Abstract
Introduction
Diabetes therapy should balance glycemic control with risk of adverse events. This sub-analysis of the A1chieve study evaluated clinical safety and effectiveness of insulin detemir in different age-groups (≤40 years, >40–65 years, and >65 years) of insulin-experienced and insulin-naïve people with type 2 diabetes.
Methods
A1chieve was an international, open-label, non-interventional, 24-week study in 66,726 people with type 2 diabetes starting/switching to therapy with biphasic insulin aspart 30, insulin detemir or insulin aspart (alone/in combination) in routine clinical practice. This sub-analysis evaluated clinical safety and effectiveness in patients starting/switching to insulin detemir (±oral glucose-lowering drugs).
Results
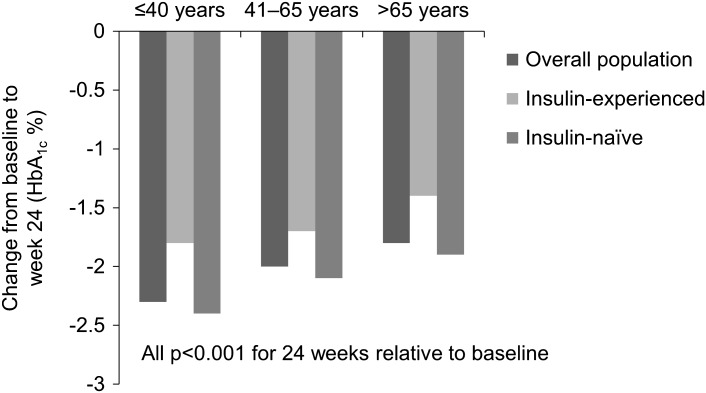
In total, 15,241 patients were included in the sub-analysis. In all age-groups, the proportion of participants experiencing any, major or nocturnal hypoglycemia was significantly (all p < 0.05) reduced relative to baseline, except in insulin-naïve patients for any and nocturnal hypoglycemia, where there was a significant increase or no significant change in patients aged >65 years and >40–65 years, respectively, and no significant change in major hypoglycemia in insulin-naïve patients aged ≤40 years. Seven serious adverse drug reactions were reported. Body weight was significantly reduced in patients aged ≤40 years and >40–65 years and significantly increased in insulin-naïve patients aged >65 years at 24 weeks. At 24 weeks, glycated hemoglobin was reduced by 2.3%, 2.0%, and 1.8%, in the ≤40 years, >40–65 years, and >65 years age-groups, respectively (all p < 0.001). Fasting and post-prandial plasma glucose were significantly reduced and health-related quality of life (HRQoL) significantly improved across all patient cohorts (all p < 0.001).
Conclusion
After 24-week treatment with insulin detemir, all age-groups of insulin-experienced and insulin-naïve patients had significantly improved glycemic control and HRQoL. The proportion of patients experiencing hypoglycemia was reduced in all age-groups but unchanged in insulin-naïve patients aged >40–65 years and increased in insulin-naïve patients aged >65 years. The safety and effectiveness of insulin detemir may benefit all age-groups.
Keywords: Hypoglycemia, Insulin detemir, Type 2 diabetes
Introduction
The prevalence of diabetes is increasing worldwide [1]. Good glycemic control is an important goal of diabetes treatment to prevent and/or delay long-term microvascular complications [2, 3]. Current guidelines from the International Diabetes Federation (IDF) and joint American Diabetes Association (ADA)/European Association for the Study of Diabetes (EASD) recommend the glycated hemoglobin (HbA1c) target of <7.0%, with some individualization according to age [4–6]. For example, the IDF recommends the HbA1c target of 7.0–7.5% for people with type 2 diabetes aged 70 years or older [4], and California Healthcare Foundation/American Geriatrics Society Panel guidelines recommend the target of <7.0% for people with diabetes aged 65 years or older [7].
The ADA/EASD position statement [5] and IDF guidelines [4] for type 2 diabetes recommend initiating insulin therapy with basal (long-acting) insulin if glycemic target is not achieved with metformin alone or in combination with other oral medications. Insulin detemir (Levemir®, Novo Nordisk A/S, Bagsvaerd, Denmark) is a long-acting insulin analog with a duration of action of up to 24 h [8–10]. Randomized, parallel studies have shown that insulin detemir, given as add-on therapy to oral glucose-lowering drugs (OGLDs) in people with uncontrolled type 2 diabetes, is associated with reduced risk of hypoglycemia compared with neutral protamine Hagedorn (NPH) [11, 12]. Furthermore, large observational studies in people with type 2 diabetes have shown that insulin detemir results in significantly reduced HbA1c, fasting plasma glucose (FPG) and within-patient FPG variability [13, 14], with low rates of adverse events and hypoglycemia, and no weight gain [13, 14].
A1chieve was an international non-interventional study evaluating the clinical safety and effectiveness of insulin analogs in people with type 2 diabetes in everyday clinical practice [15, 16]. The study demonstrated the safety and effectiveness of these insulin analog therapies [15], and significant improvements in health-related quality of life (HRQoL) [16]. This sub-analysis of data from A1chieve evaluated the clinical safety and effectiveness of insulin detemir in three age-groups. Additionally, the data were analyzed by pre-study insulin experience.
