Abstract
Keloids extend beyond the borders of the original wound invading normal skin. Usually appear as firm nodules, often pruritic and painful, and generally do not regress spontaneously. Most often occur on the chest, shoulders, upper back, back of the neck, and earlobes. The aim of the paper is to discuss a case of keloid, review the pathophysiology and also to highlight the differences between keloid and hypertrophic scars. A 26-year-old female complains of swelling on ear lobe since 3 years. Swelling was firm, non-tender, dumbell-shaped with central wooden stick still present, measuring 3 cm in diameter medial to the inferior part of the helix. A clinical diagnosis of keloid was given. Histopathological sections showed hyperorthokeratinized stratified squamous epithelium with deep dermal sclerosis showing large dense bundle of glassy collagen diagnostic of Keloid. Special stain like Van Gieson's was used to identify collagen bundles. The sections were also subjected to immunohistochemical markers such as α-SMA (alpha Smooth muscle actin), Desmin, and S-100. Despite decades of research, the pathophysiology of keloids remains incompletely understood. Recent studies indicate that TGF-β (Transforming growth factor beta) and PDGF (Platelet-derived growth factor) play an integral role in the formation of keloids. In future, development of selective inhibitors of TGF-β might produce new therapeutic tools with enhanced efficacy and specificity for the treatment of keloids. Patients with a previous history of keloid or other risk-factors should avoid body piercing and elective cosmetic procedures. Keloid scars should be sent for histopathology in order to avoid missing potentially malignant conditions particularly those showing unusual features.
Keywords: Ear lobe, fibrogenic response, glassy collagen, hypertrophic scars, keloid
INTRODUCTION
Trauma that creates tissue loss gives rise to the repair process and eventually ends with scar tissue.[1] The formation of a scar is a process that has evolved over millions of years for the purpose of restoring functionality, but not esthetic quality. In some individuals, an aberrant healing process results in excessive scar formation that may extend well beyond the original boundaries of the wound, resulting in a significant and troubling cosmetic defect called keloid.[2]
The term keloid was originally described in 1800s as “cheloid,” derived from the Greek word “chele” means “crab claw.” By definition and in distinction from a hypertrophic scar, keloids extend beyond the borders of the original wound and invading into/around normal skin. Usually appear as firm nodules, often pruritic and painful, and generally do not regress spontaneously.[2]
Individuals of all ethnic backgrounds can form keloid and hypertrophic scars as a familial predisposition was believed to exist. However, keloid formation is approximately 15 times greater in highly pigmented ethnic groups than in whites. The pathogenesis of keloid scar is complex which involves both genetic and environmental factors.[3]
Keloids often occur on the chest, shoulders, upper back, back of the neck, and more frequently on earlobes with the posterior aspect of the ear lobule being the most common site involved, which is under minimal tension. In addition keloids appear rarely on the palms or soles, where we would expect significant skin.[2]
Here, a case of keloid is presented and also, an attempt has been made to review the differences between keloid and hypertrophic scar with pathophysiology of keloid formation.
CASE REPORT
A 26-year-old female complains of swelling on right ear lobe since 3 years. Patient had got her ear pierced when she was 3 years old and had not developed any swelling following piercing. Additional ear piercing was done at the age of 23 years, which was 1 cm above the previous site, subsequent to which patient developed swelling, which continued to grow until it reached to the present size. The swelling was firm, non-tender, dumbell-shaped, measuring 3 cm in diameter, present medial to the inferior part of the helix [Figure 1]. A clinical diagnosis of keloid or an irritational fibroma was given.
Figure 1.
