Abstract
Background:
The present study was aimed to develop and optimize in situ gel for the treatment of periodontal disease.
Materials and Methods:
Temperature-sensitive in situ gel containing 0.1% w/v Chlorhexidine hydrochloride was formulated by cold method using different polymers. Preliminary study was carried out to optimize different types and concentration of polymers such as Poloxamer 188, Poloxamer 407, Gellan gum, and Carbopol 934P. Central composite design was employed for optimization of the effect of independent variables such as Poloxamer 407 and Carbopol 934P on responses such as gelation temperature, spreadability, cumulative percentage release at 2 h, and time for 50% drug release (t50 %). Each formulations were evaluated for clarity, pH, gelation temperature, spreadability, drug content, in vitro drug release, t50 %, and cumulative percentage drug release at 2 h.
Results:
Results of evaluation parameters revealed that the drug release, gelation temperature was considerably decreased with increasing t50 % as the concentration of each polymer was increased. The desirability function was utilized to find out optimized formulation of the factorial design. Formulation F6 showed the highest overall desirability of 0.6283 and, therefore, this formulation was considered to be the optimized formulation. The % relative error was calculated, which showed that observed responses were in close agreement with the predicted values calculated from the generated regression equations.
Conclusion:
The clarity, pH, drug content of all formulations was found to be satisfactory. Further, all the formulations showed sustained drug release for a period of 6 h, which satisfied to treat periodontal disease.
Keywords: Carbopol 934P, chlorhexidine hydrochloride, in situ gel, periodontal disease, poloxamer 407
INTRODUCTION
Periodontal disease is term used to ascribe to some pathological conditions characterized by degeneration and inflammation of gums, periodontal ligaments, alveolar bone, and dental cementum.[1] It is a localized inflammatory reaction caused by bacterial infection of a periodontal pocket accompanying with sub-gingival plaque.[2] Although bacteria are the principal cause of periodontal disease, the appearance of microbial pathogenic factors alone may not be enough to cause periodontitis. Periodontal pathogens generate destructive by-products and enzymes that break extracellular matrices as well as host cell membranes to produce nutrients for their growth. In doing so, they start damage directly or indirectly by triggering host-mediated responses that lead to self-injury.[1] In the early phase of the disease, inflammation is localized to the gingiva called gingivitis but extends to deeper tissues in periodontitis, leading to gingival swelling, bleeding, and bad breath. In the late phase of the disease, the supporting collagen of the periodontium is disintegrated, alveolar bone begins to resorb, and gingival epithelium migrates along the tooth surface forming a ‘periodontal pocket.’[2,3]
In situ gel forming formulations current a novel idea of deliver drugs to patients as a liquid dosage form, yet achieve sustained release of drug for the desired period.[4] Different delivery systems based on polymers have been developed, which are able to increase the residence time of the formulation at absorption site of drugs. In recent years, there has been an increasing interest in water-soluble polymers that are able to form gels after application to delivery site. These so-called in situ gelling polymers are highly advantageous compared with other polymers because, in contrast to very strong gels, they can be easily applied in liquid form to the site of drug absorption. At the site of drag absorption, they swell to form a strong gel that is capable of prolonging the residence time of the active substance.[5]
Chlorhexidine, a bisbiguanide compound, has been shown to possess a broad-spectrum of topical anti-microbial activity.[6] It has been used by dental professionals for plaque control and for the treatment of gingival inflammation.[7] Chlorhexidine was primarily used in mouth-rinses and was recommended in the hygiene phase of treatment as an adjunct to tooth-brushing. Most attention, however, has been focused on the use of chlorhexidine during the operative and immediate post-operative phases of non-surgical and surgical periodontal treatment.[8]
The Poloxamer consist of more than 30 different non-ionic surface-active agents. These polymers are ABA-type triblock co-polymers composed of PEO (A) and PPO units (B).[9] Pluronic are commercially available in a range of molecular weights, composition ratios, and forms, it would be useful to mention the nomenclature rules for these copolymers. The letter in the notation stands for liquid (L), paste (P), or flakes (F), whereas the first two numbers indicate the molecular weight of the PPO block and the last number the weight fraction of the PEO block. For example, the commonly used in biomedical applications F127 has a weight percentage of 70% PEO and a molecular weight of PPO around 4000.[10]
Carbopol is a well-known pH-dependent polymer, which stays in solution form at acidic pH but forms a low viscosity gel at alkaline pH.[11] Carbopols, which are very high molecular weight polymers of acrylic acid, have been used mainly in liquid or semi-solid pharmaceutical formulations, such as gels, suspensions and emulsions, as a thickening agent, in order to modify the flow characteristics.[12]
MATERIALS AND METHODS
Materials
Chlorhexidine hydrochloride was obtained as a gift sample from Cadila Healthcare Pvt. Ltd. Ankleshwar, Gujarat. Poloxamer188 was purchased from Hi media Laboratories Pvt. Ltd. Mumbai, India. Poloxamer 407 was purchased from Zeel pharmaceutical Pvt. Ltd. Mumbai, India.
