95]. Immune reconstruction inflammatory syndrome can cause pericardial effusions and pericarditis in patients co-infected with HIV and tuberculosis [98]. Pericardiocentesis has been found to be a safe and effective treatment of tuberculosis pericardial effusions in HIV-infected patients [99].
Monitoring and therapy
Baseline echocardiography and ECG measurements should be taken on all HIV-infected patients with evidence of heart failure, Kaposi's sarcoma, tuberculosis, or other pulmonary infections. Pericardiocentesis is indicated for pericardial effusion when there are clinical signs of tamponade (such as elevated jugular venous pressure, dyspnea, hypotension, persistent tachycardia, or pulsus paradoxus), or echocardiographic signs of tamponade (such as continuous-wave Doppler echocardiographic evidence of respiratory variation in valvular inflow, septal bounce, right ventricular diastolic collapse, and a large effusion).
Patients with pericardial effusion without tamponade should be evaluated for malignancy and opportunistic infections, such as tuberculosis. HAART should be considered if it has not already been instituted. Repeat echocardiography is recommended after one month or sooner if indicated (Figure 3) [20,62].
Infective endocarditis
Infective endocarditis has been reported in adults with HIV infection, most commonly in intravenous drug users, and usually causes right-sided endocarditis. The most common organism associated with endocarditis in HIV-infected adult patients is Staphylococcus aureus. Endocarditis caused by Aspergillus fumigatus, Candida species, and Cryptococcus neoformans are more common in intravenous drug users with HIV than in those without HIV. Generally, HIV does not appear to significantly influence the response to treatment or outcome (Table 1) [20].
Late-stage AIDS patients with poor nutritional status and severely compromised immune systems may experience a more fulminant course and a higher mortality. However, several patients have been successfully treated with antibiotics. Surgical indications in HIV-infected patients with endocarditis include persistent bacteremia despite intravenous antibiotics to which the organism is sensitive, hemodynamic instability, persistent embolization and severe valvular destruction in patients with a reasonable life expectancy after surgery.
Endocarditis in HIV-infected children is rare. There is a report of a two month old HIV-infected Ugandan boy who presented with disseminated Staphylococcus aureus infection with a large obstructing vegetation on the free wall of the left ventricle in association with a purulent pericardial effusion and an empyema. Echocardiogram showed no structural abnormalities other than a patent foramen ovale [100].
Nonbacterial thrombotic endocarditis
Marantic or non-bacterial thrombotic endocarditis involves deposition of large, friable, sterile vegetations predominantly on the cardiac valves. These vegetations have been associated with disseminated intravascular coagulation and systemic embolization. Vegetations are rarely diagnosed before death, but when they are, clinically important emboli are likely [20]. Marantic endocarditis is rare in children, but was described in a child newly diagnosed with HIV at age 14 months. The child developed pneumonia, Staphylococcus sepsis, and later developed acute cardiac failure with valvular dysfunction, hepatosplenomegaly, ascites and failure to thrive. An echocardiogram showed bright echoes within the chordea of the tricuspid valve and the tips of the leaflets. After a complicated course, the child died of pulmonary insufficiency at age 34 months [101]. It is likely that this type of endocarditis is more likely to be identified in patients with delayed HIV diagnosis, limited or no access to ART and those with progressive disease. In the early HIV epidemic, several case series in adults suggested a high incidence of this uncommon disorder; however, few cases have since been reported.
Cardiovascular malignancy
Malignancy affects many adult AIDS patients, generally in the later stages of disease. Cardiac malignancy may be a primary tumour or a metastatic secondary site. Although lymphomas have been associated with malignancy in HIV-infected children, the incidence is low and cardiac malignancy is rare in children with HIV infection. The Children's Cancer Group and the Paediatric HIV Clinic at the National Cancer Institute reported 65 tumours diagnosed between 1982 and 1997 in 64 HIV-infected children [102], although these patients were not on treatment. Non-Hodgkin's lymphoma accounted for 65% of these tumours. In this study, almost one-third of the children with this disease had normal or moderate immune suppression. Leiomyosarcoma occurred in 17% and Kaposi's sarcoma in 5%.
Kaposi's sarcoma (angiosarcoma) affected up to 35% of AIDS patients early in the HIV epidemic and is associated with human herpesvirus 8. Its incidence is inversely related to CD4 count. Although sarcoma is infrequently described as a primary cardiac tumour, autopsy studies have found that 28% of HIV-infected patients with widespread Kaposi's sarcoma had cardiac involvement [4]. Kaposi's sarcoma is often an endothelial cell neoplasm with a predilection in the heart for sub-pericardial fat around the coronary arteries [4,20]. Combination antiretroviral therapy has markedly decreased the incidence of Kaposi's sarcoma from that in the pre-HAART era [20].
Children with HIV infection may harbour human herpesvirus 8, the virus associated with Kaposi's sarcoma. Kaposi's sarcoma is endemic in eastern equatorial Africa. It can cause a lymphadenopathic type of Kaposi's sarcoma that is found mainly in children, which may have a fulminant course and ultimately also invade organ systems. Two children were reported in the United States early in the epidemic, both died before one year of age and had progressive HIV infection with severe immune deficiency. There were lesions of Kaposi's sarcoma in the lymph glands and spleen and in one case in the thymus [103].
Primary cardiac malignancy associated with HIV infection is generally caused by cardiac lymphoma. Lymphoma, an AIDS-defining illness, has a higher incidence in HIV-infected populations. Non-Hodgkin's lymphomas are 25–60 times more common in HIV-infected individuals. They are the first manifestation of AIDS in up to 4% of new cases [4]. This disease is not specifically associated with severe immune suppression. Patients with primary cardiac lymphoma can present with signs of heart failure, chest pain, or arrhythmias. Cardiac lymphoma can cause rapid progression to cardiac tamponade, heart failure, myocardial infarction, tachyarrhythmias, conduction abnormalities, or superior vena cava syndrome. Malignant cells can be found in the pericardial fluid. Systemic multi-agent chemotherapy with and without concomitant radiation or surgery has benefitted some patients, but overall, the prognosis is poor [4]. Treatment with HAART has not substantially affected the incidence of HIV-related non-Hodgkin's lymphomas [20,104].
Isolated right ventricular disease
Isolated right ventricular hypertrophy is rare in HIV-infected individuals, with or without right ventricular dilation. It is generally related to pulmonary disease that increases pulmonary vascular resistance. Possible causes include pulmonary arteritis from the immunological effects of HIV disease, multiple bronchopulmonary infections, or microvascular pulmonary emboli caused by thrombus or contaminants in injected drugs such as Talc [3]. Right ventricular diastolic dysfunction has been reported in asymptomatic patients studied with Doppler tissue imaging [105].
Vasculitis
Vasculitis may occur in patients with fever of unknown origin, unexplained arthritis or myositis, unexplained multisystem disease, glomerulonephritis, or peripheral neuropathy (especially mononeuritis multiplex), and in unexplained gastrointestinal, cardiac or central nervous system ischemia. Several types have been described in HIV-infected patients, but all types show diffuse inflammation of the vessel walls [106]. Successful immunomodulatory therapy has been reported, chiefly with systemic corticosteroid therapy [106]. The HIV protein, transactivator of transcription (Tat), has been implicated in the pathogenesis of vasculitis [106].
Sudden cardiac death
Sudden cardiac death is becoming increasingly common as the HIV-infected population ages. In one study, sudden cardiac death accounted for 86% of all cardiac-related deaths (30 of 35). The mean rate of sudden cardiac death was 2.6 per 1000 person-years (95% confidence interval: 1.8–3.8), which was 4.5-fold as high as that expected in an age-matched uninfected population [107]. One report found that patients dying from sudden cardiac death were older than those dying from AIDS (mean age at death, 49 vs. 45 years, p=0.02), had a higher prevalence of prior MI (17% vs. 1%, p<0.001), cardiomyopathy (23% vs. 3%, p<0.001), heart failure (30% vs. 9%, p=0.004), and arrhythmias (20% vs. 3%, p=0.003) [107].
