Abstract
During the formation of the nervous system, axonal growth cones navigate through the complex environment of the developing embryo to innervate their targets. Growth cones achieve this formidable feat by responding to attractive or repulsive guidance cues expressed at specific points along the trajectory of their growth, which impart the directional information required for accurate pathfinding. While much is known about guidance molecules and their receptors, many questions remain unanswered. Which signal transduction pathways are activated within the growth cone after encountering a guidance cue? How is this related to rearrangement of the growth cone cytoskeleton? Do different cues use different signal transduction pathways? This chapter will review some of the work that has addressed these fundamental questions, with a specific focus on the role of the cyclic nucleotides, cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP), in axon guidance.
General Introduction
Throughout embryonic development, elongating axons are in constant communication with the extracellular environment, which provides signals necessary for axonal growth and survival. The local environment also provides specific positional information, facilitating directional pathfinding. With respect to this, significant advances have been made towards understanding how locally expressed molecules can act as axon guidance cues, mediating attraction or repulsion. This has culminated in the discovery of many families of axon guidance molecules whose roles have been conserved to a remarkable extent during evolution. These include the netrins, Slits, Semaphorins and ephrins.1 The tip of the developing axon, known as the growth cone, is pivotal in the process of recognising guidance cues expressed in the environmental milieu and integrating this information into a coordinated response.
To do this, the growth cone must be able to control its cytoskeletal assembly and disassembly, membrane dynamics and adhesion to the extracellular matrix. A number of signal transduction pathways have been shown to underlie this, including the mitogen activated protein kinases (MAPK) and the Rho GTPase family.1,2 Another signal transduction system involved in regulating growth cone cytoskeletal dynamics in response to axon guidance cues centres on the cyclic nucleotides, cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP). The first indication that cyclic nucleotides may be involved in axon guidance came from studies of embryonic chick dorsal root ganglion (DRG) neurites exposed to gradients of cGMP or dB cAMP (a lipid soluble cAMP analogue) in vitro.3 These neurites turned towards the source of these molecules, suggesting that local asymmetries in cAMP and cGMP within the growth cone may control axonal turning. Subsequent studies have demonstrated that cyclic nucleotides play an important role in guidance in response to many common cues. This review will provide a brief overview of the structure of cyclic nucleotides and their mechanism of signal transduction, before focussing on the current understanding of the role played by cyclic nucleotides in response to guidance cues and the functional implications for this in nervous system development.
Molecular Structure of Cyclic Nucleotides
cAMP
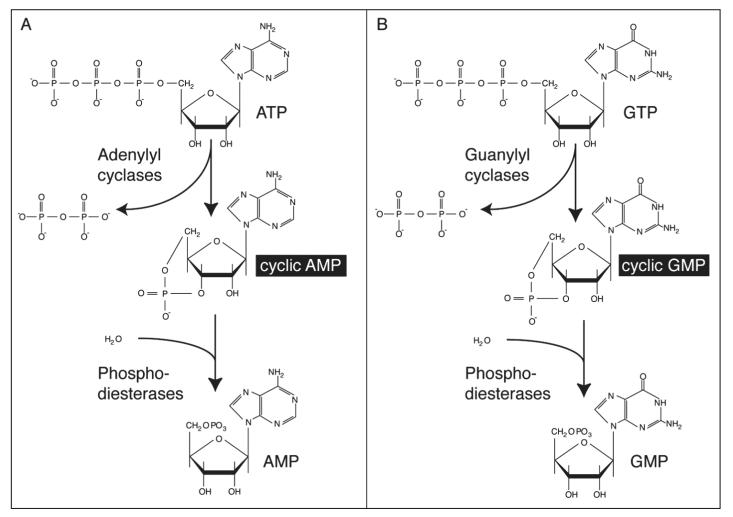
cAMP is a small cytoplasmic molecule whose function as an intracellular messenger has been conserved during evolution. Indeed, cAMP is found ubiquitously in both prokaryotes and eukaryotes. Membrane-bound enzymes called adenylyl cyclases catalyse the formation of cAMP from ATP, whilst cAMP phosphodiesterases prevent the accumulation of cAMP by converting it to AMP (Fig. 1A).
Figure 1.
Molecular structure of cyclic nucleotides. A) Cyclic AMP (cAMP) is generated from ATP through the action of the enzyme adenylyl cyclase and is degraded by cAMP phosphodiesterases, which catalyse its conversion to AMP. B) Similarly, guanylyl cyclases convert GTP to cyclic GMP (cGMP), and cGMP phosphodiesterases convert this to GMP.
cGMP
cGMP is another common intracellular messenger, whose cycle of synthesis and degradation is similar to that of cAMP. Guanylyl cyclases, which may be soluble or membrane-bound, convert GTP to cGMP, and cGMP phosphodiesterases catalyse the conversion of cGMP to GMP (Fig. 1B).