Gellan gum was purchased from Sisco Research Laboratories Pvt. Ltd. Mumbai, India. Carbopol 934P were obtained from Noveon Ltd. Mumbai, India. Triethanolamine were obtained from Burgoyne burbidges and co. Mumbai, India.
Optimization of types and concentration of polymers
Different grades of Poloxamer i.e., Poloxamer 188, Poloxamer 407, and Gellan gum was used for the preparation of in situ gel formulation. The periodontal in situ gel was prepared using different Poloxamer 188, Poloxamer 407 concentration by cold method. This method involved slow addition of polymer in cold water with continuous stir. The formed mixtures were stored overnight at 4°C and studied for their gelation temperature to select optimum concentration of Poloxamer grades for effective in situ gel formulation.
Method of preparation of in situ gel
The method of preparation of in situ gel involved slow addition of polymers (i.e., Poloxamer 188, Poloxamer 407), and methyl paraben were solubilized in required quantity of cold deionized water. An appropriate amount of (0.1% w/v) Chlorhexidine HCl was solubilized in polymer solution with continuous stirring until uniform drug solution obtained. 0.1% w/v Chlorhexidine thermosensitive hydrogel is a strong candidate as a local drug delivery system for periodontal treatment.[13] Required quantities of Carbopol 934P were added into the solution with continuous stirring by mechanical stirrer) until uniform solution obtained. The final solution was kept overnight in refrigerator at 5°C to completely dissolve polymers in solution, and then addition of small amount of triethanolamine (TEA) was added to adjust the pH 7.[14]
Experimental design
Central composite design (CCD) was employed for systemic study of joint influence of the effect of independent variables [Poloxamer 407 (X1) and Carbopol 934P (X2)] on responses such as gelation temperature (°C), spreadability (gm·cm/sec), cumulative percentage release (CPR) at 2 h and time for 50% drug release (t50%). Based on preliminary trials, two factors were determined as follows: Poloxamer 407 (X1): 15–19% w/v and Carbopol 934P (X2): 0.20-0.40% w/v. In this design, two factors with five levels were probed to investigate the main effects and interaction of the two factors on two responses [Table 1]. The design consists of nine runs (4 factorial points, 4 star points, and 1 center point) and four replicated runs (center points) yielding 13 experiments in total. The main purpose of the replication runs was to increase the precision and to minimize experimental error. The data obtained for the four responses in each trial were fitted to classical second order polynomial model. The mathematical model was expressed as follow, second order polynomial model:
Table 1.
Layout of Experimental design batches
![]()
Where y is the measured response, b0 is an intercept, and b1-b5 is the regression co-efficients, X1, X2 represents the main effect, the quadratic effect, and X1 X2 is the interaction effect. The effects were evaluated statistically at 0.1 level (α = 1.414). Data were further analyzed by Microsoft Excel® 2007 for regression analysis. Analysis of variance (ANOVA) was implemented to assure that there was no significant difference between the developed full model and the reduced model. Response surface plots were plotted to study response variations against two independent variables using Design Expert® Version 8 software.[15,16]
Desirability function
The desirability value is a satisfaction index ranging between 0 and 1, characterizing the level of a response considering a particular objective. For the optimization of a process involving multiple responses, the individual responses have to be combined to define a product with the desired characteristics. The desirability value[17,18] is used to classify experiments according to the degree of satisfaction. For each response (Yi), the desirability function D (Yi) assigns values from 0 and 1 to the possible values of Yi; D (Yi) =0 corresponding to a completely undesirable value of Yi, and D (Yi) =1 corresponding to a completely desirable or ideal response value. Two desirability functions D (Yi) were used depending on whether the response Yi had to be maximized or minimized up to a target value (T).
