Abstract
Introduction
Hypoglycemia is a complication in the management of type 2 diabetes, and elderly people are at greater risk of experiencing hypoglycemia events than younger patients. Insulin analogs achieve glycemic control with minimal risk of hypoglycemia and may therefore be a good treatment option for all patients.
Methods
A1chieve was an international, multicenter, prospective, open-label, non-interventional, 24-week study in people with type 2 diabetes who started/switched to therapy with biphasic insulin aspart 30, insulin detemir or insulin aspart (alone/in combination) in routine clinical practice. This sub-analysis evaluated clinical safety and effectiveness of insulin aspart as part of a basal-bolus regimen (±oral glucose-lowering drugs) in three age-groups (≤40, >40–65, and >65 years) of insulin-experienced and insulin-naive people with type 2 diabetes.
Results
In total, 4,032 patients were included in the sub-analysis. After 24 weeks of insulin aspart treatment, significant improvements versus baseline were observed in all age-groups for: proportion of people with ≥1 hypoglycemia events (18.3–27.1% and 11.0–12.7%, at baseline and 24 weeks, respectively), ≥1 major hypoglycemia events (3.3–6.7% and 0–0.2%), and ≥1 nocturnal hypoglycemia events (9.2–13.7% and 2.9–4.9%); glycated hemoglobin (9.6–9.8% and 7.4%); fasting plasma glucose (change from baseline ranged from −3.6 to −4.4 mmol/l); and post-breakfast post-prandial plasma glucose (change from baseline ranged from −5.5 to −5.9 mmol/l). Fourteen serious adverse drug reactions were reported. Health-related quality of life was significantly improved for all age-groups (all, p < 0.001).
Conclusion
All age-groups showed improved glycemic control and reduced risk of hypoglycemia when starting/switching to insulin aspart therapy within a basal-bolus regimen; this may be particularly important for elderly patients given their greater risk of hypoglycemia versus younger patients.
Keywords: Elderly, Insulin aspart, Type 2 diabetes
Introduction
The worldwide prevalence of diabetes was estimated at 366 million in 2011 (8.3% of the population), and is predicted to rise to 552 million (9.9%) by 2030 [1]; type 2 diabetes accounts for approximately 95% of these cases [2].
Treatment in patients with type 2 diabetes varies with age; for example, elderly patients may be more likely than younger patients to have comorbidities and need polypharmacy, which, in the case of some drugs, may disrupt glycemic control, reduce quality of life, and increase the risk of severe hypoglycemia [3–6]. Furthermore, elderly patients may be unable to adequately self-monitor blood glucose levels due to poor dexterity, and cognitive and visual impairments [7]. The differences between patient age-groups emphasize the need for individualized care in these different groups. With their more favorable clinical profiles (lower risk of hypoglycemia, flexible dosing, improved convenience, and greater treatment satisfaction) compared with human insulin [8, 9], insulin analogs may be a better choice for starting or optimizing insulin therapy for most patients, including the elderly [3].
Insulin aspart (NovoRapid®; Novo Nordisk A/S, Bagsvaerd, Denmark) is a rapid-acting insulin analog that can be administered immediately before or after a meal [10], and a large body of evidence supports the clinical utility of insulin aspart when administered as part of a basal-bolus regimen [11–17]. In addition to the data from randomized clinical trials, observational studies have demonstrated that basal-bolus regimens are effective in everyday practice in type 1 and type 2 diabetes [18–20]. There is a lack of specific clinical studies in different age-groups to elucidate the risks and benefits of existing treatments in older compared with younger patients, and therefore, large observational studies can be invaluable for providing data from this cohort of patients.
A1chieve was an international non-interventional study that was conducted to examine the safety and effectiveness of initiating or switching to insulin analogs (alone/in combination with other anti-diabetes medication) as part of routine clinical care among patients with type 2 diabetes [18]. In this sub-group analysis of the A1chieve study, we aimed to investigate the safety and effectiveness of insulin aspart administered at mealtime(s) as required, together with basal insulin (insulin detemir, neutral protamine Hagedorn or insulin glargine) with or without oral glucose-lowering drugs (OGLDs) in three age-groups (≤40, >40–65 and >65 years of age) with type 2 diabetes.
Materials and Methods
All local requirements for Health Authorities or Ethics Committee approvals were obtained, if applicable. In every country, participants signed informed consent forms and were free to withdraw from the study at any time. The study was conducted in accordance with the Declaration of Helsinki of 1964, as revised in 2008 [21] and the Guidelines for Good Pharmacoepidemiology Practice [22].
Study Design
This was a sub-analysis of a 24-week, international, prospective, multicenter, non-interventional, observational study, which was conducted in 28 countries encompassing seven geographical regions: China, South Asia (Bangladesh, India, Pakistan), East Asia (Indonesia, Korea, Malaysia, Philippines, Singapore, Taiwan), North Africa (Algeria, Morocco, Tunisia, Libya), Middle East (Egypt, Iran, Jordan, Turkey, Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, United Arab Emirates, Yemen), Latin America (Argentina, Mexico), and Russia [18].
In the A1chieve study, the safety and effectiveness of initiating or switching to treatment with insulin analogs (alone/in combination with other anti-diabetes medication) was evaluated in patients with type 2 diabetes receiving routine clinical care between January 2009 and June 2010. Choice of insulin analog and the insulin dose were based on the clinical judgment of the treating physician, and with patient agreement. Insulin analogs (all manufactured by Novo Nordisk A/S, Bagsvaerd, Denmark) were used in accordance with the label approved by the regulatory authority. Further details, inclusion and exclusion criteria, and study design have been reported elsewhere [18].
