Abstract
Context:
Oral lichen planus (OLP) affects 0.5-1% of the total world's population. The histological features of oral lichen planus were first described by Dubreuill in 1906. Despite the advent of various techniques, the etiology of lichen planus remains obscure, although many theories for the etiology have been proposed.
Aims:
By studying OLP electron microscopically, we shall be emphasizing on the cells and its interactions in specific/altered surroundings which would help us in hypothesizing the effects of its specific cell-to-cell interactions.
Materials and Methods:
A total of 20 cases of oral lichen planus were selected and categorized into erosive and nonerosive forms based upon clinical pattern and confirmed as lichen planus by histopathological analysis. Tissue specimens thus obtained were cut into two halves and fixed in appropriate fixatives, i.e., neutral buffered formalin for paraffin-embedded hematoxylin and eosin stained sections and 2.5% glutaraldehyde and 2% paraformaldehyde for electron microscopic purpose respectively.
Results:
Ultrastructural comparison among the two forms showed significant differences between them. The basal layer showed cytoplasmic processes, intercellular spaces, desmosomes, nuclei, and signs of degeneration. The erosive form showed elongated, narrow or irregular cytoplasmic projections whereas the nonerosive showed short and broad based projections.
Conclusions:
The present study confirms the ultrastructural findings of basal cells in OLP with previous authors findings. Besides this, the categorization of the ultrastructural differences between erosive and nonerosive has raised the question of difference in the probable cellular and molecular mechanism between erosive and nonerosive forms.
Keywords: Basal cells, lichen planus, ultrastructure
INTRODUCTION
Lichen planus is a common mucocutaneous disease affecting the skin and areas in the oral cavity.[1] This lesion was first described by Wilson in 1869 and he compared the skin lesions of lichen planus to the lichens (algae/fungi) growing on flat rocks. This characteristic appearance contributed to the advent of the term “lichen planus”.[2]
Andreasen divided oral lichen planus into six types: Reticular, papular, plaque-like, erosive, atrophic, and bullous on the basis of its clinical presentation. OLP may occur in the oral cavity commonly on buccal mucosa, tongue, and gingiva and uncommonly on palatal mucosa.[3]
Despite the advent of various techniques, the etiology of lichen planus remains obscure, even today as the disease was first described more than 100 years ago. Although many theories for the etiology have been proposed and there appears to be some relationship with the patient's emotional state, none has been adequately substantiated.[4] In order to understand lichen planus a holistic approach would be required to divulge the pathogenesis since the epithelium and its subjacent connective tissue seem to be acting in tandem in bringing out these vast histopathological/clinical features.
Understanding the structural organization of cells is an essential prerequisite in order to know how cells function. Cell biology began with the advent of light microscopy and it is still an essential tool. Light microscopy permits diagnosis of the disease but contributes little to the understanding of etiology or the true nature of the tissue alteration. Also light microscopy is limited in the fineness of the detail it can reveal. Microscopes using other types of rays such as electron microscopes can resolve much smaller structures than is possible with visible light.[5]
The purpose of the present study was to examine representative specimens of OLP ultramicroscopically and to correlate the findings in order to study further the nature of the disease. By studying OLP electron microscopically, we shall be emphasizing on the larger picture of the cells and their interactions in specific/altered surroundings. This would also help us in hypothesizing the effects of these specific cell-to-cell interactions.
MATERIALS AND METHODS
A total of 20 cases of oral lichen planus were selected and categorized into erosive and nonerosive forms based upon clinical pattern and confirmed as lichen planus by histopathological analysis. One case of normal gingival tissue was obtained at the time of extraction of the impacted third molar as control. Tissue specimens thus obtained were cut into two halves and fixed in appropriate fixatives, i.e., neutral buffered formalin for paraffin-embedded hematoxylin and eosin stained sections and 2.5% glutaraldehyde and 2% paraformaldehyde for electron microscopic purpose respectively. For formalin-fixed paraffin-embedded tissue sections, 4 μm sections were cut and were stained with hematoxylin and eosin for confirmatory lesional diagnosis. Postfixation was done in osmium tetraoxide. Staining was done with uranyl acetate and lead citrate. Examination was done using a Morgagni (Fie) transmission electron microscope (TEM).