Evaluation of trans-dermal films
clarity
The in situ gel solutions was prepared and evaluated visually for clarity against white and black backgrounds in light.[19]
pH measurement
The two areas of critical importance are the effect of pH on solubility and stability. The pH of in situ gel formulation should be such that the formulation was be stable at that pH and at the same time, there would be no irritation to the patient upon administration of the formulation. in situ gel formulations should have pH range in between 6.2 and 7.4. The developed in situ gel formulations were evaluated for pH by using calibrated digital pH meter. The pH meter was calibrated before each use with standard pH 4, 7, and 9.2 buffer solutions. The formulation temperature was maintained at 25°C.[20,21]
Gelation temperature
Gelation temperature was assessed using a modified Miller and Donovan technique.[22] Two mL aliquots of the gel were transferred to test tubes sealed with parafilm and immersed in a water bath at 4°C. The temperature of the bath was increased in increments of 1°C and left to equilibrate for 15 min at each new setting. The samples were examined for gelation, which was deemed to have occurred when the meniscus would no longer move upon tilting through 90°C. All measurements were performed in triplicate (n = 3).[23]
Drug content
The prepared in situ gel formulations were analyzed for drug content by transferring 1 mL of formulation in 100 mL volumetric flask. In this volumetric flask, 50 mL of phosphate buffer with pH 6.8 was added, followed by continuous shaking until the gel was totally dispersed to give a clear solution. Final volume was adjusted to 100 ml with the help of phosphate buffer pH 6.8 and filtered the solution. Drug concentration in filtrated solution was determined spectrophotometrically at 257 nm using UV-Visible spectrophotometer (Shimadzu 1700, Japan).[20]
Instrumental analysis
Fourier transform infrared spectroscopy
Pre-formulation studies regarding the drug-polymer interaction are, therefore, very critical in selecting appropriate polymers. The FTIR spectra of the samples were recorded using KBr pellets method. The samples were then placed in the sample holder of the instrument. These were analyzed by FTIR to study the interference of polymer for drug analysis. The integrity and compatibility of the pure drug and polymer were evaluated with the help of IR spectra of the pure drug, and polymer was carried out using FTIR spectrophotometer.[24]
Differential scanning calorimetry
DSC study was carried out using DSC-60 instrument (Shimadzu corporation, Japan) to check the formation as well as the compatibility of ingredients. Thermogram of pure drug (Chlorhexidine hydrochloride) and optimized formulation was scanned for DSC. Accurately weighed samples were placed on aluminum plates sealed with aluminum seals and heated at constant temperature of 10°C/min over a temperature range of 0-300°C.[25,26]
Viscosity
The viscosity determination of the prepared optimized in situ gel formulation was determined using Brookfield Digital Viscometer (LVDV III U, Brookfield Engineering Labs. USA). The optimized in situ gel formulation was taken in a beaker and maintained at room temperature. The measurements were carried out using spindle no. 62 at the speed of 50 rpm in the sample, and the viscosity was measured at 10 min after the rotation of the spindle.[20,26]
Spreadability test
Spreadability was measured by apparatus, which was suitably modified in the laboratory and used for the study. It consists of a wooden block, which was provided by a pulley at one end. A ground glass slide was fixed on this block. An excess of gel (about 1 gm) under study was placed on this ground slide. The gel was then sandwiched between this slide and another glass slide having the dimension of fixed ground slide and provided with the hook. One kg weight was placed on the top of the two slides for five minutes to expel air and to provide a uniform film of the gel between the slides. Excess of the gel was scrapped off from the edges. The top plate was then subjected to pull of 80 gms. With the help of string attached to the hook and the time (in seconds) required by the top slide to cover a distance of 7.5 cm be noted. A shorter interval indicates better spreadability. The time required to separate the two slides and spreadability measured by using following equation[27,28]
![]()
Where,
M = weight tide to upper slide
L = length moved on the glass slide
T = time taken to separate two slides.
