Abstract
BACKGROUND
Preexposure prophylaxis with antiretroviral drugs has been effective in the prevention of human immunodeficiency virus (HIV) infection in some trials but not in others.
METHODS
In this randomized, double-blind, placebo-controlled trial, we assigned 2120 HIV-negative women in Kenya, South Africa, and Tanzania to receive either a combination of tenofovir disoproxil fumarate and emtricitabine (TDF–FTC) or placebo once daily. The primary objective was to assess the effectiveness of TDF–FTC in preventing HIV acquisition and to evaluate safety.
RESULTS
HIV infections occurred in 33 women in the TDF–FTC group (incidence rate, 4.7 per 100 person-years) and in 35 in the placebo group (incidence rate, 5.0 per 100 person-years), for an estimated hazard ratio in the TDF-FTC group of 0.94 (95% confidence interval, 0.59 to 1.52; P = 0.81). The proportions of women with nausea, vomiting, or elevated alanine aminotransferase levels were significantly higher in the TDF–FTC group (P = 0.04, P<0.001, and P = 0.03, respectively). Rates of drug discontinuation because of hepatic or renal abnormalities were higher in the TDF–FTC group (4.7%) than in the placebo group (3.0%, P = 0.051). Less than 40% of the HIV-uninfected women in the TDF–FTC group had evidence of recent pill use at visits that were matched to the HIV-infection window for women with seroconversion. The study was stopped early, on April 18, 2011, because of lack of efficacy.
CONCLUSIONS
Prophylaxis with TDF–FTC did not significantly reduce the rate of HIV infection and was associated with increased rates of side effects, as compared with placebo. Despite substantial counseling efforts, drug adherence appeared to be low. (Supported by the U.S. Agency for International Development and others; FEM-PrEP ClinicalTrials.gov number, NCT00625404.)
Recent studies have shown that daily oral preexposure prophylaxis with 300 mg of tenofovir disoproxil fumarate (TDF), an oral prodrug of tenofovir, alone or in combination with 200 mg of emtricitabine (FTC) (TDF–FTC [Truvada], Gilead Sciences) can reduce the risk of sexually acquired human immunodeficiency virus (HIV) infection in men and women.1–3 Consequently, an advisory committee of the Food and Drug Administration recently recommended that the label indications for Truvada be changed to include HIV prevention.4
We report the primary results of the Preexposure Prophylaxis Trial for HIV Prevention among African Women (FEM-PrEP), a randomized, double-blind, placebo-controlled trial of once-daily oral TDF–FTC among high-risk women in Africa. The study was stopped early, on April 18, 2011, because of a lack of efficacy.
METHODS
PARTICIPANTS
From June 11, 2009, to April 15, 2011, we recruited women at four study centers in Kenya, South Africa, and Tanzania. To be eligible, women in good health who were between the ages of 18 and 35 years had to test negative for HIV antibody, be at increased risk for HIV infection, not be pregnant or breast-feeding, be willing to use an effective nonbarrier contraceptive method, show an ability to swallow a vitamin tablet similar in size to that of a study tablet, and pass an informed-consent quiz. Women were considered to be at increased risk for HIV infection if they had had one or more vaginal sex acts in the previous 2 weeks or more than one sex partner in the previous month. Women were excluded if they tested positive for the hepatitis B virus surface antigen or had evidence of abnormal hepatic or renal function. (Details are provided in the study protocol, available with the full text of this article at NEJM.org.) At most sites, the Priorities for Local AIDS Control Efforts (PLACE) method was adapted to identify geographic areas of high risk and prioritize these regions for recruitment efforts.5 All women provided written informed consent.
Extensive sociobehavioral research and community activities were part of the trial and informed several trial procedures, including recruitment, retention, informed consent, and adherence counseling. The trial was approved by all applicable ethics and regulatory committees.
VISIT PROCEDURES
Participants attended clinic visits at the time of screening and enrollment and at 4-week intervals thereafter for up to 60 weeks (52 weeks of taking the study drug, followed by 8 weeks off the study drug). At each visit, participants received a month’s supply of the assigned study drug; underwent rapid HIV antibody testing, pregnancy testing, and assessment for adverse events; and received counseling (with respect to risk reduction, study-drug adherence, and contraceptive use), free condoms, and free (or referral for) effective nonbarrier contraception. In addition, self-reported adherence was assessed, returned pills were counted, and blood samples were obtained and stored. At predefined visits, testing of hepatic and renal function was performed, and a questionnaire on sexual behavior was administered. The administration of the study drug was temporarily or permanently discontinued in case of pregnancy, sero-conversion to HIV positivity, or protocol-defined biochemical abnormalities.
OBJECTIVES AND END POINTS
The primary objective was to assess the effectiveness and safety of TDF–FTC in preventing HIV acquisition. The secondary objectives reported here include assessing the effect of TDF–FTC on the CD4+ T-cell count and plasma HIV RNA level (viral load), the rate of HIV infection with resistance to TDF–FTC, changes in risk behavior, adherence to the study regimen, and effects on pregnancy outcomes.