This sub-analysis included patients starting or switching to treatment with insulin aspart administered at mealtime(s) plus basal insulin (insulin detemir, neutral protamine Hagedorn, or insulin glargine) with or without OGLDs. As the basal component of the regimen could be human insulin or an insulin analog, basal insulin dose is expressed as U (or IU). The decision to prescribe OGLDs, stop OGLDs, or switch the type of OGLDs prescribed during the study was made by the treating physician.
Assessments
Assessments were at baseline (time when the treating physician prescribed insulin aspart as part of a basal-bolus regimen), approximately 12 weeks after baseline (results not reported here), and study end (approximately 24 weeks after baseline).
The primary endpoint was the incidence of serious adverse drug reactions (SADRs), including major hypoglycemia events. Other safety assessments included the change in the number of hypoglycemia events between baseline and 24 weeks. These were based on patient recall of events within the last 4 weeks of the pre-scheduled clinical visit. A hypoglycemia event was defined as an event with one of the following characteristics: symptoms of hypoglycemia that resolved with oral carbohydrate intake, glucagon or intravenous glucose; or symptomatic or asymptomatic plasma glucose <3.1 mmol/l. Major hypoglycemia events were defined as hypoglycemia events with severe central nervous system symptoms consistent with hypoglycemia, in which the patient was unable to treat himself/herself. Nocturnal hypoglycemia events were defined as individualized symptomatic events consistent with hypoglycemia, that occurred while the patient was asleep, between bedtime after the evening insulin injection and before getting up in the morning [before morning determination of fasting plasma glucose (FPG) and before morning injection, if relevant].
The effectiveness of therapy was determined from measurements made by the treating physician team at each assessment visit; data were collected from the physicians’ clinical notes, and participants’ recall and self-monitoring diary/meter, as available. Effectiveness outcomes encompassed change from baseline after 24 weeks in blood glucose control measures [glycated hemoglobin (HbA1c; most recent during the preceding 4 weeks), FPG (pre-breakfast), and post-prandial plasma glucose (PPG; 90 to 120 min after the beginning of breakfast)], body weight, and health-related quality of life (HRQoL). HRQoL was measured by self-report at baseline and after 24 weeks using the EQ-5D questionnaire [23], which evaluates five domains of patient health/lifestyle (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression). Scores in these five domains were converted to a single utility value (UK VAS set), with ‘1.00’ indicating ‘full health’ and ‘0.00’ indicating the state ‘deceased’ [24, 25].
Due to the observational nature of the study and lack of protocol enforcement to report all effectiveness outcomes, results are reported here as per available reports.
Statistical Analyses
Analyses were performed in all patients with a baseline visit and who were treated with insulin aspart as part of a basal-bolus regimen at baseline. For those patients who withdrew from the study, data collected until the date of withdrawal was used for analysis. Sub-group analyses were conducted according to age (≤40, >40–65 and >65 years), and pre-study insulin experience (insulin-experienced and insulin-naive).
The sample size (full cohort) was based on the number of people (20,000) exposed for 6 months required to confirm at 95% confidence a frequency of any one adverse drug reaction of ≥15 events/100,000 person-years. This would detect a rate of major hypoglycemia as reported in any published clinical trial.
Changes from baseline in efficacy measures were evaluated using Student’s paired t test. For hypoglycemia, the percentage of patients reporting at least one event was analyzed using McNemar’s test. All statistical analyses were two-sided, using a pre-specified 5% significance level, and were performed by Novo Nordisk using SAS® Version 9.1.3 (SAS® Institute Inc., Cary, NC, USA).
Results
Study Participants
Data for 4,032 people (6% of the total A1chieve population) with type 2 diabetes who received insulin aspart as part of a basal-bolus regimen were collected: 571 patients aged ≤40 years, 2,801 patients aged >40–65 years, and 660 patients aged >65 years (Table 1). Most patients (61.3%) starting basal-bolus regimens were previously treated with other insulin regimens (Table 1). Previous insulin therapies included human soluble insulin, neutral protamine Hagedorn, premixed human insulin, insulin glargine, and others, such as premixed insulin lispro.
Table 1.
Baseline patient and disease characteristics by age-group
| Baseline variable | Age-group | ||
|---|---|---|---|
| ≤40 years | >40–65 years | >65 years | |
| N | 571 | 2,801 | 660 |
| Insulin status, n | |||
| Insulin-naive | 211 | 1,106 | 244 |
| Insulin-experienced | 360 | 1,695 | 416 |
| Gender (male/female), %a | 56.6/43.4 | 55.1/44.9 | 47.5/52.5 |
| Mean (SD) age, years | 30.3 (8.7) | 53.3 (6.5) | 71.8 (5.2) |
| Mean (SD) body weight, kgb | 71.9 (17.5) | 79.5 (17.7) | 72.3 (14.6) |
| Mean (SD) BMI, kg/m2 c | 25.7 (5.4) | 28.9 (6.1) | 27.2 (5.1) |
| Mean (SD) age at diagnosis, yearsd | 25.9 (8.7) | 43.7 (7.6) | 57.3 (9.5) |
| Mean (SD) diabetes duration, yearsd | 5.2 (4.9) | 9.6 (6.2) | 14.5 (8.8) |
Due to the observational nature of the study, data were not collected or not recorded for some patients
BMI body-mass index
a n = 2,799, n = 659 for the >40–65 and >65 years age-groups, respectively
b n = 549, n = 2,700, n = 635 for the ≤40, >40–65 and >65 years age-groups, respectively
c n = 517, n = 2,621, n = 612 for the ≤40, >40–65 and >65 years age-groups, respectively
d n = 552, n = 2,785, n = 657 for the ≤40, >40–65 and >65 years age-groups, respectively
Physicians cited the need to improve glycemic control (92.9–95.1% in the three age-groups) as the most frequent reason for prescribing insulin aspart as part of a basal-bolus regimen. Between 30.5% and 39.6% of physicians gave patient dissatisfaction with current therapy, need to reduce the risk of hypoglycemia and need to reduce plasma glucose variability as reasons for initiating or switching to insulin aspart therapy. There was no obvious difference in these reasons between age-groups.