In vitro drug release study
The in vitro drug release study of Chlorhexidine HCl from in situ gel was determined using a Franz-diffusion cell. The cellophane membrane was fixed on the receptor cell.[29] The donor cell was filled with 1 g of in situ gel formulation. The receptor compartment was filled with phosphate buffer pH 6.8 and constantly stirred with a small magnetic bar at a speed of 200-250 rpm during the experiments to confirm homogeneity, and temperature was maintained at 37 ± 1°C by circulating hot water through the jacket of Franz-diffusion cell. The 0.5 mL samples were withdrawn at scheduled time intervals (0, 15, 30, 45, 60, 90, 120, 180, 240, 300, 360 min) and were replaced with same volume of pH 6.8 phosphate buffer to maintain the sink condition. Samples were analyzed at 257 nm on UV-visible spectrophotometer.[20]
Stability study
Drug decomposition or degradation occurs during stability, because of chemical alteration of the active ingredients or due to product instability, lowering the concentration of the drug in the dosage form. The stability of pharmaceutical preparation should be evaluated by accelerated stability studies. Stability studies of periodontal in situ gel were carried out to determine the effect of contents on the stability of the drug. The accelerated stability studies were carried out according to ICH guidelines by storing the samples at 40 + 2°C and 75 + 5% RH for 1 month using stability chamber (Remi, India). The optimized formulation were evaluated for clarity, pH measurement, drug content, gelation temperature, spreadability, CPR at 6 h, CPR at 2 h, and t50%.[30]
RESULTS AND DISCUSSION
Fourier transform infrared spectroscopy
FTIR study was performed to evaluate compactability between drug and polymer utilized in study. IR spectrum of Chlorhexidine HCl is characterized by principal absorption peaks at 2542, 2956.67 cm–1 (N-H stretch, salt of secondary amine), 1519.80 cm–1 (N-H bending, secondary aromatic amine), 1259.43 cm–1 (C–N stretch, secondary aromatic amine), 1022.20, 1155.28 cm–1 (C-N stretch) shown in Figure 1. IR spectrum of poloxamer 188 is characterized by principal absorption peaks at 2883.38 cm–1 (C-H stretch aliphatic), 1348.15 cm–1 (in-plane O-H bend), and 1107.06 cm–1 (C-O stretch) shown in Figure 2. IR spectrum of poloxamer 407 is characterized by principal absorption peaks at 2893.02 cm–1 (C-H stretch aliphatic), 1355.86 cm–1 (in-plane O-H bend), and 1124.42 cm–1 (C-O stretch) shown in Figure 3. IR spectrum of Carbopol 934P is characterized by principal absorption peaks at 3089.75 cm–1 (O-H stretching), 1706.88 cm–1 (carboxyl group) shown in Figure 4. The IR spectrum of physical mixture of drug and polymers is characterized by principal absorption peaks at 2935.45 cm–1 (N-H stretch, salt of secondary amine), 1529.45 cm–1 (N-H bending, secondary aromatic amine), 1155.28 cm–1 (C-N stretch).
Figure 1.
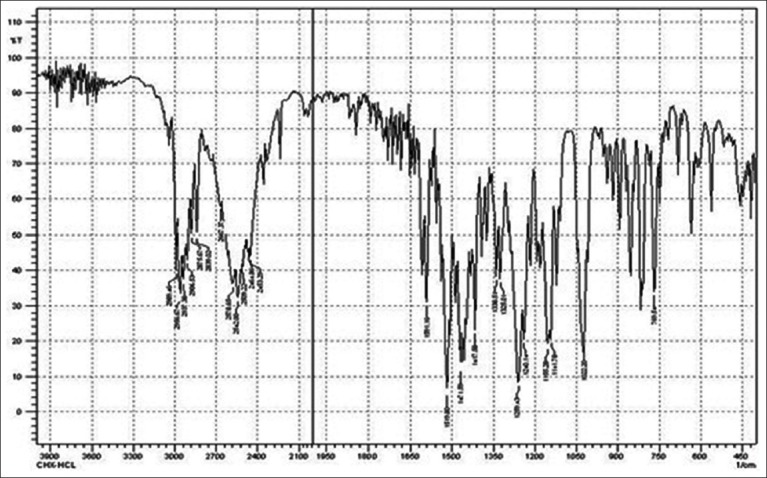
FTIR spectrum of chlorhexidine HCl
Figure 2.