The primary effectiveness end point was incident infection with HIV type 1 (HIV-1) or type 2 (HIV-2), as determined by the presence of antibodies on two consecutive rapid tests conducted on plasma. Primary safety end points included grade 3 or higher elevations in the alanine aminotransferase or aspartate aminotransferase level, grade 3 or higher reductions in phosphorus, and grade 2 or higher elevations in the creatinine level; assessments were based on the grading table of the Division of AIDS and on local normal ranges.6 HIV-1 resistance was determined with the use of genotypic testing (TRUGENE, Siemens) and phenotypic testing (PhenoSense, Monogram Biosciences).
DRUG-LEVEL ANALYSIS
We measured levels of tenofovir and FTC in plasma samples, using protein precipitation and liquid chromatography with tandem mass spectrometry detection with deuterated internal standards. The lower limit of quantification was 0.25 ng per milliliter. Rates of intraassay and interassay accuracy ranged from 96 to 112%, and rates of intraassay and interassay precision ranged from 5 to 13%.
STUDY OVERSIGHT
The trial was supported by a grant from the Agency for International Development and by the Bill and Melinda Gates Foundation for community preparatory work in some sites. The study was designed and the protocol written by the two principal investigators and lead statistician in collaboration with the other investigators and input from an external protocol advisory committee. Gilead Sciences donated the study drugs (TDF–FTC and placebo) and was allowed to review the draft manuscript and provide comments but had no other role in the trial. The data were analyzed by the last author. The first and last authors, along with the data-management group, vouch for the accuracy and completeness of the data and the fidelity of this report to the study protocol.
STATISTICAL ANALYSIS
The study was designed to test the hypothesis that once-daily TDF–FTC is more than 30% effective in preventing HIV acquisition. An enrollment of 3900 women was expected to provide the 72 infections required to achieve a power of 90% on the basis of a one-sided alpha level of 0.025, an assumed cumulative probability of infection at 1 year of 3% in the placebo group, and an assumed relative reduction in risk of 70%.
The primary effectiveness analysis was based on a confidence interval for the hazard ratio for infection in the TDF–FTC group, as compared with the placebo group, that was obtained from a proportional-hazards regression model stratified according to study site. This analysis excluded women who were subsequently determined to have been infected with HIV at enrollment on the basis of polymerase-chain-reaction assay and those who did not undergo HIV follow-up testing. We determined the coverage error of the confidence interval that was reported at each interim analysis by using the Lan–DeMets spending function with O’Brien–Fleming–type boundaries in EAST software (Cytel).7 Since the trial was stopped because of futility, unadjusted two-sided 95% confidence intervals and P values are reported here.
Primary safety analyses, which excluded women for whom no follow-up safety data were available and those who returned all their pills unused, were based on exact log-rank tests of between-group differences in the rates of grade 3 or higher alanine aminotransferase, aspartate aminotransferase, and phosphorus levels and grade 2 or higher creatinine levels.
We also conducted a nested case–control analysis using data on plasma drug levels. In the TDF–FTC group, for every participant with seroconversion to HIV positivity, we matched three participants without HIV infection (controls), according to the study site and the duration of participation in the study.
RESULTS
RECRUITMENT AND FOLLOW-UP
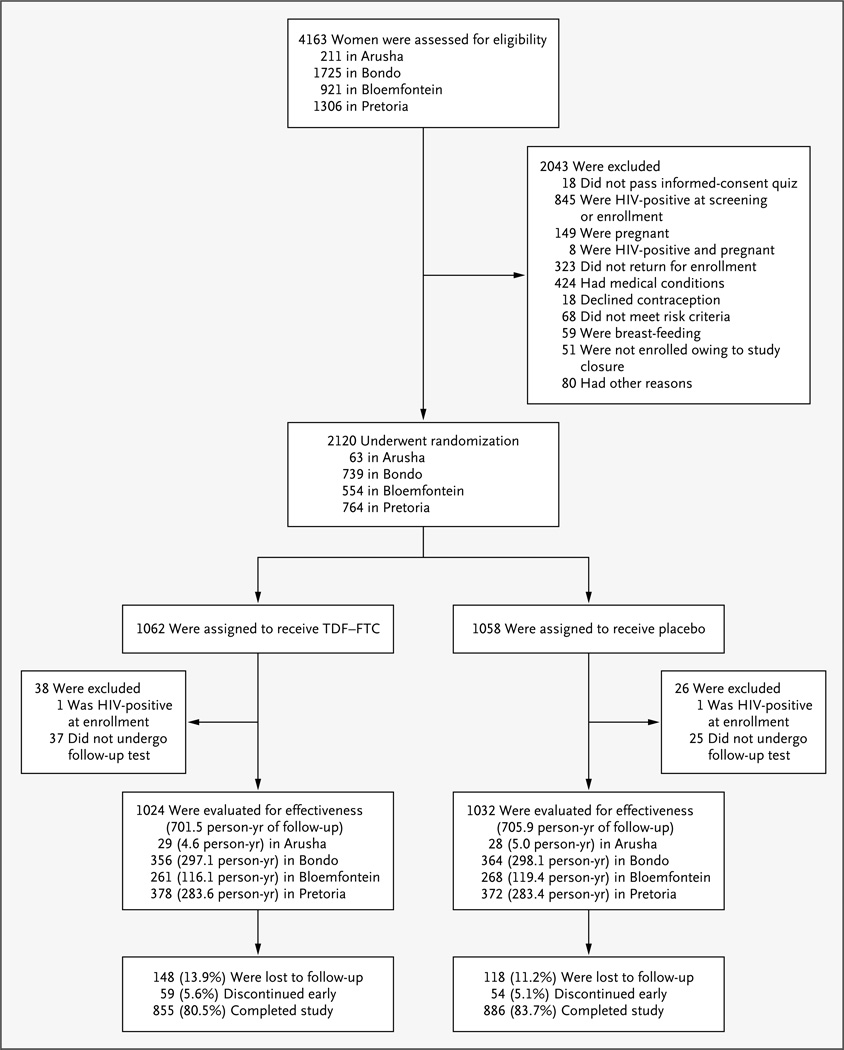
During the nearly 2-year recruitment period, 4163 women were screened and 2120 underwent randomization (Fig. 1). Demographic characteristics and other baseline data were generally similar in the two groups (Table 1). The mean age was 24.2 years. Among the women who were tested at baseline, 5.7% had gonorrhea and 14.0% had chlamydial infection; 41.8% had bacterial vaginosis. Participants reported an average of 3.7 vaginal sex acts, 1.9 sex acts without a condom, and 1.0 sex partners in the 7 days before enrollment; 12.6% reported exchanging sex for money or gifts with a nonprimary partner in the previous 4 weeks. At enrollment, most participants were using an injectable contraceptive (66.1%) or oral contraceptive (30.1%) to meet the study requirement, with more use of oral contraceptives in the TDF–FTC group than in the placebo group (32.0% vs. 28.2%).