Materials and Methods
A1chieve was an international, multicenter, prospective, open-label, non-interventional, 24-week study in people with type 2 diabetes who had been receiving anti-diabetes medication before starting, or switching to, insulin therapy with biphasic insulin aspart 30 (NovoMix 30®, Novo Nordisk A/S, Bagsvaerd, Denmark), insulin detemir or insulin aspart (NovoRapid®, Novo Nordisk A/S, Bagsvaerd, Denmark) (alone or in combination) in routine clinical practice [15]. The study included patients attending diabetes care clinics where insulin therapy was initiated or modified at the discretion of the treating physician, based on their clinical judgment. The patients were enrolled between January 2009 and June 2010 in 28 countries, which were grouped into seven geographical regions: China; South Asia (Bangladesh, India, Pakistan); East Asia (Indonesia, Korea, Malaysia, Philippines, Singapore, Taiwan); North Africa (Algeria, Morocco, Tunisia, Libya); Middle East/Gulf (Egypt, Iran, Jordan, Turkey, Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, United Arab Emirates, Yemen); Latin America (Argentina, Mexico); and Russia [15]. The study was conducted in accordance with the Guidelines for Good Pharmacoepidemiology Practice [17] and Declaration of Helsinki of 1964, as revised in 2008 [18]. All local requirements for Health Authorities or Ethics Committee approvals, if applicable, were acquired. All participants signed informed consent forms and could withdraw from the study at any time.
This sub-analysis included patients starting or switching to treatment with insulin detemir alone or in combination with OGLDs. To reflect routine clinical practice as much as possible, inclusion and exclusion criteria were minimal. Further details on inclusion and exclusion criteria, and study design were previously published [15]. Insulin detemir was used according to the label approved by the regulatory authority.
Assessments and Outcome Measures
Assessment sessions were defined as baseline, interim [approximately 12 weeks from baseline (results not reported here)] and study end (approximately 24 weeks from baseline). The primary objective was to assess the safety profile of insulin detemir by evaluating the incidence of serious adverse drug reactions (SADRs), including major hypoglycemia events. An additional safety assessment was change in the number of hypoglycemia events between baseline and 24 weeks, which was based on patient recall of events within the last 4 weeks prior to the study visit.
A hypoglycemia event was defined as an event with symptoms of hypoglycemia that resolved with oral carbohydrate intake, glucagon or intravenous glucose, or any symptomatic or asymptomatic event where plasma glucose was <3.1 mmol/l or 56 mg/dl. Nocturnal hypoglycemia events were defined as individualized symptomatic events consistent with hypoglycemia, occurring during sleep, after the evening insulin injection and before getting up in the morning; and if relevant, before morning determination of FPG and the morning insulin injection. Major hypoglycemia events were defined as events with severe central nervous system symptoms consistent with hypoglycemia in which the patient was unable to self-treat and had one of the following characteristics: plasma glucose <3.1 mmol/l or 56 mg/dl, or reversal of symptoms after either food intake, glucagon or intravenous glucose administration.
The secondary objective was to investigate the clinical effectiveness of insulin detemir. Effectiveness measurements comprised change in HbA1c, FPG levels before breakfast, post-prandial plasma glucose (PPG) levels after breakfast, body weight, and HRQoL between baseline and 24 weeks. To assess the impact of insulin detemir on HRQoL, this was assessed at baseline and after 24 weeks by self-report using the EQ-5D questionnaire [19], which evaluates five domains of patient health/lifestyle (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression). Scores in these five domains were converted to a single utility value (UK VAS set), with ‘1.00’ indicating ‘full health’ and ‘0.00’ indicating the state ‘deceased’ [16].
Statistical Analysis
Safety and effectiveness outcome measures were analyzed by age-group (≤40 years, >40–65 years, and >65 years) and by pre-study insulin experience (insulin-experienced and insulin-naïve). All variables were analyzed using the full analysis set (FAS), defined as all patients with a baseline visit who used insulin detemir at least once. For hypoglycemia, the proportion of participants reporting at least one event was analyzed using McNemar’s test. The proportion of participants reporting hypoglycemia at baseline and 24 weeks was also analyzed according to sulfonylurea use at the study visits. Changes from baseline in effectiveness measures were assessed using Student’s paired t test. Data analysis was performed by Novo Nordisk using SAS® Version 9.1.3 (SAS® Institute Inc., Cary, North Carolina, USA). All statistical tests were two-sided, using a pre-specified 5% significance level.
Results
Study Participants
Baseline characteristics of the participants are shown in Table 1. A total of 15,241 people with type 2 diabetes (22.8% of the overall A1chieve study population) were treated with insulin detemir (±OGLDs), including 1,467 aged ≤40 years, 10,967 aged >40–65 years, and 2,807 aged >65 years. Most study participants were insulin-naïve before the study.
Table 1.