RESULTS
Basal cells showed regular stratification in the normal epithelium [Figure 1]. Ultrastructural comparison among the two forms showed significant differences between them [Table 1]. The basal layer showed cytoplasmic processes, intercellular spaces, desmosomes, nuclei, and signs of degeneration. The erosive form showed elongated, narrow or irregular cytoplasmic projections whereas the other showed short and broad based projections [Figure 2]. The intercellular spaces were clearly widened in nonerosive but were wider in erosive form [Figures 3 and 4]. In many cases lymphocytes penetrated the intercellular spaces of the basal cell layers [Figure 5]. The number of desmosomes was decreased especially in erosive form. The cytoplasm was organelle-poor but tonofilament bundles were abundant in these cases [Figure 4]. Apoptotic changes were more marked in erosive form [Figure 6]. Similar changes were observed in the spinous layer but to a lesser extent. Basal lamina exhibited frequent breaks in the erosive form. Cells with characteristic of small or medium-sized lymphocytes, macrophages and mast cells were identified. Macrophages were round or oval with an oval nucleus containing several indentations [Figure 7]. The macrophages were frequently seen in close proximity to lymphocytes. The mast cells were characteristic with membrane-bound secretory granules filling up a large part of cytoplasm [Figure 8]. In both forms they showed the signs of activation but more marked in the erosive form [Figure 9]. Lymphocytes were frequently seen between basal layers and penetrating the basal lamina. A Langerhans cell was also observed in close proximity to keratinocyte [Figure 10]. Degenerative changes in the basal cells were more extensive in the erosive form of oral lichen planus.
Figure 1.
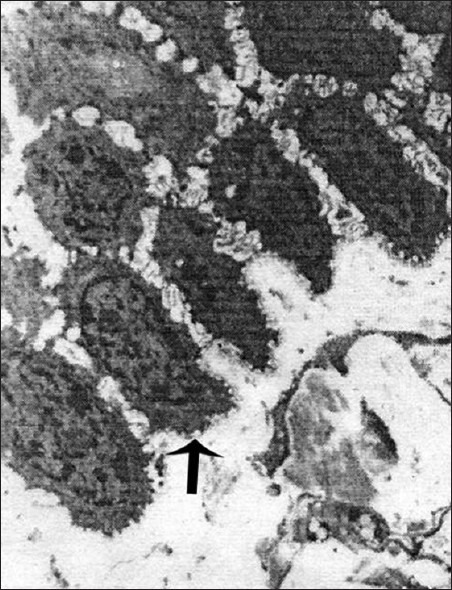
Electron micrograph showing regular stratification of the basal cells in normal epithelium – ×1400
Table 1.
Ultrastructural differences between erosive and non-erosive oral lichen planus
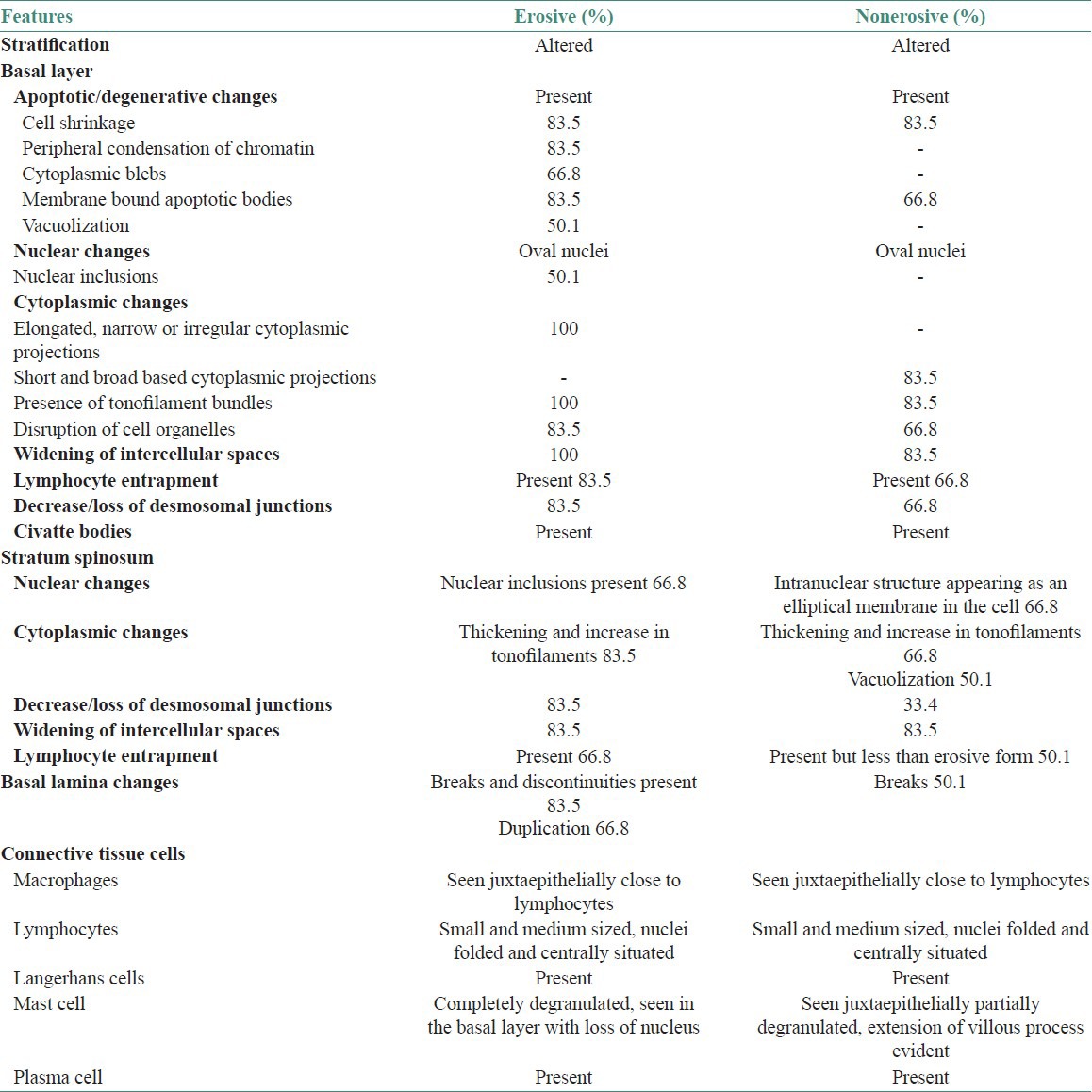
Figure 2.