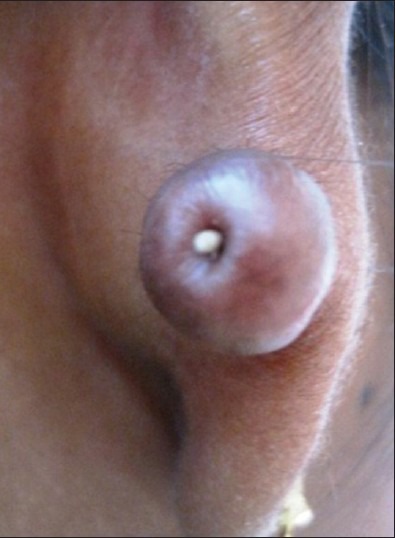
Keloid on the right ear lobe
Keloid was surgically excised after obtaining informed consent from the patient. Histopathological sections showed hyperorthokeratinized stratified squamous epithelium and superficial dermis showed fibroblastic cells arranged parallel to epidermal surface with diffuse chronic inflammatory cells. Mild to deep dermal sclerosis showing large dense bundle of glassy collagen, was characteristically seen. A final diagnosis of Keloid was given [Figure 2].
Figure 2.
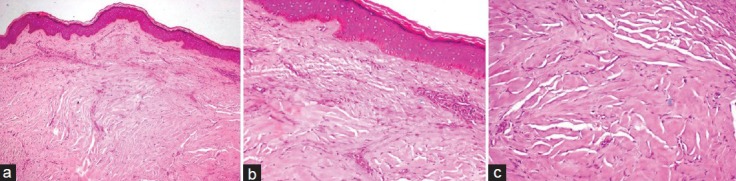
(a) H and E section showing hyperorthokeratinized stratified squamous epithelium with collagen spanning full thickness of the dermis (×100), (b) Epidermal changes seen are flattening of epithelium, hyperorthokeratosis, hypergranulosis, and spongiosis, regular palisading basal cell organization with prominent vacuolar changes (×200), (c) Dermal changes seen are abnormally large, dense, broad, glassy, and eosinophilic, focally fragmented collagen arranged haphazardly (×400)
Special stain like Van Gieson's was used to identify collagen bundles. Van Gieson's stained slide showed yellow colored epidermis and thick collagen bundles stained red in the dermis. Under polarized light, collagen bundles showed reddish to orange birefringence indicating thick fibers [Figure 3]. The sections were also subjected to immunohistochemical markers such as α-SMA (alpha smooth muscle actin), desmin, and S-100. The presence of brown colored end products at the site of target antigen was considered as positive. Expression of α-SMA was seen in myofibroblasts, blood vessels and fine collagen fibers. Scattered diffuse staining of S-100 was seen and expression of desmin was negative [Figure 4]. Follow-up of patient after 1 year showed no evidence of recurrence.
Figure 3.
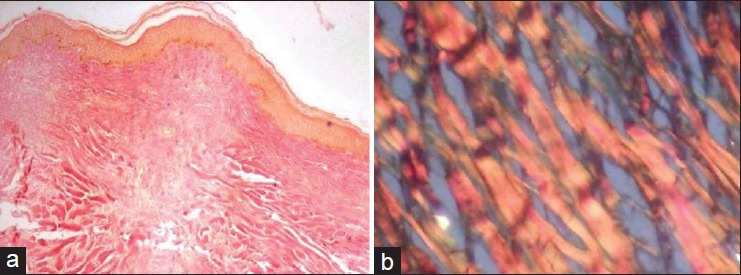
(a) Van Gieson's stained section showing yellow colored epidermis and thick collagen bundles in dermis showing red color (×40), (b) Under polarized microscopy collagen bundles showing thick reddish orange birefringence (×200)
Figure 4.
