Abstract
Background:
Basement membrane heparan sulfate proteoglycan (perlecan) has been demonstrated in precancer lesions and carcinomas of oral cavity. It helps in malignant transformation of epithelial cells. The aim of our study was to understand the immuno-localization of perlecan in oral dysplastic epithelium and oral carcinomas.
Materials and Methods:
A total of 50 cases comprising 10 normal mucosa, 20 dysplastic mucosa, and 20 oral squamous cell carcinomas (OSCC) were included in the retrospective study. They were examined for the presence of perlecan protein core by immunohistochemistry using monoclonal antibody. Interpretation of the pattern of staining was done, and majority of the observations were taken for statistical analysis.
Results:
In normal epithelium, perlecan was found to be present in basal layer at the cell border. In dysplastic epithelium, it was present in suprabasal layers also. With the increase in severity of dysplasia, its expression was more in suprabasal layers, and the immuno-localization was found to be at cell border and cytoplasm. In OSCC cases, perlecan was present in stroma and tumor islands.
Conclusion:
It was deduced from the above results that perlecan helps potentially in dysplastic changes of epithelial cells. It gets accumulated within the cell and intercellular spaces and serves as a reservoir for various growth factors. In OSCC, it breaks down and releases growth factors, which help in tumor progression, angiogenesis, and metastasis of the carcinoma.
Keywords: Dysplasia, growth factors, intercellular space, oral cancer, perlecan
INTRODUCTION
A basement membrane-type heparan sulfate proteoglycan (400 to 500 kDa) is present in virtually all vascularized tissue with a distribution that is primarily confined to basement membranes, including those of oral mucosa. It is also known as perlecan. Perlecan name derives from its electron microscopic structure, i.e., beads or pearls on a string. Perlecan is synthesized by basal cells and fibroblasts adjacent to the basal lamina.[1,2] Perlecan is also synthesized by vascular endothelial and smooth muscle cells present in the extracellular matrix. It has been demonstrated in recent years that perlecan is distributed not only in the basement membranes but also in the stromal space of various pathophysiological conditions.[2,3] The complex pleiotropy of perlecan suggests that this gene product is involved in several developmental processes, at both early and late stages of embryogenesis, as well as in pervasive human diseases such as cancer and diabetes.[4]
One of the 13 major histological characteristics of the oral epithelial dysplasia listed in the WHO Histological Typing of Cancer and Precancer of the oral mucosa that had been originally proposed by the WHO Collaborating Centre for Oral Precancerous lesions[5] is “reduction of cellular cohesion,” which was thought to be able to make epithelial cells behave autonomously. However, the reduction of cellular cohesion, in other words, the enlargement of the intercellular space results from accumulation of proteoglycans. It is of great interest to investigate what is accumulated in the widened intercellular space and how it helps in dysplastic transformation of epithelial cells.[2] Perlecan expression is limited to cell borders of basal cells in normal mucosa and its expression increases in dysplastic mucosa. The dysplastic cells need more amount of perlecan for its proliferation. Immuno-localization of perlecan using a monoclonal antibody against its core protein helps in understanding the dysplastic changes in the tissue. The purpose of our study was to observe the pattern of expression of perlecan in normal oral epithelium, dysplastic mucosa, and OSCC, as well as to know how it contributes to malignant transformation of epithelial cells.
MATERIALS AND METHODS
Formalin-fixed paraffin-embedded tissues were retrieved from the archives of Department of Oral Pathology and Microbiology, V K Institute of Dental Sciences. Ten cases of normal mucosa (Group 1), 20 cases of dysplastic mucosa (Group 2), and 20 cases of OSCC (Group 3) were included in the study. Cases of normal oral mucosa were used as controls. Two sections, each of 5 μ thickness, from paraffin-embedded tissues of groups 1, 2, and 3 were obtained. One section was stained with hematoxylin and eosin and another was immunostained with perlecan monoclonal antibody.