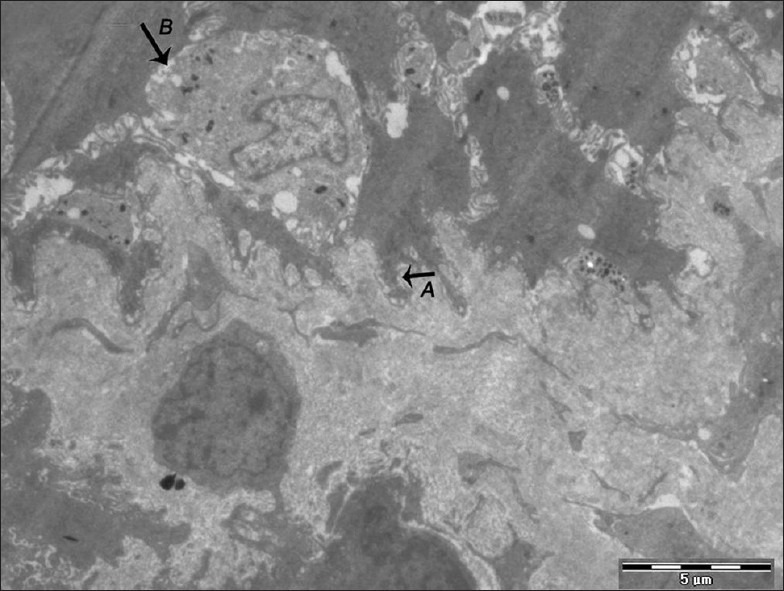
Electron micrograph of erosive OLP showing basal cells with elongated, irregular cytoplasmic projections (A). Presence of the degenerating cell is also seen with broken-up plasma membrane (B) – ×880
Figure 3.
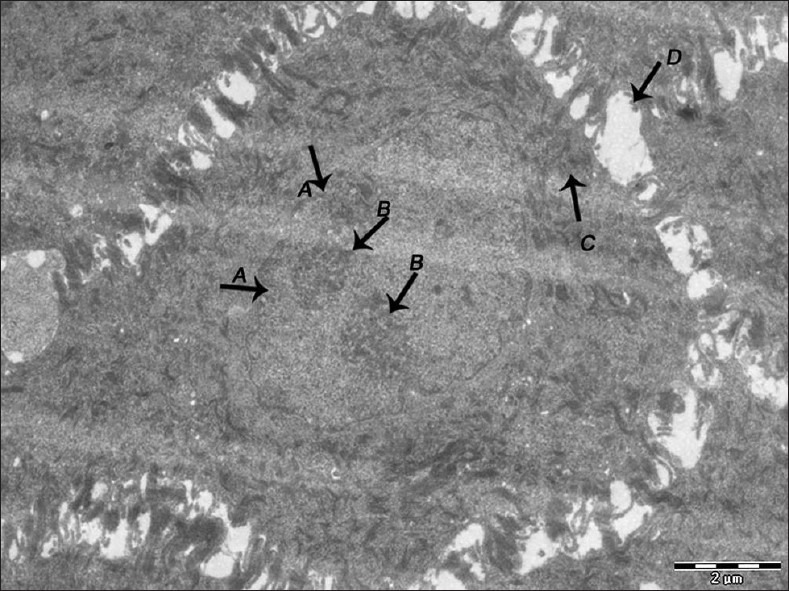
Electron micrograph of nonerosive OLP showing nuclear membrane infolding (A), prominent nucleoli (B), tonofilament accumulation in the cytoplasm of the cells of spinous layer (C). Widened intercellular spaces between the cells of spinous layer (D) – ×1600
Figure 4.