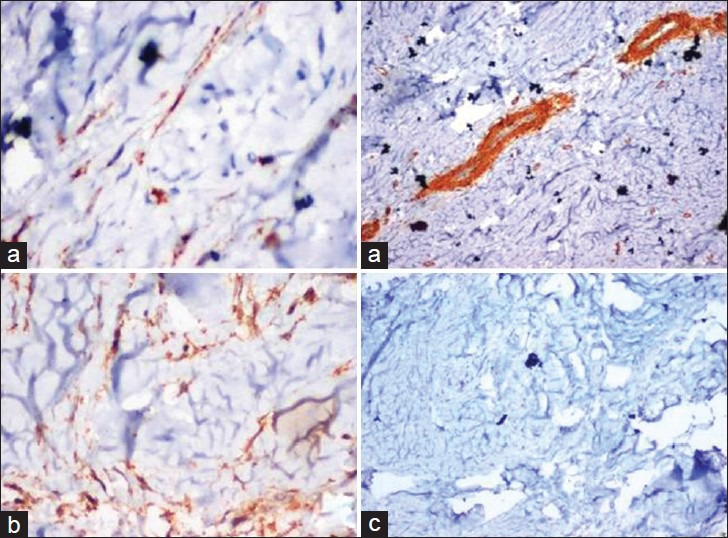
(a) Keloid collagen showed positivity for α-SMA, which is expressed in myofibroblasts, blood vessels and fine collagen, giving an appearance of “Tram track.” (×200/×100), (b) Keloid collagen showed scattered diffuse staining of S-100 (×200), (c) Keloid collagen showed desmin negativity (×100)
DISCUSSION
The clinical controversy as to whether hypertrophic scars and keloids are different entities or merely the opposite ends of a spectrum of wound-healing behavior has still been an issue.
Differences between hypertrophic scar and a keloid
In 1960s Mancini and Peacock[4] differentiated excessive scarring into hypertrophic and keloid scar formation. Both scar types rise above skin level, while hypertrophic scars do not extend beyond the initial site of injury, the keloids typically project beyond the original wound margins.[4]
It is important to correctly identify the type of scar, since it might result in inappropriate management of pathologic scar formation, and occasionally contribute to inappropriate decision making related to elective or cosmetic surgery.
Hence clinical, histopathological, immunohistochemical and electron microscopic differences between hypertrophic scar and a keloid are extensively reviewed in the literature.
Clinical differences
Hypertrophic scar usually show a rapid growth phase for up to 6 months, and then gradually regresses over a period of a few years, eventually leading to flat scars with no further symptoms but keloid typically persist for long periods of time, and do not regress spontaneously.[4]
As a familial predisposition, all individuals can form keloid and hypertrophic scars. However, the incidence of keloid scar formation is much higher in black-skinned individuals than in whites.[5]
The most common sites of predilection for hypertrophic scar are shoulders, neck, presternum, knees, and ankles whereas; keloids are frequently seen on anterior chest, earlobes, upper arms, and cheeks. Keloids have a higher tendency to recur following excision (45-100%), whereas new hypertrophic scar formation is rare after its excision (10%).[6]
Histopathological differences
Histopathologically, both hypertrophic scars and keloids contain an overabundance of dermal collagen. Keloid is characterized by the presence of thick, hyalinized collagen bundles or “keloid collagen” arranged in a haphazard pattern within the mucinous ground substance, showing relatively few fibroblasts. Conversely, little or no keloidal collagen is found in hypertrophic scar. Hypertrophic scar shows collagen fibers orientated parallel to the long axis of the epidermal surface and contains nodules of high density of fibroblasts and collagen, which are located in the middle or deeper layer of the scar. The absence of such nodules is characteristic of keloids.[3,4,6]
Moshref and Mufti[3] have extensively studied the possible biological and diagnostically relevant differences between keloid and hypertrophic scar using histopathological and immunohistochemical features. The differences were the presence of small aggregating blood vessels just below the epidermis appearing to grow out in keloids, while in the hypertrophic scars the blood vessels were oriented vertically around the nodules. Moderate degree of perivascular chronic inflammatory infiltrate was seen in keloids showing 73% of mast cells in reticular dermis, whereas only 20-30% of hypertrophic scars showed mast cells.[3]
Immunohistochemical differences
Myofibroblasts are differentiated fibroblasts found in granulation tissue and fibrotic lesions. They differ from normal fibroblasts by their characteristic cytoplasmic bundles of microfilaments, nuclear indentations and cell-to-cell or cell-to-stroma connections. The presence or absence of myofibroblasts was demonstrated by α-SMA immunostaining. “Keloid collagen” showed positivity for α-SMA expressing myofibroblasts in 33.3% of keloid scars, while the collagen nodules of hypertrophic scars contained no α-SMA expressing myofibroblasts, although, they were cellular.[3] Thus myofibroblasts are considered to play an important role in pathogenesis of Keloid.