Baseline patient and disease characteristics by age-group
| Baseline variable | Age ≤40 years | Age >40–65 years | Age >65 years |
|---|---|---|---|
| Entire cohort, n | 1,467 | 10,967 | 2,807 |
| Insulin status, n | |||
| Insulin-experienced | 258 | 2,286 | 840 |
| Insulin-naïve | 1,209 | 8,681 | 1,967 |
| Gender (male/female) (%)a | 63.4/36.6 | 55.4/44.6 | 43.4/56.6 |
| Mean (SD) age (years) | 34.9 (5.8) | 53.0 (6.5) | 71.6 (5.0) |
| Mean (SD) body weight (kg)b | 77.4 (16.1) | 77.3 (16.7) | 69.5 (14.6) |
| Mean (SD) BMI (kg/m2)c | 28.0 (5.2) | 28.5 (5.4) | 26.9 (5.0) |
| Mean (SD) age at diagnosis (years)d | 30.9 (5.6) | 45.0 (7.1) | 59.3 (9.0) |
| Mean (SD) diabetes duration (y)d | 4.3 (3.3) | 8.0 (5.2) | 12.3 (7.8) |
Due to the observational nature of this study, not all baseline data were recorded
BMI body mass index
a n = 1,464, n = 10,950, n = 2,805 for the ≤40, >40–65 and >65 years age-groups, respectively
b n = 1,401, n = 10,454, n = 2,589 for the ≤40, >40–65 and >65 years age-groups, respectively
c n = 1,323, n = 9,726, n = 2,392 for the ≤40, >40–65 and >65 years age-groups, respectively
d n = 1,422, n = 10,812, n = 2,763 for the ≤40, >40–65 and >65 years age-groups, respectively
Safety Measures
Insulin Dose
In all three age-groups, mean total insulin dose appeared to increase slightly between baseline (when patients started or switched to insulin detemir) and 24 weeks (Table 2).
Table 2.
Insulin dose at baseline (when patients started or switched to insulin detemir) and 24 weeks
| Mean (SD) insulin dose (U/kg) | Age ≤40 years | Age >40–65 years | Age >65 years | |||
|---|---|---|---|---|---|---|
| Baseline | 24 weeks | Baseline | 24 weeks | Baseline | 24 weeks | |
| Entire cohort | 0.29 (0.16) | 0.37 (0.20) | 0.26 (0.15) | 0.37 (0.20) | 0.27 (0.16) | 0.38 (0.21) |
| n | 1,401 | 1,171 | 10,453 | 8,755 | 2,589 | 2,048 |
| Insulin-experienced | 0.35 (0.20) | 0.45 (0.27) | 0.35 (0.19) | 0.45 (0.23) | 0.36 (0.19) | 0.45 (0.24) |
| n | 244 | 201 | 2,163 | 1,812 | 766 | 585 |
| Insulin-naïve | 0.28 (0.14) | 0.36 (0.18) | 0.24 (0.13) | 0.35 (0.18) | 0.23 (0.13) | 0.35 (0.19) |
| n | 1,157 | 970 | 8,290 | 6,943 | 1,823 | 1,463 |
Due to the observational nature of this study not all measures were reported or collected
All Hypoglycemia Events
At 24 weeks, the proportion of participants experiencing hypoglycemia in the entire cohort and in insulin-experienced patients was significantly reduced from baseline in all age-groups (Table 3). In insulin-naïve patients, the proportion experiencing hypoglycemia was significantly reduced from baseline in the ≤40 years age-group and significantly increased in the >65 years age-group at 24 weeks (Table 3). There was no significant change between baseline and 24 weeks in the proportion of participants experiencing hypoglycemia in the >40–65 years age-group (Table 3). At both baseline and week 24, there was no indication in any age-group that the proportion experiencing hypoglycemia was higher in participants taking sulfonylureas compared with those who were not (Table 3).
Table 3.