Figure 1. Screening, Randomization, and Follow-up of the Study Participants.
The percentages of participants who completed the study and were included in the primary analysis were calculated on the basis of the total numbers of participants in the two randomized study groups. HIV infection at the time of enrollment was further determined by subsequent polymerase-chain-reaction assay.
Table 1.
Baseline Characteristics of the Participants.*
| Characteristic | TDF–FTC (N = 1062) |
Placebo (N = 1058) |
All Participants (N = 2120) |
|---|---|---|---|
| Study site — no. (%) | |||
| Arusha, Tanzania† | 33 (3.1) | 30 (2.8) | 63 (3.0) |
| Bloemfontein, South Africa | 276 (26.0) | 278 (26.3) | 554 (26.1) |
| Bondo, Kenya | 370 (34.8) | 369 (34.9) | 739 (34.9) |
| Pretoria, South Africa | 383 (36.1) | 381 (36.0) | 764 (36.0) |
| Age — yr | |||
| Mean | 24.2 | 24.2 | 24.2 |
| Median (range) | 23 (18–35) | 23 (18–35) | 23 (18–35) |
| Education — yr | |||
| Mean | 10.4 | 10.3 | 10.3 |
| Median (range) | 11 (0–19) | 11 (0–19) | 11 (0–19) |
| Married — no. (%) | 319 (30.0) | 336 (31.8) | 655 (30.9) |
| Ever pregnant — no. (%) | 755 (71.1) | 778 (73.5) | 1533 (72.3) |
| Contraceptive method at screening — no. (%) | |||
| Oral pills | 101 (9.5) | 75 (7.1) | 176 (8.3) |
| Injectable contraceptives | 404 (38.0) | 410 (38.8) | 814 (38.4) |
| Condoms | 170 (16.0) | 175 (16.5) | 345 (16.3) |
| Implant, IUD, or female sterilization | 27 (2.5) | 26 (2.5) | 53 (2.5) |
| Other | 4 (0.4) | 2 (0.2) | 6 (0.3) |
| None | 356 (33.5) | 370 (35.0) | 726 (34.2) |
| Contraceptive method at enrollment — no. (%) | |||
| Oral pills | 340 (32.0) | 298 (28.2) | 638 (30.1) |
| Injectable contraceptives | 675 (63.6) | 726 (68.6) | 1401 (66.1) |
| Implant, IUD, or female sterilization | 47 (4.4) | 34 (3.2) | 81 (3.8) |
| Has primary partner — no. (%) | 1051 (99.0) | 1043 (98.6) | 2094 (98.8) |
| Has other, nonprimary partners — no. (%) | 286 (26.9) | 270 (25.5) | 556 (26.2) |
| Sex for money or gifts with a nonprimary partner in previous 4 wk — no. (%) | 139 (13.1) | 129 (12.2) | 268 (12.6) |
| Partners in past wk — no. | |||
| Mean | 1.0 | 1.0 | 1.0 |
| Median (range) | 1 (0–6) | 1 (0–11) | 1.0 (0–11) |
| Vaginal sex acts in past wk — no. | |||
| Mean | 3.7 | 3.7 | 3.7 |
| Median (range) | 3 (0–28) | 3 (0–23) | 3 (0–28) |
| Sex without a condom in past wk — no. | |||
| Mean | 1.9 | 1.9 | 1.9 |
| Median (range) | 1 (0–25) | 1 (0–23) | 1 (0–25) |
| Anal sex in past 4 wk — no. (%) | 14 (1.3) | 14 (1.3) | 28 (1.3) |
| Diagnosis of sexually transmitted infection — no./total no. (%) | |||
| Gonorrhea | 56/939 (6.0) | 52/948 (5.5) | 108/1887 (5.7) |
| Chlamydial infection | 142/939 (15.1) | 122/948 (12.9) | 264/1887 (14.0) |
| Trichomoniasis | 64/943 (6.8) | 45/950 (4.7) | 109/1893 (5.8) |
| Syphilis | 21/1060 (2.0) | 15/1058 (1.4) | 36/2118 (1.7) |
| Bacterial vaginosis | 402/932 (43.1) | 383/945 (40.5) | 785/1877 (41.8) |
| Positive for hepatitis B surface antibody | 220/1060 (20.8) | 226/1056 (21.4) | 446/2116 (21.1) |
There were no significant differences between the two study groups, except for the contraceptive method used at enrollment (P = 0.04). IUD denotes intrauterine device.
