Abstract
Gene therapy is an emerging field in medical and pharmaceutical sciences because of its potential in treating chronic diseases like cancer, viral infections, myocardial infarctions, and genetic disorders. Application of gene therapy is limited because of lack of suitable methods for proper introduction of genes into cells and therefore, this is an area of interest for most of the researchers. To achieve successful gene therapy, development of proper gene delivery systems could be one of the most important factors. Several nonviral and viral gene transfer methods have been developed. Even though the viral agents have a high transferring efficiency, they are difficult to handle due to their toxicity. To overcome the safety problems of the viral counterpart, several nonviral in vitro and in vivo gene delivery systems are developed. Out of these, the most promising and latest systems include polymer-based nonviral gene carriers, dendrimers, and physical means like electroporation, microinjection, etc., Shunning of possible immunogenicity and toxicity, and the feasibility of repeated administration are some of the merits of nonviral gene delivery systems over viral gene delivery. An ideal nonviral gene carrying system should possess all these merits without any compromise to its gene transferring efficiency. The viral gene delivery systems include lytic and nonlytic vectors for drug delivery. Inspite of its toxicity they are still preferred because of their long term expression, stability, and integrity. This review explores the recent developments and relevancy of the novel gene delivery systems in gene therapy.
Keywords: Dendrimers, gene therapy, liposomes, nonviral gene delivery, viral gene delivery
INTRODUCTION
Gene therapy involves addition of an exogenous gene to an altered gene where the expression of the inserted gene takes place and thus, curing the disease caused by altered gene. It is a useful tool to treat diseases or disorders due to altered gene-like mutations or due to an inherited gene. Gene therapy is not only useful in correcting genetic disorders but also other disorders like myocardial infarction, stroke, cancer, and viral infections. Application of gene therapy is limited as there is lack of suitable methods for proper introduction of genes into cells and so this is an area of interest for most of the researchers. Figure 1 show major steps involved in the gene delicery process.
Figure 1.
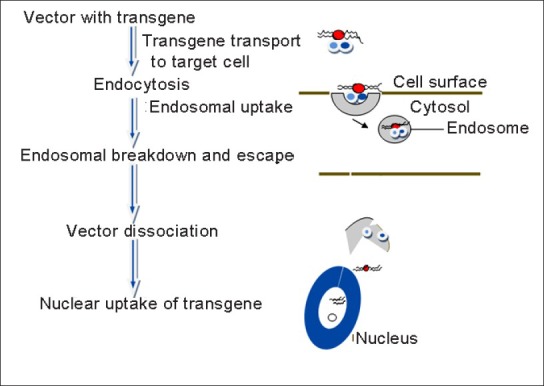
Steps involved in gene delivery process
Exogenous deoxyribonucleic acid (naked DNA) cannot effectively enter into the nuclei of cells, therefore the major research that is going on in this area is specific carriers for these DNA which can as well target the altered DNA. The carriers can be broadly classified into viral agents and nonviral agents. Viral agents have high transferring efficiency, but they are difficult to handle mainly due to toxicity; whereas nonviral agents are less toxic with less transferring efficiency. So research works based on how to make viral agents less toxic without losing their high transferring efficiency and nonviral agents with maximum transferring efficiency without making them toxic is widely carried out nowadays. Delivery systems utilizing these viral or nonviral agents for efficient gene delivery are called gene delivery systems.
An ideal gene delivery system should meet three criteria: The carriers should protect the transgene from nuclease enzymes inside intracellular matrixes; should transport the transgene from plasma membrane to the target cell nucleus; and should not cause any toxic effect.[1]
VIRAL GENE DELIVERY SYSTEMS
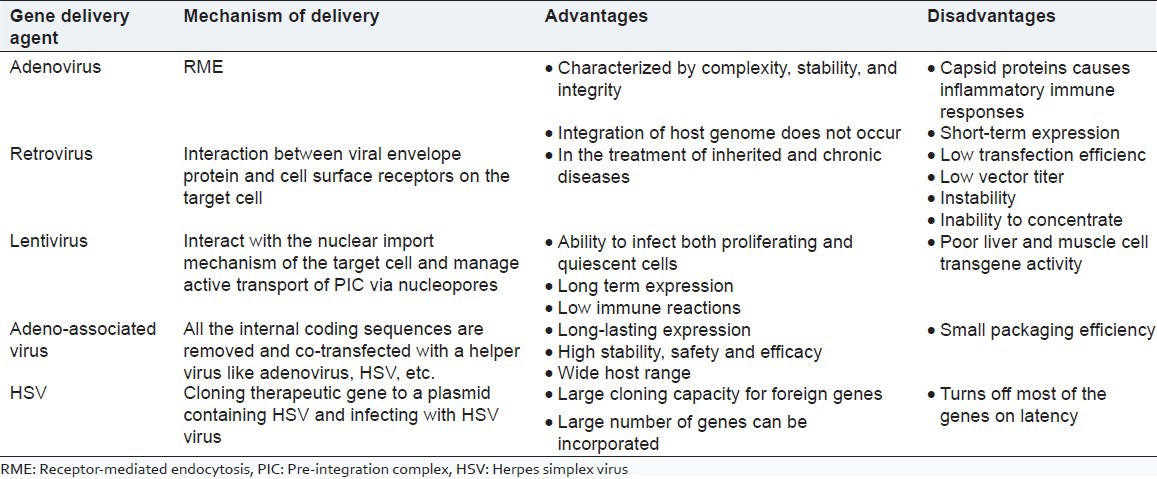
Viral gene delivery can be by modifications in adenoviruses, retroviruses, lentiviruses, adeno-associated viruses (AAVs), etc., These vectors can be classified into two:
Nonlytic: Those that produce virions but leave host cell intact. For example, adenoviruses and lentiviruses.