Antibodies
Primary antibody: [anti-basement membrane-type heparan sulfate proteoglycan (HSPG) perlecan core protein. Specificity: Mouse monoclonal anti-human perlecan. Clone No: 85-9] was obtained from Division of Oral Pathology, Department of Tissue Regeneration and Reconstruction, Niigata University Graduate School of Medical and Dental Sciences 2-5274 Gakkochi-dori, Niigata 951-8514, JAPAN. Secondary antibody containing Super Sensitive Polymer DAB detection kit was purchased from Biogenex (Sikandrabad, India).
Immunohistochemistry
Paraffin sections were subjected to immunohistochemical staining for perlecan core protein. For antigen retrieval, deparaffinized sections were kept in staining trough filled with citric acid (pH 6.0) and were boiled in pressure cooker for 5 min. The sections were rinsed in 0.01 M phosphate-buffered saline (PBS; 7.4). After that, sections were introduced to peroxide block for 10 min, followed by power block for 10 min, at room temperature in a humidifying chamber. Sections were not washed with PBS after exposing them with power block. Then, sections were incubated with primary antibodies for 1 h at 1:100 dilutions. After rinsing with two changes of PBS, sections were subjected to super enhancer. Then, they were incubated for 30 min with secondary antibodies, which conjugated with peroxidase-labeled dextran polymers. After rinsing those with PBS, sections were treated with 3, 3¢-diaminobenzidine for 20 min. Finally, sections were counterstained with hematoxylin. For control study on antibodies, the primary antibodies were replaced with pre-immune rabbit IgG or mouse IgG subclasses (Biogenex). Immunoreaction of groups 1, 2, and 3 were interpreted and observations were taken for statistical analysis.
RESULTS
In the current study, cases of normal oral mucosa were taken as control group, which comprised 10 in number. Perlecan was expressed faintly in the basal layer of 70% of normal epithelium. Within the basal layer, it was immuno-localized at the cell border in all the cases. Chi square test was done for statistical analysis [Table 1, Figures 1a–c]. In the second group, which constituted of dysplastic mucosa, there were 20 cases. Perlecan expression was significant in the basal layer and was also present in suprabasal layers [Table 1, Figures 2a–c]. In mild and moderate dysplasia, perlecan was expressed in the basal layer in most of the cases where as it was expressed in suprabasal layers in severe dysplasia cases. Immuno-localization of perlecan protein was mainly at the cell borders in almost all the cases of dysplastic mucosa. In severe dysplasia cases, it was immuno-localized at the cell border and cytoplasm; and a coalescent staining was seen [Figure 2c]. In OSCC group, which included 20 cases, perlecan immunopositivity was found in connective tissue stroma of almost all the cases, which was a significant find. [Table 2, Figures 3a–c]. In a few cases, immunopositivity was seen in and around the tumor islands. In the tumor islands, immuno-localization of perlecan was at the cell border, and in a few areas there was a coalescent staining at cell border and cytoplasm [Figure 3c]. With the increase in level of carcinoma, perlecan immunostaining showed descendency. No specific pattern was observed for immunostaining of perlecan in the stroma.
Table 1.
Perlecan expression within layers of epithelium
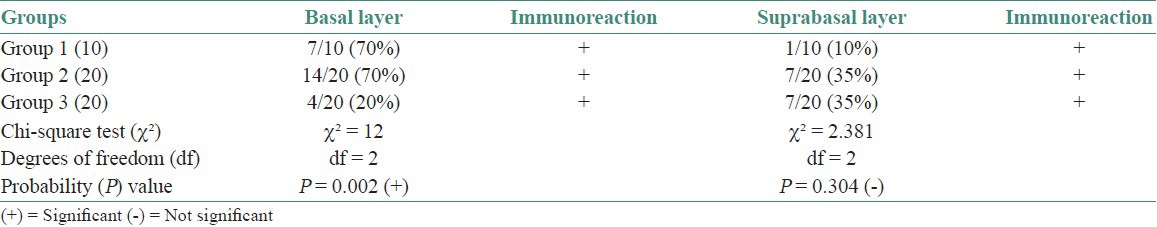
Figure 1.