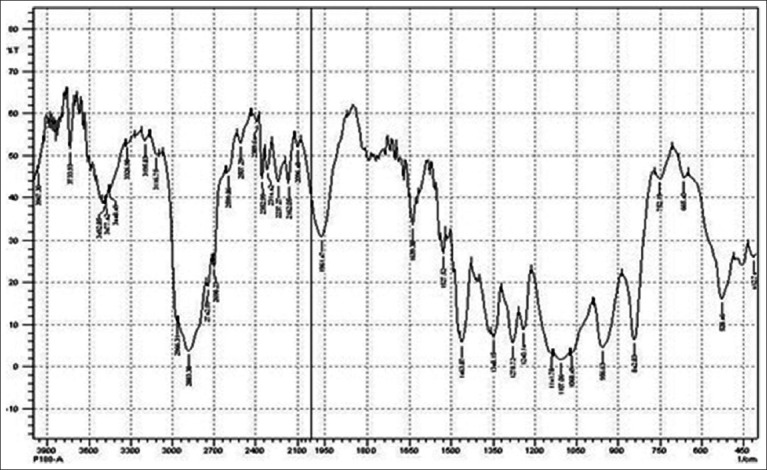
FTIR spectrum of poloxamer 188
Figure 3.
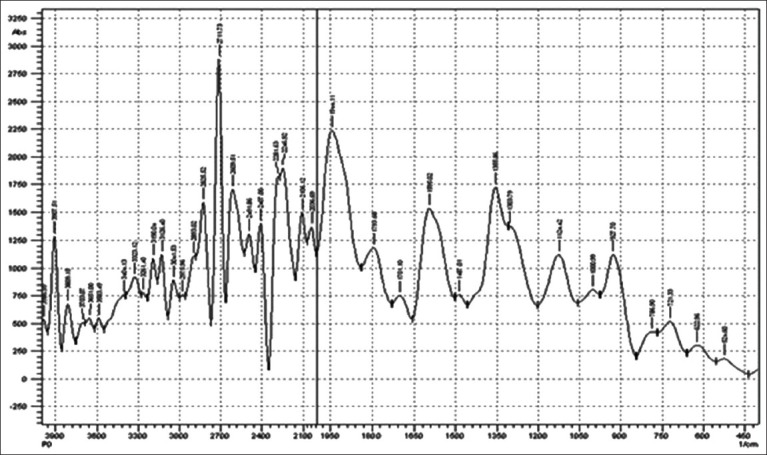
FTIR spectrum of poloxamer 407
Figure 4.
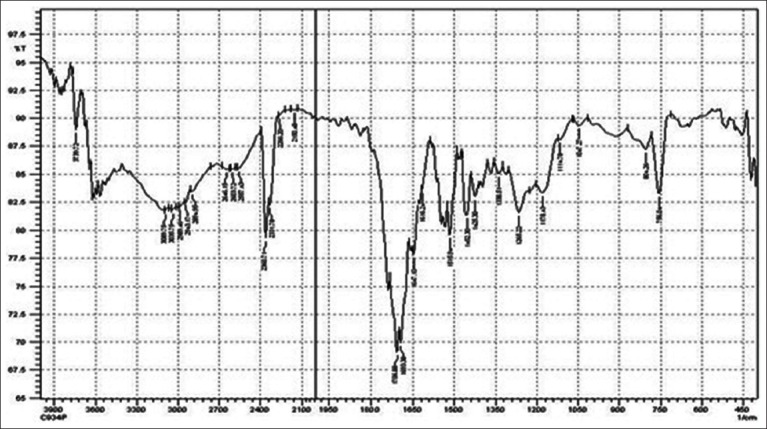
FTIR spectrum of carbopol 934P
The interaction between the drug and the polymers often leads to identifiable changes in the FTIR profile of solid systems. FTIR spectra for pure drug, polymers, and physical mixture of drug and polymers have been depicted in Figures 1-5. The spectrum of physical mixture of drug and polymers was equivalent to the addition spectrum of pure drug, indicating no interaction occurring in the simple physical mixture of drug and polymer as shown in Figure 5.
Figure 5.
