Abstract
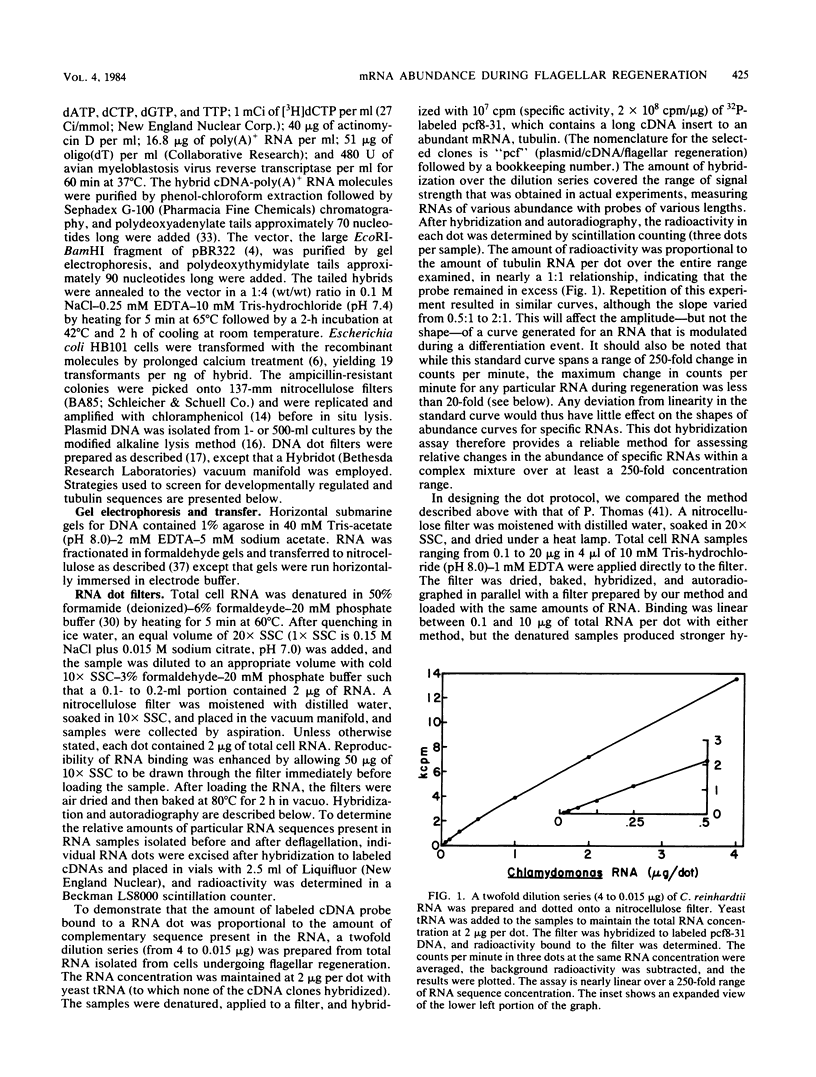
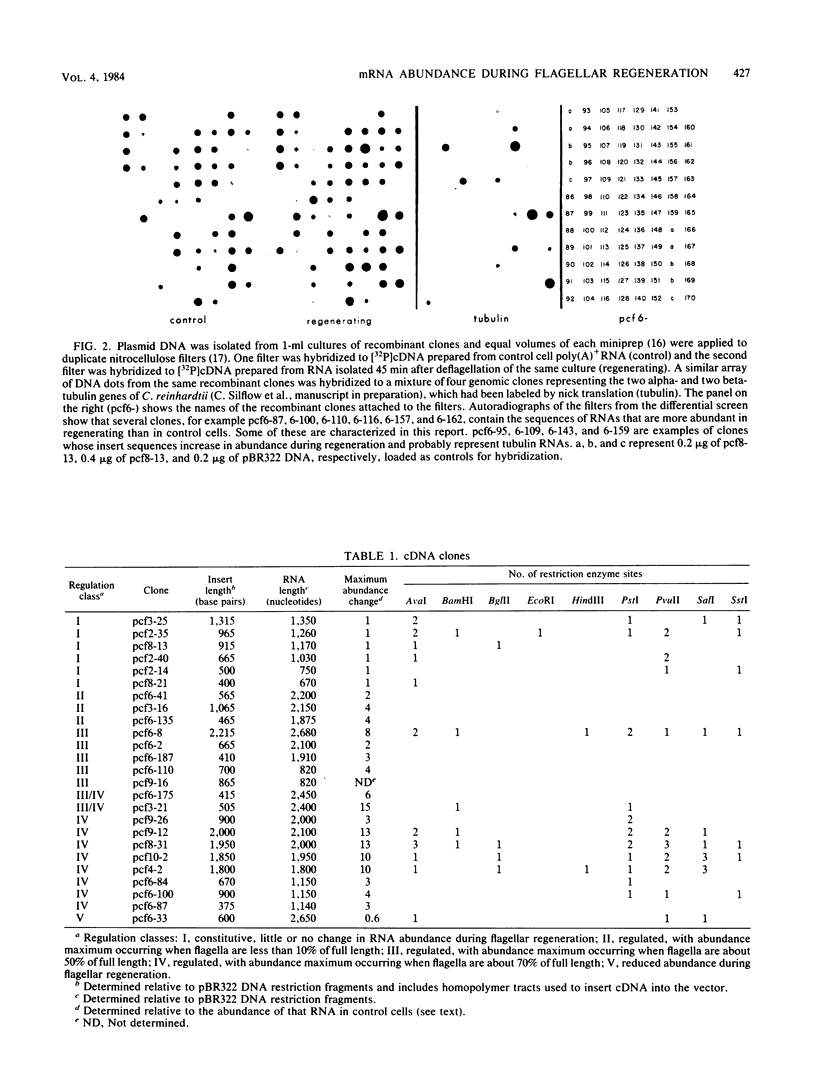
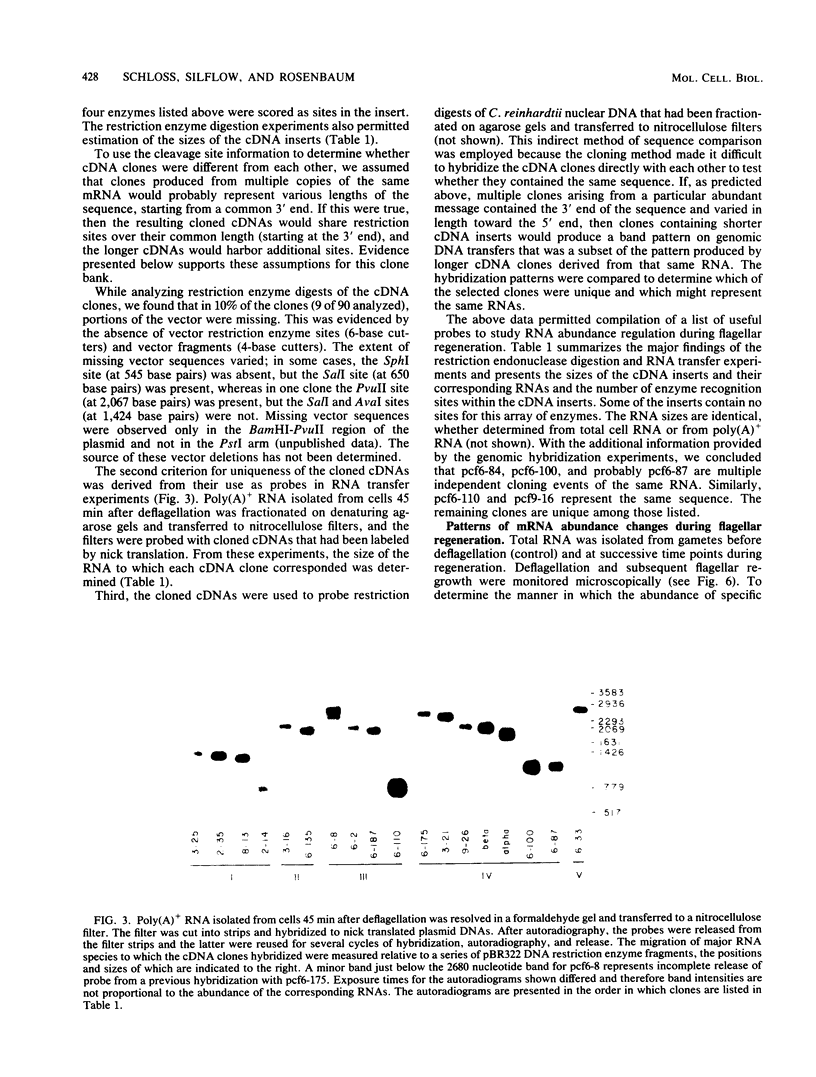
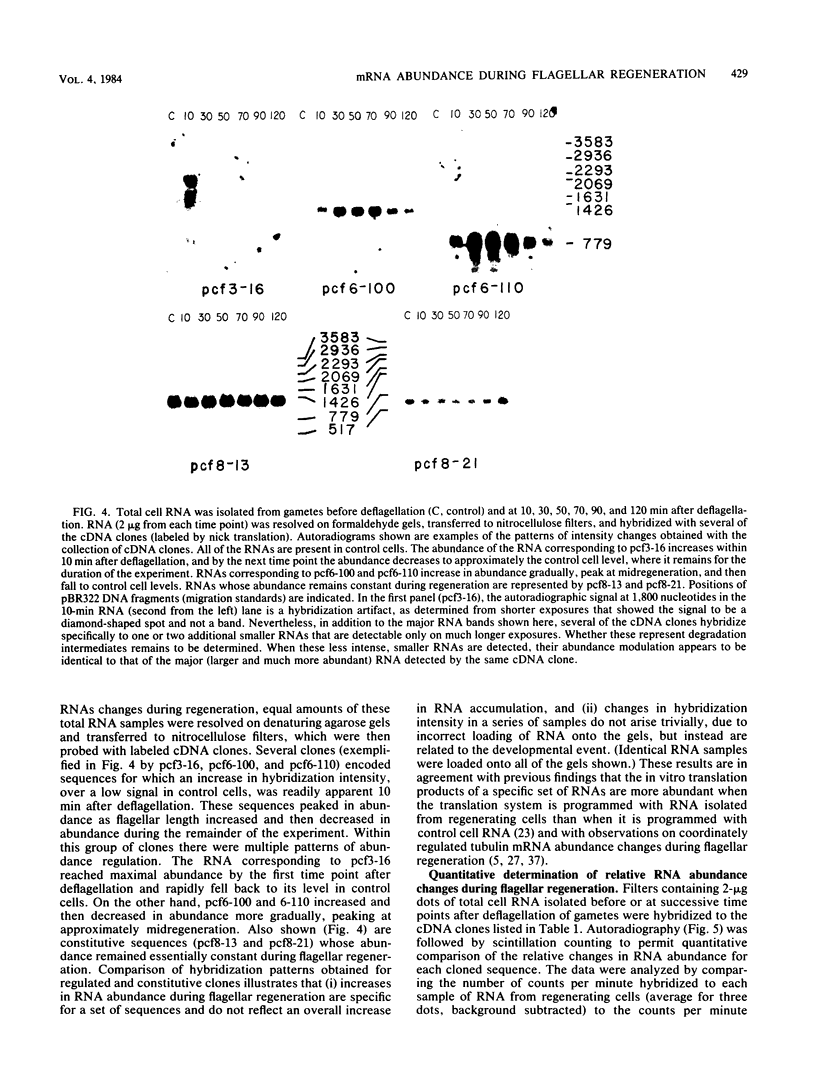
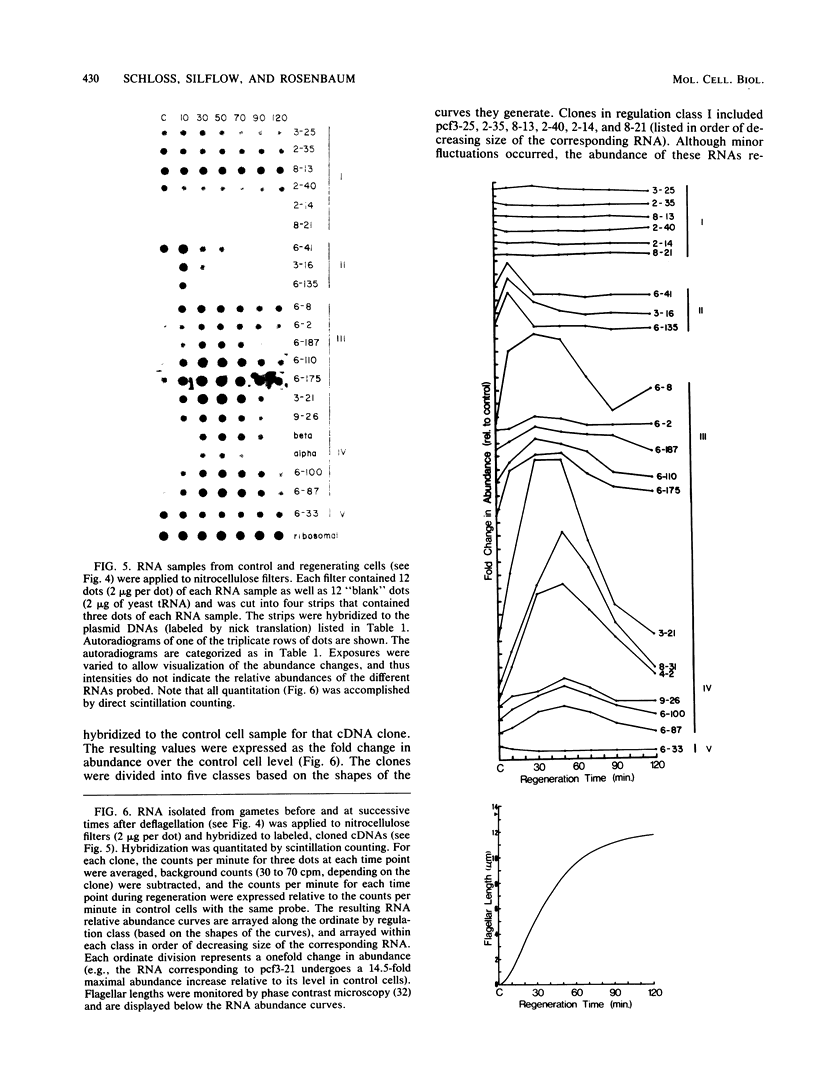
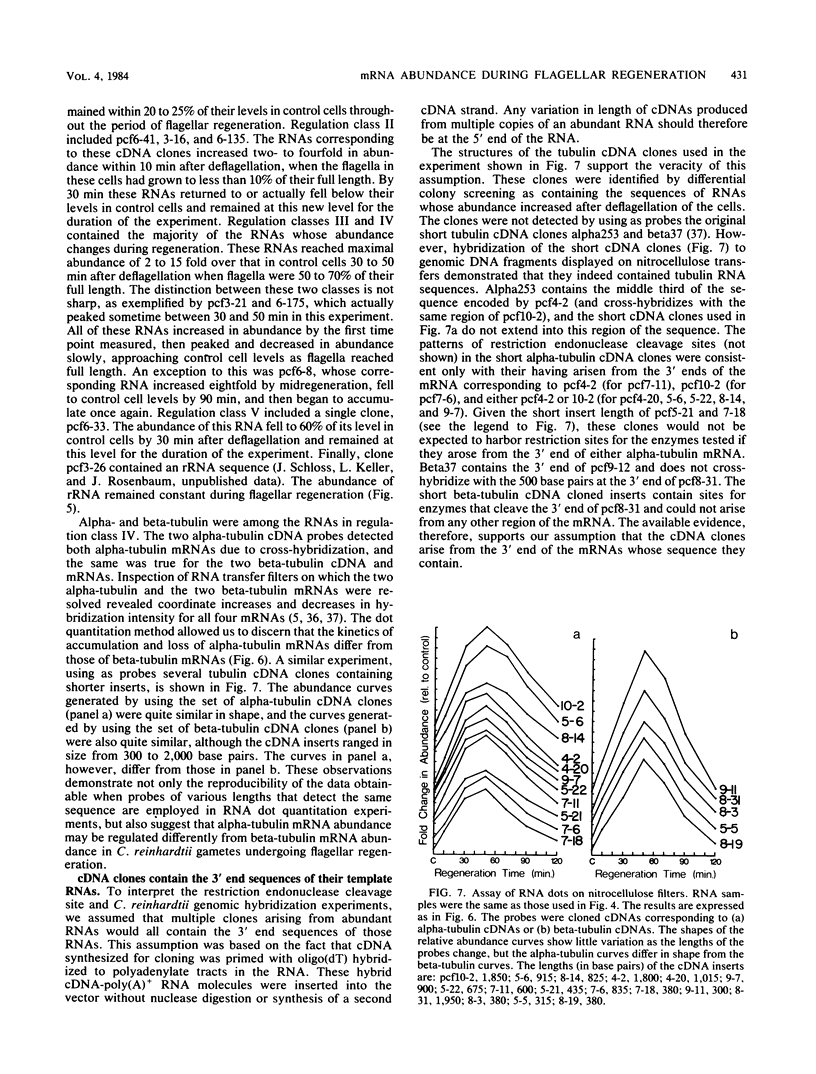
Flagellar amputation in Chlamydomonas reinhardtii induces the accumulation of a specific set of RNAs, many of which encode flagellar proteins. We prepared a cDNA clone bank from RNA isolated from cells undergoing flagellar regeneration. From this bank, we selected clones that contain RNA sequences that display several different patterns of abundance regulation. Based on quantitation of the relative amounts of labeled, cloned cDNAs hybridizing to dots of RNA on nitrocellulose filters, the cloned sequences were divided into five regulatory classes: class I RNAs remain at constant abundance during flagellar regeneration; classes II, III, and IV begin to increase in abundance within a few minutes after deflagellation, reach maximal abundance at successively later times during regeneration, and return to control cell levels within 2 to 3 h; and class V RNA abundance decreases during flagellar regeneration. Alpha- and beta-tubulin mRNAs are included in regulatory class IV. The abundance kinetics of alpha-tubulin mRNAs differ slightly from those of beta-tubulin mRNAs. The availability of these clones makes possible studies on the mechanisms controlling the abundance of a wide variety of different RNA species during flagellar regeneration in Chlamydomonas.