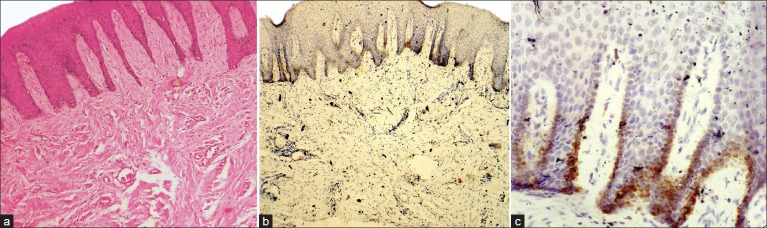
Immunohistochemical expression of Perlecan in Normal Epithelium of Oral mucosa (a) Apparently Normal Epithelium of Oral mucosa H and E ×100, (b) Immunohistochemical expression of Perlecan in basal layer of Normal Oral mucosa IHC ×100, (c) Immunohistochemical expression of Perlecan at cell surface IHC ×400
Figure 2.
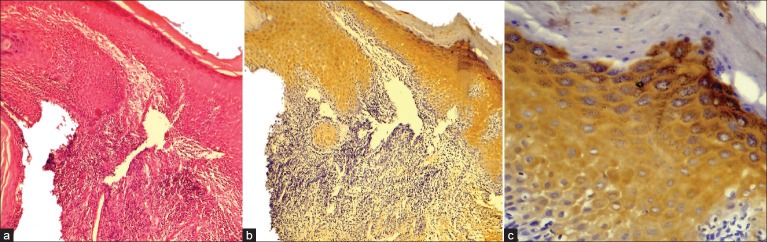
Immunohistochemical expression of Perlecan in Oral Dysplastic Epithelium (a) Oral Dysplastic Epithelium H and E ×100, (b) Immunohistochemical expression of Perlecan in suprabasal (stratum spinosum, stratum granulosum) layers IHC ×100, (c) Coalescent Immunohistochemical expression of Perlecan within the cell, at the cell surface & intercellular spaces IHC ×400. Note: Stratum corneum does not show immunoexpression of Perlecan.
Table 2.
Perlecan expression in the connective tissue
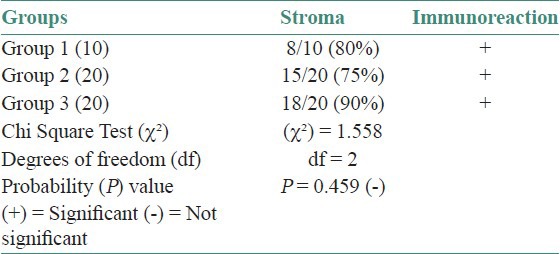
Figure 3.
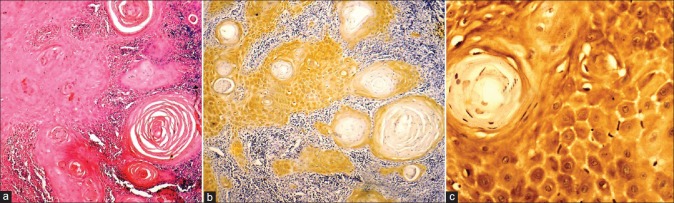
Immunohistochemical expression of Perlecan in Oral Squamous Cell Carcinoma (a) Well Differentiated Squamous Cell Carcinoma H and E ×100, (b) Immunohistochemical expression of Perlecan in tumor islands & stroma IHC ×100, (c) Immunohistochemical expression of Perlecan in cytoplasm & cell surface IHC ×400
DISCUSSION
Perlecan consists of a core protein to which long chains of glycosaminoglycans are attached. The core protein consists of five distinct structural domains. Perlecan core protein can modulate matrix assembly, cell proliferation, lipoprotein binding, and cell adhesion.[4] In the current study, it could be deduced that in normal oral mucosa, basal cells beget perlecan in normal epithelium. In a study done by Horiguchi, perlecan has been localized to the basement membrane of fetal skin during the first trimester of the life and as early as 54 days of intrauterine development. The presence of perlecan at the earliest time at which basement membrane is formed supports the hypothesis that this large proteoglycan contributes to the early structural integrity of the epidermal–connective tissue interface.[6] Perlecan plays an important role in cellular growth, differentiation, adhesion, and motility by its interaction with growth factors and cytokines. Recently, perlecan has been localized within various pathophysiological conditions. Its expression in odontogenic epithelium was studied by Hiroko et al. where they demonstrated that perlecan was present in the stellate reticulum of enamel organs of developing tooth germs. Their study has suggested that perlecan plays a role in tooth morphogenesis.[7,8]
In the second group of our study, which constituted of dysplastic mucosa, perlecan expression was significant in basal layer and suprabasal layers. In mild and moderate dysplasia, perlecan was expressed in the basal layer in most of the cases whereas it was expressed in suprabasal layers in severe dysplasia cases. Saku et al. have conducted a study on perlecan expression in dysplastic epithelium where they found its location in prickle cell layer of moderate and severe dysplasia whereas in our study it was observed that suprabasilar immunostaining of perlecan was mainly seen in cases of severe dysplasia. A recent study conducted by Saku et al. on perlecan expression