Study Treatments and Dose
Mean (standard deviation; SD) basal insulin dose started at 0.31 (0.18) U (or IU)/kg and increased slightly to 0.36 (0.20) U (or IU)/kg after 24 weeks in the ≤40 years age-group. Similar increases from starting dose were observed in the >40–65 years age-group [0.31 (0.16) U (or IU)/kg at baseline and 0.37 (0.19) U (or IU)/kg after 24 weeks] and the >65 years age-group [0.29 (0.16) U (or IU)/kg at baseline and 0.36 (0.20) U (or IU)/kg after 24 weeks]. The mean starting basal insulin dose and the basal insulin dose after 24 weeks was higher in insulin-experienced patients [0.39–0.41 U (or IU)/kg in the three age-groups after 24 weeks] than in insulin-naive patients [0.26–0.32 U (or IU)/kg in the three age-groups after 24 weeks].
Mean (SD) bolus insulin dose increased slightly from baseline to 24 weeks in all age-groups [≤40 years age-group: 0.42 (0.22) U/kg to 0.48 (0.26) U/kg; >40–65 years age-group: 0.37 (0.19) U/kg to 0.42 (0.21) U/kg; >65 years age-group: 0.38 (0.21) U/kg to 0.43 (0.22) U/kg]. The mean starting bolus insulin dose and the bolus insulin dose after 24 weeks was higher in insulin-experienced patients (0.43–0.50 U/kg in the three age-groups after 24 weeks) than in insulin-naive patients (0.39–0.44 U/kg in the three age-groups after 24 weeks).
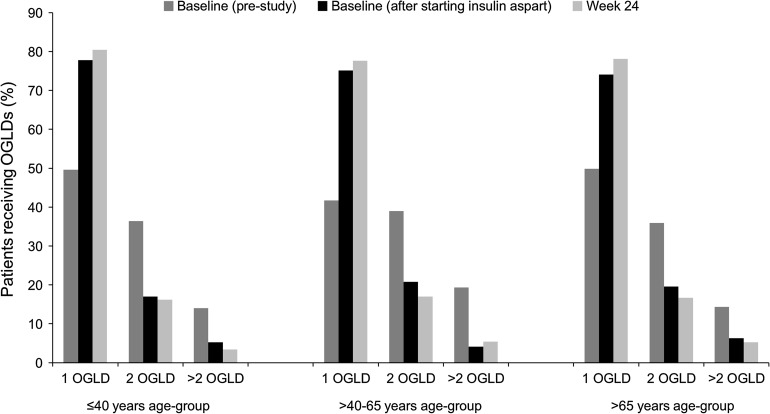
The number of concomitant OGLDs used was generally reduced as patients entered the study and remained stable or reduced slightly during the course of the 24 weeks of insulin aspart therapy as part of a basal-bolus regimen (Fig. 1); there was no major difference in OGLD use between the age-groups. A similar pattern was observed in insulin-naive and insulin-experienced patients, but a greater proportion of insulin-naive patients than insulin-experienced patients were on two or more OGLDs at baseline. However, after 24 weeks of insulin aspart therapy as part of a basal-bolus regimen, the proportion of patients using two or more OGLDs was very similar in the insulin-naive and insulin-experienced groups, in all age-groups.
Fig. 1.
Oral glucose-lowering drug use among patients starting or switching to a basal-bolus insulin regimen with insulin aspart in the A1chieve study. n = 264 pre-study, n = 194 at baseline and n = 204 at 24 weeks in ≤40 years age-group. n = 2,062 pre-study, n = 1,479 at baseline and n = 1,467 at 24 weeks in >40–65 years age-group. n = 440 pre-study and n = 270 at baseline and 24 weeks in >65 years age-group. OGLD oral glucose-lowering drug
Metformin and/or sulfonylureas were the predominant OGLDs in all age-groups at study initiation and after 24 weeks of treatment with insulin aspart; >70% of patients in all age-groups were prescribed metformin after 24 weeks.
SADRs
Of the 4,032 people with type 2 diabetes who received insulin aspart as part of a basal-bolus regimen, there were 14 reports of SADRs: five hypoglycemia episodes in the ≤40 years age-group; five in the >40–65 years age-group, including two hypoglycemia episodes, one report of diabetic ketoacidosis, one report of a fall, and one report of a pelvic fracture; and four in the >65 years age-group, including two hypoglycemia episodes, one report of inadequate diabetes control, and one episode of hypoglycemia unconsciousness. All SADRs occurred in insulin-experienced patients. Eleven of these events were probably related to treatment (with good reasons and sufficient documentation to assume a causal relationship) and three were possibly related (a causal relationship was conceivable and could not be dismissed).
Hypoglycemia
In each age-group (in the entire cohort), there was a significant reduction from baseline in overall hypoglycemia, major hypoglycemia, and nocturnal hypoglycemia after 24 weeks of treatment with insulin aspart as part of a basal-bolus regimen (Table 2). There were no reports of major hypoglycemia at 24 weeks in the >40–65 years age-group and >65 years age-group (Table 2).
Table 2.