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird R. C., Zimmerman A. M. Induction of tubulin synthesis during cilia regeneration in growing Tetrahymena. Exp Cell Res. 1980 Jul;128(1):199–205. doi: 10.1016/0014-4827(80)90403-6. [DOI] [PubMed] [Google Scholar]
- Blumberg D. D., Lodish H. F. Changes in the messenger RNA population during differentiation of dictyostelium discoideum. Dev Biol. 1980 Aug;78(2):285–300. doi: 10.1016/0012-1606(80)90337-1. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Brunke K. J., Young E. E., Buchbinder B. U., Weeks D. P. Coordinate regulation of the four tubulin genes of Chlamydomonas reinhardi. Nucleic Acids Res. 1982 Feb 25;10(4):1295–1310. doi: 10.1093/nar/10.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Britten R. J. Regulation of gene expression: possible role of repetitive sequences. Science. 1979 Jun 8;204(4397):1052–1059. doi: 10.1126/science.451548. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dobberstein B., Blobel G., Chua N. H. In vitro synthesis and processing of a putative precursor for the small subunit of ribulose-1,5-bisphosphate carboxylase of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1082–1085. doi: 10.1073/pnas.74.3.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin M. B., Dawid I. B. Use of a cloned library for the study of abundant poly(A)+RNA during Xenopus laevis development. Dev Biol. 1980 May;76(2):449–464. doi: 10.1016/0012-1606(80)90393-0. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Guttman S. D., Glover C. V., Allis C. D., Gorovsky M. A. Heat shock, deciliation and release from anoxia induce the synthesis of the same set of polypeptides in starved T. pyriformis. Cell. 1980 Nov;22(1 Pt 1):299–307. doi: 10.1016/0092-8674(80)90177-4. [DOI] [PubMed] [Google Scholar]
- Guttman S. D., Gorovsky M. A. Cilia regeneration in starved tetrahymena: an inducible system for studying gene expression and organelle biogenesis. Cell. 1979 Jun;17(2):307–317. doi: 10.1016/0092-8674(79)90156-9. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Hastings K. E., Emerson C. P., Jr cDNA clone analysis of six co-regulated mRNAs encoding skeletal muscle contractile proteins. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1553–1557. doi: 10.1073/pnas.79.5.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATES J. R., JONES R. F. THE CONTROL OF GAMETIC DIFFERENTIATION IN LIQUID CULTURES OF CHLAMYDOMONAS. J Cell Physiol. 1964 Apr;63:157–164. doi: 10.1002/jcp.1030630204. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowit J. D., Fulton C. Programmed synthesis of tubulin for the flagella that develop during cell differentiation in Naegleria gruberi. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2877–2881. doi: 10.1073/pnas.71.7.