Self-reported hypoglycemia events in the preceding 4 weeks of the study visit at baseline and after 24 weeks of treatment with insulin detemir
| Measurement | Proportion of patients with at least one event, % (event/person-year) | |||||
|---|---|---|---|---|---|---|
| Age ≤40 years | Age >40–65 years | Age >65 years | ||||
| Baseline | 24 weeks | Baseline | 24 weeks | Baseline | 24 weeks | |
| Hypoglycemia (overall) | ||||||
| Entire cohort | 6.4 (1.89) | 3.3*** (0.91) | 7.3 (2.56) | 4.7*** (1.42) | 10.0 (3.75) | 6.6*** (1.88) |
| n | 1,467 | 1,279 | 10,967 | 9,677 | 2,807 | 2,334 |
| Insulin-experienced | 16.3 (6.40) | 6.3*** (2.16) | 19.9 (7.98) | 6.0*** (1.69) | 23.9 (9.67) | 8.1*** (2.18) |
| n | 258 | 223 | 2,286 | 1,991 | 840 | 680 |
| Insulin-naïve | 4.3 (0.92) | 2.7* (0.65) | 3.9 (1.13) | 4.4 (1.35) | 4.1 (1.22) | 6.0** (1.76) |
| n | 1,209 | 1,056 | 8,681 | 7,686 | 1,967 | 1,654 |
| Sulfonylurea | 6.2 (1.57) | 3.0* (0.87) | 6.0 (1.91) | 4.9 (1.40) | 7.1 (2.69) | 7.1 (2.04) |
| n | 942 | 657 | 8,261 | 5,517 | 1,913 | 1,199 |
| No sulfonylurea | 6.9 (2.45) | 3.5 (0.96) | 11.2 (4.53) | 4.5* (1.44) | 16.3 (6.02) | 6.2** (1.72) |
| n | 525 | 622 | 2,706 | 4,160 | 894 | 1,135 |
| Hypoglycemia (major)a | ||||||
| Entire cohort | 0.8 (0.12) | 0** (0) | 1.1 (0.23) | 0.0*** (0.00) | 1.6 (0.37) | 0.0*** (0.01) |
| Insulin-experienced | 3.5 (0.55) | 0** (0) | 3.5 (0.85) | 0.1*** (0.01) | 4.0 (1.02) | 0.1*** (0.02) |
| Insulin-naïve | 0.2 (0.03) | 0 (0) | 0.4 (0.07) | 0.0*** (0.00) | 0.5 (0.09) | 0** (0) |
| Hypoglycemia (nocturnal)a | ||||||
| Entire cohort | 3.1 (0.53) | 1.0*** (0.26) | 3.5 (0.81) | 1.7*** (0.41) | 4.5 (1.10) | 2.3*** (0.47) |
| Insulin-experienced | 7.4 (1.51) | 2.7* (0.93) | 11.3 (2.84) | 1.7*** (0.42) | 12.3 (3.10) | 2.4*** (0.44) |
| Insulin-naïve | 2.2 (0.32) | 0.7*** (0.12) | 1.5 (0.27) | 1.7 (0.41) | 1.2 (0.25) | 2.2* (0.48) |
Due to the observational nature of this study not all measures were reported or collected
* p < 0.05 for proportion of patients with at least one event at 24 weeks relative to baseline
** p < 0.01 for proportion of patients with at least one event at 24 weeks relative to baseline
*** p < 0.001 for proportion of patients with at least one event at 24 weeks relative to baseline
a n for each cohort same as for hypoglycemia (overall) data
Major Hypoglycemia Events
In the entire cohort and in insulin-experienced patients, the proportion of participants experiencing major hypoglycemia was significantly reduced at 24 weeks relative to baseline (Table 3). In insulin-naïve patients, the proportion of patients experiencing major hypoglycemia was significantly reduced from baseline in the >40–65 and >65 years age-groups at 24 weeks, and no major hypoglycemia was reported in the ≤40 years age-group at 24 weeks (Table 3).
Nocturnal Hypoglycemia Events
The proportion of participants experiencing nocturnal hypoglycemia was significantly lower at 24 weeks versus baseline in all three age-groups in the entire cohort and in insulin-experienced patients (Table 3). In insulin-naïve patients, the proportion experiencing nocturnal hypoglycemia was significantly reduced from baseline in the ≤40 years age-group and significantly increased in the >65 years age-group, with no significant change in the >40–65 years age-group (Table 3).
SADRs
There were seven reports of SADRs in the 15,241 people with type 2 diabetes receiving insulin detemir (±OGLDs). None of these were in the ≤40 years age-group. Six SADRs were reported in the >40–65 years age-group (three episodes of hyperglycemia and three episodes of hypoglycemia). Of these, two episodes of hyperglycemia and two episodes of hypoglycemia were probably related to insulin detemir treatment (with good reasons and sufficient documentation to assume a causal relationship), and one episode of hyperglycemia and one episode of hypoglycemia were possibly related (a causal relationship was conceivable and could not be dismissed). One SADR (an episode of hypoglycemia) was reported in the >65 years age-group, which was probably related to insulin detemir treatment.
Body Weight
In the ≤40 and >40–65 years age-groups, body weight was significantly reduced at 24 weeks; in the >65 years age-group, body weight was significantly increased (Table 4). In insulin-experienced patients, significant reduction in body weight was observed in the >40–65 and >65 years age-groups, and there was no significant change in the ≤40 years age-group (Table 4). In insulin-naïve patients, significant reduction in body weight was observed in the ≤40 and >40–65 years age-groups, and the >65 years age-group showed significant weight gain (Table 4).
Table 4.