Lytic: Those that produce virions and destroy host cell. For example, human adenovirus and herpes simplex virus (HSV).
An active vector is produced by introducing a defective vector with a deleted capsid protein gene into ‘packaging cell line’ (PCL) which are specialized engineered cell lines. Effective replication of the cod gene occurs with the aid of replicase enzyme and capsid proteins are produced from capsid genes encoded in the PCL. This method of producing a viral gene vector is called ‘complementation’.
Adenoviral agents are double-stranded DNA viruses which highly infest the target cell and are stable in ether and ethanol. They enter the target cells through receptor-mediated endocytosis (RME). Adenovirus infect cells by binding the viral fiber capsid protein by Coxsackie virus and adenovirus receptor (CAR) and binding of viral pentose base by αv integrins on cell surface.[2] Adenoviruses Ad2 and Ad5 of group C are the mostly studied ones. First generation was developed by deleting the E1 region to reduce the immune response but produced coinfection with other viruses. Therefore, the second generation was developed by deleting E2 or E4, but still there were immune responses. Third generation was also developed called ‘gutless vectors’, but had to be complemented for replication. At last, replication complemented adenovirus HEK293 was developed.[3] Direct injection or bolus delivery is the easiest way of viral gene delivery. They are considered to be the most effective vectors in gene delivery.[1]
Adenoviral gene delivery can reprogram human fibroblasts to induced pluripotent stem cells.[4] Most of the adenoviral gene delivery is being aimed for cancer chemotherapy. The first initiatives included that for inherited monogenic diseases (cystic fibrosis, muscular dystrophy, and hemophilia) and infectious diseases like AIDS.[5] Tropism-modification strategies for targeted gene delivery using adenoviral vectors are of great importance these days.[6]
Recent advances in adenoviral gene delivery include:
Antiviral and antitumor immune responses in immune competent mice upon P. 029* oncolytic adenovirus therapy of malignant glioma.[7]
Hypertension and reduction in renal failure and injury in rats with reduced renal mass on eNOS gene delivery.[8]
Adenoviral neutral endopeptidase gene delivery for the treatment of prostate cancer in combination with paclitaxel.[9]
Attenuation of pulmonary hypertension by targeted gene delivery of BMPR2.[10]
Retroviral gene delivery is done by the interaction between viral envelope and cell surface receptor to infect a target cell. Retroviruses are small ribonucleic acid (RNA) viruses consisting of only genes required for replication and long terminal repeats (LTRs) transgene with therapeutic gene produced by replacing certain genes that code for capsid, envelope glycoprotein, reverse transcriptase etc., Unlike adenoviral vectors, retroviral vectors realize transfection through transgene integration to the target cell genome. The most commonly used retroviruses are the Moloney murine leukemia virus species.[1] Retroviral gene delivery systems include delivery into male germ line stem cells,[11] efficient gene delivery to mammalian cells using 293T cell-based systems,[12] etc.
Lentiviruses (e.g., HIV-1) do not replicate as they do not have retrovirus-like proteins and their advantage is that they can be used in the transgene expression of nerve cell. Lentiviruses interact with the nuclear import mechanism of the target cell and manage active transport of pre-integration complex (PIC) via nucleopores.[1]
Applications of lentiviral gene delivery include:
Distinct roles of nucleus accumbens dopamine D2 and D3 receptors in novelty- and light-induced locomotor activity revealed using lentiviral-mediated gene delivery.[13]
Lentiviral gene delivery using fibrin hydrogels in vitro and in vivo.[14]
Maturation of human peripheral blood lymphocytes by lentiviral vectors for ex vivo gene delivery.[15]
Gene delivery to pancreatic exocrine cells in vivo and in vitro.[16]
AAV is a small single-stranded DNA virus causing mild immune response in humans and other primates derived from Parvoviridae family. AAV vectors are used for gene therapy as these affect only the dividing cells. AAV gene therapy in retina is almost a success.[17]
Of the twelve known sub types of AAV, the most common vector is derived from AAV2 which is not known to cause any pathologic response.[18] It is produced by inserting therapeutic gene in between two ‘inverted terminal repeats’ (ITRs). As all the internal coding sequences are removed, they become replication deficient and so cotransfected with other viruses like adenoviruses, HSV, etc.