Hypoglycemia at baseline, and after 24 weeks of treatment with insulin aspart as part of a basal-bolus regimen
| Measurement | % patients with at least one event (event/person-year) | |||||
|---|---|---|---|---|---|---|
| Age ≤40 years | Age >40–65 years | Age >65 years | ||||
| Baseline | 24 weeks | Baseline | 24 weeks | Baseline | 24 weeks | |
| Hypoglycemia (overall) | ||||||
| Entire cohort | 27.1 (12.3) | 12.7*** (4.1) | 18.6 (8.8) | 11.1*** (3.6) | 18.3 (10.3) | 11.0*** (3.4) |
| n | 571 | 474 | 2,801 | 2,532 | 660 | 582 |
| Insulin-experienced | 40.3 (19.0) | 14.1*** (5.2) | 27.6 (13.6) | 13.1*** (4.0) | 24.3 (14.7) | 11.2*** (3.3) |
| n | 360 | 298 | 1,695 | 1,528 | 416 | 366 |
| Insulin-naive | 4.7 (0.8) | 10.2* (2.4) | 4.9 (1.6) | 8.1** (3.0) | 8.2 (2.9) | 10.6 (3.5) |
| n | 211 | 176 | 1,106 | 1,004 | 244 | 216 |
| Hypoglycemia (major)a | ||||||
| Entire cohort | 6.7 (1.5) | 0.2*** (0.0) | 3.6 (0.9) | 0*** (0) | 3.3 (0.6) | 0*** (0) |
| Insulin-experienced | 10.3 (2.4) | 0.3*** (0.0) | 5.8 (1.4) | 0*** (0) | 4.1 (0.8) | 0*** (0) |
| Insulin-naive | 0.5 (0.1) | 0 (0) | 0.4 (0.2) | 0* (0) | 2.0 (0.3) | 0* (0) |
| Hypoglycemia (nocturnal)a | ||||||
| Entire cohort | 13.7 (3.8) | 4.9*** (1.1) | 9.2 (2.6) | 4.1*** (0.7) | 9.8 (3.5) | 2.9*** (0.6) |
| Insulin-experienced | 20.6 (5.8) | 6.7*** (1.6) | 14.2 (4.1) | 5.2*** (0.9) | 13.9 (5.3) | 3.0*** (0.6) |
| Insulin-naive | 1.9 (0.3) | 1.7 (0.2) | 1.5 (0.4) | 2.4 (0.4) | 2.9 (0.5) | 2.8 (0.6) |
* p < 0.05 vs. baseline; ** p < 0.01 vs. baseline; *** p<0.001 vs. baseline
a n for each cohort same as for hypoglycemia (overall) data
Rates of hypoglycemia, major hypoglycemia and nocturnal hypoglycemia were numerically higher at baseline among insulin-experienced than insulin-naive patients (Table 2). Rates of hypoglycemia and nocturnal hypoglycemia were significantly reduced in all three age-groups of insulin-experienced patients after 24 weeks of treatment with insulin aspart as part of a basal-bolus regimen (Table 2). Across all age-groups, the proportion of insulin-experienced patients reporting at least one hypoglycemia event was 24.3–40.3% at baseline versus 11.2–14.1% at 24 weeks, and at least one nocturnal hypoglycemia event was 13.9–20.6% at baseline versus 3.0–6.7% at 24 weeks. There were no reports of major hypoglycemia events at 24 weeks in the >40–65 and >65 years age-groups, and significant reductions in the proportion of patients reporting major hypoglycemia events between baseline and 24 weeks in the ≤40 years age-group.
There was a significant increase from baseline to 24 weeks in the proportion of insulin-naive patients reporting at least one hypoglycemia event, except in the >65 years age-group (4.7%, 4.9% and 8.2% of patients at baseline compared with 10.2%, 8.1% and 10.6% of patients at 24 weeks in the ≤40, >40–65, and >65 years age-groups, respectively; Table 2). However, rates of nocturnal hypoglycemia did not significantly increase with insulin aspart treatment in insulin-naive patients of any age-group, and there were no reports of major hypoglycemia at 24 weeks (Table 2).
The proportion of patients taking sulfonylureas who reported hypoglycemia events at baseline was 9.6% (n = 115), 11.7% (n = 1,109), and 15.1% (n = 232) in the ≤40 years age-group, >40–65 years age-group, and >65 years age-group, respectively. At 24 weeks, there was no significant change from baseline in the proportion of patients reporting hypoglycemia events: 3.6% (n = 28), 7.1% (n = 254) and 4.7% (n = 43), respectively.
Glucose Control
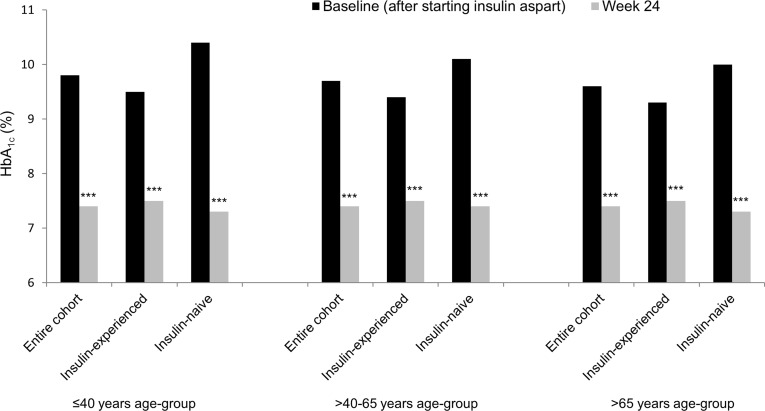
Baseline HbA1c levels were high in all age-groups in insulin-naive and insulin-experienced patients (Fig. 2). Mean (SD) change in HbA1c between baseline and week 24 in insulin-experienced patients was −2.0% (2.0%) in the ≤40 years age-group, −2.0% (1.6%) in the >40–65 years age-group, and −1.8% (1.9%) in the >65 years age-group. Mean (SD) change in HbA1c between baseline and week 24 in insulin-naive patients was −3.1% (2.1%) in the ≤40 years age-group, −2.8% (2.0%) in the >40–65 years age-group, and −2.8% (2.1%) in the >65 years age-group (Fig. 2).