In Arusha, the trial was initiated in November 2010, a few months before the unexpected closure.
Among enrolled participants, 62 (2.9%) had no follow-up HIV testing; it was determined that 2 participants (1 in each study group) had preexisting HIV infection, leaving 2056 women who contributed 1407.4 person-years to the primary effectiveness analysis (Fig. 1). A total of 266 women (12.5%) were lost to follow-up, with a determination that a third of these women had relocated without returning for a final visit. Another 113 women (5.3%) discontinued the study early (most for personal reasons unrelated to the study), leaving 1741 of 2120 women (82.1%) who contracted HIV infection or completed follow-up and were included in the primary end-point analysis.
EFFECTIVENESS
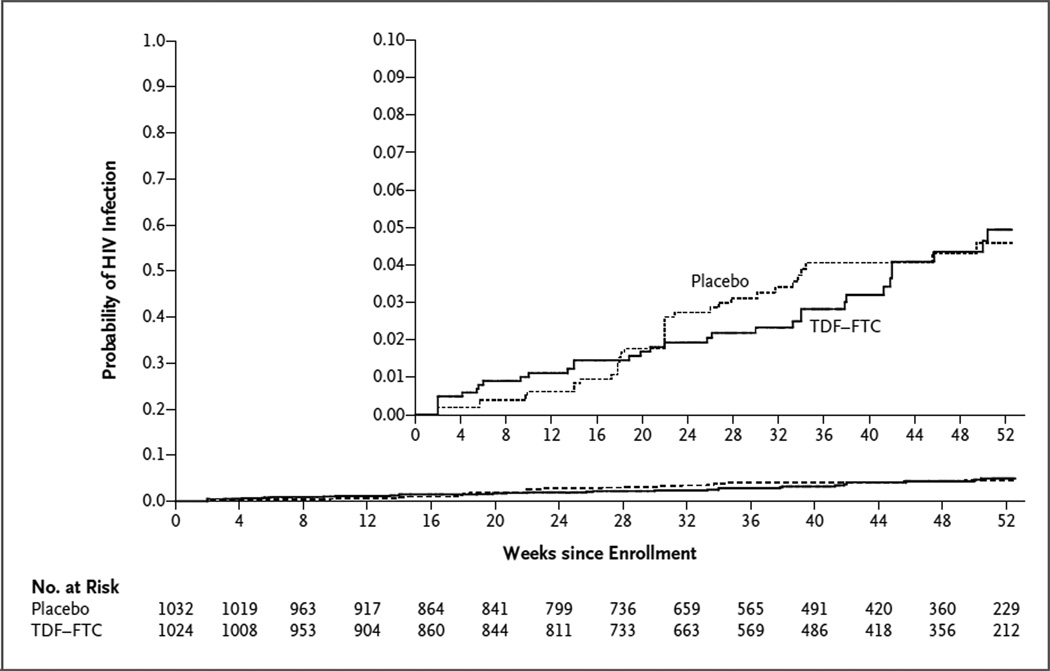
Of the 68 HIV infections that occurred before the visit at 52 weeks (or the first visit after April 18, 2011), 33 occurred in the TDF–FTC group (incidence rate, 4.7 per 100 person-years) and 35 in the placebo group (incidence rate, 5.0 per 100 person-years), leading to an estimated hazard ratio in the TDF–FTC group of 0.94 (95% confidence interval [CI], 0.59 to 1.52; P = 0.81) (Table 2). A prespecified sensitivity analysis in which data were censored when the study drug was no longer available (i.e., owing to an interruption or a missed visit) also showed a between-group difference that was not significant (hazard ratio, 0.82; 95% CI, 0.49 to 1.36; P = 0.44). When all HIV infections occurring after randomization (i.e., through week 8 after the regular study visit) were included in the analysis, there were 34 infections in the TDF–FTC group and 39 in the placebo group (hazard ratio, 0.87; 95% CI, 0.55 to 1.38; P = 0.56). The estimated cumulative probability of infection at 12 months was 0.049 in the TDF–FTC group and 0.046 in the placebo group (Fig. 2).
Table 2.