HSV is an enveloped, double-stranded DNA virus which is pathogenic to humans. They are highly useful in clinical neurology. Transfection can be achieved by:
Therapeutic gene is cloned into plasmid of HSV origin and a packaging signal, and then infected to a helper virus.
Gene is cloned into a plasmid containing specific HSV virus and plasmid is cotransfected into cells with HSV virus.
Pox viruses and alphaviruses
Pox viruses derived from vaccinia virus and alphaviruses derived from Togaviridae are also used as vectors. Therapeutic gene can be directly introduced into vaccinia virus. There is no need of any inactivation. Replication incompetent alphaviruses with transgene is combined with helper plasmid capable of replication. Alpha viruses have high host cell toxicity, therefore widely researched for cancer chemotherapy.[3] Similar viruses found to be useful in gene therapy include:
Semliki forest virus (SFV)
Sindbis virus (SIV)
Venezuelan equine encephalitis virus (VEE)
A summary of various viral gene delivery systems are presented in Table 1.
Table 1.
Viral gene delivery systems
NONVIRAL GENE DELIVERY SYSTEMS
The easiest way of gene delivery is by naked DNA. Naked DNA transfer has brought some sort of success in areas like intramuscular injection for DNA vaccination, vascular endothelial growth factor (VEGF) coded gene therapy in myocardial ischemia and angiogenesis in critical limbs, intratumoral injection in some cancer therapy, etc., But it is difficult for the naked DNA to enter cells, due to their large size owing to phosphate group and hydrophilic nature. Also, nuclease enzyme fragments them. Studies to transfer naked DNA other than with viral agents and eliminating the above factors led to the development of gene delivery by nonviral agents. Advantages of nonviral systems are their less toxicity and their ease of preparation. They are easy to handle and they can be incorporated using wide variety of agents and techniques, polymers, micronization, etc., Researchers are trying to increase their transfection efficiency by incorporating chemical or biological entities like enzymes or using physical methods like adsorption.
Nonviral vectors like plasmid, oligodeoxynucleotides, and polymerase chain reaction (PCR) products are transported through a suitable system into the target gene of the cell. Different nonviral gene delivery systems [Figure 2] include liposomal delivery, microinjection, gene gun, cationic systems, adsorption, using polymers, dendrimers, encapsulation, ultrasound, electrostatic interaction, cationic systems, hydrodynamic therapy, etc.
Figure 2.
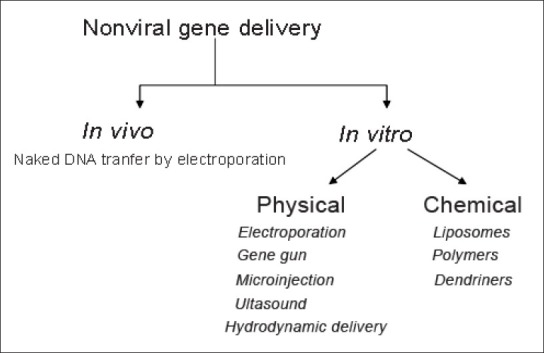
Various nonviral gene delivery systems
The major barriers in nonviral therapy include extracellular and intracellular barriers and that for production, formulation and storage.
Electroporation
It is method in which small pores are produced by transmembrane potential induction of small electric impulses. Electropores are produced in approximately 10 ns and the pore size is about 10 nm. It is through these pores that the DNA can enter the cells. Electrodes producing impulses are placed at target organs in situ and electroporation is affected by injecting naked DNA. Electroporation was found to be effective in transfecting muscle, brain tumors, etc., This method is highly dependent on electric impulses and on concentration and amount of DNA.[19] In vivo electroporation is found to be more effective. But the disadvantage is destruction of cells due to heat produced by high voltage application.