Mechanisms of Signal Transduction
cAMP Pathway
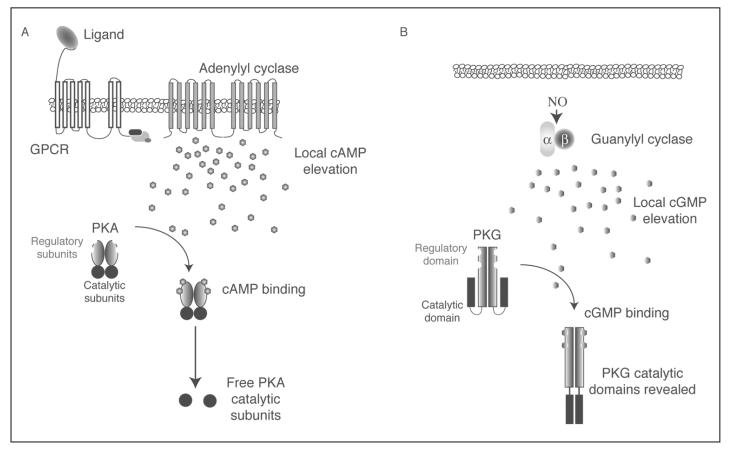
cAMP plays a central role in the mediation of many cellular events, and much of our understanding of how cAMP signalling occurs has been generated in a nonneuronal context. Binding of an extracellular ligand to a G protein-coupled receptor (GPCR) leads to disassembly of the heterotrimeric G protein complex from the receptor, allowing the stimulatory Gsα subunit to activate adenylyl cyclase. This leads to a local elevation of intracellular cAMP (Fig. 2A).4 The primary physiological target of cAMP is cAMP-dependent protein kinase (PKA). This tetrameric complex consists of two catalytic and two regulatory subunits. Binding of two cAMP molecules to each regulatory domain abolishes the inhibition of the catalytic subunits, allowing them to phosphorylate downstream targets. These include a very broad range of substrates, as PKA has targets in the cytoskeleton, nucleus, cytoplasm, mitochondria and cell membrane. It should also be noted that cAMP also exhibits some PKA-independent effects, including activating cyclic nucleotide gated ion channels and binding to the cAMP-interacting proteins EPAC1 and EPAC2. These guanine nucleotide exchange factors regulate the small GTP binding protein Rap1, which is involved in regulating cell adhesion.5 The relevance of these nonPKA mediated functions in axon guidance, however, remains undefined.
Figure 2.
Mechanisms of signal transduction. A) Adenylyl cyclases are dimeric transmembrane proteins that interact with G protein-coupled receptors (GPCRs). Binding of ligand to receptor results in the release of the Gαs subunit of the G protein, which activates adenylyl cyclase. The local elevation of cAMP results in increased binding of cAMP to the regulatory subunits of cAMP-dependent protein kinase (PKA), and subsequent release of the active catalytic kinase domains of PKA. PKA can modulate cytoskeletal dymanics via multiple pathways. B) Soluble guanylyl cyclase is activated by nitric oxide (NO), resulting in a local increase in cGMP concentration. cGMP-dependent protein kinase (PKG) is a homodimer, with each subunit possessing an autoinhibitory regulatory domain, 2 cGMP binding sites and a catalytic domain. Binding of cGMP releases autoinhibition and reveals the catalytic domain. PKG is able to modulate cytoskeletal dynamics.
cGMP Pathway
The cGMP signalling cascade is similar to that of cAMP. cGMP is produced by guanylyl cyclases, which may be soluble or membrane-bound. The best-known activators of (soluble) guanylyl cyclases are small gaseous molecules like nitric oxide. The main target of cGMP is cGMP-dependent protein kinase (PKG). This homodimeric protein is comprised of multiple functional domains. In the absence of cGMP, the autoinhibitory domain suppresses the activity of the catalytic domain. Binding of two cGMP molecules to each inhibitory domain causes a conformational shift that reveals the catalytic site (Fig. 2B).