QT interval and PR prolongation
HIV infection is associated with QT prolongation and Torsades de Pointes ventricular tachycardia. There is an increased risk of sudden death late in HIV infection and specifically with AIDS. The incidence of QT prolongation increases as the disease progresses to AIDS [108]. Hepatitis C is independently associated with increased QT duration. One study found that the risk of QT prolongation (that is, QTc values of 470 ms or higher) was 16% with HIV alone and 30% with both HIV and hepatitis C infections [109]. The risk of increased QT duration is also higher in patients treated with ART as well as anti-tuberculosis medications, such as levofloxacin, moxifloxacin, and bedaquoline [51].
Different protease inhibitor-based regimens have a similar, minimal effect on the QT interval, but significantly prolong the PR interval by a difference of 3 ms in non-boosted protease inhibitor regimen to 5.11 ms in boosted protease inhibitor regimen. The intervals do normalize on withdrawal of the protease inhibitor therapy and prolongation is not associated with NNRTIs. The clinical significance is not well established [110]. It is thought that PR prolongation may lead to a higher likelihood of complete heart block during immune reconstitution inflammatory syndrome during initiation of ART.
Autonomic dysfunction
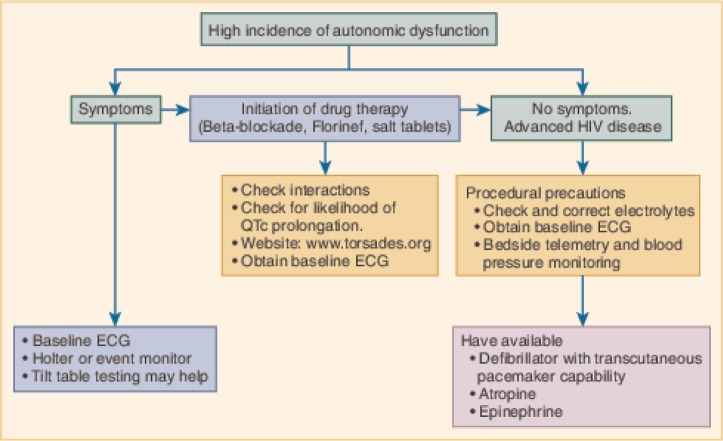
Preliminary clinical signs of autonomic dysfunction in HIV-infected patients include syncope and presyncope, diarrhoea, diminished sweating, bladder dysfunction, and impotence. One study found heart rate variability Valsalva ratio, cold pressor testing, hemodynamic responses to isometric exercise, tilt-table testing, and standing showed that autonomic dysfunction occurred in HIV-infected individuals and was pronounced in AIDS patients. AIDS patients receiving HAART were relatively protected. Patients with HIV-associated nervous system disease had the greatest abnormalities in autonomic function (Figure 4) [111].
Figure 4.
Evaluation and management of dysautonomia. ECG = electrocardiography. Reproduced with permission from “Fisher SD, Lipshultz SE. Chapter 72: Cardiovascular abnormalities in HIV-infected individuals. In: Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine, Ninth Edition. Editors: Bonow RO, Mann DL, Zipes DP, Libby P. Philadelphia: Elsevier Saunders. 1618–27. 2011 ISBN: 978-1-4377-0398-6.”
Complications of therapy
Antiretroviral medications have greatly reduced mortality by delaying the progression to AIDS and increased quality of life of HIV-infected patients [52]. However, these same therapies are associated with a number of complications [20,57,80,112,113].
As previously detailed, altered body composition and hyperlipidemia are associated with PIs. Nucleoside reverse transcriptase inhibitors may lead to increasing the child's cardiometabolic risk [112,113]. Lipid abnormalities vary with different PIs. Ritonavir had the most adverse effects on lipids, with a mean increase in total cholesterol concentration of 2.0 mmol/L and a mean increase in triglyceride concentration of 1.83 mmol/L [57,80,114]. More modest increases of total cholesterol concentration without marked triglyceride increases were found in patients taking indinavir and nelfinavir. Combination with saquinavir (including atanazavir and saquinavir in salvage therapy) did not further elevate total cholesterol concentrations. Protease inhibitors significantly increased lipoprotein (a) in patients with elevated pre-treatment values (>20 mg/dL), which is another risk marker for atherosclerotic cardiovascular disease [57,80,114]. In some cases, switching PIs may reverse both elevations in triglyceride concentrations and abnormal fat deposition. Low-level aerobic exercise may also help reverse lipid abnormalities [20,53]. Zidovudine or azidothymidine (AZT) has been implicated in skeletal muscle myopathies. In culture, AZT causes a dose-dependent destruction of human myotubes. Human cultured cardiac muscle cells treated with AZT developed mitochondrial abnormalities, and nucleoside reverse transcriptase inhibitors in general have been associated with altered mitochondrial DNA replication and cardiac structure [20,115]; it is uncertain whether altered mitochondrial DNA replication is the cause of cardiomyopathy. However, cardiac myopathies have not been evident in clinical data. Some patients with LV dysfunction may improve when AZT therapy is stopped [20]. Some evidence has been presented associating ARTs and mitochondrial toxicity [116]. This and additional factors may predispose children infected with HIV to reduced aerobic capacity. HIV-infected children and adolescents had lower cardiorespiratory fitness, lower extremity strength, and flexibility than did their uninfected counterparts. Additionally, HAART exposure for greater than five-years and higher total body fat percentage independently had a negative effect on aerobic capacity [60].
Intravenous pentamidine, used to treat Pneumocystis jirovecii pneumonia in patients intolerant of trimethoprim-sulfamethoxazole, has been associated with Torsades de Pointes and refractory ventricular tachycardia [20]. Pentamidine should be reserved for patients with a QTc interval below 480 ms. Multiple medication reactions and interactions have occurred during HIV treatment and are a major cause of cardiac emergencies in HIV-infected patients (Table 3) [62,65,117].
Table 3.
Cardiac interactions and side effects of drugs commonly used in HIV therapy
| Class | Cardiac drug interactions | Cardiac side effects |
|---|---|---|
|
Antiretroviral
Nucleoside (and nucleotide) reverse transcriptase inhibitors Abacavir (ABC), Didanosine (ddI), Emtricitabine (FTC) Lamivudine (3TC), Stavudine (d4T), Tenofovir (TDF), Zalcitabine (ddC), Zidovudine (ZDV, AZT) |
Zidovudine and dipyridamole Stavdine and DDI |
Rare: lactic acidosis, hypotension Accelerated risk with cardiopulmonary bypass Zidovudine: skeletal muscle myopathy, myocarditis Mitochondrial toxicity with lipodystrophy |
| Non-nucleoside reverse transcriptase inhibitors Delavirdine (DLV), Efavirenz (EFV), Nevirapine (NVP), Rilpivirine (RPV) |
Calcium channel blockers, warfarin, β-blockers, nifedipine, quinidine, steroids, theophylline. Delavirdine can cause serious toxic effects if given with antiarrhythmic drugs and calcium channel blockers |
Arrhythmia |
| Protease inhibitors Amprenavir (APV), Atazanavir (ATV), Darunavir (DRV), Fosamprenavir (FPV) Indinavir (IDV), Lopinavir/ritonavir (LPV/r), Nelfinavir (NFV), Ritonavir (RTV), Saquinavir (SQV), Tipranavir (TPV) |
Metabolized by cytochrome P450 and interact with other drugs metabolized through this pathway, such as selected antimicrobials, antidepressant and antihistamine agents, cisapride, HMG CoA reductase inhibitors (lovastatin, simvastatin), and sildenafil. Potentially dangerous interactions that require close monitoring or dose adjustment can occur with amiodarone, disopyramide, flecainide, lidocaine, mexiletine, propafenone, and quinidine. Ranolazine (1.8–2.3× increase in Ranolazine level) Ritonavir is the most potent cytochrome activator (CYP3A) and P-glycoprotein inhibitor and is most likely to interact. Indinavir, amprenavir, and nelfinavir are moderate. Saquinavir has the lowest probability to interact Calcium channel blockers, prednisone, quinine, β-blockers (1.5- to 3-fold increase). Decreases theophylline concentrations |