Gene gun
Gene gun is also called ballistic DNA delivery or DNA-coated particle bombardment. DNA-coated with gold, tungsten, or silver microparticles are targeted to a tissue with certain speed with a pressurized inert gas like helium. These enter few millimeters into the cells by momentum and cause cellular DNA release. Major disadvantage is that it causes greater immune response than microinjection and the advantages include no receptor is required, the size of DNA is not a problem, and the production of DNA-coated metal particles are easy to produce.[1]
It was found in a recent study that gene gun and electroporation are almost equally efficient in DNA vaccination for Alzheimer's with similar immune responses.[20] Electroporation efficiency is found to be increased on using a small DNA fragment for in vivo gene transfer.[21] Gene gun delivery of DNA-coated gold particles enhanced the efficiency of DNA vaccine for Schistosomiasis.[22] It is also found that DNA vaccine delivery by electroporation and gene gun enhanced the efficiency.[23]
Microinjection
Microinjection is the process by which substances are inserted into cells at a microscopic or borderline macroscopic level using a glass pipette and performed with the help of a specialized optical microscope called micromanipulator. Cell membrane or nuclear membrane is penetrated by simple mechanical process using a needle of 0.5-5 μm diameter.[24] This technology is extremely time-consuming and is a tedious work. It can infest only few numbers of cells in an experiment. Newer techniques developed require less time but is highly expensive.[25] Recently developed, controlled flow device includes pneumatic picopumps.[26]
Ultrasound
Nowadays ultrasound-mediated gene delivery is found to be very effective. Ultrasound application of microbubbles causes an increase in gene delivery. Here, ultrasound is applied on microbubbles modified with plasmid DNA. Its effects depend on the amount of DNA mounted, time of application of ultrasound waves, frequency, etc.[1] High-intensity focused ultrasound (HIFU) is found to liquefy tissues.
Recent studies showed that plasmid binding cationic microbubbles are more efficient than neutral microbubbles in gene delivery.[27] Phenomenon of increased uptake using microbubble assisted ultrasound gene delivery is called sonoporation. It has been found to be effective in vitro and in vivo but its mechanism is not known.[28]
Hydrodynamic delivery
This technique mainly depends on the characteristics of blood vessels and other fluids in the body and the flow characteristics. Large volume of DNA is injected into blood pressure veins creating hydrodynamic pressure leading to increased permeability of capillary endothelium and pore formation in the membrane encircling parenchyma cells. Parenchyma cells are targeted as they are in close proximity to endothelial cells and when the endothelial wall is breached, DNA can easily enter parenchyma.[1] Most of the research on hydrodynamic delivery targets liver cells. Liver-targeted delivery system with apolipoprotein B siRNA was studied and found to decrease serum cholesterol level.[29]
Liposomal delivery
Liposomes are vesicular structures formed by the assembly of lipid molecules themselves. They contain a hydrophilic head as well as hydrophobic tail which will align in tail-to-tail fashion and adjoining ends, upon modification making it hydrophilic by exposing the hydrophilic head into aqueous systems and rendering them more efficient gene transferring capacity. Liposomes can easily bind to cell surface and can cause gene transfer. Most widely used liposome includes dioleoylphosphatidylethanolamime (DOPE). Upon acidification it promotes membrane fusion. Most of these liposomes are made pH sensitive to bind with endosomes upon acidification. Lipoproteins combined with DNA called lipoplexes can be modified into cationic, anionic, neutral, or a mixture of all. Cationic lipids are found to be still more effective in transfection as they are made neutral and can easily capture plasmids.[30] Lipid emulsions are also used in gene delivery which is found to be superior to liposomal delivery.[31]
Liposomal gene delivery systems can be prepared by hydrating the lipid film in the presence of DNA, high molecular weight DNA is entrapped in egg phosphatidylcholine liposomes and metaphase chromosomes incorporated in egg phosphatidylcholine-cholesterol (7:2, mol/mol) liposomes. Another approach include cationic lipids added to hydrophobic plasmid DNA in monomer or micellar form to get hydrophobic plasmid DNA-cationic lipid complex which is expected to produce efficient gene delivery system when compred to simple liposomal gene delivery. Advancements in liposome research also include enhancement of the lifetime of liposomes by decreasing activity of phagocytic cells of reticuloendothelial system on them. Targeted cell delivery can also be achieved by coupling monoclonal antibodies onto the surface of liposomes by covalent or noncovalent methods. Fusogenic liposomes for intracellular delivery are also an advanced form.[32] Recent advances in liposomal delivery include cancer chemotherapy,[33] nanoparticle liposomes which is a most promising area,[34] brain delivery,[35] etc.
Polymers
Polymers are long chain compounds with either identical monomers (homopolymers), or with different monomers (heteropolymers), or with two different polymers (copolymers). They can be natural (e.g., chitosan, dextran) or synthetic which can be hydrophilic (swells in water; e.g. polyvinyl alcohol, polyvinyl acetate, polyethylene glycol, polyacrylic acid, etc.) and hydrophobic (do not swell in water; e.g. silicones, polyvinyl acetate). Polymers usually used in pharmaceutical preparations include those for controlled release such as cellulose acetate phthalate (CAP), hydroxypropyl methylcellulose (HPMC), ethyl cellulose (EC), eudragit, etc.