Functional Implications during Nervous System Development
The Role of Cyclic Nucleotides in Chemotropic Responses
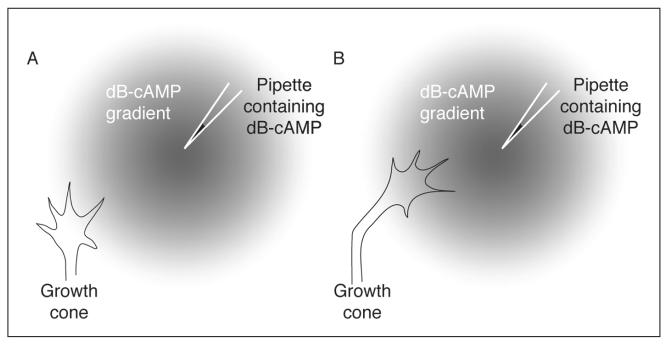
The finding that local asymmetries in cyclic nucleotides within the growth cone may mediate turning decisions3 has been further explored using in vitro turning assays first elaborated in the Poo laboratory.6 To conduct a growth cone turning assay, a defined microscopic gradient of a chemical is produced near a growth cone by repetitive pulsatile application of a concentrated source of the cue from a nearby pipette. In turning assays conducted with embryonic Xenopus spinal neurons, a gradient of dB-cAMP induces turning towards the pipette (Fig. 3).6 Furthermore, local elevation of intracellular cAMP by photolytic release of caged cAMP induces turning of Xenopus spinal neurons towards the side of the growth cone in which cAMP is elevated.7 This illustrates that a cytoplasmic gradient of cAMP across the growth cone is sufficient to initiate a turning response.
Figure 3.
Growth cone turning assay. In the growth cone turning assay elaborated by Poo and colleagues,6 a microscopic gradient of dB-cAMP (a lipid-soluble cAMP analogue) was produced through pulsatile ejection from a pipette. Growth cones from embryonic Xenopus spinal neurons encountering the gradient (A) turned and grew towards the pipette (B).
Poo and colleagues have gone on to translate these findings into a developmental context by demonstrating that cyclic nucleotides are capable of modulating the response of growth cones to guidance cues. For example, embryonic Xenopus spinal neurons are attracted to a gradient of brain-derived neurotrophic factor (BDNF), but inhibiting cAMP production within the growth cone converts this attraction into repulsion.8 Similarly, the attractive response of these neurons to netrin-19 and achetylcholine (ACh)8 can be switched to repulsion by inhibiting PKA, while the repulsive response from myelin associated glycoprotein (MAG)10 can be reversed by raising the intracellular levels of cAMP. On the other hand, attraction of Xenopus spinal neurons to neurotrophin-3 (NT-3) and repulsion from Sema III requires cGMP signalling. Thus, increasing levels of growth cone cGMP converts repulsive turning induced by Sema III into attraction, whereas lowering cGMP levels converts NT-3 induced attraction into repulsion.10 The repulsion of embryonic rat DRG axons from Slit2 also appears to function via a cGMP-dependent pathway.11
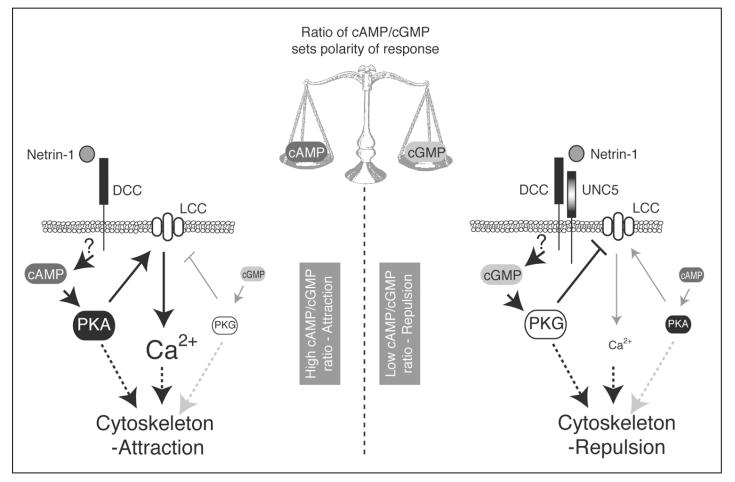
These observations led to the idea that guidance cues can be classified into two groups, in which levels of either cAMP (netrin-1, BDNF, ACh, MAG) or cGMP (Sema III, NT-3, Slit2) are critical determinants for the polarity of the turning response.2 However, recent results have caused this concept to be revised with respect to the guidance cue netrin-1. By modulating the ratio of cAMP to cGMP in embryonic Xenopus spinal axons exposed to a gradient of netrin-1, Nishiyama et al12 have demonstrated that it is the ratio between these cyclic nucleotides that is the key in determining the polarity of the turning response. A high cAMP/cGMP ratio favours growth cone attraction, while a low ratio favours repulsion. This was shown by comparing axons expressing the DCC receptor (which mediates attraction to netrin-1) to those axons expressing DCC as well as over-expressing UNC5 (the DCC-UNC5 complex mediates repulsion to netrin-1). In those axons expressing DCC, inhibiting cAMP or elevating cGMP, thus lowering the cAMP/cGMP ratio, converted attraction to repulsion. Conversely, in axons over-expressing UNC-5, repulsion was converted to attraction by raising the cAMP/cGMP ratio. Furthermore, when varying ratios of the membrane-permeable analogues Sp-8-Br-cAMPS and 8-Br-cGMP (Table 1) were bath-applied to the cultures, high proportions of the cAMP analogue promoted attraction to netrin-1 gradients, while high proportions of the cGMP analogue favoured repulsion.12
Table 1. Activators and inhibitors of cyclic nucleotide signalling pathways.