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai E. Y., Walsh C., Wardell D., Fulton C. Programmed appearance of translatable flagellar tubulin mRNA during cell differentiation in Naegleria. Cell. 1979 Aug;17(4):867–878. doi: 10.1016/0092-8674(79)90327-1. [DOI] [PubMed] [Google Scholar]
- Lasky L. A., Lev Z., Xin J. H., Britten R. J., Davidson E. H. Messenger RNA prevalence in sea urchin embryos measured with cloned cDNAs. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5317–5321. doi: 10.1073/pnas.77.9.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre P. A., Nordstrom S. A., Moulder J. E., Rosenbaum J. L. Flagellar elongation and shortening in Chlamydomonas. IV. Effects of flagellar detachment, regeneration, and resorption on the induction of flagellar protein synthesis. J Cell Biol. 1978 Jul;78(1):8–27. doi: 10.1083/jcb.78.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre P. A., Silflow C. D., Wieben E. D., Rosenbaum J. L. Increased levels of mRNAs for tubulin and other flagellar proteins after amputation or shortening of Chlamydomonas flagella. Cell. 1980 Jun;20(2):469–477. doi: 10.1016/0092-8674(80)90633-9. [DOI] [PubMed] [Google Scholar]
- Marcaud L., Hayes D. RNA synthesis in starved deciliated Tetrahymena pyriformis. Eur J Biochem. 1979 Jul;98(1):267–273. doi: 10.1111/j.1432-1033.1979.tb13185.x. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- Merlino G. T., Chamberlain J. P., Kleinsmith L. J. Effects of deciliation of tubulin messenger RNA activity in sea urchin embryos. J Biol Chem. 1978 Oct 10;253(19):7078–7085. [PubMed] [Google Scholar]