DNA packed polymers increase the stability of DNA and enhances endocytosis or cellular uptake and enters nucleus releasing DNA. Delivery of gene by polymers is by electrostatic interactions with polyplexes of nanosize for gene therapy. The basics of polymer based nonviral gene delivery are displayed in Figure 3.
Figure 3.
Polymer-based nonviral gene delivery
Synthetic gene delivery systems should fulfill the following requirements for its efficiency as delivery system.
Protect the phosphate DNA.
Condense to suitable length for the entry into cell.
Protect DNA from nucleases.
Newer methods like electrostatic interaction, adsorption, encapsulation, etc., are employed to fulfill the above mentioned requirements. Electrostatic interaction between negatively charged DNA and polymer to form complexes, mainly, nanocomplexes enhance DNA uptake and decreases nuclease activity on DNA. DNA encapsulation with the biodegradable polymer helps to enhance gene delivery and also provides controlled delivery by altering physicochemical properties. Adsorption is a combination of encapsulation and electrostatic interaction.[1]
Gene delivery using polymers can be by poly (l-lysine) (PLL)-based systems, poly ethylenimine (PEI)-based systems, biodegradable polycations-based, polysaccharide-based, or other polycation-based.[36] Toxicogenomics of cationic polymers is such that these linear or branched cationic polymers interact with anionic nucleotides, easily bind and condense with them forming polyplexes causing endosomal uptake and gene delivery. Polyethyleneimine and polypropylenimine are the major and widely studied cationic polymers for DNA, oligonucleotides, and siRNA delivery. Nonionic polymers are also capable of gene delivery, but much is not explored yet.[37] Chitosan-carbon nanoparticles are also studied for gene delivery.
Dendrimers
Dendrimers are 100-200 Å diametric particles with a central core and large number of branching chains arising from them. They have a lot of functional groups on their surface to which the gene to be targeted can be attached, either electrostatically or covalently. Another method is by encapsulation.[1] A nanosphere made of polyamidoamine (PAMAM) dendrimer-capped mesoporous silica can be used as a gene transfer agent.[38] Studies on dendrimers both theoretical and computational aspects are going on nowadays expecting highly efficient gene delivery.[39] Carbosilane dendrimers for antisense oligonucleotides,[40] siRNA delivery using PAMAM[41] are also widely studied.
RECENT ADVANCES IN GENE DELIVERY SYSTEMS
Targeted delivery using magnetic gene vectors is an arising and promising area. Polyethylenimine and chitosan-coated magnetic micelles (CP-mag-micelles) are on the path of its development, intented on gene delivery and magnetic resonance imaging (MRI).[42] Polymer and lipid-coated magnetic nanocrystals for in vivo gene delivery for cancer is in its development stages.[43] Gene delivery using chlorotoxin-labelled magnetic nanovectors for glioma,[44] polyethylenimine-based magnetic iron oxide vectors,[45] and nanomagnetic particles in mouse fibroblasts[46] are widely studied nowadays. Multifunctional envelope-type nanodevice using octaarginine (R8) liposomes were also developed.[47] Ultrasonic gene delivery using micelle and nanoparticles have gained importance recently.[48] Targeted gene delivery to hepatocytes using galactose-bearing cationic polymers[49] is also in development. Chitosan-based nanocarriers are in the pace of development.[50] Most recent development is that of nanoexosomes of 30-100 nm for diagnosis and therapy.[51] Figure 4 displays the steps involved in the process of dendrimer based gene delivery.
Figure 4.