| Compound | Mode of Action | References | ||
|---|---|---|---|---|
| cAMP | Activators | dB-cAMP | cAMP analogue that activates PKA | 3,6,30,31,33,34 |
| 8-Br-cAMP | cAMP analogue that activates PKA | 32 | ||
| Sp-cAMP cAMP | analogue that specifically activates PKA |
8,10-12,22,23, 26-29,31 |
||
| forskolin | Activator of adenylyl cyclases | 6-8,13,28,32,33 | ||
| Inhibitors | Rp-cAMP | cAMP analogue that specifically inhibits PKA |
7-10,22,23,26,31 | |
| KT5720 | Specific inhibitor of PKA |
7-9,12,13,23,27, 30,34 |
||
| H89 | Specific inhibitor of PKA | 31,34 | ||
| PKI | Myristoylated inhibitor protein of PKA | 22 | ||
| IBMX | Non-specific inhibitor of cAMP and cGMP phosphodiesterases |
6 | ||
| cGMP | Activators | dB-cGMP | cGMP analogue that activates PKG | 6,31 |
| 8-Br-cGMP | cGMP analogue that activates PKG | 10,12,22 | ||
| Sp-cGMP | cGMP analogue that specifically activates PKG |
10,23,26 | ||
| PP-9 | Activator of soluble guanylyl cyclases | 10 | ||
| SNAP | Nitric oxide donor, activates soluble guanylyl cyclases |
11 | ||
| Inhibitors | Rp-cGMP | cGMP analogue that specifically inhibits PKG |
10,22,23,26 | |
| KT5823 | Specific inhibitor of PKG | 11,12,31,34 | ||
| LY83583 | Blocks cGMP production, inhibits extracellular Ca2+, blocks effects of nitric oxide |
6 |
Listed are several activators and inhibitors of components of cAMP- and cGMP-dependent signalling pathways. The references indicate the studies in which these compounds have been used to address the role of cyclic nucleotide signalling in axons.
Thus, the ratio of the cyclic nucleotides cAMP and cGMP within the growth cone is thought to underlie turning responses to netrin-1 in embryonic Xenopus spinal neurons (Fig. 4). However, caution must be used when generalising from these data, as it is clear that the role of cyclic nucleotides during guidance varies greatly depending on the types of signal, axonal population and age of neuron involved. For instance, in cultured embryonic rat spinal commissural neurons, intracellular cAMP levels do not increase after stimulation of the growth cone with netrin-1.13
Figure 4.
Model for netrin-1-induced turning of embryonic Xenopus spinal neurites. In response to netrin-1, embryonic Xenopus spinal neurites exhibit either attraction or repulsion. The ratio of cAMP to cGMP within the growth cone is postulated to determine which outcome is seen. For attraction, binding of netrin-1 to DCC elicits a rise in intracellular cAMP. PKA is subsequently activated and may stimulate an increase in calcium (Ca2+) by activating L-type Ca2+ channels (LCC) in the membrane. For repulsion, binding of netrin-1 to the DCC-UNC5 complex results in a rise in cGMP levels and PKG activity. PKG may inactivate LCC, lowering growth cone Ca2+. How differential patterns of intracellular calcium induced by cAMP/cGMP induce attractive or repulsive turning responses remains unclear. Adapted from reference 12.