Fig. 2.
Mean plasma glycated hemoglobin among patients starting or switching to a basal-bolus insulin regimen with insulin aspart in the A1chieve study. Entire cohort: n = 383 in ≤40 years age-group; n = 2,117 in >40–65 years age-group; n = 452 in >65 years age-group. Insulin-experienced cohort: n = 249 in ≤40 years age-group; n = 1,306 in >40–65 years age-group; n = 289 in >65 years age-group. Insulin-naive cohort: n = 134 in ≤40 years age-group; n = 811 in >40–65 years age-group; n = 163 in >65 years age-group. HbA 1c glycated hemoglobin. ***p < 0.001 vs. baseline
The proportion of patients (insulin-naive plus insulin-experienced) with HbA1c <7.0% at baseline was similar between age-groups at baseline (3.5–6.9%) and at 24 weeks (32.8–35.9%). A greater proportion of patients appeared to achieve HbA1c <7.0% following 24 weeks of treatment with insulin aspart as part of a basal-bolus regimen than at baseline (Table 3). In the >65 years age-group, 12.0% and 14.3% of patients had baseline HbA1c <7.5% in the insulin-naive and insulin-experienced cohorts, respectively. Following 24 weeks of treatment with insulin aspart as part of a basal-bolus regimen, 63.0% and 55.0% patients aged >65 years achieved HbA1c <7.5%.
Table 3.
Outcomes after 24 weeks of treatment with insulin aspart as part of a basal-bolus regimen
| Measurement | Age ≤40 years | Age >40–65 years | Age >65 years | |||
|---|---|---|---|---|---|---|
| Baseline | 24 weeks | Baseline | 24 weeks | Baseline | 24 weeks | |
| % patients with HbA1c <7.0%a | ||||||
| Entire cohort | 4.1 | 32.8 | 3.5 | 35.9 | 6.9 | 34.5 |
| n | 517 | 411 | 2,543 | 2,258 | 580 | 495 |
| Insulin-experienced | 5.3 | 32.2 | 3.7 | 35.7 | 7.7 | 32.0 |
| n | 322 | 270 | 1,551 | 1,386 | 364 | 322 |
| Insulin-naive | 2.1 | 34.0 | 3.2 | 36.1 | 5.6 | 39.3 |
| n | 195 | 141 | 992 | 872 | 216 | 173 |
| Measurement | Age ≤40 years | Age >40–65 years | Age >65 years | |||
|---|---|---|---|---|---|---|
| Baseline | Change after 24 weeks | Baseline | Change after 24 weeks | Baseline | Change after 24 weeks | |
| FPG before breakfast | ||||||
| Entire cohort, mmol/l | 11.3 (4.5) | −4.4*** (4.5) | 10.9 (3.6) | −3.9*** (3.7) | 10.7 (4.2) | −3.6*** (4.3) |
| Entire cohort, mg/dl | 203.2 (81.3) | −79.4*** (81.9) | 196.6 (65.4) | −71.0*** (66.2) | 192.4 (74.8) | −65.0*** (77.0) |
| n | 384 | 2,210 | 493 | |||
| Insulin-experienced, mmol/l | 10.4 (3.9) | −3.6*** (3.9) | 10.3 (3.3) | −3.3*** (3.4) | 10.3 (4.0) | −3.1*** (4.1) |
| Insulin-experienced, mg/dl | 187.9 (70.6) | −65.2*** (70.6) | 185.0 (60.1) | −59.8*** (60.8) | 186.2 (72.2) | −55.8*** (73.9) |
| n | 242 | 1,334 | 309 | |||
| Insulin-naive, mmol/l | 12.7 (5.1) | −5.8*** (5.2) | 11.9 (3.8) | −4.9*** (3.9) | 11.3 (4.3) | −4.5*** (4.4) |
| Insulin-naive, mg/dl | 229.3 (91.3) | −103.6*** (93.7) | 214.3 (69.1) | −88.0*** (70.2) | 202.9 (78.0) | −80.3*** (79.7) |
| n | 142 | 876 | 184 | |||
| PPG after breakfast | ||||||
| Entire cohort, mmol/l | 14.8 (5.0) | −5.9*** (4.9) | 14.4 (4.7) | −5.5*** (4.7) | 14.7 (5.1) | −5.6*** (5.3) |
| Entire cohort, mg/dl | 266.1 (89.7) | −106.6*** (87.5) | 260.2 (84.1) | −99.9*** (83.9) | 264.4 (92.5) | −101.6*** (95.3) |
| n | 303 | 1,660 | 371 | |||
| Insulin-experienced, mmol/l | 14.1 (4.7) | −5.2*** (4.5) | 13.6 (4.3) | −4.8*** (4.3) | 14.3 (5.1) | −5.2*** (5.2) |
| Insulin-experienced, mg/dl | 253.7 (83.8) | −92.8*** (80.9) | 245.2 (78.1) | −87.1*** (78.2) | 257.9 (92.2) | −93.5*** (94.5) |
| n | 199 | 999 | 232 | |||
| Insulin-naive, mmol/l | 16.1 (5.3) | −7.4*** (5.2) | 15.7 (4.9) | −6.6*** (4.9) | 15.3 (5.1) | −6.4*** (5.3) |
| Insulin-naive, mg/dl | 289.9 (95.9) | −132.9*** (93.8) | 282.9 (87.7) | −119.2*** (88.5) | 275.3 (92.4) | −115.2*** (95.5) |
| n | 104 | 661 | 139 | |||
| Body weight (kg) | ||||||
| Entire cohort | 72.0 (16.8) | 0.4 (3.8) | 79.8 (17.3) | −0.4*** (3.9) | 73.5 (14.7) | 0.1 (3.6) |
| n | 423 | 2,317 | 518 | |||
| Insulin-experienced | 71.8 (16.6) | 0.6** (3.6) | 82.3 (17.0) | −0.5*** (3.7) | 75.3 (14.3) | −0.1 (3.4) |
| n | 269 | 1,410 | 329 | |||
| Insulin-naive | 72.3 (17.0) | −0.1 (4.1) | 76.0 (17.1) | −0.2 (4.2) | 70.3 (15.1) | 0.5 (4.0) |
| n | 154 | 907 | 189 | |||
| HRQoL (UK VAS)b | ||||||
| Entire cohort | 0.799 (0.236) | 0.062*** (0.267) | 0.710 (0.239) | 0.116*** (0.242) | 0.657 (0.270) | 0.141*** (0.251) |
| n | 257 | 1,560 | 432 | |||
| Insulin-experienced | 0.753 (0.237) | 0.119*** (0.275) | 0.695 (0.232) | 0.115*** (0.239) | 0.640 (0.263) | 0.156*** (0.242) |
| n | 129 | 869 | 255 | |||
| Insulin-naive | 0.845 (0.226) | 0.005 (0.247) | 0.729 (0.246) | 0.116*** (0.245) | 0.681 (0.277) | 0.118*** (0.263) |
| n | 128 | 691 | 177 | |||
Due to the observational nature of the study, data were not collected or not recorded for some patients. All values are mean (SD)
FPG fasting plasma glucose; HbA 1c glycated hemoglobin; HRQoL health-related quality of life; PPG post-prandial plasma glucose; VAS visual analog scale