Change in glycemic control, body weight and health-related quality of life after 24 weeks of treatment with insulin detemir
| Measurement | Age ≤40 years | Age >40–65 years | Age >65 years | |||
|---|---|---|---|---|---|---|
| Baseline | Change after 24 weeks | Baseline | Change after 24 weeks | Baseline | Change after 24 weeks | |
| HbA1c | ||||||
| Entire cohort | ||||||
| % | 9.6 (1.7) | −2.3*** (1.7) | 9.5 (1.6) | −2.0*** (1.6) | 9.4 (1.8) | −1.8*** (1.7) |
| mmol/mol | 81.4 (18.6) | −48.6*** (18.6) | 80.3 (17.5) | −45.4*** (17.5) | 79.2 (19.7) | −43.2*** (18.6) |
| n | 1,093 | 7,767 | 1,563 | |||
| Insulin-experienced | ||||||
| % | 9.3 (1.8) | −1.8*** (1.7) | 9.3 (1.7) | −1.7*** (1.7) | 9.2 (2.0) | −1.4*** (1.9) |
| mmol/mol | 78.1 (19.7) | −43.2*** (18.6) | 78.1 (18.6) | −42.1*** (18.6) | 77.1 (21.9) | −38.8*** (20.8) |
| n | 183 | 1,492 | 409 | |||
| Insulin-naïve | ||||||
| % | 9.7 (1.7) | −2.4*** (1.7) | 9.5 (1.6) | −2.1*** (1.6) | 9.5 (1.7) | −1.9*** (1.6) |
| mmol/mol | 82.5 (18.6) | −49.7*** (18.6) | 80.3 (17.5) | −46.5*** (17.5) | 80.3 (18.6) | −44.3*** (17.5) |
| n | 910 | 6,275 | 1,154 | |||
| Baseline | 24 weeks | Baseline | 24 weeks | Baseline | 24 weeks | |
|---|---|---|---|---|---|---|
| % patients with HbA1c <7.0%a | ||||||
| Entire cohort | 1.8 | 33.9 | 2.5 | 31.6 | 5.6 | 29.5 |
| n | 1,318 | 1,166 | 9,451 | 8,421 | 2,179 | 1,849 |
| Insulin-experienced | 3.9 | 30.3 | 5.2 | 30.9 | 10.9 | 28.7 |
| n | 229 | 195 | 1,883 | 1,667 | 626 | 499 |
| Insulin-naïve | 1.4 | 34.6 | 1.9 | 31.8 | 3.5 | 29.9 |
| n | 1,089 | 971 | 7,568 | 6,754 | 1,553 | 1,350 |
| Baseline | Change after 24 weeks | Baseline | Change after 24 weeks | Baseline | Change after 24 weeks | |
|---|---|---|---|---|---|---|
| FPG before breakfast (mmol/l, mg/dl) | ||||||
| Entire cohort | 11.4 (3.4), | −4.4*** (3.2), | 10.9 (3.2), | −3.9*** (3.2), | 10.5 (3.5), | −3.4*** (3.7), |
| 205 (61) | −78*** (58) | 197 (58) | −70*** (59) | 190 (63) | −62*** (67) | |
| n | 1,043 | 7,823 | 1,726 | |||
| Insulin-experienced | 10.7 (3.3), | −3.6*** (3.5), | 10.0 (3.3), | −2.7*** (3.4), | 9.3 (3.4), | −2.0*** (3.8), |
| 193 (59) | −65*** (63) | 179 (59) | −49*** (62) | 168 (61) | −36*** (68) | |
| n | 171 | 1,513 | 470 | |||
| Insulin-naïve | 11.5 (3.4), | −4.5*** (3.2), | 11.2 (3.2), | −4.2*** (3.1), | 11.0 (3.4), | −4.0*** (3.6), |
| 207 (61) | −81*** (57) | 201 (57) | −75*** (57) | 198 (62) | −71*** (65) | |
| n | 872 | 6,310 | 1,256 | |||
| PPG after breakfast (mmol/l, mg/dl) | ||||||
| Entire cohort | 15.7 (4.3), | −6.2*** (4.2), | 14.8 (4.1), | −5.2*** (4.2), | 14.2 (4.4), | −4.0*** (4.7), |
| 283 (77) | −112*** (75) | 266 (75) | −94*** (75) | 255 (80) | −73*** (84) | |
| n | 680 | 5,348 | 1,029 | |||
| Insulin-experienced | 14.3 (4.7), | −5.1*** (4.4), | 13.9 (4.2), | −4.0*** (4.2), | 13.2 (4.5), | −2.8*** (4.6) |
| 257 (84) | −92*** (80) | 250 (76) | −71*** (76) | 238 (81) | −50*** (83) | |
| n | 114 | 993 | 267 | |||
| Insulin-naïve | 16.0 (4.2), | −6.5*** (4.1), | 15.0 (4.1), | −5.5*** (4.1), | 14.5 (4.4), | −4.5*** (4.6), |
| 288 (75) | −116*** (73) | 269 (74) | −99*** (74) | 261 (79) | −81*** (83) | |
| n | 566 | 4,355 | 762 | |||
| Body weight (kg) | ||||||
| Entire cohort | 77.3 (15.7) | −0.5*** (3.7) | 77.8 (16.4) | −0.5*** (4.0) | 70.5 (14.7) | 0.2* (3.7) |
| n | 1,166 | 8,603 | 2,012 | |||
| Insulin-experienced | 80.1 (17.0) | −0.3 (3.9) | 77.7 (16.4) | −0.8*** (3.6) | 71.7 (14.8) | −0.5** (3.5) |
| n | 201 | 1,784 | 575 | |||
| Insulin-naïve | 76.7 (15.4) | −0.6*** (3.7) | 77.8 (16.4) | −0.4*** (4.1) | 70.0 (14.6) | 0.4*** (3.7) |
| n | 965 | 6,819 | 1,437 | |||
| HRQoL (UK VAS)b | ||||||
| Entire cohort | 0.625 (0.293) | 0.204*** (0.281) | 0.691 (0.261) | 0.149*** (0.258) | 0.680 (0.244) | 0.109*** (0.243) |
| n | 759 | 6,213 | 1,958 | |||
| Insulin-experienced | 0.719 (0.265) | 0.141*** (0.265) | 0.723 (0.223) | 0.106*** (0.239) | 0.654 (0.245) | 0.113*** (0.243) |
| n | 119 | 1,276 | 575 | |||
| Insulin-naïve | 0.608 (0.295) | 0.215*** (0.283) | 0.683 (0.270) | 0.160*** (0.261) | 0.691 (0.243) | 0.108*** (0.243) |
| n | 640 | 4,937 | 1,383 | |||
All values are mean (SD) except for % patients with HbA1c < 7.0%. Due to the observational nature of this study not all measures were reported or collected
HbA 1c glycated hemoglobin, FPG fasting plasma glucose, PPG post-prandial plasma glucose, HRQoL health-related quality of life, VAS visual analog scale