In addition, immunohistochemical investigations have shown a high amount of activated immune-cell infiltrate in the excised keloid, consisting of CD3+, CD4+, CD 45R0. It was found that there was a significantly higher CD4 (+): CD8 (+) ratio in keloid tissue, suggesting that an imbalance in these inflammatory cell sub-populations along with mast cells may contribute to keloid formation.[3]
Electron microscopic features
Fibroblasts in keloid showed a well-developed rough endoplasmic reticulum. The banded collagen fibrils of keloid were organized into thick fibers that were separated from the membrane surface of the fibroblast by a diffuse amorphous substance surrounding the surface of the cell. This was evident in keloids only and not found in hypertrophic scars or normal dermis.[1]
The electron microscopic observations revealed this to be an unusual pericellular structure surrounding keloid fibroblasts. The chemical makeup of this pericellular structure is unknown.[1] Hembry et al., reported that in tight skin mice, this pericellular material disappeared 3 weeks after wound healing whereas in keloids, it can remain for as long as 2 years. However, the author concludes that more work on the characterization and function of this pericellular material will be needed for the understanding of the biological behavior of keloid fibroblasts.[7]
Pathophysiology of a keloid
Understanding the normal sequence of wound healing is important before knowing the pathophysiology and treatment of keloids. Normal wound healing occurs in three phases: (1) The inflammatory phase, (2) the proliferative or granulation phase, and (3) the maturation or re-modeling phase.[8]
Keloid represent aberrations in the fundamental processes of wound healing, in which there is an obvious imbalance between the anabolic and catabolic phases, in addition keloids seem to be a more sustained and aggressive fibrotic disorder.[4]
Evidence to date strongly suggests more prolonged inflammatory period, with immune cell infiltrate present in the scar tissue of keloids which may contribute to increased fibroblastic activity with greater and more sustained extracellular matrix deposition.[4]
Factors that are responsible for the pathophysiology of keloid are:
Inflammation
It is suggested that the type of immune response and severity of inflammation predisposes individuals to keloid scarring. T-helper CD41 cells have been implicated as major immunoregulators in wound healing. The development of a Th2 response (with production of interleukin [IL-4, IL-5, IL-10 and IL-13]) has been strongly linked to fibrogenesis in keloids.[4]
Fibrogenic response
Central to the formation of hypertrophic scar and keloid scar tissue is an alteration of the fibroblast phenotype. Indeed, when compared with normal fibroblasts, keloid fibroblasts show increased numbers of growth-factor receptors and respond more briskly to growth factor like TGF-β, which may upregulate these abnormal cells from the beginning of wound healing.[4]
TGF-β1, β2, and β3 are three isoforms that exists. TGF-β1 is thought to be profibrotic, whereas, TGF-β3 may have anti-fibrotic functions. The overproduction of the subtype TGF-β1 is associated with an excessive deposition of scar tissue and fibrosis.[9]
TGF-β modulates the expression of matrix metalloproteinase (MMPs), which is capable of cleaving all the components of the extra cellular matrix and the basement membrane. Expression of these MMPs in healthy tissues is low, but a special characteristic of keloid invasiveness is increased migratory activity associated with higher MMP-1 (interstitial collagenase) and MMP-2 (gelatinase-A) production.[9]
Other growth factors such as platelet-derived growth factor (PDGF), and insulin like growth factor 1 (IGF-1), are also known to regulate cell proliferation, differentiation and growth. TGF-β seems to turn on PDGF receptors and IGF-1 receptors in unusually high numbers in keloid fibroblasts.[10]
Genetics and hormonal factors