Implicated in premature atherosclerosis, dyslipidemia, insulin resistance, diabetes mellitus, fat wasting, and redistribution Abacavir may be associated with increased risk of MI13 |
| Integrase strand transfer inhibitors (INSTIs) Elvitegravir (EVG), Raltegravir (RAL) |
||
| CCR5 antagonists Maraviroc |
||
| Fusion inhibitor Enfuvirtide |
– | |
| Anti-infective antibiotics |
Rifampin:
Reduces therapeutic effect of digoxin by inducing intestinal P-glycoprotein, reduces protease inhibitor concentration and effect Erythromycin: Cytochrome P450 metabolism and drug interactions Trimethoprim-sulfamethoxazole: (Bactrim) increases warfarin effects |
Erythromycin:
Orthostatic hypotension, ventricular tachycardia, bradycardia, Torsades (with drug interactions) Clarithromycin: QT prolongation and Torsades de Pointes Trimethoprim-sulfamethoxazole: Orthostatic hypotension, anaphylaxis, QT prolongation, Torsades de Pointes, hypokalemia Sparfloxacin (fluoroquinolones): QT prolongation |
| Antifungal agents |
Amphotericin B:
Digoxin toxicity Ketoconazole or itraconazole: Cytochrome P450 metabolism and drug interactions—increases levels of sildenafil, warfarin, HMG CoA reductase inhibitors, nifedipine, digoxin |
Amphotericin B:
Hypertension, arrhythmia, renal failure, hypokalemia, thrombophlebitis, bradycardia, angioedema, dilated cardiomyopathy. Liposomal formulations still have the potential for electrolyte imbalance and QT prolongation Ketoconazole, fluconazole, itraconazole: QT prolongation and torsades de pointes |
| Antiviral agents |
Ganciclovir:
Zidovudine |
Foscarnet:
Reversible cardiac failure, electrolyte abnormalities Ganciclovir: Ventricular tachycardia, hypotension |
| Antiparasitic |
Pentamidine:
Hypotension, QT prolongation, arrhythmias (Torsades de Pointes), ventricular tachycardia, hyperglycemia, hypoglycemia, sudden death. These effects are enhanced by hypomagnesemia and hypokalemia |
|
| Chemotherapy agents |
Vincristine, doxorubicin:
Decrease digoxin level |
Vincristine:
Arrhythmia, myocardial infarction, cardiomyopathy, autonomic neuropathy Recombinant human interferon-alpha: Hypertension, hypotension, tachycardia, acute coronary events, dilated cardiomyopathy, arrhythmias, sudden death, atrioventricular block, peripheral vasodilation. Contraindicated in patients with unstable angina or recent myocardial infarction Interleukin-2: Hypotension, arrhythmia, sudden death, myocardial infarction, dilated cardiomyopathy, capillary leak, thyroid alterations Anthracyclines (doxorubicin, daunorubicin, mitoxantrone): Myocarditis, cardiomyopathy Liposomal anthracyclines: As above for doxorubicin and also vasculitis |
| Pentoxifylline |
Pentoxifylline:
Decreased triglyceride levels, arrhythmias, chest pain Megace: Edema, thrombophlebitis, hyperglycemia |
|
| Megestrol acetate (Megace) | Epoetin alpha (erythropoietin): Hypertension, ventricular dysfunction | |
| Methadone | Prolonged QT interval | |
| Amphetamines | Increased heart rate and blood pressure |
Modified with permission from Fisher SD, Lipshultz SE. Chapter 72: Cardiovascular abnormalities in HIV-infected individuals. In: Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine, Ninth Edition. Editors: Bonow RO, Mann DL, Zipes DP, Libby P. Philadelphia: Elsevier Saunders. 1618–27. 2011 ISBN: 978-1-4377-0398-6.
Mother-to-child transmission has been reduced in the United States to approximately 1–2% (CDC). Intrauterine exposures to these potent ARTs have been shown to have some effects on the child [118]. At birth, children exposed to HIV and ARTs were lighter than a comparison group with no exposures to ARTs and showed accelerated growth during the first two years of life. Additionally, these children had less subcutaneous fat and decreasing mid-upper arm circumference over time when compared to national standards [119].
Perinatal transmission of HIV-infection
Although HIV transmission can be minimized if mothers are given ART in the second and third trimesters or short courses before parturition, most children with HIV are infected in the perinatal period [120]. Current therapies, some including up to six months of neonatal AZT, can limit the incidence of perinatal transmission to <2%. A worldwide UNAIDS goal is to eliminate perinatal transmission by the end of 2014.
Rates of congenital cardiovascular malformations ranged from 5.6 to 8.9% in cohorts of HIV-uninfected and HIV-infected children born to HIV-infected mothers. Although these rates were not higher than in similarly screened normal populations, they were 5 to 10 times as high as those reported in population-based epidemiological studies [120].
In the same cohorts, serial echocardiograms performed at four- to six-month intervals showed subclinical cardiac abnormalities to be common, persistent, and often progressive [5,62,68]. Some patients had dilated cardiomyopathy (LV contractility 2 standard deviations or more below the mean of a normative population and LV end-diastolic dimension 2 standard deviations or more above the mean) whereas others had mildly increased cardiac mass for height and weight. Depressed LV function correlated with immune dysfunction at baseline but not over time. This correlation suggests that the CD4 cell count may not be a useful surrogate marker of HIV-associated LV dysfunction. Disease can progress rapidly or slowly in children with perinatally-transmitted HIV-1 infection [62]. Rapid progressors have higher heart rates, higher respiratory rates, and lower fractional shortening on serial examinations than do non-rapid progressors and HIV-uninfected children who are similarly screened. Rapid progressors also have higher HIV-1 viral loads, higher five-year cumulative mortality, and lower CD8+ (cytotoxic) T-cell counts.
Studies of non-HIV-infected infants born to HIV-infected mothers have reported that foetal exposure to ART is associated with reduced LV dimension, LV mass, and septal wall thickness along with higher LV fractional shortening and contractility during the first two years of life [121]. In utero exposure to ART may initially improve LV function while impairing myocardial growth. Although LV function is improved, it is still below normal [68]. These effects are more pronounced in girls [68].
Conclusions
Cardiac monitoring recommendations
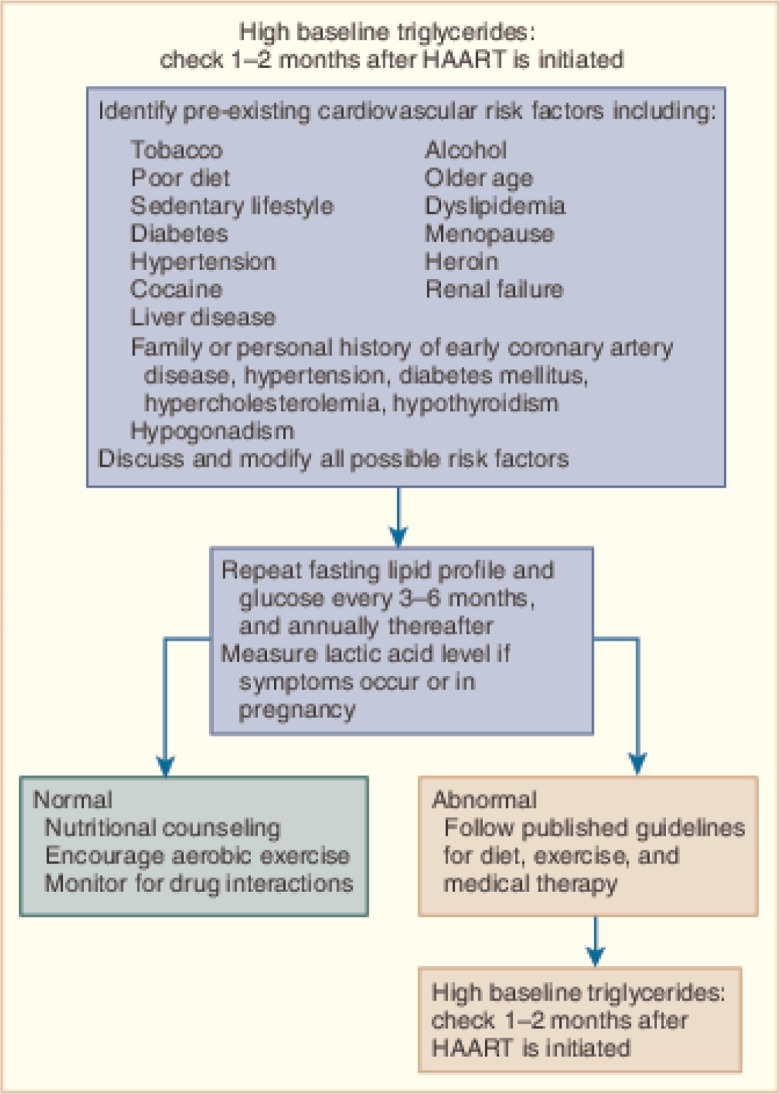
Routine, systematic cardiac evaluation, including a comprehensive history and thorough cardiac examination, is essential care for HIV-infected children and adults. The history should include traditional risk factors, environmental exposures, prior opportunistic infections, and therapeutic and illicit drug use. Laboratories should include a lipid profile, fasting glucose, and HIV viral load (Figure 5). Routine blood pressure monitoring is important because HIV-infected individuals can experience hypertension at a younger age and more frequently than in the general population [20,33,52].