* p < 0.05 for 24 weeks relative to baseline
** p < 0.01 for 24 weeks relative to baseline
*** p < 0.001 for 24 weeks relative to baseline
aStatistical analysis was not performed on these data
bUK VAS score was calculated using data from EQ-5D scores (1.00 = full health; 0 = dead)
Effectiveness Measures
Glycemic Measures
Significant reductions in HbA1c were achieved in all age-groups and in insulin-experienced and insulin-naïve patients at 24 weeks (Table 4; Fig. 1). The percentage of participants with HbA1c <7.0% appeared to increase between baseline and 24 weeks in all three age-groups in insulin-experienced and insulin-naïve (Table 4). In the >65 years age-group, the percentage with HbA1c <7.5% was 12.0% at baseline and 53.8% at 24 weeks. The proportion of this age-group with HbA1c <7.5% at baseline appeared to be higher in insulin-experienced patients (20.0%) compared with insulin-naïve patients (8.8%). At 24 weeks, the percentage of the >65 years age-group with HbA1c <7.5% appeared to be similar in insulin-experienced (51.5%) and insulin-naïve patients (54.7%).
Fig. 1.
Effectiveness results by age-group and pre-study insulin experience
Significant reductions in FPG were observed between baseline and 24 weeks for all age-groups and in both insulin-experienced and insulin-naïve patients (Table 4). PPG levels were also significantly lowered in all age-groups at 24 weeks (Table 4). In both insulin-experienced and insulin-naïve patients, all age-groups showed significant improvements in FPG and PPG at 24 weeks (Table 4).
HRQoL
All age-groups, whether insulin-experienced or insulin-naïve, had significantly improved UK VAS scores at 24 weeks (Table 4).
Discussion
This sub-analysis of the A1chieve study reports the results for people with poorly controlled type 2 diabetes of different age-groups who switched to, or started, insulin therapy with insulin detemir. The data showed that 24-week treatment with insulin detemir (±OGLDs) in routine clinical practice resulted in improved glycemic control for all age-groups, in both insulin-experienced and insulin-naïve patients. HbA1c levels were significantly reduced in all age-groups overall and in insulin-experienced and insulin-naïve patients after 24-week treatment with insulin detemir. FPG and PPG levels were significantly improved across all age-groups and in insulin-naïve or insulin-experienced patients.
Insulin detemir was well tolerated in all age-groups. Not surprisingly, baseline hypoglycemia appeared to be more frequent in insulin-experienced patients than insulin-naïve patients, and in patients aged >65 years than younger patients. The improvement in the incidence of hypoglycemia when switching to insulin detemir is important for all age-groups but especially for patients aged >65 years, given the increased risk of severe hypoglycemia [20, 21]. The data for this age-group also show that major hypoglycemia is significantly reduced at week 24 compared with baseline. Between baseline and 24 weeks, the proportion of patients in the >65 years age-group with HbA1c <7.0% increased from 5.6% to 29.5%. While this HbA1c level may not be a target in the elderly population, better control of diabetes could have resulted in a higher frequency of hypoglycemia.
The observed increase in HRQoL in all age-groups may reflect the overall improvements in incidence of hypoglycemia reported for insulin detemir treatment [22, 23]. It is surprising that overall hypoglycemia and nocturnal hypoglycemia were significantly reduced at 24 weeks compared with baseline in insulin-naïve patients in the ≤40 years age-group, while there was no change in the >40–65 years age-group and a significant increase in the >65 years age-group. There is no clear explanation for the reduced hypoglycemia in the ≤40 years age-group, but it could be related to pre-study OGLD use; for example, sulfonylureas are associated with an increased risk of hypoglycemia [24]. There was a reduction in sulfonylurea use between baseline and week 24 in this age-group that could potentially have contributed to a lower rate of hypoglycemia. However, a similar decrease in sulfonylurea use was observed in the other age-groups and there was no evidence for a higher rate of hypoglycemia in participants taking sulfonylureas compared with those who were not. Therefore, an alternative explanation may be needed.