* p < 0.05 vs. baseline; ** p < 0.01 vs. baseline; *** p < 0.001 vs. baseline
aStatistical analysis was not performed on these data
bUK VAS score was calculated using data from EQ-5D scores (1.00 = full health; 0 = deceased)
Baseline FPG was high in all age-groups in insulin-naive and insulin-experienced patients, and significantly improved in all age-groups after 24 weeks treatment with insulin aspart as part of a basal-bolus regimen (Table 3). Baseline FPG values were higher in insulin-naive than insulin-experienced patients, and improvements were numerically greater in insulin-naive patients than in insulin-experienced patients (Table 3). Furthermore, baseline FPG was slightly higher at younger ages, and the reductions after 24 weeks were correspondingly greater in the younger age-group (Table 3). Likewise, baseline post-breakfast PPG was high in all age-groups and higher in insulin-naive than insulin-experienced patients (Table 3). After 24 weeks treatment with insulin aspart as part of a basal-bolus regimen, statistically significant improvements in PPG were observed in all sub-groups and these were greater in insulin-naive groups than insulin-experienced groups (Table 3).
Body Weight
Baseline body weight was higher in the >40–65 years age-group than the other two age-groups (Table 3). Weight remained stable after 24 weeks treatment with insulin aspart as part of a basal-bolus regimen in the ≤40 and >65 years age-groups, but there was a significant weight loss in patients aged >40–65 years (Table 3). In insulin-experienced patients, there was a significant weight increase in the ≤40 years age-group, a significant weight loss in the >40–65 years age-group, and no significant change in weight in the >65 years age-group (Table 3). By contrast, weight remained stable after 24 weeks treatment with insulin aspart as part of a basal-bolus regimen in all age-groups in the insulin-naive cohort (Table 3).
Health-Related Quality of Life
Statistically significant improvement in UK VAS scores after 24 weeks were observed in all age-groups except insulin-naive patients in the ≤40 years age-group (Table 3). This younger age-group had considerably higher UK VAS scores (i.e., better HRQoL) at baseline than the older age-groups, regardless of previous therapy.
Discussion
Insulin aspart, administered at mealtime(s) as part of a basal-bolus regimen, was associated with significant improvements in glycemic control in patients with type 2 diabetes across a wide age spectrum in this sub-analysis of the observational A1chieve study. These improvements in glycemic control were achieved despite a reduction in the number of concomitant OGLDs used. Older age and basal-bolus insulin therapy were identified as significant predictors of response to insulin therapy (defined as HbA1c <7.5% and/or >1% HbA1c reduction 12 months post-insulin initiation) at 1 year in a recent UK retrospective study [26]; this current analysis shows good response in everyday practice across the range of patient ages.
The cohort of patients aged >65 years who were recruited in this non-interventional study experienced lower rates of hypoglycemia at baseline than patients aged ≤40 years, and similar rates to those aged >40–65 years. Patients in the insulin-experienced ≤40 years age-group had particularly high rates of hypoglycemia, nocturnal hypoglycemia and major hypoglycemia at baseline. However, the proportion of patients reporting hypoglycemia episodes decreased on the basal-bolus regimen with insulin aspart in all age-groups, and great reassurance can be drawn from the very low rates of major hypoglycemia after 24 weeks. Furthermore, sulfonylurea use did not appear to increase the rate of hypoglycemia in any age-group; indeed improvements in hypoglycemia were achieved despite between 13.7% and 17.3% of patients in all age-groups receiving sulfonylureas at 24 weeks. While guidelines recommend discontinuation of sulfonylureas on commencement of more complex insulin regimens (such as basal-bolus insulin regimens) in order to minimize the risk of hypoglycemia episodes, this does not always occur in practice [27]. The beneficial effect of insulin aspart as part of a basal-bolus regimen on hypoglycemia may therefore reflect the overall reduction in polypharmacy.
Elderly patients are at greater risk of hypoglycemia events than patients of a younger age [3]. This, together with the association of hypoglycemia with the use of basal-bolus therapy, may have raised concerns over the application of this regimen in elderly patients. However, the results from this study showed that elderly patients in both the entire cohort and the insulin-experienced cohort had lower rates of hypoglycemia following 24 weeks of a basal-bolus regimen with insulin aspart versus baseline. Indeed, the rate in the oldest age-group was similar to the younger age-groups after 24 weeks of treatment (in both insulin-experienced and insulin-naive populations).