Cyclic Nucleotide Signalling Pathways in Axons
An obvious conclusion to draw from these data is that many axon guidance cues elicit changes in growth cone behaviour by activating cyclic nucleotide signal transduction pathways. Although much remains unknown, the components of these pathways are gradually being identified, and it is apparent that growth cones use the classical PKA/PKG signalling pathways to activate and transduce signals via cyclic nucleotides. For example, mice deficient in adenylyl cyclase I activity display patterning defects in the somatosensory cortex of the brain,14 and studies in Drosophila have shown that both membrane-bound receptor guanylyl cyclases (motor axons)15 and soluble guanylyl cyclases (retinal axons)16 have the potential to mediate axon pathfinding. Activation of soluble guanylyl cyclases via the lipid 12-HPETE, a metabolite in the arachidonic acid pathway, has also been implicated in the response of Xenopus spinal axons to netrin-1 in vitro.12
The targets of cAMP and cGMP, PKA and PKG respectively, are also intimately involved in the control of axon guidance. Activators and inhibitors of PKA and PKG (Table 1) often mimic the responses seen with cAMP and cGMP analogues indicating that the cyclic nucleotides exert their effects on growth cone turning via these enzymes. One potential mechanism to link guidance cues to PKA activation is by the direct recruitment of PKA to the guidance receptor via scaffolding proteins like PKA anchoring proteins (AKAPs). In Drosophila motor axons, the protein Nervy has been suggested to act as an AKAP, coupling cAMP-PKA signalling to the plexin-A receptor to regulate Sema-1a mediated axonal repulsion.17 However, a recent report casts doubt on this suggestion, indicating that the axon guidance defects seen in Nervy mutants may arise from changes in gene expression rather than cytoplasmic control of PKA anchoring.18
Perhaps the most important role of PKA in the context of axon guidance is its capacity to regulate components of the cytoskeleton, including actin filaments, intermediate filaments and microtubules.5,19 For example, the Ena/VASP family of proteins, which act as enhancers of actin filament formation, are substrates for PKA.20,21 The Rho family of small GTPases, including Rho, Rac and Cdc42, control many aspects of actin function, and are also regulated by PKA, both directly (Rho) and indirectly (Rac, Cdc42).21 For instance cAMP-mediated regulation of Rho activity has been implicated in the response of embryonic chick DRG axons to the chemokine SDF1,22 and of Xenopus spinal axons to the peptide PACAP.7 Furthermore, the regenerative capacity of injured rat DRG axons can be enhanced through elevation of cAMP (see below), possibly via modulation of RhoA and Rac1 activity.23 A third potential cytoskeletal target for PKA is myosin, an actin-associated motor protein, via the action of myosin light chain kinase (MLCK). SDF1 has been suggested to alter MLCK activity in a cAMP-dependent fashion.22 Thus, by activating PKA, cAMP may be able to regulate actin dynamics in a variety of ways, and so influence axon pathfinding during development.
The role of PKG in mediating aspects of neuronal development has received far less attention than PKA, and as such, how it acts to mediate cytoskeletal dynamics during axon guidance remains unclear. However, the target motif for PKG is similar to that of PKA, and the two are thought to have overlapping substrate specificities. Thus, PKG also has the potential to modulate cytoskeletal behaviour in an analogous fashion. Members of the Ena/VASP family, for instance, have been implicated as substrates of PKG in vitro.24,25
Cyclic nucleotide signalling is also unequivocally linked to Ca2+ signalling, which itself plays a central role in growth cone dynamics. Indeed, local asymmetries in Ca2+ across the growth cone have been shown to elicit turning responses in cultured axons similar to those induced by cyclic nucleotides. For instance, a localised Ca2+ signal in the growth cone generated by photolytic release of caged Ca2+ or induction of Ca2+ release from internal stores is sufficient to induce growth cone turning in embryonic Xenopus spinal neurons,26,27 and preventing cytoplasmic Ca2+ elevation abolishes netrin-1 induced attractive and repulsive responses.27 The Ca2+ and cyclic nucleotide signalling cascades have the potential to interact and cross regulate each other at a number of different levels. In some instances, cyclic nucleotides act upstream of Ca2+, directly regulating the level of Ca2+ within the growth cone. An example of this is seen in Xenopus spinal neurons, where the cAMP/cGMP ratio can directly affect the activity of L-type Ca2+ channels (LCC) to alter intracellular Ca2+ signals induced by netrin-1. When signalling via DCC, netrin-1 stimulates a high cAMP/cGMP ratio and thus elicits PKA activation. PKA activates LCC in the plasma membrane and Ca2+ channels in the endoplasmic reticulum, eventually leading to a steep Ca2+ gradient across the growth cone, favouring attraction. When signalling via DCC-UNC5, netrin-1 stimulates the cGMP pathway, which, through PKG, closes LCCs and inhibits Ca2+ release from internal stores, therefore creating a gradient of Ca2+ across the growth cone which is lowest on the side facing the guidance cue, resulting in repulsion.12 In this model cyclic nucleotide signalling directly regulates Ca2+ signalling to determine bi-directional turning responses (Fig. 4).
Recent studies have shown that Ca2+ gradients regulate another switch-like mechanism by activating Ca2+-calmodulin-dependent protein kinase II (CaMKII) and calcineurin (CaN)-phosphatase (PP1) to mediate attraction and repulsion respectively.26 However, in this study, the cAMP pathway was shown to act downstream of Ca2+ signalling by negatively regulating CaN-PP1, emphasising the complexity and potential for multiple interactions between these two signal transduction cascades. Moreover, it should be noted that some axon guidance cues also affect cyclic nucleotide levels without affecting the Ca2+ pathway. For example, in Xenopus spinal neurons Sema3A has no effect on calcium currents and PACAP induces attractive turning independently of Ca2+, although both of these cues require cyclic nucleotide signalling.7,12
Thus, these studies have started to elucidate the role of cyclic nucleotides in the control of growth cone cytoskeletal dynamics in response to guidance cues. While it is apparent that cue-induced activation of cAMP or cGMP can elicit cytoskeletal changes that manifest as attractive or repulsive turning in vitro, a great deal remains to be discovered. The ways in which adenylyl and guanylyl cyclases are activated is, in many cases, completely unknown, and, in vivo, the relevant cytoskeletal targets for PKA and PKG within the growth cone remain poorly defined. Furthermore, although many studies have focussed on the effect of netrin-1 on embryonic Xenopus spinal neurons, it is clear that not all cues act in the same fashion, nor even that all developing axonal populations respond to this cue in the same way. More work is needed to clarify the molecular components of the cyclic nucleotide signalling pathways in growth cones and to discover how they are activated and regulated to coordinate axon pathfinding during axonogenesis.