in keratocystic odontogenic tumor reports that perlecan protein was localized on the cell border from parabasal layer to subkeratinized layers of lining epithelium,[9] which is in accordance with our finding of perlecan expression in severe dysplasia cases. The immuno-localization of perlecan on the cell border is thought to possibly suggest its actual localization in the intercellular space of epithelial cells. As the intracellular and intercellular deposition of perlecan became more with the increase in degree of dysplasia; it seems that biosynthesis of perlecan is accelerated in the process of malignant transformation of epithelial cells, which is still limited to epithelial layers. As a result of enhanced intercellular deposition of perlecan, the intercellular space was apparently widened and this may be interpreted histopathologically as “loss of intercellular adherence.”[2,5] Strong immunoreaction of perlecan in cytoplasm of dysplastic cells suggests that dysplastic cell is in the active phase of proliferation with lot of proteoglycans inside it. It is known from the previous studies that perlecan binds to various molecules through its domains, but how these molecules assist in dysplastic transformation is intricate to resolve.
White and Gohari studied the volume of intercellular space during hamster cheek pouch carcinogenesis at the ultrastructural level. Their results indicated increase in separation of epithelial cells during carcinogenesis, although it is not yet known whether this results from the loss of cohesion between specialized structures, i.e., desmosomes or non-specialized membrane areas.[10] The intercellular accumulation of perlecan in dysplastic epithelia seems to disturb cellular communication between epithelial cells, especially in the lateral direction such as from basal cells to basal cells or from prickle cells to prickle cells. Such a reduction in communicating abilities of stratified squamous epithelial cells may contribute to their concordant differentiation towards keratinization.[2] A perfect example of the intercellular deposit of perlecan is the enamel pulp of the tooth germ. The stellate reticulum seems to have resulted from an excessive amount of proteoglycans, including perlecan, in the intercellular space. Proteoglycans tend to gather water molecules around them, which are thought to participate in the widening of the intercellular space.[8] Results of the study conducted by Hirako et al. in ameloblastoma also indicate that ameloblastoma cells synthesize perlecan (HSPG) and deposit it in their intercellular space. The intercellular perlecan, might act as a carrier for transport of nutrients to tumor cells within ameloblastomatous foci. Various other studies conducted on odontogenic tumors have demonstrated that enamel proteins such as amelogenin and enamelin are co-localized with other extra cellular matrix (ECM) molecules, especially those that are basement membrane associated such as HSPG, type IV collagen, laminin, and fibronectin. [11,12] Reviewing the above studies, it can be hypothesized that perlecan in oral dysplastic epithelium helps in tumor progression by acting as a carrier for transport of nutrients in the intercellular spaces along with other ECM molecules.