Interestingly, in patients aged >65 years, those switching to insulin detemir had significant weight loss while those starting insulin detemir had significant weight gain at 24 weeks. The weight changes were significant but small, and therefore, may not be clinically important. Furthermore, this difference between insulin-naïve and insulin-experienced patients was not observed in the other age-groups, and in fact weight generally was reduced at 24 weeks. In other observational studies in people with type 2 diabetes, 24-week [13] and 26-week [14] treatment with insulin detemir led to small significant reductions in weight.
Observational studies such as A1chieve allow a large number of outcomes to be assessed in a large population, and sub-analyses can be performed to evaluate which patients benefit from each treatment. Unlike RCTs, observational studies are not randomized and are more susceptible to selection bias. However, the large number of patients assessed in A1chieve may help to minimize possible confounding factors. This study did not include a control group or control for concomitant medication or dietary intake, which is difficult to measure, and some outcomes relied on self-reported information, participant recall, or diverse diaries. A limitation with the hypoglycemia data is that they were based on patient recall of hypoglycemia in the 4 weeks preceding the study visits, which could have led to an underestimation of mild hypoglycemia events. The incidence of hypoglycemia, especially mild events, could also be underestimated in non-Western countries where blood glucose may not be measured very frequently. However, the advantage of this study is the real-world clinical setting, including actual practice patterns and a broader population than a RCT. Despite the limitation that reporting of hypoglycemia relied on patient recall, the significant improvement in glycemic control (with HbA1c reduced by 1.4–2.4%), with a modest proportion of patients experiencing hypoglycemia, is of clinical relevance. In general, RCTs have either a participant age limit or exclude elderly people with diabetes who have certain complications. Hence, it is noteworthy that data from even the smallest sub-group of this large observational study will help to elucidate the safety and effectiveness of insulin detemir in different age-groups.
Consistent with the results of other studies [13, 14] and the overall A1chieve population [15], the results of this sub-analysis suggest that insulin detemir is well tolerated, and improves glycemic control and HRQoL in most groups of people with poorly controlled type 2 diabetes versus their previous insulin regimen. Improvements in glycemic control and HRQoL were observed across a wide range of age-groups, and both in patients initiating insulin therapy with insulin detemir and those switching from other insulin regimens. The proportion of participants reporting hypoglycemia events was reduced in all age-groups in the entire cohort and in insulin-experienced patients, and the reduced proportion of elderly people with type 2 diabetes reporting major hypoglycemia is particularly important.
Acknowledgments
The A1chieve study and this manuscript were funded by Novo Nordisk A/S (Bagsvaerd, Denmark). Editorial assistance in the preparation of this manuscript was provided by Emily Chu at ESP Bioscience (Crowthorne, UK). Support for this assistance was funded by Novo Nordisk A/S. Vinay Prusty is the guarantor for this article, and takes responsibility for the integrity of the work as a whole.
Conflict of interest
Rachid Malek is the national coordinator of A1chieve and a member of the advisory board for Novo Nordisk. Guillermo Gonzalez-Galvez is an advisory board member for MSD, BMS, Sanofi, Roche, Boehringer Ingelheim, and Novo Nordisk, and a speaker for Novo Nordisk. Nabil El Naggar is a speaker for Novo Nordisk, MSD, Novartis, AstraZeneca, Aventis, BMS, Eli Lilly, GSK, and Merck, and an advisory board member for MSD, Eli Lilly, and BMS. Leon Litwak is a member of the Latin-American board of Eli Lilly and a member of the national board of Novo Nordisk, Novartis, Sanofi-Aventis, BMS, and AstraZeneca. He is a principal investigator of clinical trials for Eli Lilly, Novo Nordisk, Novartis, GSK, Takeda, PPD, Pfizer, MSD, Sanofi-Aventis, Boehringer Ingelheim, BMS, and Astra Zeneca. He is also a speaker for Eli Lilly, Novo Nordisk, Novartis, Sanofi-Aventis, BMS, AstraZeneca, GSK, Takeda, PPD, Pfizer, MSD, and Boehringer Ingelheim. Siddharth Shah is an advisory board member for Novo Nordisk. Vinay Prusty is an employee of Novo Nordisk A/S.
Compliance with ethics guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2008. Informed consent was obtained from all patients for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Footnotes
The A1chieve trial was registered with ClinicalTrials.gov (NCT00869908).
References
- 1.International Diabetes Federation. IDF Diabetes Atlas 2012 Update, 5th edn. Brussels, Belgium; 2012.
- 2.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.UK Prospective Diabetes Study (UKPDS) Group Intensive blood–glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. doi: 10.1016/S0140-6736(98)07019-6. [DOI] [PubMed] [Google Scholar]
- 4.International Diabetes Federation. IDF Clinical Guidelines Task Force. Global guideline for type 2 diabetes. Brussels, Belgium; 2012.