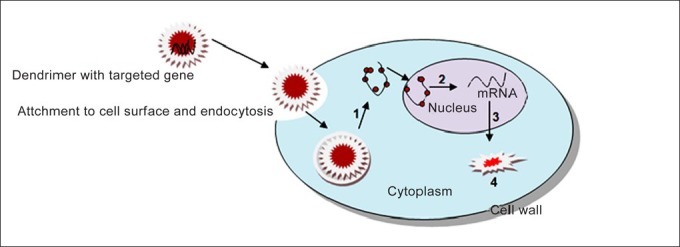
Dendrimer based gene delivery: (1) Endosomal release, (2) transcription, (3) translation, and (4) formation of new protein which produces the therapeutic effect
CONCLUSION
A wide variety of gene delivery systems have been developed already and many are in the pace of development. A large number of clinical trials are also going on of which some are in its final stage. The developed and developing systems can be classified as viral and nonviral. Some of the hybrid systems are also in the path of development which can be the future of gene delivery. Gene delivery is an emerging area which can be the further scope for treatment of many fatal diseases and has been successful up to a certain limit till date.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Cevher E, Sezer AD, Çağlar ES. Sezer AD, editor. Gene delivery systems: Recent progress in viral and non-viral therapy. Recent Advances in Novel Drug Carrier Systems Intech. 2012:337–470. [Google Scholar]
- 2.Franceschi RT, Ge C. Gene Delivery by adenoviruses. Methods Mol Biol. 2008;455:137–47. doi: 10.1007/978-1-59745-104-8_10. [DOI] [PubMed] [Google Scholar]
- 3.Gardlík R, Pálffy R, Hodosy J, Lukács J, Turna J, Celec P. Vectors and delivery systems in gene therapy. Med Sci Monit. 2005;11:RA110–21. [PubMed] [Google Scholar]
- 4.Zhou W, Freed CR. Adenoviral gene delivery can reprogram human fibroblasts to induced pluripotent stem cells. Stem Cells. 2009;27:2667–74. doi: 10.1002/stem.201. [DOI] [PubMed] [Google Scholar]
- 5.Breyer B, Jiang W, Cheng H, Zhou L, Paul R, Feng T, et al. Adenoviral vector-mediated gene transfer for human gene therapy. Curr Gene Ther. 2001;1:149–62. doi: 10.2174/1566523013348689. [DOI] [PubMed] [Google Scholar]
- 6.Coughlan L, Alba R, Parker AL, Bradshaw AC, McNeish IA, Nicklin SA, et al. Tropism-modification strategies for targeted gene delivery using adenoviral vectors. Viruses. 2010;2:2290–355. doi: 10.3390/v2102290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roth P, Silginer M, Goodman SL, Hasenbach K, Thies S, Schraml PM. Inhibition of TGF-beta signaling in glioma cells by the integrin inhibitor. [Last accessed on 2012 Nov 27];Neuro Oncol. 2012 14(iii):1–94. Available from: [Google Scholar]
- 8.Savard S, Lavoie P, Villeneuve C, Agharazii M, Lebel M, Larivière R. eNOS gene delivery prevents hypertension and reduces renal failure and injury in rats with reduced renal mass. Nephrol Dial Transplant. 2012;27:2182–90. doi: 10.1093/ndt/gfr641. [DOI] [PubMed] [Google Scholar]
- 9.Iida K, Zheng R, Shen R, Nanus DM. Adenoviral neutral endopeptidase gene delivery in combination with paclitaxel for the treatment of prostate cancer. Int J Oncol. 2012 doi: 10.3892/ijo.2012.1586. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reynolds AM, Holmes MD, Danilov SM, Reynolds PN. Targeted gene delivery of BMPR2 attenuates pulmonary hypertension. Eur Respir J. 2012;39:329–43. doi: 10.1183/09031936.00187310. [DOI] [PubMed] [Google Scholar]
- 11.Nagano M, Shinohara T, Avarbock MR, Brinster RL. Retrovirus-mediated gene delivery into male germ line stem cells. FEBS Lett. 2000;475:7–10. doi: 10.1016/s0014-5793(00)01606-9. [DOI] [PubMed] [Google Scholar]
- 12.Swift S, Lorens J, Achacoso P, Nolan GP. Rapid production of retroviruses for efficient gene delivery to mammalian cells using 293T cell-based systems. Curr Protoc Immunol. 2001 doi: 10.1002/0471142735.im1017cs31. [DOI] [PubMed] [Google Scholar]
- 13.Fernandes AR, Easton AC, De Souza Silva MA, Schumann G, Müller CP, Desrivières S. Lentiviral-mediated gene delivery reveals distinct roles of nucleus accumbens dopamine D2 and D3 receptors in novelty- and light-induced locomotor activity. Eur J Neurosci. 2012;35:1344–53. doi: 10.1111/j.1460-9568.2012.08028.x. [DOI] [PubMed] [Google Scholar]
- 14.Kidd ME, Shin S, Shea LD. Fibrin hydrogels for lentiviral gene delivery in vitro and in vivo . J Control Release. 2012;157:80–5. doi: 10.1016/j.jconrel.2011.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang S, Karne NK, Goff SL, Black MA, Xu H, Bischof D, et al. A simple and effective method to generate lentiviral vectors for ex vivo gene delivery to mature human peripheral blood lymphocytes. Hum Gene Ther Methods. 2012;23:73–83. doi: 10.1089/hgtb.2011.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houbracken I, Baeyens L, Ravassard P, Heimberg H, Bouwens L. Gene delivery to pancreatic exocrine cells in vivo and in vitro. BMC Biotechnol. 2012;12:74. doi: 10.1186/1472-6750-12-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anonymous. Adeno-associated virus. [Last accessed on 2012 Nov 30]. Available from: http://www.en.wikipedia.org/wiki/Adeno.associated_virus .