Effectiveness Results.*
| Variable | TDF-FTC | Placebo | Hazard Ratio (95% Cl)† |
P Value | ||||
|---|---|---|---|---|---|---|---|---|
| No. of Participants |
No. of Events |
Rate | No. of Participants |
No. of Events | Rate | |||
|
no. of events/ 100 person-yr |
no. of events/ 100 person-yr |
|||||||
| Type of analysis | ||||||||
| Primary analysis | 1024 | 33 | 4.7 | 1032 | 35 | 5.0 | 0.94 (0.59–1.52) | 0.81 |
| Data censored when study drug was last available for use ‡ | 1025 | 27 | 4.2 | 1031 | 34 | 5.1 | 0.82 (0.49–1.36) | 0.44 |
| After adjustment for covariates§ | 1020 | 33 | 4.7 | 1028 | 34 | 4.9 | 0.95 (0.59–1.54) | 0.84 |
| All data after randomization¶ | 1024 | 34 | 4.2 | 1032 | 39 | 4.7 | 0.87 (0.55–1.38) | 0.56 |
| Prespecified subgroup | ||||||||
| Study site | ||||||||
| Arusha, Tanzania | 29 | 0 | 0.0 | 28 | 0 | 0.0 | NA | NA |
| Bloemfontein, South Africa | 261 | 3 | 2.6 | 268 | 4 | 3.4 | 0.77 (0.17–3.43) | 0.73 |
| Bondo, Kenya | 356 | 13 | 4.4 | 364 | 14 | 4.7 | 0.93 (0.44–1.97) | 0.85 |
| Pretoria, South Africa | 378 | 17 | 6.0 | 372 | 17 | 6.0 | 1.00(0.51–1.95) | 0.99 |
| Age | ||||||||
| ≥25 yr | 422 | 11 | 3.5 | 421 | 12 | 3.8 | 0.91 (0.40–2.06) | 0.82 |
| <25 yr | 602 | 22 | 5.7 | 611 | 23 | 5.9 | 0.97 (0.54–1.73) | 0.91 |
| Sexually transmitted infection at baseline | ||||||||
| Gonorrhea, chlamydial infection, or syphilis | ||||||||
| Yes | 186 | 6 | 4.7 | 156 | 8 | 8.0 | 0.54(0.19–1.56) | 0.25 |
| No | 722 | 23 | 4.6 | 771 | 22 | 4.1 | 1.12 (0.63–2.01) | 0.70 |
| Bacterial vaginosis | ||||||||
| Yes | 389 | 14 | 5.1 | 372 | 19 | 7.6 | 0.67 (0.34–1.34) | 0.26 |
| No | 511 | 16 | 4.7 | 550 | 11 | 2.9 | 1.62 (0.75–3.49) | 0.22 |
| Use of injectable contraceptive | ||||||||
| Yes | 650 | 18 | 4.0 | 710 | 28 | 5.7 | 0.70 (0.39–1.27) | 0.24 |
| No | 374 | 15 | 6.0 | 322 | 7 | 3.3 | 1.74 (0.71–4.26) | 0.23 |
| Availability of study drug after randomization | ||||||||
| >80% | 812 | 22 | 3.9 | 861 | 31 | 5.1 | 0.75 (0.44–1.30) | 0.31 |
| ≤80% | 212 | 11 | 8.4 | 171 | 4 | 4.0 | 1.96 (0.62–6.17) | 0.25 |
NA denotes not applicable.
Hazard ratios were calculated with the use of a Cox proportional-hazards model, stratified according to site.
This analysis was performed 30 days after the last dose of the study drug was dispensed or at the time of early, permanent drug discontinuation. For this analysis, one participant who was erroneously given TDF-FTC at enrollment was included in the analysis of the TDF-FTC group.
The covariates were age (<25 yr or ≥25 yr); the presence or absence of gonorrhea, chlamydial infection, or syphilis; the presence or absence of bacterial vaginosis; the number of sexual partners per week (≤1 or >1); status with respect to sex without a condom in the past 4 weeks; use or nonuse of an injectable contraceptive; and the acceptability of a vitamin pill administered between screening and enrollment. Eight women were excluded from this analysis because of missing covariate data.
This analysis includes events after the conclusion of regular follow-up visits (i.e., 8 weeks after the discontinuation of the study drug).
Figure 2. Kaplan–Meier Estimates of the Cumulative Probability of HIV Infection.
Shown are the numbers of participants who were at risk at the start of each 4-week interval. The estimated cumulative probability of HIV infection at 12 months was 0.049 in the TDF–FTC group and 0.046 in the placebo group.
SAFETY
Less than 1% of women had grade 3 or higher hepatic abnormalities or grade 2 or higher creatinine abnormalities, with no significant differences between the two study groups (Table 3). Rates of grade 3 or higher phosphorus abnormalities were also similar in the two groups (4.4% in the TDF–FTC group and 3.9% in the placebo group, P = 0.59). With respect to other prespecified safety end points, only the proportions of women with nausea (P = 0.04), vomiting (P<0.001), and any alanine aminotransferase elevation (P = 0.03) were significantly higher in the TDF–FTC group, with rates of vomiting and nausea more frequent in the first few months of follow-up. (Details regarding the safety analysis are provided in the Supplementary Appendix, available at NEJM.org.)
Table 3.