Modulation of Guidance Cue Activity via Cyclic Nucleotides
The capacity to control cue-induced turning by manipulating the levels of cyclic nucleotides within the growth cone also implies that the polarity of turning in response to guidance signals may be modulated by other extrinsic or intrinsic factors that alter cAMP or cGMP levels. This may underlie how some axons change responsiveness to the same guidance cue over time. For example, a switch in responsiveness from attraction to repulsion has been documented for embryonic Xenopus retinal axons exposed to netrin-1 in vitro. When cultured on a fibronectin substrate, these neurites turn towards netrin-1, but when cultured on high levels of laminin-1, repulsive turning is seen. Experimental evidence suggests this is due to laminin-1 decreasing cAMP levels within the growth cone.28 This may be relevant in vivo, as, within the retina, these axons are attracted to the netrin-1 expressing optic nerve head (ONH). At the ONH, laminin-1 is expressed at the retinal surface, suggesting that a laminin-1-mediated reduction in cAMP levels at this point may cause repulsion from the area of netrin-1/laminin-1 coexpression, so driving axons from the retinal surface into the optic nerve.28 Intrinsic factors might also contribute to this process as the change in netrin-1 responsiveness of old (repulsive) versus young (attractive) neurons, concomitant with a decline in cAMP levels, is also seen in pathway-naïve neurons. Cyclic AMP elevators and adenosine A2b receptor agonists can rejuvenate the behaviour of the old growth cones, causing them to regain attraction to netrin-1, whereas antagonists cause young growth cones to be repelled. Thus, age-related intrinsic changes might also modulate cAMP levels to control the developmental switch in netrin-1 responsiveness.29 A second example of the modulation of cyclic nucleotide levels by extrinsic factors affecting responses to axon guidance cues is seen in developing chick axons. In vitro, repulsion of these axons induced by Slit2, Sema3A or Sema3C can be reduced by application of SDF1, which through its receptor, CXCR4 (a GPCR), elicits an increase in cAMP in these neurons.22
Cyclic Nucleotides and Axon Regeneration
The evidence supporting a role for cyclic nucleotides in axon guidance has mainly come from in vitro experiments, such as the growth cone turning assay. Whether cyclic nucleotides are relevant for axonal guidance responses in vivo, where multiple signals are likely to impinge simultaneously on a growth cone, remains unclear. However, recent data have demonstrated an important role for cAMP in the regulation of axonal growth cone responses in response to axonal lesioning in vivo, implicating cyclic nucleotides as potential targets to enhance spinal cord regeneration.
In the adult mammalian peripheral nervous system, injured axons are able to regenerate, whereas those of the central nervous system (CNS) are not. The damaged myelin surrounding the site of injury accounts for at least some of the failure of CNS axons to regenerate by producing inhibitory molecules like myelin-associated glycoprotein (MAG) and Nogo. In vitro studies have shown that elevating cAMP levels converts MAG induced repulsion into attraction in Xenopus spinal neurons,10 and enhances the ability of neonatal mammalian axons to grow on substrates of MAG and myelin.30,31 Together with the growth promoting effect of cAMP on cultured embryonic axons,32 these observations make cAMP an attractive candidate to potentially overcome inhibitory regenerative responses in vivo. This has been addressed by two recent studies investigating the response of rat DRG CNS axons after spinal cord injury. After lesioning of the dorsal column, the centrally projecting axons from the DRG normally fail to regenerate into the lesion. However, injection of the lipid soluble analogue db-cAMP into rat DRG neurons prior to lesioning results in extensive regeneration into the lesion site.33,34 Removal of DRG neurons treated with db-cAMP in vivo, followed by culturing in vitro, demonstrates that these neurons have an increased intrinsic growth capacity and the ability to overcome the effects of inhibitory factors like MAG.33,34 Although these studies are somewhat artificial in that db-cAMP was applied prior to injury, they show that regulating cyclic nucleotide levels in vivo may be a potentially useful approach in promoting regeneration after spinal cord injury and stress the importance of understanding cyclic nucleotide signalling in neuronal behaviour.