- 18.Ponnazhagan S, Curiel DT, Shaw DR, Alvarez RD, Siegal GP. Adeno-associated virus for cancer gene therapy. Cancer Res. 2001;61:6313–21. [PubMed] [Google Scholar]
- 19.Gehl J. Electroporation: Theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol Scand. 2003;177:437–47. doi: 10.1046/j.1365-201X.2003.01093.x. [DOI] [PubMed] [Google Scholar]
- 20.Davtyan H, Ghochikyan A, Movsesyan N, Ellefsen B, Petrushina I, Cribbs DH, et al. Delivery of a DNA vaccine for Alzheimer's disease by electroporation versus gene gun generates potent and similar immune responses. Neurodegener Dis. 2012;10:261–4. doi: 10.1159/000333359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng J, Zhao Y, Mai J, Guo W, Xu Y. Short noncoding DNA fragment improve efficiencies of in vivo electroporation-mediated gene transfer. J Gene Med. 2012;14:563–9. doi: 10.1002/jgm.2667. [DOI] [PubMed] [Google Scholar]
- 22.Cao Y, Zhao B, Han Y, Zhang J, Li X, Qiu C, et al. Gene gun bombardment with DNA-coated golden particles enhanced the protective effect of a DNA vaccine based on thioredoxin glutathione reductase of Schistosoma japonicum. J Biomed Biotecnol. 2012;1:1–10. doi: 10.1155/2013/952416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ginsberg AA, Shen X, Hutnick NA, Weiner DB. Improvement of DNA vaccines by electroporation. [Last accessed on 2012 Nov 26]. Available from: http://www.researchgate.net/publication/225843247_Improvement_of_DNA_Vaccines_by_Electroporation .
- 24.Anonymous. Microinjection. [Last accessed on 2012 Nov 27]. Available from: http://en.wikipedia.org/wiki/Microinjection .
- 25. [Last accessed on 2012 Nov 27]. Available from: http://www.lib.store.yahoo.net/lib/yhst-131428861332406/How to Choose the Optimal Gene Delivery Method.pdf 1-10 .
- 26.Dean DA. Gene delivery by direct injection (microinjection) using a pulsed-flow system. CSH Protoc. 2006:2006. doi: 10.1101/pdb.prot4653. pii: pdb.prot4653. [DOI] [PubMed] [Google Scholar]
- 27.Wang DS, Panje C, Pysz MA, Paulmurugan R, Rosenberg J, Gambhir SS, et al. Cationic versus neutral microbubbles for ultrasound-mediated gene delivery in cancer. Radiology. 2012;264:721–32. doi: 10.1148/radiol.12112368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delalande A, Postema M, Mignet N, Midoux P, Pichon P. Ultrasound and microbubble-assisted gene delivery: Recent advances and ongoing challenges. Ther Deliv. 2012;3:1199–215. doi: 10.4155/tde.12.100. [DOI] [PubMed] [Google Scholar]
- 29.Kang JH, Tachibana Y, Obika S, Harada-Shiba M, Yamaoka T. Efficient reduction of serum cholesterol by combining a liver-targeted gene delivery system with chemically modified apolipoprotein B siRNA. J Control Release. 2012;163:119–24. doi: 10.1016/j.jconrel.2012.08.030. [DOI] [PubMed] [Google Scholar]
- 30.Ropert C. Liposomes as a gene delivery system. Braz J Med Biol Res. 1999;32:163–9. doi: 10.1590/s0100-879x1999000200004. [DOI] [PubMed] [Google Scholar]
- 31.Hashida M, Kawakami S, Yamashita F. Lipid carrier systems for targeted drug and gene delivery. Chem Pharm Bull (Tokyo) 2005;53:871–80. doi: 10.1248/cpb.53.871. [DOI] [PubMed] [Google Scholar]
- 32.Cullis PR, Chonn A. Recent advances in liposome technologies and their applications for systemic gene delivery. Adv Drug Deliv Rev. 1998;30:73–83. doi: 10.1016/s0169-409x(97)00108-7. [DOI] [PubMed] [Google Scholar]
- 33.Hsu JL, Chao C, Xie X, Hung M. Advances in liposome-based targeted gene therapy of cancer. In: Liu X, Pestka S, Shi Y, editors. Recent Advances in Cancer Research and Therapy. Burlington: Elsevier; 2012. pp. 113–33. [Google Scholar]
- 34.Zhou M, Wang L, Su W, Tong L, Liu Y, Fan Y, et al. Assessment of therapeutic efficacy of liposomal nanoparticles mediated gene delivery by molecular imaging for cancer therapy. J Biomed Nanotechnol. 2012;8:742–50. doi: 10.1166/jbn.2012.1442. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, Zhao L, Wu J, Dong H, Xu F, Gong G, et al. Current advances in vehicles for brain gene delivery. Curr Gene Ther. 2012;12:423–36. doi: 10.2174/156652312802762590. [DOI] [PubMed] [Google Scholar]