In the OSCC group, perlecan immunopositivity was found in connective tissue stroma of almost all the cases. In well-differentiated squamous cell carcinomas (WDSCC), more number of tumor islands showed positivity compared with moderately differentiated squamous cell carcinomas (MDSCC) and poorly differentiated carcinomas (PDSCC). In the tumor islands, immuno-localization of perlecan was at the cell border, and in a few areas there was a coalescent staining at cell border and cytoplasm. Intensity of immune reaction was reduced with the higher grades of carcinoma. Batmunkh et al. studied expression of agrin (basement membrane heparan sulfate proteoglycan) in cholangiocarcinoma and hepatocellular carcinoma by immunohistochemistry. They derived that agrin was abundant in the tumor-specific basement membrane in well-differentiated areas of cholangiocarcinoma, whereas with immunostaining it was fragmented, decreased, or even disappeared in less differentiated areas.[13] Shuji et al. studied expression of endoglycosidic heparanase and sydecan-1 in esophageal carcinomas. They inferred that there was loss of syndecan-1 in advanced head and neck carcinomas. Syndecan is also a cell surface-bound HSPG and endoglycosidic heparanase cleaves HSPGs. There is increased expression of heparanase in invasive esophageal carcinomas.[14] Considering the above studies, it is suggested that with the increase in grade of carcinoma, HSPGs get cleaved by heparanase. Similarly, in the present study perlecan expression was scarce with the higher grades of carcinoma and its pattern became haywire. It can be hypothesized that with the increase in degree of carcinoma; more heparanase enzyme acts on perlecan and from the breakdown of perlecan; FGF, TGF-β, and growth factors are released. All these factors promote the tumor growth and perlecan expression goes down with the severity of carcinoma.
Several lines of evidence by immunohistochemistry have shown that basal cells express specific cell membrane molecules such as integrins, type II interleukin 1 receptor, epidermal growth factor, fibroblast growth factor, and lectin. These trapped growth factors and molecules may function in a way that will lead to the proliferation of dysplastic cells. Lectin, a sugar moiety associated with cell adhesion, has been demonstrated in oral epithelial dysplasias and squamous lining of jaw cysts. In fact, Wakulich et al. have shown that the expression levels of fibroblast growth factor (FGF)-2 are in accordance with severity of epithelial dysplasias, which were found in the vicinity of head and neck squamous cell carcinoma.[15–17] Taking these studies and our study into account, it can be predicted that because of the presence of perlecan in the cytoplasm and intercellular space, FGF, tumor growth factor (TGF)-β, lectins, integrins, and interleukins can help in progress of dysplasia.
Considering the results of our study and other studies until now, it is almost established that perlecan expression increases with the increase in levels of dysplasia in oral epithelium and diminishes with the higher grades of carcinoma by lysing itself and releasing growth factors. Its presence in cytoplasm and intercellular space definitely helps in dysplastic proliferation. Further studies are required to use perlecan as a marker for oral epithelial dysplasia and OSCC.
ACKNOWLEDGMENTS
We are thankful to Professor T. Saku of Niigata University Graduate School of Medical and Dental Sciences, Japan, for providing us with perlecan monoclonal primary antibody.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Garant PR. Illinois: Quintessence Publishing Co, Inc; 2003. Oral Cells and Tissues. [Google Scholar]
- 2.Ikarashi T, Ida-Yonemochi H, Ohshiro K, Cheng J, Saku T. Intraepithelial expression of perlecan, a basement membrane-type heparan sulfate proteoglycan reflects dysplastic changes of the oral mucosal epithelium. J Oral Pathol Med. 2004;33:87–95. doi: 10.1111/j.1600-0714.2004.00026.x. [DOI] [PubMed] [Google Scholar]
- 3.Murdoch AD, Liu B, Schwarting R, Tuan RS, Iozzo RV. Widespread expression of perlecan proteoglycan in basement membranes and extracellular matrices of human tissue as detected by a novel monoclonal antibody against domain III and by in situ hybridization. J Histochem Cytochem. 1994;42:239–49. doi: 10.1177/42.2.7507142. [DOI] [PubMed] [Google Scholar]