Figure 5.
Cardiovascular considerations when initiating highly active antiretroviral therapy (HAART). Reproduced with permission from “Fisher SD, Lipshultz SE. Chapter 72: Cardiovascular abnormalities in HIV-infected individuals. In: Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine, Ninth Edition. Editors: Bonow RO, Mann DL, Zipes DP, Libby P. Philadelphia: Elsevier Saunders. 1618–27. 2011 ISBN: 978-1-4377-0398-6.”
Unless patients have symptoms such as palpitations, syncope, stroke, or dysautonomia, routine ECG and Holter monitoring are not warranted. These tests can be useful for baseline and monitoring before, during, and after therapies, such as pentamidine, methadone, or antibiotics that may prolong the QT interval [108].
Asymptomatic cardiac disease related to HIV can be fatal. When present, cardiac symptoms are often disguised by secondary effects of HIV infection. Thus, systematic echocardiographic monitoring is warranted [64,65,122,123]. An international consensus panel recommended echocardiographic monitoring, with a baseline, for any patient at high risk or with any clinical manifestation of CVD, in addition to studies every 1–2 years or as clinically indicated. Patients with cardiac symptoms should begin directed therapy and receive a formal cardiac assessment, including baseline ECG, echocardiography, and Holter monitoring [124]. Brain natriuretic peptide concentrations may help diagnose ventricular dysfunction [125,126].
Serum troponin assays are indicated in patients with LV dysfunction. Elevated concentrations of serum troponin warrant consideration of endomyocardial biopsy and cardiac catheterization. Therapy with intravenous immunoglobulin should be considered for biopsy-proven myocarditis [2]. Echocardiography should be repeated after two weeks of therapy to encourage continued therapy if improvement has occurred and adapt a more aggressive approach if LV dysfunction persists or worsens.
Stress testing and coronary assessment such as CT angiography or cardiac catheterization should be considered in the appropriate clinical settings [33,44,54,62]. Guidelines for using implantable cardioverter-defibrillators should be followed in this population, especially in patients after MI being treated for HIV infection [33].
As a chronic disease, HIV-related CVD is a vital area of research. If HIV can be used as a model of chronic immunosuppression in a large population, findings may translate to other populations. Understanding genetic predispositions to QT prolongation may guide therapy. Understanding the causes of cardiomyopathy may benefit diverse research efforts, such as the effects of cytokines, mitochondria, and neurohormonal pathways. Observations, such as increased mortality related to LV mass and very mild LV dysfunction might enhance diagnostic testing in at-risk populations affected by other poorly understood cardiomyopathies.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
All authors contributed to the content and design of this review and have read and approved the final version.
References
- 1.Currie PF, Jacob AJ, Foreman AR, Elton RA, Brettle RP, Boon NA. Heart muscle disease related to HIV infection: prognostic implications. BMJ. 1994;309:1605. doi: 10.1136/bmj.309.6969.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipshultz SE, Orav EJ, Sanders SP, Colan SD. Immunoglobulins and left ventricular structure and function in pediatric HIV infection. Circulation. 1995;92:2220–5. doi: 10.1161/01.cir.92.8.2220. [DOI] [PubMed] [Google Scholar]
- 3.Saidi A, Bricker JT. Pulmonary hypertension in patients infected with HIV. In: Lipshultz SE, editor. Cardiology in AIDS. New York: Chapman & Hall; 1998. pp. 187–94. [Google Scholar]
- 4.Jenson HB, Pollock BH. Cardiac cancers in HIV-infected patients. In: Lipshultz SE, editor. Cardiology in AIDS. New York: Chapman & Hall; 1998. pp. 255–63. [Google Scholar]
- 5.Lipshultz SE, Easley KA, Orav EJ, Kaplan S, Starc TJ, Bricker JT, et al. Cardiac dysfunction and mortality in HIV-infected children: the prospective P2C2 HIV multicenter study. Pediatric pulmonary and cardiac complications of vertically transmitted HIV infection (P2C2 HIV) study group. Circulation. 2000;102:1542–8. doi: 10.1161/01.cir.102.13.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morse CG, Kovacs JA. Metabolic and skeletal complications of HIV infection: the price of success. JAMA. 2006;296:844–54. doi: 10.1001/jama.296.7.844. [DOI] [PubMed] [Google Scholar]
- 7.Lipshultz SE, Fisher SD, Miller TL, Sharma TS, Milton AN. The cardiovascular manifestations of HIV infection. Dialog Cardiovasc Med. 2007;12(1):5–23. [Google Scholar]
- 8.Zareba KM, Miller TL, Lipshultz SE. Cardiovascular disease and toxicities related to HIV infection and its therapies. Expert Opin Drug Saf. 2005;4(6):1017–25. doi: 10.1517/14740338.4.6.1017. [DOI] [PubMed] [Google Scholar]
- 9.Zareba KM, Lipshultz SE. Cardiovascular complications in patients with HIV infection. Curr Infect Dis Rep. 2003;5(6):513–20. doi: 10.1007/s11908-003-0096-5. [DOI] [PubMed] [Google Scholar]
- 10.Barbaro G, Fisher SD, Lipshultz SE. Pathogenesis of HIV-associated cardiovascular complications. Lancet Infect Dis. 2001;1(2):115–24. doi: 10.1016/S1473-3099(01)00067-6. [DOI] [PubMed] [Google Scholar]
- 11.Keesler MJ, Fisher SD, Lipshultz SE. Cardiac manifestations of HIV infection in infants and children. Ann N Y Acad Sci. 2001;946:169–78. doi: 10.1111/j.1749-6632.2001.tb03911.x. [DOI] [PubMed] [Google Scholar]
- 12.Langston C, Cooper ER, Goldfarb J, Easley KA, Husak S, Sunkle S, et al. Human immunodeficiency virus-related mortality in infants and children: data from the pediatric pulmonary and cardiovascular complications of vertically transmitted HIV P2C2 study. Pediatrics. 2001;107(2):328–38. doi: 10.1542/peds.107.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Starc TJ, Lipshultz SE, Kaplan S, Easley KA, Bricker JT, Colan SD, et al. Cardiac complications in children with human immunodeficiency virus infection. Pediatric pulmonary and cardiac complications of vertically transmitted HIV infection (P2C2 HIV) study group, national heart, lung, and blood institute. Pediatrics. 1999;104(2):14. doi: 10.1542/peds.104.2.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipshultz SE, Easley KA, Orav EJ, Kaplan S, Starc TJ, Bricker JT, et al. Left ventricular structure and function in children infected with human immunodeficiency virus: the prospective P2C2 HIV multicenter study. Pediatric pulmonary and cardiac complications of vertically transmitted HIV infection (P2C2 HIV) study group. Circulation. 1998;97(13):1246–56. doi: 10.1161/01.cir.97.13.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epstein JE, Eichbaum QG, Lipshultz SE. Cardiovascular manifestations of HIV infection. Compr Ther. 1996;22(8):485–91. [PubMed] [Google Scholar]
- 16.Lane-McAuliffe EM, Lipshultz SE. Cardiovascular manifestations of pediatric HIV infection. Nurs Clin North Am. 1995;30(2):291–316. [PubMed] [Google Scholar]
- 17.Luginbuhl LM, Orav EJ, McIntosh K, Lipshultz SE. Cardiac morbidity and related mortality in children with HIV infection. JAMA. 1993;269(22):2869–75. [PubMed] [Google Scholar]
- 18.Patel N, Abdelsayed S, Veve M, Miller CD. Predictors of clinically significant drug-drug interactions among patients treated with Non-nucleoside reverse transcriptase inhibitor-, protease inhibitor-, and raltegravir-based antiretroviral regimens. Ann Pharmacother. 2011;45(3):317–24. doi: 10.1345/aph.1P576. [DOI] [PubMed] [Google Scholar]
- 19.Mas CM, Miller TL, Cordero C, Dauphin D, White MD, Vila CK, et al. The effects of fetal and childhood exposure to antiretroviral agents. J AIDS Clin Res. 2011;S2:001. [Google Scholar]
- 20.Fisher SD, Kanda BS, Miller TL, Lipshultz SE. Cardiovascular disease and therapeutic drug-related cardiovascular consequences in HIV-infected patients. Am J Cardiovasc Drugs. 2011;11(6):383–94. doi: 10.2165/11594590-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 21.Dubé MP, Lipshultz SE, Fichtenbaum CJ, Greenberg R, Schecter AD, Fisher SD. Effects of HIV infection and antiretroviral therapy on the heart and vasculature. Circulation. 2008;118(2):e36–40. doi: 10.1161/CIRCULATIONAHA.107.189625. [DOI] [PubMed] [Google Scholar]
- 22.Fisher SD, Miller TL, Lipshultz SE. Impact of HIV and highly active antiretroviral therapy on leukocyte adhesion molecules, arterial inflammation, dyslipidemia, and atherosclerosis. Atherosclerosis. 2006;185(1):1–11. doi: 10.1016/j.atherosclerosis.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 23.UNAIDS. Report on the Global AIDS epidemic. 2012. http://www.unaids.org.