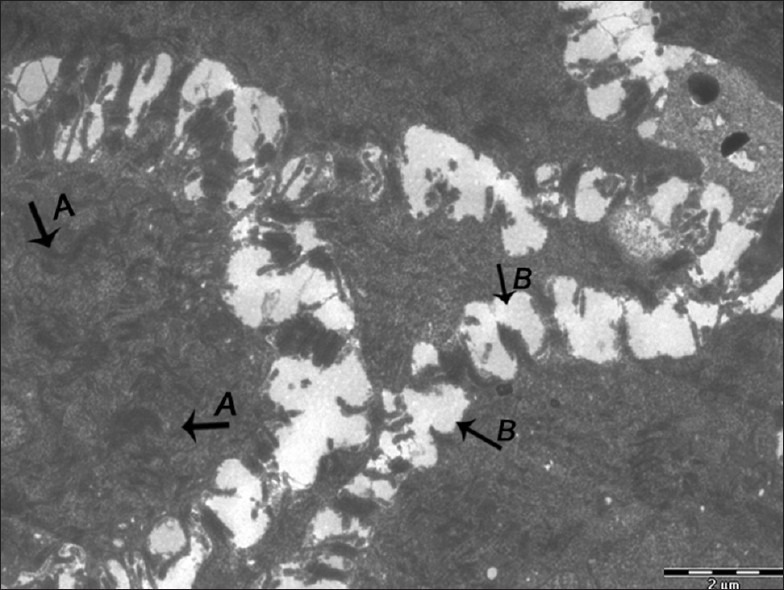
Electron micrograph of erosive OLP showing clumping of tonofilaments (A). Widened intercellular spaces (B) – ×1800
Figure 5.
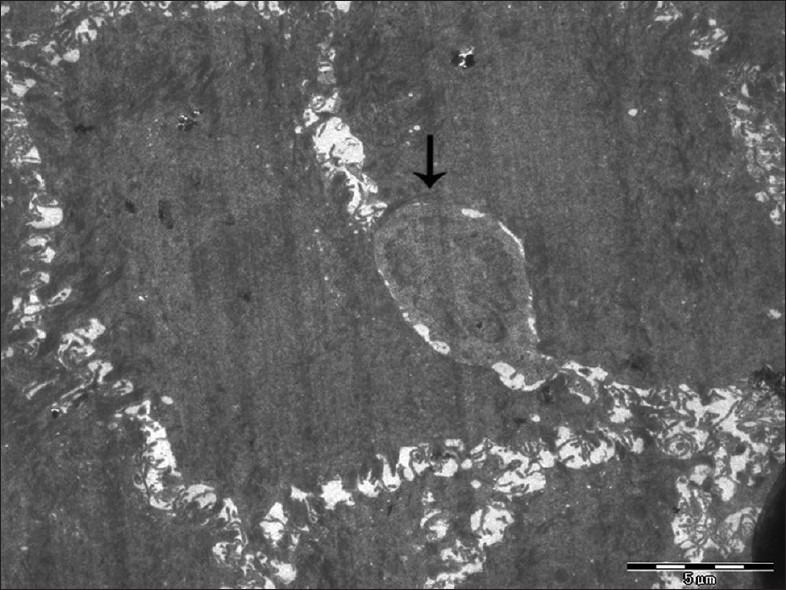
Electron micrograph of erosive OLP showing intraepithelial lymphocyte with an indented nucleus (arrow) – ×880
Figure 6.
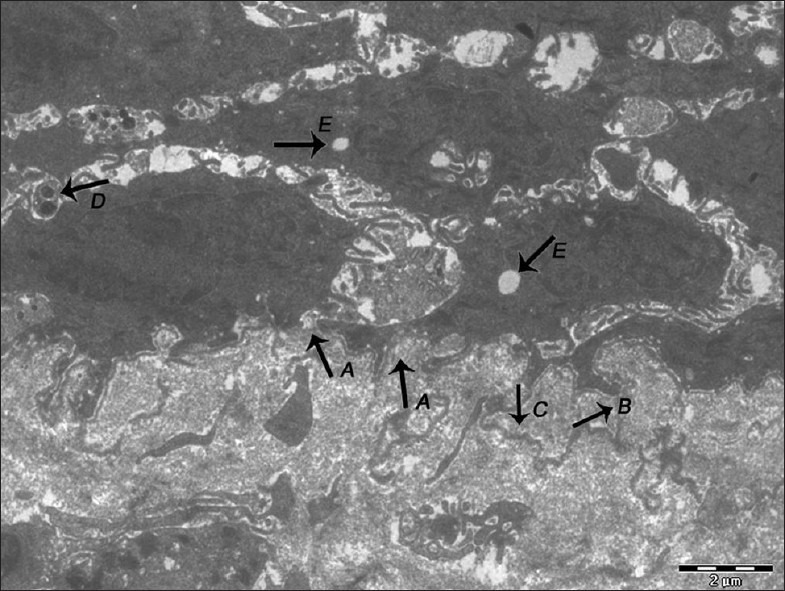
Electron micrograph of erosive OLP showing basal lamina exhibiting breaks (A), duplication (B), loss of hemidesmosomes and separation of basal lamina from the surface of the basal epithelial cells (C). Keratinocytes undergoing apoptosis showing the presence of membrane-bound apoptotic bodies (D). Vacuolization is evident in the cytoplasm of keratinocyte (E) – ×1400
Figure 7.