Safety Outcomes.*
| Variable | TDF–FTC (N = 1025) | Placebo (N = 1033) | P Value† | ||
|---|---|---|---|---|---|
|
no. of participants (%) |
no. of events |
no. of participants (%) |
no. of events | ||
| Any adverse event | 760 (74.1) | 2373 | 747 (72.3) | 2247 | 0.37 |
| Nausea | 50 (4.9) | 52 | 32 (3.1) | 33 | 0.04 |
| Vomiting | 37 (3.6) | 38 | 12 (1.2) | 12 | <0.001 |
| Diarrhea | 17 (1.7) | 18 | 17 (1.6) | 21 | 1.00 |
| Fatigue | 17 (1.7) | 17 | 8 (0.8) | 8 | 0.07 |
| Respiratory tract infection | 96 (9.4) | 108 | 108 (10.5) | 125 | 0.42 |
| Nasopharyngitis | 19 (1.9) | 21 | 36 (3.5) | 42 | 0.03 |
| Fracture | 1 (0.1) | 1 | 2 (0.2) | 2 | 1.00 |
| Intentional overdose | 2 (0.2) | 2 | 2 (0.2) | 2 | 1.00 |
| Depression | 0 | 0 | 1 (0.1) | 1 | 1.00 |
| Other important adverse events | |||||
| Genital ulceration | 10 (1.0) | 10 | 1 (0.1) | 2 | 0.006 |
| Any pregnancy-related event | 24 (2.3) | 28 | 12 (1.2) | 12 | 0.04 |
| Any serious adverse event‡ | 33 (3.2) | 36 | 23 (2.2) | 24 | 0.18 |
| Laboratory abnormality leading to study-drug interruption or permanent discontinuation | 48 (4.7) | 74 | 31 (3.0) | 35 | 0.05 |
| Laboratory abnormality | |||||
| Elevated ALT | |||||
| ≥Grade 1 | 117 (11.4) | 153 | 89 (8.6) | 100 | 0.03 |
| ≥Grade 3§ | 6 (0.6) | 6 | 8 (0.8) | 8 | 0.79 |
| Elevated AST | |||||
| ≥Grade 1 | 180 (17.6) | 229 | 153 (14.8) | 188 | 0.09 |
| ≥Grade 3§ | 3 (0.3) | 3 | 1 (0.1) | 1 | 0.62 |
| Elevated creatinine | |||||
| ≥Grade 1 | 68 (6.6) | 89 | 54 (5.2) | 69 | 0.19 |
| ≥Grade 2§ | 4 (0.4) | 4 | 2 (0.2) | 2 | 0.45 |
| Decreased phosphorus | |||||
| ≥Grade 2 | 201 (19.6) | 253 | 195 (18.9) | 258 | 0.65 |
| ≥Grade 3§ | 45 (4.4) | 50 | 40 (3.9) | 43 | 0.59 |
| no. of participants | no. of cells | no. of participants | no. of cells | ||
| Participants with seroconversion¶ | |||||
| CD4+ count | |||||
| At first evidence of infection | 11 | 14 | 0.16 | ||
| Mean | 443.5 | 602.9 | |||
| Median | 388 | 560 | |||
| At seroconversion visit | 31 | 31 | 0.44 | ||
| Mean | 561.2 | 613.5 | |||
| Median | 511 | 576 | |||
| At 16 wk after seroconversion | 26 | 21 | 0.94 | ||
| Mean | 579.3 | 586.5 | |||
| Median | 580 | 491 | |||
| no. of participants | log10 value | no. of participants | log10 value | ||
| Viral load | |||||
| At first evidence of infection | 33 | 35 | 0.61 | ||
| Mean | 4.9 | 5.1 | |||
| Median | 5.2 | 5.3 | |||
| At seroconversion visit | 32 | 35 | 0.92 | ||
| Mean | 5.2 | 5.2 | |||
| Median | 5.0 | 5.1 | |||
| At 16 wk after seroconversion | 27 | 22 | 0.70 | ||
| Mean | 4.4 | 4.3 | |||
| Median | 4.6 | 4.5 | |||
Listed are adverse events for all participants to whom the study drug was provided, regardless of HIV infection status at enrollment as determined by subsequent testing. ALT denotes alanine aminotransferase, and AST aspartate aminotransferase.
P values are based on Fisher’s exact tests for adverse events, exact log-rank tests for primary safety end points, and t-tests for CD4+ cell counts and log10 viral loads.
A complete list of serious adverse events is provided in Table S2 in the Supplementary Appendix.
This grade of abnormality was a prespecified primary safety end point.
Data were missing for some participants with seroconversion because of a lack of stored blood specimens before seroconversion, participant refusal of additional blood tests, invalid test results, or missed visits.
Less than 5% of women had hepatic or renal abnormalities requiring discontinuation of the study drug (Table S4 in the Supplementary Appendix), with a trend toward a significant between-group difference (4.7% in the TDF–FTC group vs. 3.0% in the placebo group, P = 0.051).
There were 36 serious adverse events reported in the TDF–FTC group and 24 in the placebo group. (A complete list is provided in Table S2 in the Supplementary Appendix.) One participant in the TDF–FTC group was hospitalized with diarrhea and severe dehydration and later died. Another participant in the placebo group died of unknown causes several months after her last clinic visit. We were unable to obtain any additional details with respect to these two events.
The percentage of women with pregnancy-related adverse events was higher in the TDF–FTC group (P = 0.04), but there were also 74 pregnancies in this group (incidence rate, 11.2 per 100 person-years), as compared with 51 in the placebo group (incidence rate, 7.5 per 100 person-years). In a preliminary analysis, there was no evidence of a difference between groups in rates of abortions or other potentially teratogenic effects. The between-group difference in pregnancy rates was not significant after adjustment for study site, age, and the imbalance in the use of oral contraceptives at baseline (hazard ratio, 1.32; P = 0.13). Pregnancy rates were highest among participants who chose to use oral contraceptives at baseline (incidence rate, 29.0 per 100 person-years), with no significant difference between the two study groups.