Consistent with earlier reports [5, 6], quality of life was lower at baseline for older patients versus the youngest age-group. However, elderly patients experienced the greatest magnitude of changes in HRQoL following 24 weeks of basal-bolus therapy with insulin aspart. It is likely that the significant improvements in quality of life reflect the observed improvements in glycemic control and benefits on hypoglycemia risk [28, 29], but may also reflect other factors, such as reduction in OGLD use or the flexible treatment regimen offered by insulin aspart.
The limitations of this observational study design have been discussed elsewhere [18], and include: the lack of randomization and absence of a control arm, the absence of control for concomitant medication, dietary or lifestyle changes, and lack of data relating to the progress of disease-related complications (e.g., diabetic nephropathy). Some variables (e.g., hypoglycemia events) were also based on participant recall and the incidence, especially of minor events, could be under-estimated. Despite the limitation with reporting of hypoglycemia episodes, the improvement in glycemic control across all age-groups after 24 weeks of basal-bolus therapy (reduction in HbA1c levels between 1.8% and 3.1%), coupled with the modest rates of hypoglycemia reported by patients, suggest that insulin aspart as part of a basal-bolus regimen is an effective and tolerable treatment that can reduce risk for hypoglycemia events. Further, most randomized controlled trials exclude elderly patients with diabetes through imposed age limitations, or stringent exclusion criteria. Therefore, the sub-group of elderly patients from this large observational study will provide valuable information regarding the use of basal-bolus regimens in elderly patients.
This international study offered the opportunity to view results from a regional perspective. However, the smaller number of patients in the oldest and youngest age-groups receiving insulin aspart as part of a basal-bolus regimen would make interpretation of individual region results problematic. The overall proportion of participants in A1chieve that received the basal-bolus regimen was low [18] despite poor general glycemic control and the obvious need for more intensive insulin regimens. This may highlight a general reluctance among physicians to adopt an intensive regimen, such as a basal-bolus regimen, or limited awareness of the potential benefits in use of insulin analogs in less developed countries.
In conclusion, insulin aspart administered within a basal-bolus regimen as part of routine clinical practice had efficacy and good tolerability in all age-groups as either a starting insulin regimen, or when patients were switched from other regimens. The results also show that the use of insulin aspart as part of a basal-bolus regimen is effective and tolerable in elderly people with diabetes, although treatment should be individualized.
Acknowledgments
The A1chieve study and this manuscript were funded by Novo Nordisk A/S (Bagsvaerd, Denmark). Editorial assistance on this manuscript was provided by Martin Gilmour and Daniella Otway at ESP Bioscience (Crowthorne, UK), funded by Novo Nordisk A/S. Jian-Wen Chen is the guarantor for this article, and takes responsibility for the integrity of the work as a whole.
Author contribution
All authors contributed to: (1) the analysis and interpretation of data; (2) revising the article critically for important intellectual content; and (3) final approval of the version to be published. Jian-Wen Chen also contributed to the study design.
Conflict of interest
Zafar A. Latif and Nabil El Naggar have no conflicts of interest. Jian-Wen Chen is an employee of Novo Nordisk A/S. Zanariah Hussein has been involved in clinical trials as well as delivered lectures for Novo Nordisk, Eli Lilly, and Sanofi-Aventis. Leon Litwak is a member of the national board of Novo Nordisk, Novartis, Sanofi-Aventis, BMS and AstraZeneca. He is also a speaker and Principal Investigator of clinical trials for Eli Lilly, Novo Nordisk, Novartis, GSK, Takeda, PPD, Pfizer, MSD, Sanofi-Aventis, Boehringer Ingelheim, BMS and AstraZeneca. Pradana Soewondo is an advisory board member for Novo Nordisk, Sanofi-Aventis, and Novartis.
Ethical standard
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2008. Informed consent was obtained from all patients for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Footnotes
The A1chieve trial was registered with ClinicalTrials.gov (NCT00869908).