- 24.Fisher SD, Easley KA, Orav EJ, Colan SD, Kaplan S, Starc TJ, et al. Mild dilated cardiomyopathy and increased left ventricular mass predict mortality: the prospective P2C2 HIV multicenter study. Am Heart J. 2005;150(3):439–47. doi: 10.1016/j.ahj.2005.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipshultz SE. Dilated cardiomyopathy in HIV-infected patients. N Engl J Med. 1998;339(16):1153–5. doi: 10.1056/NEJM199810153391609. [DOI] [PubMed] [Google Scholar]
- 26.Harmon WG, Dadlani GH, Fisher SD, Lipshultz SE. Myocardial and pericardial disease in HIV. Curr Treat Options Cardiovasc Med. 2002;4(6):497–509. doi: 10.1007/s11936-002-0043-z. [DOI] [PubMed] [Google Scholar]
- 27.Fitch K, Grinspoon S. Nutritional and metabolic correlates of cardiovascular and bone disease in HIV-infected patients. Am J Clin Nutr. 2011;94(6):1721S–8S. doi: 10.3945/ajcn.111.012120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carr A, Samaras K, Chisholm DJ, Cooper DA. Abnormal fat distribution and use of protease inhibitors. Lancet. 1998;351(9117):1736. doi: 10.1016/S0140-6736(05)77775-8. [DOI] [PubMed] [Google Scholar]
- 29.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352(1):48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 30.Palella FJ, Jr, Phair JP. Cardiovascular disease in HIV infection. Curr Opin HIV AIDS. 2011;6(4):266–71. doi: 10.1097/COH.0b013e328347876c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friis-Moller N, Weber R, Reiss P, Thiébaut R, Kirk O, d'Arminio Monforte A, et al. Cardiovascular disease risk factors in HIV patients–association with antiretroviral therapy. Results from the DAD study. AIDS. 2003;17(8):1179–93. doi: 10.1097/01.aids.0000060358.78202.c1. [DOI] [PubMed] [Google Scholar]
- 32.Mary-Krause M, Cotte L, Simon A, Partisani M, Costagliola D. Increased risk of myocardial infarction with duration of protease inhibitor therapy in HIV-infected men. AIDS. 2003;17(17):2479–86. doi: 10.1097/00002030-200311210-00010. [DOI] [PubMed] [Google Scholar]
- 33.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, et al. Practice guideline: 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44–e164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 34.Arpadi SM, Bethel J, Horlick M, Sarr M, Bamji M, Abrams EJ, et al. Longitudinal changes in regional fat content in HIV-infected children and adolescents. AIDS. 2009;23(12):1501–9. doi: 10.1097/QAD.0b013e32832b7e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacobson DL, Patel K, Siberry GK, Van Dyke RB, DiMeglio LA, Geffner ME, et al. Body fat distribution in perinatally HIV-infected and HIV-exposed but uninfected children in the era of highly active antiretroviral therapy: outcomes from the Pediatric HIV/AIDS Cohort Study. Am J Clin Nutr. 2011;94(6):1485–95. doi: 10.3945/ajcn.111.020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geffner ME, Patel K, Miller TL, Hazra R, Silio M, Van Dyke RB, et al. Factors associated with insulin resistance among children and adolescents perinatally infected with HIV-1 in the pediatric HIV/AIDS cohort study. Horm Res Paediatr. 2011;76(6):386–91. doi: 10.1159/000332957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller TL, Borkowsky W, DiMeglio LA, Dooley L, Geffner ME, Hazra R, et al. Metabolic abnormalities and viral replication are associated with biomarkers of vascular dysfunction in HIV-infected children. HIV Med. 2012;13(5):264–75. doi: 10.1111/j.1468-1293.2011.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller TL, Orav EJ, Lipshultz SE, Arheart KL, Duggan C, Weinberg GA, et al. Risk factors for cardiovascular disease in children infected with human immunodeficiency virus-1. J Pediatr. 2008;153(4):491–497. doi: 10.1016/j.jpeds.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanchez Torres AM, Munoz Muniz R, Madero R, Borque C, Garcia-Miguel MJ, De Jose Gomez MI. Prevalence of fat redistribution and metabolic disorders in human immunodeficiency virus-infected children. Eur J Pediatr. 2005;164(5):271–6. doi: 10.1007/s00431-004-1610-y. [DOI] [PubMed] [Google Scholar]
- 40.Brambilla P, Bricalli D, Sala N, Renzetti F, Manzoni P, Vanzulli A, et al. Highly active antiretroviral-treated HIV-infected children show fat distribution changes even in absence of lipodystrophy. AIDS. 2001;15(18):2415–22. doi: 10.1097/00002030-200112070-00009. [DOI] [PubMed] [Google Scholar]
- 41.Moscicki AB, Ellenberg JH, Murphy DA, Jiahong X. Associations among body composition, androgen levels, and human immunodeficiency virus status in adolescents. J Adolesc Health. 2006;39(2):164–73. doi: 10.1016/j.jadohealth.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 42.Miller TL, Mawn BE, Orav EJ, Wilk D, Weinberg GA, Nicchitta J, et al. The effect of protease inhibitor therapy on growth and body composition in human immunodeficiency virus type 1-infected children. Pediatrics. 2001;107(5):E77. doi: 10.1542/peds.107.5.e77. [DOI] [PubMed] [Google Scholar]
- 43.Aldrovandi GM, Lindsey JC, Jacobson DL, Zadzilka A, Sheeran E, Moye J, et al. Morphologic and metabolic abnormalities in vertically HIV-infected children and youth. AIDS. 2009;23(6):661–72. doi: 10.1097/QAD.0b013e3283269dfb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boccara F, Teiger E, Cohen A, Ederhy S, Janower S, Odi G, et al. Percutaneous coronary intervention in HIV infected patients: immediate results and long term prognosis. Heart. 2006;92:543–4. doi: 10.1136/hrt.2005.068445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vigano A, Mora S, Testolin C, Beccio S, Schneider L, Bricalli D, et al. Increased lipodystrophy is associated with increased exposure to highly active antiretroviral therapy in HIV-infected children. J Acquir Immune Defic Syndr. 2003;32(5):482–9. doi: 10.1097/00126334-200304150-00003. [DOI] [PubMed] [Google Scholar]
- 46.Arpadi SM, Cuff PA, Horlick M, Wang J, Kotler DP. Lipodystrophy in HIV-infected children is associated with high viral load and low CD4+ -lymphocyte count and CD4+ -lymphocyte percentage at baseline and use of protease inhibitors and stavudine. J Acquir Immune Defic Syndr. 2001;27(1):30–4. doi: 10.1097/00126334-200105010-00005. [DOI] [PubMed] [Google Scholar]
- 47.Gerschenson M, Shiramizu B, LiButti DE, Shikuma CM. Mitochondrial DNA levels of peripheral blood mononuclear cells and subcutaneous adipose tissue from thigh, fat and abdomen of HIV-1 seropositive and negative individuals. Antivir Ther. 2005;10(Suppl 2):M83–9. [PubMed] [Google Scholar]
- 48.Sztam KA, Jiang H, Jurgrau A, Deckelbaum RJ, Foca MD. Early increases in concentrations of total, LDL, and HDL cholesterol in HIV-infected children following new exposure to antiretroviral therapy. J Pediatr Gastroenterol Nutr. 2011;52(4):495–8. doi: 10.1097/MPG.0b013e3181f5e9d4. [DOI] [PubMed] [Google Scholar]
- 49.Rhoads MP, Lanigan J, Smith CJ, Lyall EG. Effect of specific ART drugs on lipid changes and the need for lipid management in children with HIV. J Acquir Immune Defic Syndr. 2011;57(5):404–12. doi: 10.1097/QAI.0b013e31821d33be. [DOI] [PubMed] [Google Scholar]
- 50.Jacobson DL, Williams P, Tassiopoulos K, Melvin A, Hazra R, Farley J. Clinical management and follow-up of hypercholesterolemia among perinatally HIV-infected children enrolled in the PACTG 219C study. J Acquir Immune Defic Syndr. 2011;57(5):413–20. doi: 10.1097/QAI.0b013e31822203f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2012. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.