Conclusion and Perspectives
The importance of cyclic nucleotides in mediating responses of axonal growth cones to guidance cues during development is now well established. It is clear that guidance cues can activate the cAMP and/or the cGMP signalling cascades, potentiating changes in the growth cone cytoskeleton and leading to attractive or repulsive responses. Furthermore, cAMP and cGMP are themselves able to modulate responses of growth cones to environmental signals, so directly controlling the polarity of growth. In this way extrinsic and intrinsic factors may be able to temporally regulate axonal responsiveness. However, we only possess a very general hypothesis of the role of these molecules during development of neuronal axons, as the use of cyclic nucleotide signalling seems to differ widely among axonal populations. It may be that intracellular signal transduction pathways in general depend on developmental context, age and the neuronal type involved. More work is required to clarify the molecular components of the cAMP and cGMP pathways and the targets of PKA and PKG that elicit cytoskeletal changes. From a clinical perspective, these developmental studies have the potential to be of great benefit in understanding why, after axonal injury, neurons of the CNS are unable to regenerate in vivo.
References
- 1.Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298(5600):1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- 2.Song H, Poo M. The cell biology of neuronal navigation. Nat Cell Biol. 2001;3(3):E81–88. doi: 10.1038/35060164. [DOI] [PubMed] [Google Scholar]
- 3.Gundersen RW, Barrett JN. Characterization of the turning response of dorsal root neurites toward nerve growth factor. J Cell Biol. 1980;87(3 Pt 1):546–554. doi: 10.1083/jcb.87.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linder JU, Schultz JE. The class III adenylyl cyclases: Multi-purpose signalling modules. Cell Signal. 2003;15(12):1081–1089. doi: 10.1016/s0898-6568(03)00130-x. [DOI] [PubMed] [Google Scholar]
- 5.Howe AK. Regulation of actin-based cell migration by cAMP/PKA. Biochim Biophys Acta. 2004;1692(2-3):159–174. doi: 10.1016/j.bbamcr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Lohof AM, Quillan M, Dan Y, et al. Asymmetric modulation of cytosolic cAMP activity induces growth cone turning. J Neurosci. 1992;12(4):1253–1261. doi: 10.1523/JNEUROSCI.12-04-01253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guirland C, Buck KB, Gibney JA, et al. Direct cAMP signaling through G-protein-coupled receptors mediates growth cone attraction induced by pituitary adenylate cyclase-activating polypeptide. J Neurosci. 2003;23(6):2274–2283. doi: 10.1523/JNEUROSCI.23-06-02274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song HJ, Ming GL, Poo MM. cAMP-induced switching in turning direction of nerve growth cones. Nature. 1997;388(6639):275–279. doi: 10.1038/40864. [DOI] [PubMed] [Google Scholar]
- 9.Ming GL, Song HJ, Berninger B, et al. cAMP-dependent growth cone guidance by netrin-1. Neuron. 1997;19(6):1225–1235. doi: 10.1016/s0896-6273(00)80414-6. [DOI] [PubMed] [Google Scholar]
- 10.Song H, Ming G, He Z, et al. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281(5382):1515–1518. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen-Ba-Charvet KT, Brose K, Marillat V, et al. Sensory axon response to substrate-bound Slit2 is modulated by laminin and cyclic GMP. Mol Cell Neurosci. 2001;17(6):1048–1058. doi: 10.1006/mcne.2001.0994. [DOI] [PubMed] [Google Scholar]
- 12.Nishiyama M, Hoshino A, Tsai L, et al. Cyclic AMP/GMP-dependent modulation of Ca2+ channels sets the polarity of nerve growth-cone turning. Nature. 2003;423(6943):990–995. doi: 10.1038/nature01751. [DOI] [PubMed] [Google Scholar]
- 13.Bouchard JF, Moore SW, Tritsch NX, et al. Protein kinase A activation promotes plasma membrane insertion of DCC from an intracellular pool: A novel mechanism regulating commissural axon extension. J Neurosci. 2004;24(12):3040–3050. doi: 10.1523/JNEUROSCI.4934-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdel-Majid RM, Leong WL, Schalkwyk LC, et al. Loss of adenylyl cyclase I activity disrupts patterning of mouse somatosensory cortex. Nat Genet. 1998;19(3):289–291. doi: 10.1038/980. [DOI] [PubMed] [Google Scholar]