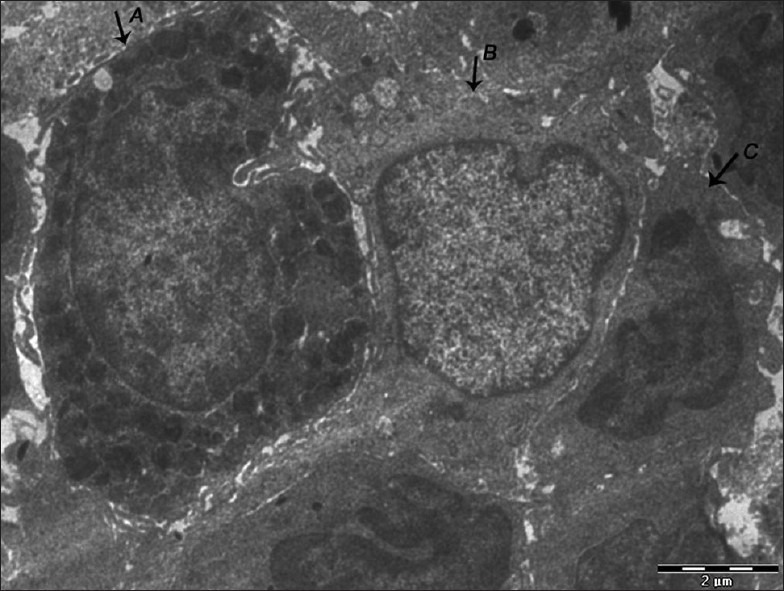
Electron micrograph of erosive OLP showing the presence of mast cell (A), langerhans cell (B), and macrophage (C) close to each other – ×1800
Figure 8.
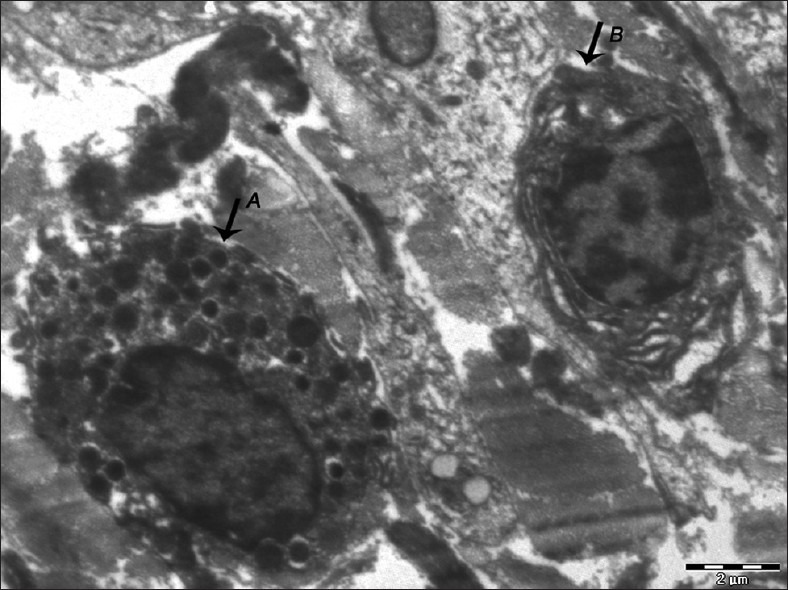
Electron micrograph of erosive OLP showing a mast cell (A) close to a lymphocyte (B) – ×1400
Figure 9.