- Minami S. A., Collis P. S., Young E. E., Weeks D. P. Tubulin induction in C. reinhardii: requirement for tubulin mRNA synthesis. Cell. 1981 Apr;24(1):89–95. doi: 10.1016/0092-8674(81)90504-3. [DOI] [PubMed] [Google Scholar]
- Neal M. W., Florini J. R. A rapid method for desalting small volumes of solution. Anal Biochem. 1973 Sep;55(1):328–330. doi: 10.1016/0003-2697(73)90325-4. [DOI] [PubMed] [Google Scholar]
- Piperno G., Huang B., Luck D. J. Two-dimensional analysis of flagellar proteins from wild-type and paralyzed mutants of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1600–1604. doi: 10.1073/pnas.74.4.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rave N., Crkvenjakov R., Boedtker H. Identification of procollagen mRNAs transferred to diazobenzyloxymethyl paper from formaldehyde agarose gels. Nucleic Acids Res. 1979 Aug 10;6(11):3559–3567. doi: 10.1093/nar/6.11.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remillard S. P., Witman G. B. Synthesis, transport, and utilization of specific flagellar proteins during flagellar regeneration in Chlamydomonas. J Cell Biol. 1982 Jun;93(3):615–631. doi: 10.1083/jcb.93.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum J. L., Moulder J. E., Ringo D. L. Flagellar elongation and shortening in Chlamydomonas. The use of cycloheximide and colchicine to study the synthesis and assembly of flagellar proteins. J Cell Biol. 1969 May;41(2):600–619. doi: 10.1083/jcb.41.