- 15.Ayoob JC, Yu HH, Terman JR, et al. The Drosophila receptor guanylyl cyclase Gyc76C is required for semaphorin-1a-plexin A-mediated axonal repulsion. J Neurosci. 2004;24(30):6639–6649. doi: 10.1523/JNEUROSCI.1104-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibbs SM, Becker A, Hardy RW, et al. Soluble guanylate cyclase is required during development for visual system function in Drosophila. J Neurosci. 2001;21(19):7705–7714. doi: 10.1523/JNEUROSCI.21-19-07705.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terman JR, Kolodkin AL. Nervy links protein kinase a to plexin-mediated semaphorin repulsion. Science. 2004;303(5661):1204–1207. doi: 10.1126/science.1092121. [DOI] [PubMed] [Google Scholar]
- 18.Ice RJ, Wildonger J, Mann RS, et al. Comment on “Nervy links protein kinase A to plexin-mediated semaphorin repulsion”. Science. 2005;309(5734):558. doi: 10.1126/science.1109259. (author reply 558) [DOI] [PubMed] [Google Scholar]
- 19.Tasken K, Aandahl EM. Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol Rev. 2004;84(1):137–167. doi: 10.1152/physrev.00021.2003. [DOI] [PubMed] [Google Scholar]
- 20.Lebrand C, Dent EW, Strasser GA, et al. Critical role of Ena/VASP proteins for filopodia formation in neurons and in function downstream of netrin-1. Neuron. 2004;42(1):37–49. doi: 10.1016/s0896-6273(04)00108-4. [DOI] [PubMed] [Google Scholar]
- 21.Krause M, Dent EW, Bear JE, et al. Ena/VASP proteins: Regulators of the actin cytoskeleton and cell migration. Annu Rev Cell Dev Biol. 2003;19:541–564. doi: 10.1146/annurev.cellbio.19.050103.103356. [DOI] [PubMed] [Google Scholar]
- 22.Chalasani SH, Sabelko KA, Sunshine MJ, et al. A chemokine, SDF-1, reduces the effectiveness of multiple axonal repellents and is required for normal axon pathfinding. J Neurosci. 2003;23(4):1360–1371. doi: 10.1523/JNEUROSCI.23-04-01360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bandtlow CE. Regeneration in the central nervous system. Exp Gerontol. 2003;38(1-2):79–86. doi: 10.1016/s0531-5565(02)00165-1. [DOI] [PubMed] [Google Scholar]
- 24.Gertler FB, Niebuhr K, Reinhard M, et al. Mena, a relative of VASP and Drosophila Enabled, is implicated in the control of microfilament dynamics. Cell. 1996;87(2):227–239. doi: 10.1016/s0092-8674(00)81341-0. [DOI] [PubMed] [Google Scholar]
- 25.Waldmann R, Nieberding M, Walter U. Vasodilator-stimulated protein phosphorylation in platelets is mediated by cAMP- and cGMP-dependent protein kinases. Eur J Biochem. 1987;167(3):441–448. doi: 10.1111/j.1432-1033.1987.tb13357.x. [DOI] [PubMed] [Google Scholar]
- 26.Wen Z, Guirland C, Ming GL, et al. A CaMKII/calcineurin switch controls the direction of Ca(2+)-dependent growth cone guidance. Neuron. 2004;43(6):835–846. doi: 10.1016/j.neuron.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 27.Hong K, Nishiyama M, Henley J, et al. Calcium signalling in the guidance of nerve growth by netrin-1. Nature. 2000;403(6765):93–98. doi: 10.1038/47507. [DOI] [PubMed] [Google Scholar]
- 28.Hopker VH, Shewan D, Tessier-Lavigne M, et al. Growth-cone attraction to netrin-1 is converted to repulsion by laminin-1. Nature. 1999;401(6748):69–73. doi: 10.1038/43441. [DOI] [PubMed] [Google Scholar]
- 29.Shewan D, Dwivedy A, Anderson R, et al. Age-related changes underlie switch in netrin-1 responsiveness as growth cones advance along visual pathway. Nat Neurosci. 2002;5(10):955–962. doi: 10.1038/nn919. [DOI] [PubMed] [Google Scholar]
- 30.Cai D, Shen Y, De Bellard M, et al. Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a cAMP-dependent mechanism. Neuron. 1999;22(1):89–101. doi: 10.1016/s0896-6273(00)80681-9. [DOI] [PubMed] [Google Scholar]
- 31.Cai D, Qiu J, Cao Z, et al. Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J Neurosci. 2001;21(13):4731–4739. doi: 10.1523/JNEUROSCI.21-13-04731.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng JQ, Zheng Z, Poo M. Long-range signaling in growing neurons after local elevation of cyclic AMP-dependent activity. J Cell Biol. 1994;127(6 Pt 1):1693–1701. doi: 10.1083/jcb.127.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neumann S, Bradke F, Tessier-Lavigne M, et al. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron. 2002;34(6):885–893. doi: 10.1016/s0896-6273(02)00702-x. [DOI] [PubMed] [Google Scholar]
- 34.Qiu J, Cai D, Dai H, et al. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron. 2002;34(6):895–903. doi: 10.1016/s0896-6273(02)00730-4. [DOI] [PubMed] [Google Scholar]