- 52.NHLBI AIDS working group. 2012. Sep 6–7, http://www.nhlbi.nih.gov/meetings/workshops/AIDSworking.htm.
- 53.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–38. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Longenecker C, Hoit B. Imaging atherosclerosis in HIV: carotid intima-media thickness and beyond. Translational research: J Lab Clin Med. 2012;159(3):127–39. doi: 10.1016/j.trsl.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 55.Miller TL, Somarriba G, Orav EJ, Mendez AJ, Neri D, Schaefer N, et al. Biomarkers of vascular dysfunction in children infected with human immunodeficiency virus-1. J Acquir Immune Defic Syndr. 2010;55(2):182–8. doi: 10.1097/QAI.0b013e3181e222c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Foster SB, Lu M, Glaze DG, Reuben JM, Harris LL, Cohen EN, et al. Associations of cytokines, sleep patterns, and neurocognitive function in youth with HIV infection. Clin Immunol. 2012;144(1):13–23. doi: 10.1016/j.clim.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DAD Study Group. Friis-Moller N, Reiss P, Sabin CA, Weber R, Monforte AD, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–35. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 58.Cruse B, Cysique LA, Markus R, Brew BJ. Cerebrovascular disease in HIV-infected individuals in the era of highly active antiretroviral therapy. J Neurovirol. 2012;18(4):264–76. doi: 10.1007/s13365-012-0092-3. [DOI] [PubMed] [Google Scholar]
- 59.Lazzaretti RK, Kuhmmer R, Sprinz E, Polanczyk CA, Ribeiro JP. Clinical research: dietary intervention prevents dyslipidemia associated with highly active antiretroviral therapy in human immunodeficiency virus type 1-infected individuals. A randomized trial. J Am Coll Cardiol. 2012;59:979–88. doi: 10.1016/j.jacc.2011.11.038. [DOI] [PubMed] [Google Scholar]
- 60.Somarriba G, Lopez-Mitnik G, Ludwig DA, Neri D, Schaefer N, Lipshultz SE, et al. Physical fitness in children infected with the human immunodeficiency virus: associations with highly active antiretroviral therapy. AIDS Res Hum Retroviruses. 2013;29(1):112–20. doi: 10.1089/aid.2012.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dubé MP, Cadden JJ. Lipid metabolism in treated HIV Infection. Best Practice Res Clin Endocrinol Metab. 2011;25(3):429–42. doi: 10.1016/j.beem.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 62.Lipshultz SE, Mas CM, Henkel JM, Franco VI, Fisher SD, Miller TL. HAART to heart: highly active antiretroviral therapy and the risk of cardiovascular disease in HIV-infected or exposed children and adults. Exp Rev Anti Infect Ther. 2012;10(6):661–74. doi: 10.1586/eri.12.53. [DOI] [PubMed] [Google Scholar]
- 63.Butt AA, Chang CC, Kuller L, Goetz MB, Leaf D, Rimland D, et al. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med. 2011;171(8):737–43. doi: 10.1001/archinternmed.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mondy KE, Gottdiener J, Overton ET, Henry K, Bush TC, et al. High prevalence of echocardiographic abnormalities among HIV-infected persons in the era of highly active antiretroviral therapy. Clin Infect Dis. 2011;52(3):378–86. doi: 10.1093/cid/ciq066. [DOI] [PubMed] [Google Scholar]
- 65.Lipshultz SE, Williams PL, Wilkinson JD, Leister EC, Van Dyke RB, Shearer WT, et al. Cardiac status of HIV-infected children treated with long-term combination antiretroviral therapy: results from the Adolescent Master Protocol of the NIH multicenter pediatric HIV/AIDS cohort study. JAMA Pediatr. 2013 Apr;22:1, 8. doi: 10.1001/jamapediatrics.2013.1206. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zareba KM, Lavigne JE, Lipshultz SE. Cardiovascular effects of HAART in infants and children of HIV-infected mothers. Cardiovasc Toxicol. 2004;4(3):271–9. doi: 10.1385/ct:4:3:271. [DOI] [PubMed] [Google Scholar]
- 67.Lipshultz SE, Easley KA, Orav EJ, Kaplan S, Starc TJ, Bricker JT, et al. Cardiovascular status of infants and children of women infected with HIV-1 (P2C2 HIV): a cohort study. Lancet. 2002;360(9330):368–73. doi: 10.1016/S0140-6736(02)09607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lipshultz SE, Shearer WT, Thompson B, Rich KC, Cheng I, Orav EJ, et al. Cardiac effects of antiretroviral therapy in HIV-negative infants born to HIV-positive mothers: NHLBI CHAART-1 (National Heart, Lung, and Blood Institute Cardiovascular Status of HAART Therapy in HIV-Exposed Infants and Children cohort study) J Am Coll Cardiol. 2011;57(1):76–85. doi: 10.1016/j.jacc.2010.08.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilkinson JD, Williams PL, Leister E, Zeldow B, Shearer WT, Colan SD, et al. Cardiac biomarkers in HIV-exposed uninfected children: the pediatric HIV/AIDS cohort study (PHACS) AIDS. 2013;27(7):1099–108. doi: 10.1097/QAD.0b013e32835cf21c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grossi PA. Update in HIV infection in organ transplantation. Curr Opin Organ Transplant. 2012;17(6):586–93. doi: 10.1097/MOT.0b013e3283592684. [DOI] [PubMed] [Google Scholar]
- 71.Pozzan G, Pagliari C, Tuon FF, Takakura CF, Kauffman MR, Duarte MI. Diffuse-regressive alterations and apoptosis of myocytes: possible causes of myocardial dysfunction in HIV-related cardiomyopathy. Int J Cardiol. 2009;132(1):90–5. doi: 10.1016/j.ijcard.2007.10.057. [DOI] [PubMed] [Google Scholar]
- 72.Bowles NE, Kearney DL, Ni J, Perez-Atayde AR, Kline MW, Bricker JT, et al. The detection of viral genomes by polymerase chain reaction in the myocardium of pediatric patients with advanced HIV disease. J Am Coll Cardiol. 1999;34(3):857–65. doi: 10.1016/s0735-1097(99)00264-8. [DOI] [PubMed] [Google Scholar]
- 73.Rogers JS, Zakaria S, Thom KA, Flammer KM, Kanno M, Mehra MR. Immune reconstitution inflammatory syndrome and human immunodeficiency virus-associated myocarditis. Mayo Clin Proc. 2008;83(11):1275–9. doi: 10.4065/83.11.1275. [DOI] [PubMed] [Google Scholar]
- 74.Yearley JH, Mansfield KG, Carville AAL, Sokos GG, Xia D, Pearson CB, et al. Antigenic stimulation in the simian model of HIV infection yields dilated cardiomyopathy through effects of TNF α. AIDS. 2008;22(5):585–94. doi: 10.1097/QAD.0b013e3282f57f61. [DOI] [PubMed] [Google Scholar]
- 75.Reinsch N, Neuhaus K, Esser S, Potthoff A, Hower M, Brockmeyer NH, et al. Prevalence of cardiac diastolic dysfunction in HIV-infected patients: results of the HIV-HEART study. HIV Clin Trials. 2010;11(3):156–62. doi: 10.1310/hct1103-156. [DOI] [PubMed] [Google Scholar]
- 76.Lipshultz SE, Orav EJ, Sanders SP, Hale AR, McIntosh K, Colan SD. Cardiac structure and function in children with human immunodeficiency virus infection treated with zidovudine. N Engl J Med. 1992;327(18):1260–5. doi: 10.1056/NEJM199210293271802. [DOI] [PubMed] [Google Scholar]