Among participants in whom HIV infection was identified, there was no significant between-group difference in the mean CD4+ counts or log10 viral loads (Table 3). We did not observe any K65R or K70E mutations in reverse transcriptase, which cause resistance to tenofovir. Among women with HIV seroconversion, one in the placebo group and three in the TDF–FTC group were infected with a viral strain with a M184V mutation in reverse transcriptase; a fourth woman with a resistant infection in the TDF–FTC group had a viral strain with an M184I mutation. Both M184V and M184I mutations cause resistance to FTC. Phenotypic testing was successful in four of the five resistant infections and confirmed resistance to FTC.
ADHERENCE
At the time of study-drug discontinuation, 95% of participants reported that they had usually or always taken the assigned drug. Pill-count data were consistent with ingestion of the study drug on 88% of the days on which it was available to the participants. In contrast, drug-level testing revealed much lower levels of adherence. In prespecified analyses, we considered that 10 ng of tenofovir per milliliter in plasma was evidence that TDF had been taken within the previous 48 hours.8 Among women with seroconversion in the TDF–FTC group, the target plasma level of tenofovir (≥10 ng per milliliter) was identified in 7 of 27 women (26%) at the beginning of the infection window (excluding 6 women for whom the window started at enrollment), in 7 of 33 (21%) at the end of the window, and in 4 of 27 (15%) at both visits (Fig. S2 in the Supplementary Appendix).
Among the uninfected control participants, the numbers of women with target-level tenofovir were somewhat higher: 27 of 78 women (35%) at the beginning of the infection window, 35 of 95 (37%) at the end of the window, and 19 of 78 (24%) at both visits. These differences were not significant after adjustment for age, unprotected sex, and use of injectable contraception (P = 0.70 at the start of the infection window, P = 0.13 at end of the window, and P = 0.68 at both visits).
RISK PERCEPTION AND SEXUAL BEHAVIOR
Most women perceived themselves to be at no or low risk for HIV infection in the coming month when asked at the baseline visit (70.0%) and at the last regular follow-up visit (74.8%). There was no evidence of increased HIV risk behavior during the trial, with modest but significant reductions in the numbers of partners (mean reduction, 0.14; P<0.001 by paired-data t-test), vaginal sex acts (mean reduction, 0.58; P<0.001), and sex acts without a condom (mean reduction, 0.46; P<0.001) reported by women at the last follow-up visit, as compared with 7 days before enrollment.
Fewer than half the study participants agreed to undergo a pelvic examination after randomization, so we were not able to confirm the self-reported data on risk behavior on the basis of data regarding sexually transmitted infections. Among participants who underwent testing at the final visit, there were no significant between-group differences in the prevalence of trichomoniasis (3.5% in the TDF–FTC group and 5.8% in the placebo group, P = 0.20), candidiasis (15.2% and 15.2%, respectively; P = 1.00), gonorrhea (4.9% and 3.2%, P = 0.25), or chlamydial infection (13.3% and 12.0%, P = 0.65).
DISCUSSION
In this study, there was no significant reduction in HIV acquisition among women in the TDF–FTC group, as compared with the placebo group. Drug-level analyses revealed that less than 40% of the HIV-uninfected women had evidence of recent pill use at visits that were matched to the HIV-infection window for women with seroconversion. If such low adherence levels prevailed for the entire cohort, the trial was substantially underpowered to detect an effect of TDF–FTC on the risk of HIV acquisition. Although women in the TDF–FTC group had higher rates of some known side effects than those in the placebo group, the prevalence of these events was modest and consistent with the overall low adherence. However, the rate of adverse events might have been higher if the level of adherence had been higher.
Relatively low adherence levels were also observed in the case–control analysis in the Preexposure Prophylaxis Initiative study involving men who have sex with men,9 in which tenofovir was detected in 44% of the uninfected controls. This finding might indicate that TDF–FTC is more forgiving of imperfect use with rectal exposure to HIV than with vaginal exposure, possibly because of a difference in concentrations of the active metabolites in rectal and vaginal tissues after oral administration.10
In the Partners Preexposure Prophylaxis study,2,11 there was a highly significant protective effect of both TDF and TDF–FTC in the overall study population and among female participants. Among uninfected controls, drug-level analyses revealed adherence rates of 81% in the TDF–FTC group and 83% in the TDF group. Thus, if there are biologic differences between drug responses in men and those in women or between HIV exposure with vaginal and rectal sex, it appears that these differences may be overcome with high rates of adherence.
In the Vaginal and Oral Interventions to Control the Epidemic (VOICE, or MTN-003) trial among women,12 treatment with daily oral TDF was stopped early because of a lack of effectiveness, a finding that is similar to ours for TDF–FTC and that contrasts with the findings for both TDF and TDF–FTC in the Partners Preexposure Prophylaxis study. One of the hypotheses is that low adherence may have played a role. In the VOICE trial, participants continue to receive daily oral TDF–FTC.