- 5.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institute for Health and Clinical Excellence. National Institute for Health and Clinical Excellence (NICE) Guidelines. Type 2 diabetes: the management of type 2 diabetes; 2008.
- 7.Olson DE, Norris SL. Diabetes in older adults. Overview of AGS guidelines for the treatment of diabetes mellitus in geriatric populations. Geriatrics. 2004;59:18–24. [PubMed] [Google Scholar]
- 8.Plank J, Bodenlenz M, Sinner F, et al. A double-blind, randomized, dose-response study investigating the pharmacodynamic and pharmacokinetic properties of the long-acting insulin analog detemir. Diabetes Care. 2005;28:1107–1112. doi: 10.2337/diacare.28.5.1107. [DOI] [PubMed] [Google Scholar]
- 9.Klein O, Lynge J, Endahl L, Damholt B, Nosek L, Heise T. Albumin-bound basal insulin analogues (insulin detemir and NN344): comparable time-action profiles but less variability than insulin glargine in type 2 diabetes. Diabetes Obes Metab. 2007;9:290–299. doi: 10.1111/j.1463-1326.2006.00685.x. [DOI] [PubMed] [Google Scholar]
- 10.Heise T, Pieber TR. Towards peakless, reproducible and long-acting insulins. An assessment of the basal analogues based on isoglycaemic clamp studies. Diabetes Obes Metab. 2007;9:648–659. doi: 10.1111/j.1463-1326.2007.00756.x. [DOI] [PubMed] [Google Scholar]
- 11.Philis-Tsimikas A, Charpentier G, Clauson P, Ravn GM, Roberts VL, Thorsteinsson B. Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes. Clin Ther. 2006;28:1569–1581. doi: 10.1016/j.clinthera.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 12.Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care. 2006;29:1269–1274. doi: 10.2337/dc05-1365. [DOI] [PubMed] [Google Scholar]
- 13.Khunti K, Caputo S, Damci T, et al. The safety and efficacy of adding once-daily insulin detemir to oral hypoglycaemic agents in patients with type 2 diabetes in a clinical practice setting in 10 countries. Diabetes Obes Metab. 2012 doi: 10.1111/j.1463-1326.2012.01665.x. [DOI] [PubMed] [Google Scholar]
- 14.Perriello G, Caputo S, De Pergola G, et al. Improved glycemic control with weight loss and a low risk of hypoglycemia with insulin detemir: insights from the Italian cohort of the PREDICTIVE study after 6-month observation in type 2 diabetic subjects. Expert Opin Pharmacother. 2011;12:2449–2455. doi: 10.1517/14656566.2011.626766. [DOI] [PubMed] [Google Scholar]
- 15.Home P, Naggar NE, Khamseh M, et al. An observational non-interventional study of people with diabetes beginning or changed to insulin analogue therapy in non-Western countries: the A1chieve study. Diabetes Res Clin Pract. 2011;94:352–363. doi: 10.1016/j.diabres.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 16.Shah S, Zilov A, Malek R, Soewondo P, Bech O, Litwak L. Improvements in quality of life associated with insulin analogue therapies in people with type 2 diabetes: results from the A1chieve observational study. Diabetes Res Clin Pract. 2011;94:364–370. doi: 10.1016/j.diabres.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 17.International Society for Pharmacoepidemiology. Guidelines for Good Pharmacoepidemiology Practices (GPP). Revision 2; 2007.
- 18.World Medical Association. Declaration of Helsinki—ethical principles for medical research involving human subjects 2008. http://www.riip-influenza.org/wp-content/uploads/2011/08/Helsinki-2008-EN.pdf. Accessed 8 Nov 2011.
- 19.EuroQol—a new facility for the measurement of health-related quality of life. The EuroQol Group. Health Policy. 1990;16:199–208. [DOI] [PubMed]
- 20.Chan TY. Estimates on the incidence of antidiabetic drug-induced severe hypoglycaemia in Hong Kong. Pharmacoepidemiol Drug Saf. 1998;7:411–414. doi: 10.1002/(SICI)1099-1557(199811/12)7:6<411::AID-PDS379>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 21.Brown AF, Mangione CM, Saliba D, Sarkisian CA. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc. 2003;51:S265–S280. doi: 10.1034/j.1600-0579.2003.00211.x. [DOI] [PubMed] [Google Scholar]
- 22.Barendse S, Singh H, Frier BM, Speight J. The impact of hypoglycaemia on quality of life and related patient-reported outcomes in type 2 diabetes: a narrative review. Diabet Med. 2012;29:293–302. doi: 10.1111/j.1464-5491.2011.03416.x. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson AM. Impact of improved glycemic control on quality of life in patients with diabetes. Endocr Pract. 2004;10:502–508. doi: 10.4158/EP.10.6.502. [DOI] [PubMed] [Google Scholar]
- 24.Bodmer M, Meier C, Krahenbuhl S, Jick SS, Meier CR. Metformin, sulfonylureas, or other antidiabetes drugs and the risk of lactic acidosis or hypoglycemia: a nested case-control analysis. Diabetes Care. 2008;31:2086–2091. doi: 10.2337/dc08-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]