- 77.Lipshultz SE, Orav EJ, Sanders SP, McIntosh K, Colan SD. Limitations of fractional shortening as an index of contractility in pediatric patients infected with human immunodeficiency virus. J Pediatr. 1994;125(4):563–70. doi: 10.1016/s0022-3476(94)70008-7. [DOI] [PubMed] [Google Scholar]
- 78.Lipshultz SE, Shearer WT, Thompson B, Rich K, Cheng I, Orav EJ, et al. Antiretroviral therapy (ART) cardiac effects in HIV-infected children: the multicenter NHLBI Cardiac Highly Active Antiretroviral Therapy (CHAART-II) study. Circulation. 2009;120:S909–10. [Google Scholar]
- 79.Hulten E, Mitchell J, Scally J, Gibbs B, Villines TC. HIV positivity, protease inhibitor exposure and subclinical atherosclerosis: a systematic review and meta-analysis of observational studies. Heart. 2009;95:1826–35. doi: 10.1136/hrt.2009.177774. [DOI] [PubMed] [Google Scholar]
- 80.Worm SW, Sabin C, Weber R, Reiss P, El-Sadr W, Dabis F, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis. 2010;201:318–30. doi: 10.1086/649897. [DOI] [PubMed] [Google Scholar]
- 81.Nayak G, Ferguson M, Tribble DR, Porter CK, Rapena R, Marchicelli M, et al. Cardiac diastolic dysfunction is prevalent in HIV-infected patients. AIDS Patient Care STDs. 2009;23(4):231–8. doi: 10.1089/apc.2008.0142. [DOI] [PubMed] [Google Scholar]
- 82.O'Brien S, Sasaki N, Eidem BW, Colan SD, Cheng I, Wilkinson JD, et al. Left ventricular diastolic dysfunction in HIV-negative infants exposed in utero to antiretroviral therapy from HIV-positive mothers: the prospective NHLBI CHAART-I study. Circulation. 2011;124:A10808. [Google Scholar]
- 83.Halley CM, Houghtaling PL, Khalil MK, Thomas JD, Jaber WA. Mortality rate in patients with diastolic dysfunction and normal systolic function. Arch Intern Med. 2011;171(12):1082–7. doi: 10.1001/archinternmed.2011.244. [DOI] [PubMed] [Google Scholar]
- 84.McMahon CJ, Nagueh SF, Pignatelli RH, Denfield SW, Dreyer WJ, Price JF, et al. Characterization of left ventricular diastolic function by tissue Doppler imaging and clinical status in children with hypertrophic cardiomyopathy. Circulation. 2004;109(14):1756–62. doi: 10.1161/01.CIR.0000124723.16433.31. [DOI] [PubMed] [Google Scholar]
- 85.McMahon CJ, Nagueh SF, Eapen RS, Dreyer WJ, Finkelshtyn I, Cao X, et al. Echocardiographic predictors of adverse clinical events in children with dilated cardiomyopathy: a prospective clinical study. Heart. 2004;90(8):908–15. doi: 10.1136/hrt.2003.020966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ingul CB, Tjonna AE, Stolen TO, Stoylen A, Wisloff U. Impaired cardiac function among obese adolescents: effect of aerobic interval training. Arch Pediatr Adolesc Med. 2010;164(9):852–9. doi: 10.1001/archpediatrics.2010.158. [DOI] [PubMed] [Google Scholar]
- 87.Wojcik M, Rudzinski A, Starzyk J. Left ventricular diastolic dysfunction in adolescents with type 1 diabetes reflects the long- but not short-term metabolic control. J Pediatr Endocrinol Metab. 2010;23(10):1055–64. doi: 10.1515/jpem.2010.167. [DOI] [PubMed] [Google Scholar]
- 88.Plazak W, Kopec G, Tomkiewicz-Pajak L, Rubis P, Dziedzic H, Suchon E, et al. Heart structure and function in patients with generalized autoimmune diseases: echocardiography with tissue Doppler study. Acta Cardiol. 2011;66(2):159–65. doi: 10.1080/ac.66.2.2071246. [DOI] [PubMed] [Google Scholar]
- 89.Cade WT, Waggoner AD, Hubert S, Krauss MJ, Singh GK, Overton ET. Reduced diastolic function and left ventricular mass in HIV-negative preadolescent children exposed to antiretroviral therapy in utero. AIDS. 2012;26(16):2053–8. doi: 10.1097/QAD.0b013e328358d4d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kelly KM, Tarwater PM, Karper JM, Bedia D, Queen SE, Tunin RS. Diastolic dysfunction is associated with myocardial viral load in simian immunodeficiency virus-infected macaques. AIDS. 2012;26(7):815–23. doi: 10.1097/QAD.0b013e3283518f01. [DOI] [PubMed] [Google Scholar]
- 91.Opravil M, Sereni D. Natural history of HIV-associated pulmonary arterial hypertension: trends in the HAART era. AIDS. 2008;22(Suppl 3):S35. doi: 10.1097/01.aids.0000327514.60879.47. [DOI] [PubMed] [Google Scholar]
- 92.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119(16):2250–94. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 93.Cicalini S, Almodovar S, Grilli E, Flores S. Pulmonary hypertension and human immunodeficiency virus infection: epidemiology, pathogenesis, and clinical approach. Clin Microbiol Infect. 2011;17(1):25–33. doi: 10.1111/j.1469-0691.2010.03286.x. [DOI] [PubMed] [Google Scholar]
- 94.Janda S, Quon B, Swiston J. HIV and pulmonary arterial hypertension: a systematic review. HIV and pulmonary arterial hypertension. HIV Med. 2011;11(10):620–34. doi: 10.1111/j.1468-1293.2010.00829.x. [DOI] [PubMed] [Google Scholar]
- 95.Lindl A, Reinschl N, Nehausl K, Esser S, Brockmeyer NH, Potthoff A, et al. Pericardial effusion of HIV-infected patients – results of a prospective multicenter cohort study in the era of antiretroviral therapy. Eur J Med Res. 2011;16:480–3. doi: 10.1186/2047-783X-16-11-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bolt RJ, Rammeloo LA, van Furth AM, van Well GT. A 15-year-old girl with a large pericardial effusion. Eur J Pediatr. 2008;167(7):811–2. doi: 10.1007/s00431-007-0559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yoon SA, Hahn YS, Hong JM, Lee OJ, Han HS. Tuberculous pericarditis presenting as multiple free floating masses in pericardial effusion. J Korean Med Sci. 2012;27(3):325–8. doi: 10.3346/jkms.2012.27.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rapose A, Sarvat B, Sarria JC. Immune reconstitution inflammatory syndrome presenting as pericarditis and pericardial effusion. Cardiology. 2008;110(2):142–4. doi: 10.1159/000110494. [DOI] [PubMed] [Google Scholar]
- 99.Reuter H, Burgess LJ, Louw VJ, Doubell AF. The management of tuberculous pericardial effusion: experience in 233 consecutive patients. Cardiovasc J S Afr. 2007;18(1):20–5. [PubMed] [Google Scholar]
- 100.Van Doorn CA, Yates R, Tsang VT. Endocarditis as the first presentation of AIDS in infancy. Arch Dis Child. 1998;79(2):179–80. doi: 10.1136/adc.79.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Steinherz LJ, Brochstein JA, Robins J. Cardiac involvement in congenital acquired immunodeficiency syndrome. Am J Dis Child. 1986;140(12):1241–4. doi: 10.1001/archpedi.1986.02140260043022. [DOI] [PubMed] [Google Scholar]
- 102.Granovsky MO, Mueller BU, Nicholson HS, Rosenberg PS, Rabkin CS. Cancer in human immunodeficiency virus-infected children: a case series from the Children's Cancer Group and the National Cancer Institute. J Clin Oncol. 1998;16(5):1729–35. doi: 10.1200/JCO.1998.16.5.1729. [DOI] [PubMed] [Google Scholar]