It is important to understand why so many women came for the monthly visits and underwent frequent blood testing but did not take their pills. We hypothesize that the women’s perception that they were at low risk for HIV infection may have contributed to the poor adherence. In addition, the high pregnancy rate among women receiving oral contraceptives may indicate difficulty with daily pill regimens. Understanding these issues and identifying characteristics predictive of high adherence will be important in developing strategies for adherence counseling for future studies and programs.
Self-reported behaviors and data regarding the prevalence of sexually transmitted infections do not suggest increased risk behavior among women in the TDF–FTC group. Although there were slightly higher rates of study-drug discontinuation owing to adverse events in the TDF–FTC group, the percentage of days on which women had a study drug available for use was high in the two groups.
There were no major safety concerns in the trial, although low adherence may have compromised our ability to identify a safety concern or an effect on the CD4+ T-cell count and viral load in participants with seroconversion. Five participants had HIV infections that were resistant to FTC, one of them in the placebo group. Among these five participants, one in the TDF–FTC group had not received the study drug for 48 weeks because of early toxicity, leaving three cases of resistant infection in the TDF–FTC group among participants who may have had access to the study drug around the time of acquiring the infection. Sero-conversion was discovered in these three participants within 12 weeks after enrollment. Thus, despite intensive testing, we cannot definitively rule out the possibility that the participants were already infected at enrollment.
It has been suggested that the use of TDF–FTC may mask HIV infection by modifying the viral load and antibody production. However, the similarity in the rates of HIV infection in the two study groups after study-drug discontinuation is not consistent with this concern.
Although we suspect that the lack of efficacy of TDF–FTC in our study population was due to low adherence, biologic factors may also be involved. The protective effect of TDF–FTC might be diminished in the presence of a very high viral load in the infecting partner, as occurs in the acute phase of HIV infection. Another possible reason for the lack of efficacy of TDF–FTC is a high cytokine level. It has been reported that the effect of vaginal tenofovir 1% gel may be lower in women with an elevated vaginal cytokine profile.13
Our trial has several limitations. The low adherence impairs our ability to make clear conclusions regarding the effectiveness and safety of TDF–FTC in the study population. In addition, 13% of the participants were lost to follow-up. Although we were able to document that a third of these women had relocated outside the study area, the absence of HIV testing at the final visit means that we cannot rule out the possibility that a difference in rates of HIV acquisition existed between the two study groups among those who were lost to follow-up.
In conclusion, prophylaxis with TDF–FTC did not reduce the rate of HIV infection and was associated with increased rates of side effects, as compared with placebo. However, we were unable to accurately assess the effect of TDF–FTC on HIV acquisition or safety because of low study-drug adherence, which may be an indication that a daily pill-taking regimen will be difficult for some populations. A better understanding of indicators of adherence among women at high risk for HIV infection is needed to ensure the effectiveness of future preexposure prophylaxis programs.
Supplementary Material
Acknowledgments
Supported by grants from the U.S. Agency for International Development and the Bill and Melinda Gates Foundation. Gilead Sciences provided the study drugs.
We thank all the women who participated in the study and all the staff who worked on the study.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 4.Mascolini M. FDA panel endorses Truvada for pre-exposure prophylaxis. International AIDS Society. 2012 May 12; ( http://www.iasociety.org/Article.aspx?elementId=14505).
- 5.Weir SS, Tate J, Hileman SB, et al. Priorities for local AIDS control efforts: a manual for implementing the PLACE method. Chapel Hill, NC: Carolina Population Center; 2005. [Google Scholar]
- 6.The Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events. Bethesda, MD: National Institute of Allergy and Infectious Diseases, Division of AIDS; 2004. Dec 28, [Google Scholar]
- 7.EAST: software for advanced clinical trial design, simulation, and monitoring, version 5.2. Cambridge, MA: Cytel Statistical Software and Services; 2008. [Google Scholar]
- 8.Patterson KB, Prince HA, Kraft E, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med. 2011;3:112re4. doi: 10.1126/scitranslmed.3003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson P, Liu L, Buchbinder S, et al. Intracellular tenofovir-DP concentrations associated with PrEP efficacy in MSM from iPrEx; Presented at the 19th Conference on Retroviruses and Opportunistic Infections; March 5–8, 2012; Seattle. abstract. [Google Scholar]
- 10.Kashuba AD, Patterson KB, Dumond JB, Cohen MS. Pre-exposure prophylaxis for HIV prevention: how to predict success. Lancet. 2011 Dec 6; doi: 10.1016/S0140-6736(11)61852-7. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donnell D, Baeten J, Hendrix C, et al. Tenofovir disoproxil fumarate drug levels indicate PrEP use is strongly correlated with HIV-1 protective effects: Kenya and Uganda; Presented at the 19th Conference on Retroviruses and Opportunistic Infections; March 5–8, 2012; Seattle. abstract. [Google Scholar]
- 12.MTN statement on decision to discontinue use of oral tenofovir tablets in VOICE, a major HIV prevention study in women. Pittsburgh: Microbicides Trial Network; 2011. Sep 28, ( http://www.mtnstopshiv.org/node/3619). [Google Scholar]
- 13.Abdool Karim S. Biological mechanisms and efficacy; Presented at the International Microbicides Conference; April 15–18, 2012; Sydney. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.