Factors that play a major role in keloid development are genetic predisposition with some form of skin trauma. Although many cases occur sporadically, a positive family history is not uncommon. There are no clearly defined genetic loci conferring risk for keloids.[2] Keloids are 15 times more likely to occur in darker skinned individuals points to genetic influences. Keloid formation mainly occurs in parts of the body with high concentrations of melanocytes, and it is rare on the soles and palms. Keloid formation has also been associated with endocrine factors. Menopause also prompts the recession of keloids, whereas women report keloid onset or enlargement during pregnancy.[8,9]
In the present case report, keloid has developed subsequently to minor insult to the skin, i.e. ear piercing. Histopathologically the present case showed epidermal changes such as flattening of epidermis as shown by 33.33% of keloid scars, hyperkeratosis, hypergranulosis, and spongiosis was also seen, which is mostly apparent in 93.33% of keloid scars, basal cell organization was regular and palisading as shown by 86.66% of keloid scars, and basal cell vacuolar change was diffusely prominent as shown by 93.33% of keloid scars (Moshref et al).[3] The dermal changes were as follows: The collagen was seen spanning full thickness of the dermis including the papillary dermis, as seen in 100% of keloid scars. The collagen was abnormally large, dense, broad, glassy, eosinophilic, contained focally fragmented complexes arranged haphazardly as seen in 100% of keloids.
Immunohistochemically, the present case was subjected to α-SMA, desmin, and S-100 markers. Keloid collagen showed α-SMA positivity, which is expressed in myofibroblasts, blood vessels and fine collagen, giving an appearance of “Tram track”. In the literature, there are wide variations regarding α-SMA expression in keloids, ranging from completely negative to 45% cases showing positivity, and 70% positive to most cases in another study. It is well accepted that myofibroblasts appear temporarily in granulation tissue during wound healing, but are present permanently in keloids and other fibrotic settings.[1] Thus myofibroblasts are thought to participate in the pathogenesis of keloids.
Desmin is negative in the present case. This indicates that the cells are myofibroblasts, and not smooth muscle cells, since α-SMA is positive and desmin is negative.[11,12]
The present case also showed scattered diffuse expression of S-100, representing langerhans cells and proliferating nerve twigs.[12]
TREATMENT
Numerous therapies for keloids have been described in the literature. Simple total excision of a keloid stimulates additional collagen synthesis, thus prompting quick recurrence of a keloid, even larger than the initial one. For this reason, intra-marginal surgical excision of keloid tissue is recommended to prevent the stimulation of additional collagen synthesis. Apart from surgery, other most commonly used treatment modalities include intra-lesional steroid injection, cryotherapy, laser removal, radiotherapy, and silicon gel sheeting. Less commonly used treatments include topical imiquimod and antimetabolites (including 5-fluorouracil and bleomycin).[2,8]
PREVENTION
The most important factor in keloid formation is prevention. The clinician should be aware of risk factors associated with keloid development, which include previous keloids, family history of keloids, tension at site of trauma and dark skin.[2]
Theopold et al., reported a case of keloid scar associated with a malignant blue nevus. The authors recommend that all keloid scars should be examined histologically in order to avoid missing potentially malignant conditions. In particular, unusual features, such as advanced age, lack of trauma preceding keloid formation, or the presence of a keloid-like lesion in uncommon sites, should prompt the clinician to seek histopathological confirmation of the diagnosis.[5]
CONCLUSION
Despite decades of research, the pathophysiology of keloids remains incompletely understood. Elucidation of the molecular pathways leading to keloid formation will undoubtedly open up a host of opportunities. Recent studies indicate that TGF-β2 and PDGF play an integral role in the formation of keloids. In future, development of selective inhibitors of TGF-β, will produce new therapeutic tools with enhanced efficacy and specificity for the treatment of keloids. Moreover, prevention of keloids is paramount, and combination therapy will likely prove to be most effective over any single modality in the treatment of keloids.