- 103.Buck BE, Scott GB, Valdes-Dapena M, Parks WP. Kaposi sarcoma in two infants with acquired immune deficiency syndrome. J Pediatr. 1983;103(6):911–3. doi: 10.1016/s0022-3476(83)80712-4. [DOI] [PubMed] [Google Scholar]
- 104.Zoufaly A, Stellbrink HJ, Heiden MA, Kollan C, Hoffmann C, van Lunzen J, et al. Cumulative HIV viremia during highly active antiretroviral therapy is a strong predictor of AIDS-related lymphoma. J Infect Dis. 2009;200(1):79–87. doi: 10.1086/599313. [DOI] [PubMed] [Google Scholar]
- 105.Karavidas A, Tsiachris D, Lazaros G, Xylomenos G, Arapi S, Potamitis N, et al. Doppler tissue imaging unmasks right ventricular function abnormalities in HIV-infected patients. Cardiol J. 2010;17(6):587–93. [PubMed] [Google Scholar]
- 106.Guillevin L. Vasculitides in the context of HIV infection. AIDS. 2008;22(3):S27–33. doi: 10.1097/01.aids.0000327513.53255.17. [DOI] [PubMed] [Google Scholar]
- 107.Tseng ZH, Secemsky EA, Dowdy D, Vittinghoff E, Moyers B, Wong JK, et al. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol. 2012;59:1891–6. doi: 10.1016/j.jacc.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sani MU, Okeahialam BN. QTc interval prolongation in patients with HIV and AIDS. J Natl Med Assoc. 2005;97:1657–61. [PMC free article] [PubMed] [Google Scholar]
- 109.Nordin C, Kohli A, Beca S, Zaharia V, Grant T, Leider J, et al. Importance of hepatitis C coinfection in the development of QT prolongation in HIV-infected patients. J Electrocardiol. 2006;39:199–205. doi: 10.1016/j.jelectrocard.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 110.Soliman EZ, Lundgren JD, Roediger MP, Duprez DA, Temesgen Z, Bickel M, et al. Boosted protease inhibitors and the electrocardiographic measures of QT and PR durations. AIDS. 2011;25(3):367–77. doi: 10.1097/QAD.0b013e328341dcc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Correia D, Rodrigues De Resende LA, Molina RJ, Ferreira BD, Colombari F, Barbosa CJ, et al. Power spectral analysis of heart rate variability in HIV-infected and AIDS patients. Pacing Clin Electrophysiol. 2006;29:53–8. doi: 10.1111/j.1540-8159.2006.00297.x. [DOI] [PubMed] [Google Scholar]
- 112.Dapena M, Jiménez B, Noguera-Julian A, Soler-Palacín P, Fortuny C, Lahoz R, et al. Metabolic disorders in vertically HIV-infected children: future adults at risk for cardiovascular disease. J Pediatr Endocrinol Metab. 2012;25(5–6):529–35. doi: 10.1515/jpem-2012-0005. [DOI] [PubMed] [Google Scholar]
- 113.Piloya T, Bakeera-Kitaka S, Kekitiinwa A, Kamya MR. Lipodystrophy among HIV-infected children and adolescents on highly active antiretroviral therapy in Uganda: a cross sectional study. J Int AIDS Soc. 2012;15(2):17427. doi: 10.7448/IAS.15.2.17427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lang S, Mary-Krause M, Cotte L, Gilguin J, Partisani M, Simon A, et al. Impact of individual antiretroviral drugs on the risk of myocardial infarction in human immunodeficiency virus-infected patients: a case-control study nested within the French Hospital Database on HIV ANRS cohort CO4. Arch Intern Med. 2010;170:1228–38. doi: 10.1001/archinternmed.2010.197. [DOI] [PubMed] [Google Scholar]
- 115.Lipshultz SE, Orav EJ, Sanders SP, Hale AR, McIntosh K, Colan SD. Cardiac structure and function in children with human immunodeficiency virus infection treated with zidovudine. N Engl J Med. 1992;327(18):1260–5. doi: 10.1056/NEJM199210293271802. [DOI] [PubMed] [Google Scholar]
- 116.Crain MJ, Chernoff MC, Oleske JM, Brogly SB, Malee KM, Borum PR, et al. Possible mitochondrial dysfunction and its association with antiretroviral therapy use in children perinatally infected with HIV. J Infect Dis. 2010;202(2):291–301. doi: 10.1086/653497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chanock SJ, Luginbuhl LM, McIntosh K, Lipshultz SE. Life-threatening reaction to trimethoprim/sulfamethoxazole in pediatric human immunodeficiency virus infection. Pediatrics. 1994;93(3):519–21. [PubMed] [Google Scholar]
- 118.Centers of Disease Control and Prevention (CDC) Achievements in public health. Reduction in perinatal transmission of HIV infection–United States, 1985–2005. MMWR Morb Mortal Wkly Rep. 2006;55(21):592–7. [PubMed] [Google Scholar]
- 119.Neri D, Somarriba GA, Schaefer NN, Chaparro AI, Scott GB, Lopez-Mitnik G, Ludwig DA, Miller TL. Growth and body composition of uninfected children exposed to human immunodeficiency virus: comparison with a contemporary cohort and United States national standards. J Pediatr. 2013 Jan 26; doi: 10.1016/j.jpeds.2012.12.034. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mofenson LM, Brady MT, Danner SP, Domingquez KL, Hazara R, Handelsman E, et al. Guidelines for the prevention and treatment of opportunistic infections among HIV-exposed and HIV-infected children: recommendations from CDC, the National Institutes of Health, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. MMWR Recomm Rep. 2009;58(RR-11):1–166. [PMC free article] [PubMed] [Google Scholar]
- 121.Hornberger LK, Lipshultz SE, Easley KA, Colan SD, Schwartz M, Kaplan S, et al. Cardiac structure and function in fetuses of mothers infected with HIV: the prospective P2C2 HIV multicenter study. Am Heart J. 2000;140(4):575–84. doi: 10.1067/mhj.2000.109645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lipshultz SE, Fisher SD, Lai WW, Miller TL. Cardiovascular risk factors, monitoring, and therapy for HIV-infected patients. AIDS. 2003;17(Suppl 1):S96–122. doi: 10.1097/00002030-200304001-00014. [DOI] [PubMed] [Google Scholar]
- 123.Lipshultz SE, Fisher SD, Lai WW, Miller TL. Cardiovascular monitoring and therapy for HIV-infected patients. Ann N Y Acad Sci. 2001;946:236–73. doi: 10.1111/j.1749-6632.2001.tb03916.x. [DOI] [PubMed] [Google Scholar]
- 124.Saidi AS, Moodie DS, Garson A, Jr, Lipshultz SE, Kaplan S, Lai WW, et al. Electrocardiography and 24-hour electrocardiographic ambulatory recording (Holter monitor) studies in children infected with human immunodeficiency virus type 1. The pediatric pulmonary and cardiac complications of vertically transmitted HIV-1 infection study group. Pediatr Cardiol. 2000;21(3):189–96. doi: 10.1007/s002460010038. [DOI] [PubMed] [Google Scholar]
- 125.Ratnasamy C, Kinnamon DD, Lipshultz SE, Rusconi P. Associations between neurohormonal and inflammatory activation and heart failure in children. Am Heart J. 2008;155:527–33. doi: 10.1016/j.ahj.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 126.Rusconi PG, Ludwig DA, Ratnasamy C, Mas R, Harmon WG, Colan SD, et al. Serial measurements of serum NT-proBNP as markers of left ventricular systolic function and remodeling in children with heart failure. Am Heart J. 2010;160:776–83. doi: 10.1016/j.ahj.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]