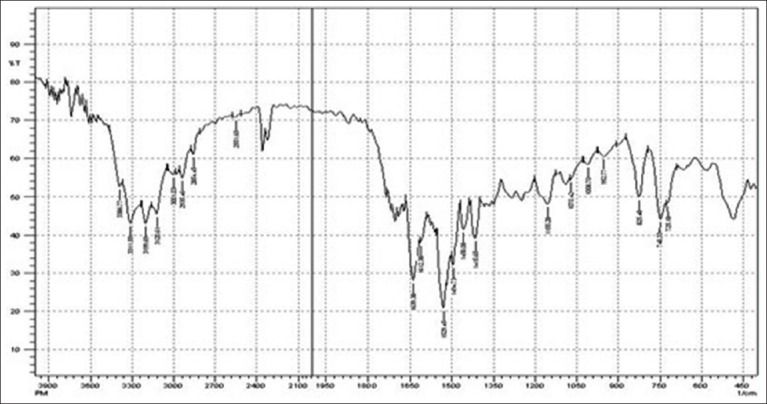
FTIR spectrum of physical mixture
Selection of polymers
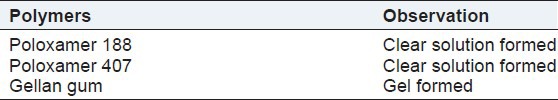
Different grades of Poloxamer i.e. Poloxamer 188, Poloxamer 407, and Gellan gum were used for the preparation of in situ gel formulation. From all polymers, Poloxamer 188 and Poloxamer 407 were formed viscous clear solution and formed gel at elevated temperature. Gellan gum was not used to prepare formulation because of its gel formation during formulation shown in Table 2. So, Poloxamer 188 and Poloxamer 407 were selected as polymer for in situ gel formulation on bases of visual examination and gelation temperature.
Table 2.
Selection of polymer
Selection of polymers concentration
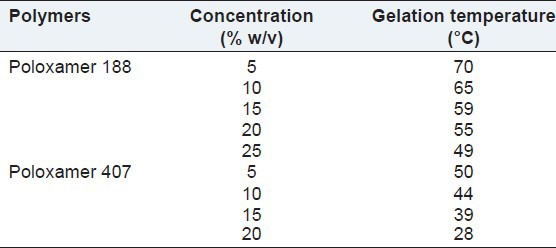
The gelation temperatures have been considered to be suitable if they are in the range of body temperature. As the temperature of the periodontal cavity is nearest 37°C, this study aimed at preparing the liquid formulations that may gel below 37°C. The gelation of Poloxamer vehicles was known to result from the change in micellar number with temperature. With increasing temperature, the number of micelles formed increases as a consequence of the negative co-efficient of solubility of block copolymer micelles. Eventually, the micelles become so tightly packed that the solution becomes immobile, and gel is formed. Conformational changes in the orientation of the methyl groups in the side chains of poly (oxypropylene) polymer chains, constituting the core of the micelle, with expulsion of the hydrating water from the micelles will contribute to the gelation phenomenon. Gelation temperatures for Poloxamer 188 and Poloxamer 407 gels were observed for the different concentration range of polymer, and it was found that the gelation temperature of formulation decreased with increasing concentration of polymer.
As the concentration of polymer increases, the gel structure becomes more closely packed with the arrangement in the lattice pattern.[23] Result revealed that the formulation containing Poloxamer 188 have found to be higher gelation temperature compared to body temperature. So, gelation temperature of Poloxamer 188 in situ gel was not fulfilling the requirement of in situ gel formulation. Only 15% w/v and 20% w/v concentration of Poloxamer 407 in situ gel showed ability to form gel in the range of body temperature shown in Table 3. So, 15-20% w/v concentration of Poloxamer 407 was used for further studies.
Table 3.
Optimization of different polymer concentration
Potential drawbacks of Poloxamer gels include their weak mechanical strength, rapid erosion, and the non-biodegradability of PEO-PPO-PEO, which prevented the use of high molecular weight polymers. Thus, it was necessary to formulate them with other bioadhesive polymers, including cellulose ethers polymers such as carboxy methylcellulose (CMC), HPMC, hydroxyl ethyl cellulose (HEC), hydroxyl propyl cellulose (HPC), acrylic polymers.[31]
Experimental design
Preliminary investigations of the process parameters exposed that factors such as Poloxamer 407 (X1) and Carbopol 934P (X2) exhibited significant influence on gelation temperature, spreadability, CPR at 2h, t50%; hence, they were utilized for further systematic studies. All selected dependent variables for all 13 batches showed a wide variation of data [Table 4]. The polynomial equations can be used to draw conclusions after considering the magnitude of co-efficients and the mathematical sign carried: Positive or negative.[16]
Table 4.