CLINICAL SIGNIFICANCE
Patients with a previous keloid or other risk factors should avoid unnecessary body piercing and elective cosmetic procedures. Keloid scars should be sent for histopathology in order to avoid missing potentially malignant conditions particularly those showing unusual features.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Ehrlich HP, Desmoulière A, Diegelmann RF, Cohen IK, Compton CC, Garner WL, et al. Morphological and immunochemical differences between keloid and hypertrophic scar. Am J Pathol. 1994;145:105–13. [PMC free article] [PubMed] [Google Scholar]
- 2.Robles DT, Berg D. Abnormal wound healing: Keloids. Clin Dermatol. 2007;25:26–32. doi: 10.1016/j.clindermatol.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Moshref SS, Mufti ST. Keloid and hypertrophic scars: Comparative histopathological and immunohistochemical study. Med Sci. 2010;17:3–22. [Google Scholar]
- 4.Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG. Hypertrophic scarring and keloids: Pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17:113–25. doi: 10.2119/molmed.2009.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Theopold C, Pritchard S, McGrouther DA, Bayat A. Keloid scar harbouring malignant blue naevus emphasises the need for excision biopsy and routine histology. J Plast Reconstr Aesthet Surg. 2009;62:93–5. doi: 10.1016/j.bjps.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 6.Verhaegen PD, van Zuijlen PP, Pennings NM, van Marle J, Niessen FB, van der Horst CM, et al. Differences in collagen architecture between keloid, hypertrophic scar, normotrophic scar, and normal skin: An objective histopathological analysis. Wound Repair Regen. 2009;17:649–56. doi: 10.1111/j.1524-475X.2009.00533.x. [DOI] [PubMed] [Google Scholar]
- 7.Hembry RM, Bernanke DH, Hayashi K, Trelstad RL, Ehrlich HP. Morphologic examination of mesenchymal cells in healing wounds of normal and tight skin mice. Am J Pathol. 1986;125:81–9. [PMC free article] [PubMed] [Google Scholar]
- 8.Wolfram D, Tzankov A, Pülzl P, Piza-Katzer H. Hypertrophic scars and keloids: A review of their pathophysiology, risk factors, and therapeutic management. Dermatol Surg. 2009;35:171–81. doi: 10.1111/j.1524-4725.2008.34406.x. [DOI] [PubMed] [Google Scholar]
- 9.Jain VK, Soundarya N, Rodrigues C, Shetty S. Bilateral tops like ear lobe keloid of unusual size: A case report and review of etiopathogenesis and treatment modalities. Int J Oral Maxillofac Pathol. 2011;2:45–50. [Google Scholar]
- 10.Messadi DV, Le A, Berg S, Huang G, Zhuang W, Bertolami CN. Effect of TGF-beta 1 on PDGF receptors expression in human scar fibroblasts. Front Biosci. 1998;3:16–22. doi: 10.2741/A246. [DOI] [PubMed] [Google Scholar]
- 11.Weiss SW, Goldblum JR. Immunohistochemistry for analysis of soft tissue tumors. In: Weiss SW, Goldblum JR, editors. Enzinger and Weiss's Soft Tissue Tumours. 5th ed. Philadelphia: Mosby Inc; 2008. pp. 633–751. [Google Scholar]
- 12.Gnepp DR. Diagnostic Surgical Pathology of the Head and Neck. Philadelphia: WB Saunders; 2001. Soft tissue tumors of head and neck; pp. 603–4. [Google Scholar]