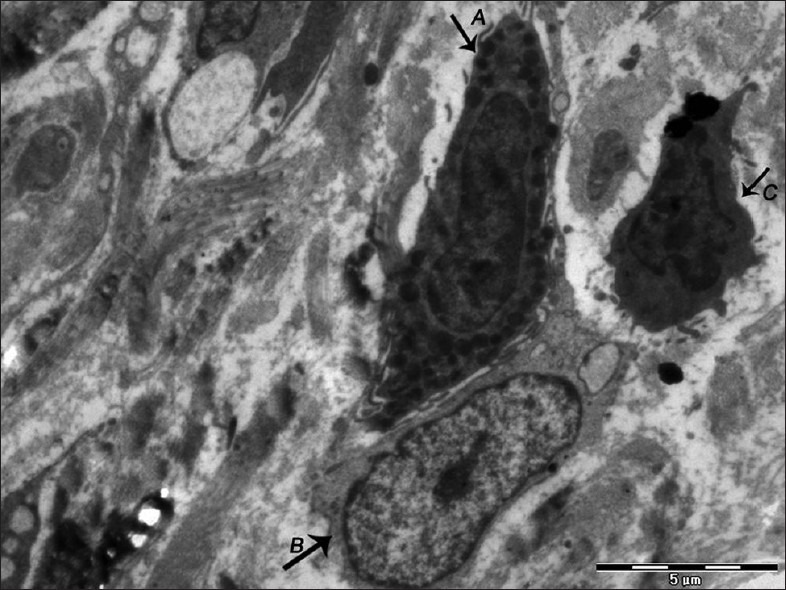
Electron micrograph of nonerosive OLP showing an active mast cell with extension of villous process and fusion of granules (A) in close proximity to lymphocyte (B) and macrophage (C) – ×1100
Figure 10.
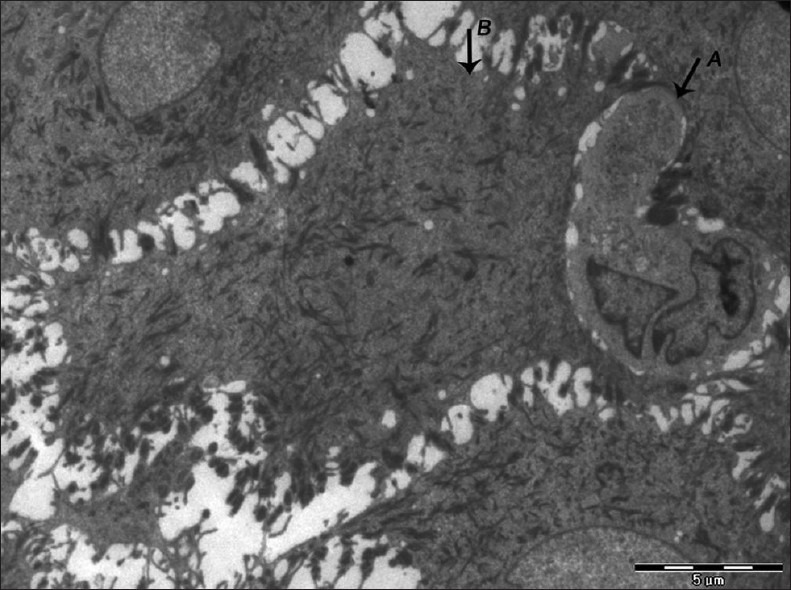
Electron micrograph of erosive OLP showing langerhans cell (A) in close proximity to keratinocyte (B) with loss of nucleus and abundant accumulation of tonofilaments – ×880
When the basal lamina is injured or becomes defective, uncontrolled interactions between epithelium and connective tissue are allowed. Degeneration of the basal epithelial cells might lead to basal lamina changes allowing stimulation of macrophages with epithelial antigens, which in turn transfer the immunological information to lymphocytes.
DISCUSSION
Oral lichen planus is a relatively common chronic inflammatory disease of the oral mucosa characterized histologically by abnormal keratinization, basal cell degeneration, and a band-like infiltrate of chronic inflammatory cells predominantly lymphocytic in nature around the basement membrane region.[6] Occasionally, lymphocytes have been noticed to be situated above in the epithelium between the basal cells.
TEM is a valuable part of microscopy which with its enormous resolving power is able to venture beyond the histological appearance and reveal the substructure or ultrastructure of individual cells.[7]
In this study, TEM was done on 20 cases of OLP which were broadly grouped into erosive and nonerosive forms based on clinical and light microscopic features.
The foremost electron microscopic study carried out on lichen planus was by Hashimoto and associates in 1966, which showed that within the subepithelial connective tissue there was vascular dilation, oedema, and infiltration of various types of inflammatory cells. The basement membrane was seen disrupted by migrating inflammatory cells and appeared wider in some areas. The spinous layer demonstrated thickening of tonofilaments, and the granular layer demonstrated an unusual method of keratohyaline formation. In this study similar findings were observed which included breaks in the basement membrane appreciated at a particular frequency per view field at a specified magnification. These breaks could be involved in facilitating the migration of lymphocytes between the epithelial cells.[8]
Analyzing these findings, further on, various other authors described the ultrastructure of the oral mucosa affected by lichen planus.