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychoudhury R., Jay E., Wu R. Terminal labeling and addition of homopolymer tracts to duplex DNA fragments by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1976 Jan;3(1):101–116. doi: 10.1093/nar/3.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAGER R., GRANICK S. Nutritional studies with Chlamydomonas reinhardi. Ann N Y Acad Sci. 1953 Oct 14;56(5):831–838. doi: 10.1111/j.1749-6632.1953.tb30261.x. [DOI] [PubMed] [Google Scholar]
- Silflow C. D., Lefebvre P. A., McKeithan T. W., Schloss J. A., Keller L. R., Rosenbaum J. L. Expression of flagellar protein genes during flagellar regeneration in Chlamydomonas. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):157–169. doi: 10.1101/sqb.1982.046.01.019. [DOI] [PubMed] [Google Scholar]
- Silflow C. D., Rosenbaum J. L. Multiple alpha- and beta-tubulin genes in Chlamydomonas and regulation of tubulin mRNA levels after deflagellation. Cell. 1981 Apr;24(1):81–88. doi: 10.1016/0092-8674(81)90503-1. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Mahowald A. P. Identification and genetic localization of mRNAs from ovarian follicle cells of Drosophila melanogaster. Cell. 1979 Mar;16(3):589–598. doi: 10.1016/0092-8674(79)90032-1. [DOI] [PubMed] [Google Scholar]