References
- 1.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Cheng D. Prevalence, predisposition and prevention of type II diabetes. Nutr Metab (Lond) 2005;18:29. doi: 10.1186/1743-7075-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ligthelm RJ, Kaiser M, Vora J, Yale JF. Insulin use in elderly adults: risk of hypoglycemia and strategies for care. J Am Geriatr Soc. 2012;60:1564–1570. doi: 10.1111/j.1532-5415.2012.04055.x. [DOI] [PubMed] [Google Scholar]
- 4.Chan TY. Estimates on the incidence of antidiabetic drug-induced severe hypoglycaemia in Hong Kong. Pharmacoepidemiol Drug Saf. 1998;7:411–414. doi: 10.1002/(SICI)1099-1557(199811/12)7:6<411::AID-PDS379>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 5.Tang WL, Wang YM, Du WM, Cheng NN, Chen BY. Assessment of quality of life and relevant factors in elderly diabetic patients in the Shanghai community. Pharmacoepidemiol Drug Saf. 2006;15:123–130. doi: 10.1002/pds.1166. [DOI] [PubMed] [Google Scholar]
- 6.Landman GW, van Hateren KJ, Kleefstra N, Groenier KH, Gans RO, Bilo HJ. Health-related quality of life and mortality in a general and elderly population of patients with type 2 diabetes (ZODIAC-18) Diabetes Care. 2010;33:2378–2382. doi: 10.2337/dc10-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mooradian AD, McLaughlin S, Boyer CC, Winter J. Diabetes care for older adults. Diabetes Spectr. 1999;12:70–77. [Google Scholar]
- 8.Home PD. The pharmacokinetics and pharmacodynamics of rapid-acting insulin analogues and their clinical consequences. Diabetes Obes Metab. 2012;14:780–788. doi: 10.1111/j.1463-1326.2012.01580.x. [DOI] [PubMed] [Google Scholar]
- 9.Freeman JS. Insulin analog therapy: improving the match with physiologic insulin secretion. J Am Osteopath Assoc. 2009;109:26–36. [PubMed] [Google Scholar]
- 10.Lindholm A, Jacobsen LV. Clinical pharmacokinetics and pharmacodynamics of insulin aspart. Clin Pharmacokinet. 2001;40:641–659. doi: 10.2165/00003088-200140090-00002. [DOI] [PubMed] [Google Scholar]
- 11.Brunner GA, Hirschberger S, Sendlhofer G, et al. Post-prandial administration of the insulin analogue insulin aspart in patients with type 1 diabetes mellitus. Diabet Med. 2000;17:371–375. doi: 10.1046/j.1464-5491.2000.00289.x. [DOI] [PubMed] [Google Scholar]
- 12.Rosenfalck AM, Thorsby P, Kjems L, et al. Improved postprandial glycaemic control with insulin aspart in type 2 diabetic patients treated with insulin. Acta Diabetol. 2000;37:41–46. doi: 10.1007/s005920070034. [DOI] [PubMed] [Google Scholar]
- 13.Home PD, Lindholm A, Riis A. Insulin aspart vs. human insulin in the management of long-term blood glucose control in type 1 diabetes mellitus: a randomized controlled trial. Diabet Med. 2000;17:762–770. doi: 10.1046/j.1464-5491.2000.00380.x. [DOI] [PubMed] [Google Scholar]
- 14.Home PD, Lindholm A, Hylleberg B, Round P. Improved glycemic control with insulin aspart: a multicenter randomized double-blind crossover trial in type 1 diabetic patients. UK Insulin Aspart Study Group. Diabetes Care. 1998;21:1904–1909. doi: 10.2337/diacare.21.11.1904. [DOI] [PubMed] [Google Scholar]
- 15.Lindholm A, McEwen J, Riis AP. Improved postprandial glycemic control with insulin aspart. A randomized double-blind cross-over trial in type 1 diabetes. Diabetes Care. 1999;22:801–805. doi: 10.2337/diacare.22.5.801. [DOI] [PubMed] [Google Scholar]
- 16.Perriello G, Pampanelli S, Porcellati F, et al. Insulin aspart improves meal time glycaemic control in patients with type 2 diabetes: a randomized, stratified, double-blind and cross-over trial. Diabet Med. 2005;22:606–611. doi: 10.1111/j.1464-5491.2005.01473.x. [DOI] [PubMed] [Google Scholar]
- 17.Rys P, Pankiewicz O, Lach K, Kwaskowski A, Skrzekowska-Baran I, Malecki MT. Efficacy and safety comparison of rapid-acting insulin aspart and regular human insulin in the treatment of type 1 and type 2 diabetes mellitus: a systematic review. Diabetes Metab. 2011;37:190–200. doi: 10.1016/j.diabet.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Home P, Naggar NE, Khamseh M, et al. An observational non-interventional study of people with diabetes beginning or changed to insulin analogue therapy in non-Western countries: the A1chieve study. Diabetes Res Clin Pract. 2011;94:352–363. doi: 10.1016/j.diabres.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 19.Krzymien J, Kobli T, Nazar M. Multicenter, open-label, nonrandomized, observational safety study in subjects using insulin aspart in basal-bolus regimen for the treatment of diabetes. Pol Arch Med Wewn. 2010;120:444–450. [PubMed] [Google Scholar]
- 20.Dornhorst A, Luddeke HJ, Honka M, et al. Safety and efficacy of insulin detemir basal-bolus therapy in type 1 diabetes patients: 14-week data from the European cohort of the PREDICTIVE study. Curr Med Res Opin. 2008;24:369–376. doi: 10.1185/030079908X260835. [DOI] [PubMed] [Google Scholar]
- 21.World Medical Association. Declaration of Helsinki—ethical principles for medical research involving human subjects 2008. http://www.riip-influenza.org/wp-content/uploads/2011/08/Helsinki-2008-EN.pdf (Accessed 8 Nov 2011).
- 22.International Society for Pharmacoepidemiology. Guidelines for Good Pharmacoepidemiology Practices (GPP). Revision 2; 2007.
- 23.EuroQol—a new facility for the measurement of health-related quality of life. The EuroQol Group. Health Policy. 1990;16:199–208. [DOI] [PubMed]
- 24.Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35:1095–1108. doi: 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Shah S, Zilov A, Malek R, Soewondo P, Bech O, Litwak L. Improvements in quality of life associated with insulin analogue therapies in people with type 2 diabetes: results from the A1chieve observational study. Diabetes Res Clin Pract. 2011;94:364–370. doi: 10.1016/j.diabres.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 26.Owen V, Seetho I, Idris I. Predictors of responders to insulin therapy at 1 year among adults with type 2 diabetes. Diabetes Obes Metab. 2010;12:865–870. doi: 10.1111/j.1463-1326.2010.01239.x. [DOI] [PubMed] [Google Scholar]
- 27.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barendse S, Singh H, Frier BM, Speight J. The impact of hypoglycaemia on quality of life and related patient-reported outcomes in type 2 diabetes: a narrative review. Diabet Med. 2012;29:293–302. doi: 10.1111/j.1464-5491.2011.03416.x. [DOI] [PubMed] [Google Scholar]
- 29.Jacobson AM. Impact of improved glycemic control on quality of life in patients with diabetes. Endocr Pract. 2004;10:502–508. doi: 10.4158/EP.10.6.502. [DOI] [PubMed] [Google Scholar]