Pullon demonstrated gross thickening and condensation of the basement membrane with breaks in its continuity. Thickening and increased electron density of the tonofibrils in the cells of both the basal layer and the stratum spinosum were noted.[4] The present study demonstrated thickening and aggregation of tonofibrils in basal and the spinous cell layers.
Tyldesley and Appleton showed that the cells of the stratum basale formed an irregular layer in which wide intercellular spaces were sometimes present. Some cells showed vacuolization of the cytoplasm. Cells of stratum spinosum were widely separated and connected through fine processes by intact desmosomes. The cytoplasm was condensed and contained aggregates of ribosomes, tonofibrils and mitochondria.[9]
Similarly, evolving the fundamentals from their articles on the changes seen in the basal cells, the present study has found changes related to the plasma membrane which shows undulations and nonconformity to the cytoplasmic structures. Features suggestive of nuclear pyknosis such as margination of chromatin which could result in chromatin clumps have also been envisioned.
The present study focuses on the ultrastructural observations in erosive and nonerosive OLP to examine the changes in the basal cell region along with various cellular interactions to postulate the underlying disease process.
Basal lamina showed alterations of two different kinds: Breaks and duplication or both. Breaks and discontinuities was encountered more frequently in erosive whereas duplication was seen in both the forms of OLP. The basal lamina delimits the connective tissue–nonconnective tissue boundary and attaches epithelial cells to the connective tissue. Injuries and defects in the basal lamina disturb the orderly organization of these tissues and therefore, may be of pathogenetic significance in OLP. Since basal lamina is an epithelial cell product, changes in basal lamina may be due to changes in the basal epithelial cells. The ultrastructure of the basal cell layer showed cytoplasmic projections, widening of intercellular spaces, decrease/loss of desmosomal junctions, nuclear inclusions, and lymphocyte entrapment. Aberrations in these characteristics were more marked in erosive than in nonerosive form. Elongated, narrow or irregular cytoplasmic projections were seen in erosive whereas short and broad based projections were evident in nonerosive form. Certain features such as cytoplasmic blebs, presence of apoptotic bodies, vacuolization, tonofilament bundles, and disruption of cell organelles were noted. These changes correspond to the signs of degeneration of basal cells. Stratum spinosum showed nuclear inclusions, increase and thickening in tonofilaments, decrease in desmosomal junction, and widening of intercellular spaces.
Few lymphocytes were seen entrapped in between basal cells and also high up in the spinous layer. Numerous small and medium-sized lymphocytes and macrophages were seen juxtaepithelially showing signs of activation. Completely or partially degranulated mast cells with extension of villous process were encountered frequently in the basal region and juxtaepithelially. Langerhans cells were seen in the basal and suprabasal region and at places they were juxtaposed next to lymphocytes. Most of these cells were seen in close proximity to each other, suggesting their involvement in the exchange of immunologic information.
Under an electron microscope, most significant differences (P < 0.01) between erosive and nonerosive OLP were observed in the following features–membrane-bound apoptotic bodies, vacuolization, disruption of cell organelles in the cytoplasm, lymphocyte entrapment between the basal cells and decrease or loss of desmosomal junctions.
Thus, based on the above-mentioned observations it may be postulated that the primary pathologic alteration in lichen planus could be a degeneration and disruption of hemidesmosomes joining basal cells of the epithelium to the basement membrane, and desmosomes linking the basal cells to each other and to the cells of lower stratum spinosum. As the hemidesmosomes degenerate, the basement membrane separates from the epithelium and fragments in areas so that the basement membrane material can be seen in the lamina propria. Loss of desmosomes and hemidesmosomes with degeneration and presence of tonofilament bundles could presumably lead eventually to cell death in selected cells of the basal layer. Destruction of basal cells could serve to facilitate the migration of inflammatory cells into the epithelium. The inflammatory infiltrate in the upper corium may represent a response to the basement membrane pathology.
Langerhans cells which have been detected in lesions of OLP by the presence of characteristic Birbeck granules via electron microscopy are antigen-presenting cells which subsequently pass information to other mononuclear cells. In OLP, there appears to be contact between the langerhans cells and mononuclear cells and the present study has shown close proximity of the langerhans cell with macrophages, T lymphocytes, and degenerated keratinocytes. Thus, it could be postulated that presentation of the immunogenic complex formed by membrane antigen with allergen to lymphocyte results in activation and proliferation of lymphocytes.
Mast cells have been previously regarded as having a role as a mediator during the inflammatory process which takes place in OLP.[10] They were associated with the release of vasoactive amines and thus related to the clinical symptom of pruritis. In the past few years, mast cells and altered mast cells have gained impetus as having a central role in the pathogenesis of OLP. The chronicity of the disease were directly associated as a phenomenon of morphologically altered mast cell.[11]
Activated mast cells were evident in the present study and were seen close to lymphocytes. In OLP, a minute local proliferation of the lymphocytes in the infiltrate may be responsible for the recruitment of these cells from the blood stream.