- Stephens R. E. Differential protein synthesis and utilization during cilia formation in sea urchin embryos. Dev Biol. 1977 Dec;61(2):311–329. doi: 10.1016/0012-1606(77)90301-3. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks D. P., Collis P. S. Induction of microtubule protein synthesis in Chlamydomonas reinhardi during flagellar regeneration. Cell. 1976 Sep;9(1):15–27. doi: 10.1016/0092-8674(76)90048-9. [DOI] [PubMed] [Google Scholar]
- Weinstock R., Sweet R., Weiss M., Cedar H., Axel R. Intragenic DNA spacers interrupt the ovalbumin gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1299–1303. doi: 10.1073/pnas.75.3.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. G., Lloyd M. M. Changes in the abundance of polyadenylated RNA during slime mould development measured using cloned molecular hybridization probes. J Mol Biol. 1979 Mar 25;129(1):19–35. doi: 10.1016/0022-2836(79)90056-1. [DOI] [PubMed] [Google Scholar]
- Witman G. B., Carlson K., Berliner J., Rosenbaum J. L. Chlamydomonas flagella. I. Isolation and electrophoretic analysis of microtubules, matrix, membranes, and mastigonemes. J Cell Biol. 1972 Sep;54(3):507–539. doi: 10.1083/jcb.54.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood K. O., Lee J. C. Integration of synthetic globin genes into an E. coli plasmid. Nucleic Acids Res. 1976 Aug;3(8):1961–1971. doi: 10.1093/nar/3.8.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]