- 4.Iozzo RV, Cohen IR, Grassel S, Murdoch AD. The biology of perlecan: The multifaceted heparan sulphate proteoglycan of basement membranes and pericellular matrices. Biochem J. 1994;302:625–39. doi: 10.1042/bj3020625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kramer IR, Lucas RB, Pindborg JJ, Sobin LH. Definition of leukoplakia and related lesions: An aid to studies on oral precancer. Oral Surg Oral Med Oral Pathol. 1978;46:518–39. [PubMed] [Google Scholar]
- 6.Horiguchi Y, Fine JD, Couchman JR. Human skin basement membrane- associated heparan sulphate proteoglycan: distinctive differences in ultrastructural localization as a function of developmental age. Br J Dermatol. 1991;124:410–4. doi: 10.1111/j.1365-2133.1991.tb00617.x. [DOI] [PubMed] [Google Scholar]
- 7.Ida-Yonemochi H, Ohshiro K, Swelam W, Metwaly H, Saku T. Perlecan, a basement membrane-type heparan sulfate proteoglycan, in the enamel organ: Its intraepithelial localization in the stellate reticulum. J Histochem Cytochem. 2005;53:763–72. doi: 10.1369/jhc.4A6479.2005. [DOI] [PubMed] [Google Scholar]
- 8.Ida-Yonemochi H, Saku T. Perlecan, a heparan sulfate proteoglycan, is a major constituent of the intraepithelial stroma functioning in tooth morphogenesis. J Oral Biosci. 2006;48:233–43. [Google Scholar]
- 9.Tsuneki M, Cheng J, Maruyama S, Ida-Yonemochi H, Nakajima M, Saku T. Perlecan-rich epithelial linings as a background of proliferative potentials of keratocystic odontogenic tumor. J Oral Pathol Med. 2008;37:287–93. doi: 10.1111/j.1600-0714.2007.00620.x. [DOI] [PubMed] [Google Scholar]
- 10.White FH, Gohari K. Alterations in the volume of the intercellular space between epithelial cells of the hamster cheek-pouch: quantitative studies of normal and carcinogen-treated tissues. J Oral Pathol. 1984;13:244–54. doi: 10.1111/j.1600-0714.1984.tb01422.x. [DOI] [PubMed] [Google Scholar]
- 11.Ida-Yonemochi H, Ikarashi T, Nagata M, Hoshina H, Takagi R, Saku T. The basement membrane-type heparan sulfate proteoglycan (perlecan) in ameloblastomas; its intercellular localization in stellate reticulum-like foci and biosynthesis by tumor cells in culture. Virchows Arch. 2002;441:165–73. doi: 10.1007/s00428-001-0556-y. [DOI] [PubMed] [Google Scholar]
- 12.Murata M, Cheng J, Horino M, Hara K, Shimokawa H, Saku T. Enamel proteins and extracellular matrix molecules are co- localized in the pseudocystic stromal space of adenomatoid odontogenic tumor. J Oral Pathol Med. 2000;29:483–90. doi: 10.1034/j.1600-0714.2000.291002.x. [DOI] [PubMed] [Google Scholar]
- 13.Batmunkh E, Tátrai P, Szabó E, Lódi C, Holczbauer Á, Páska C, et al. Comparison of the expression of agrin, a basement membrane heparan sulfate proteoglycan, in cholangiocarcinoma and hepatocellular carcinoma. Hum Pathol. 2003;38:1508–15. doi: 10.1016/j.humpath.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Mikami S, Ohashi K, Usui Y, Nemoto T, Katsube K, Yanagishita M, et al. Loss of syndecan-1 and increased expression of heparanase in invasive esophageal carcinomas. Jpn J Cancer Res. 2001;92:1062–73. doi: 10.1111/j.1349-7006.2001.tb01061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saku T, Okabe H. Differential lectin-bindings in normal and precancerous epithelium and squamous cell carcinoma of the oral mucosa. J Oral Pathol Med. 1989;18:438–45. doi: 10.1111/j.1600-0714.1989.tb01340.x. [DOI] [PubMed] [Google Scholar]
- 16.Saku T, Shibata Y, Koyama Z, Cheng J, Okabe H, Yeh Y. Lectin histochemistry of cystic jaw lesions: An aid for differential diagnosis between cystic ameloblastoma and odontogenic cysts. J Oral Pathol Med. 1991;20:108–13. doi: 10.1111/j.1600-0714.1991.tb00902.x. [DOI] [PubMed] [Google Scholar]
- 17.Wakulich C, Jackson-Boeters L, Daley TD, Wysocki GP. Immunohistochemical localization of growth factors fibroblast growth factor-1 and fibroblast growth factor-2 and receptors fibroblast growth factor receptor-2 and fibroblast growth factor receptor-3 in normal oral epithelium, epithelial dysplasias, and squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:573–9. doi: 10.1067/moe.2002.124461. [DOI] [PubMed] [Google Scholar]