Analysis of the above data suggests that mast cells may play a role in epithelial basement membrane disruption in OLP and thus lymphocytes migrate through ruptured basement membrane to enter the epithelium. Basement membrane disruption in OLP may be mediated by mast cell proteases directly or indirectly via activation of T cell secreted MMP-9.[12]
Ultrastructural differences between erosive and nonerosive have raised the question of difference in the probable cellular and molecular mechanism between erosive and nonerosive forms. It suggests an inherent instability of the epithelium to specific causative factors in the manifestations of the disease process.
The presence of basal cell changes which have been linked to the apoptotic process in the present study could highlight the manner of changes brought from the cellular interactions at the epithelial connective tissue interface. Specific interactions seen in the study prove beyond doubt an autoimmune basis for the pathogenesis of OLP. Some of these are altered basal cells and few suprabasal cells in close proximity to langerhans cells, the frequent association seen between lymphocytes and other immunologically active cells besides their close proximity to the basal cells. The present study also highlights the presence of altered mast cells from a morphological aspect which could be used to deduce its altered functional significance with respect to disease progression.
The findings of the present study are concurrent with previous studies and could be used as a basis on which studies related to molecular analysis could be paralleled. This could probably help bring in unison to the various theories postulated on the pathogenesis of OLP.
CONCLUSION
The present study confirms the ultrastructural findings of basal cells in OLP with previous authors findings. Besides this, the categorization of the ultrastructural differences between erosive and nonerosive lichen planus has raised the question of difference in the probable cellular and molecular mechanism between erosive and nonerosive forms.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Scully C, El-Kom M. Lichen planus: Review and update on pathogenesis. J Oral Pathol. 1985;14:431–58. doi: 10.1111/j.1600-0714.1985.tb00516.x. [DOI] [PubMed] [Google Scholar]
- 2.Mallaoglu N. Oral lichen planus: A review. Br J Oral and Maxillofac Surg. 2000;35:370–7. doi: 10.1054/bjom.2000.0335. [DOI] [PubMed] [Google Scholar]
- 3.Andreasen JO. Oral Lichen Planus. I: A clinical evaluation of 115 cases. Oral Surg Oral Med Oral Pathol. 1968;25:31–42. doi: 10.1016/0030-4220(68)90194-1. [DOI] [PubMed] [Google Scholar]
- 4.Pullon PA. Ultrastructure of oral lichen planus. Oral Surg Oral Med Oral Pathol. 1969;28:365–71. doi: 10.1016/0030-4220(69)90231-x. [DOI] [PubMed] [Google Scholar]
- 5.Bancroft JD, Gamble M. 5th ed. London: Churchill Livingstone; 2002. Theory and practice of histological techniques; p. 679. [Google Scholar]
- 6.Ismail SB, Kumar SK, Zain RB. Oral lichen planus and lichenoid reactions: Etiopathogenesis, diagnosis, management and malignant transformation. J Oral Sci. 2007;49:89–106. doi: 10.2334/josnusd.49.89. [DOI] [PubMed] [Google Scholar]
- 7.Edwards PC, Kelsch R. Oral lichen planus: Clinical presentation and management. J Can Dent Assoc. 2002;68:494–9. [PubMed] [Google Scholar]
- 8.Hashimoto K, DiBello, Shklar G, Lever WF. Electron microscopic studies of oral lichen planus. Gior Ital Dermat Sif. 1966;10:765–88. [PubMed] [Google Scholar]
- 9.Tyldesley WR, Appleton J. Observations on the ultrastructure of the epithelium in oral lichen planus. J Oral Pathol. 1973;2:46–57. doi: 10.1111/j.1600-0714.1973.tb01673.x. [DOI] [PubMed] [Google Scholar]
- 10.Jontell M, Hansson HA, Nygren H. Mast cells in oral lichen planus. J Oral Pathol. 1986;15:273–5. doi: 10.1111/j.1600-0714.1986.tb00622.x. [DOI] [PubMed] [Google Scholar]
- 11.Hall WB. Mast cells in desquamative gingivitis, lichen planus and pemphigoid. Oral Surg Oral Med Oral Pathol. 1969;28:646–58. doi: 10.1016/0030-4220(69)90409-5. [DOI] [PubMed] [Google Scholar]
- 12.Sugerman PB, Savange NW, Walsh LJ, Zhao ZZ, Zhou XJ, Khan A, et al. The pathogenesis of oral lichen planus. Crit Rev Oral Biol Med. 2002;13:350–65. doi: 10.1177/154411130201300405. [DOI] [PubMed] [Google Scholar]