- 36.Park TG, Jeong JH, Kim SW. Current status of polymeric gene delivery systems. Adv Drug Deliv Rev. 2006;58:467–86. doi: 10.1016/j.addr.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Akhtar S, Benter I. Toxicogenomics of non-viral drug delivery systems for RNAi: Potential impact on siRNA-mediated gene silencing activity and specificity. Adv Drug Deliv Rev. 2007;59:164–82. doi: 10.1016/j.addr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 38.Radu DR, Lai CY, Jeftinija K, Rowe EW, Jeftinija S, Lin VS. A polyamidoamine dendrimer-capped mesoporous silica nanosphere-based gene transfection reagent. J Am Chem Soc. 2004;126:13216–7. doi: 10.1021/ja046275m. [DOI] [PubMed] [Google Scholar]
- 39.Tian WD, Ma YQ. Theoretical and computational studies of dendrimers as delivery vectors. Chem Soc Rev. 2013;42:705–27. doi: 10.1039/c2cs35306g. [DOI] [PubMed] [Google Scholar]
- 40.Pedziwiatr-Werbicka E, Shcharbin D, Maly J, Maly M, Zaborski M, Gabara B, et al. Carbosilane dendrimers are a non-viral delivery system for antisense oligonucleotides: Characterization of dendriplexes. J Biomed Nanotechol. 2012;8:57–73. doi: 10.1166/jbn.2012.1369. [DOI] [PubMed] [Google Scholar]
- 41.Pavan GM, Posocco P, Tagliabue A, Maly M, Malek A, Danani A. PAMAM dendrimers for siRNA delivery: Computational and experimental insights. Chemistry. 2010;16:7781–95. doi: 10.1002/chem.200903258. [DOI] [PubMed] [Google Scholar]
- 42.Wang C, Ravi S, Martinez GV, Chinnasamy V, Raulji P, Howell M, et al. Dual-purpose magnetic micelles for MRI and gene delivery. J Control Release. 2012;163:82–92. doi: 10.1016/j.jconrel.2012.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Namiki Y, Namiki T, Yoshida H, Ishii Y, Tsubota A, Koido S, et al. A novel magnetic crystal-lipid nanostructure for magnetically guided in vivo gene delivery. Nat Nanotechnol. 2009;4:598–606. doi: 10.1038/nnano.2009.202. [DOI] [PubMed] [Google Scholar]
- 44.Kievit FM, Veiseh O, Fang C, Bhattarai N, Lee D, Ellenbogen RG, et al. Chlorotoxin labeled magnetic nanovectors for targeted gene delivery to glioma. ACS Nano. 2010;4:4587–94. doi: 10.1021/nn1008512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arsianti M, Lim M, Marquis CP, Amal R. Polyethylenimine based magnetic iron-oxide vector: The effect of vector component assembly on cellular entry mechanism, intracellular localization, and cellular viability. Biomacromolecules. 2010;11:2521–31. doi: 10.1021/bm100748p. [DOI] [PubMed] [Google Scholar]
- 46.Fouriki A, Dobson J. Nanomagnetic gene transfection for non-viral gene delivery in NIH 3T3 mouse embryonic fibroblasts. Materials. 2013;6:255–64. doi: 10.3390/ma6010255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kogure K, Akita H, Yamada Y, Harashima H. Multifunctional envelope-type nano device (MEND) as a non-viral gene delivery system. Adv Drug Deliv Rev. 2008;60:559–71. doi: 10.1016/j.addr.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 48.Husseini GA, Pitt WG. Micelles and nanoparticles for ultrasonic drug and gene delivery. Adv Drug Deliv Rev. 2008;60:1137–52. doi: 10.1016/j.addr.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Munisso MC, Mahara A, Tachibana Y, Kang JH, Obika S, Yamaoka T. Hepatocyte-specific gene delivery with galactose-bearing cationic polymers with different molecular structures. Adv Sci Techol. 2012;86:86–91. [Google Scholar]
- 50.Duceppe N, Tabrizian M. Design and development of light-sensitive chitosan-based nanocarriers for gene delivery. Adv Sci Techol. 2012;86:75–80. [Google Scholar]
- 51.Tan A, Rajadas J, Seifalian AM. Exosomes as nano-theranostic delivery platforms for gene therapy. Adv Drug Deliv Rev. 2012 doi: 10.1016/j.addr.2012.06.014. Epub ahead of print. [DOI] [PubMed] [Google Scholar]