Results of experimental design batches of variables
Effect of formulation variable on gelation temperature (Y1)
Concerning Y1, the results of multiple linear regression analysis showed that both the co-efficients b1 and b2 bear a negative sign [Table 5]. The negative of both X1 and X2 co-efficient indicates that as the concentration of X1 (Poloxamer 407) and X2 (Carbopol 934P) increases, there is decrease in the gelation temperature. The fitted equation relating the response Y1 to the transformed factor is shown in following equation,
Table 5.
Summary of regression analysis
![]()
The Y1 for all batches F1 to F13 shows good correlation co-efficient of 0.910009. Variable X1 has P value 0.0000916 (P < 0.05), and variable X2 has P value 0.038169 (P < 0.05). Variables, which have P value less than 0.05, significantly affect the gelation temperature. As the concentration of Poloxamer 407 increases, there is micelle formation, followed by micellar aggregation. The gel phase can only occur when the concentration is above the micellar concentration. When the material is in cold water, hydrogen bonding between POP chains and water keeps the hydrophobic portions of the pluronic separate. When the temperature is increased, the hydrogen bonding is disrupted, and hydrophobic interactions cause a gel to be formed. It is to be noted that the addition of increasing concentrations of Carbopol 934P from 0.2% to 0.4% further lowered the gelation temperature.[32] As the concentration of mucoadhesive polymers (Carbopol 934P) increased, the gelation temperature of gel decreased. This ability of mucoadhesive polymers to lower gelation temperature may be due to increased viscosity after dissolution of polymers. The ability of mucoadhesive polymers to lower gelation temperature could be explained by their ability to bind to the polyoxyethylene chains present in the Poloxamer 407 molecules. This would promote dehydration, causing an increase in entanglement of adjacent molecules and extensively increasing intermolecular hydrogen bonding, thus leading to gelation at lower temperature.[33] Combination of bioadhesive agents and Poloxamer 407 on gelation temperature that the packing and entanglements of micelles were promoted by adding Carbopol 934P, which accordingly lead to decrease of gelation temperature.
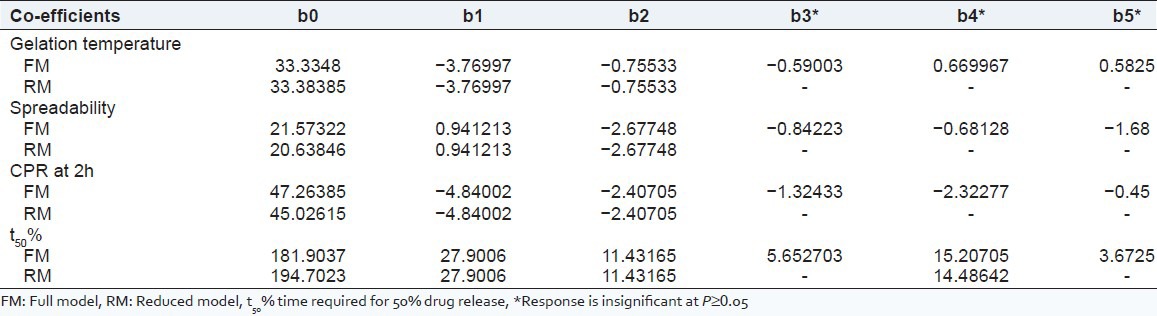
The relationship between formulation variables (X1 and X2) and Y1 was further elucidated using response surface and contour plot. The effects of X1 and X2 on Y1 are shown in Figures and b. Table 4 showed that with the increase of amount of Poloxamer 407 and Carbopol 934P, gelation temperature was reduced.
Figure 6.
(a) Response surface plot showing effect of gelation temperature of variables [Poloxamer 407 (X1) and Carbopol 934P (X2)] (b) the corresponding Contour plot
Effect of formulation variable on spreadability (Y2)
Concerning Y2, the results of multiple linear regression analysis showed that co-efficient b1 bear positive sign and co-efficient b2 bear a negative sign [Table 5]. The positive X1 co-efficient indicates that as the concentration of X1 (Poloxamer 407) increases, there is increase in the spreadability of in situ gel. The negative X2 co-efficient indicates that as the concentration of X2 (Carbopol 934P) increases, spreadability of in situ gel decreases. The fitted equation relating the response Y2 to the transformed factor is shown in following equation,
![]()
The Y2 for all batches F1 to F13 shows good correlation co-efficient of 0.749198. Variable X1 has P value 0.003824 (P < 0.05), and variable X2 has P value 0.006702 (P < 0.05). Variables, which have P value less than 0.05, significantly affect the spreadability.
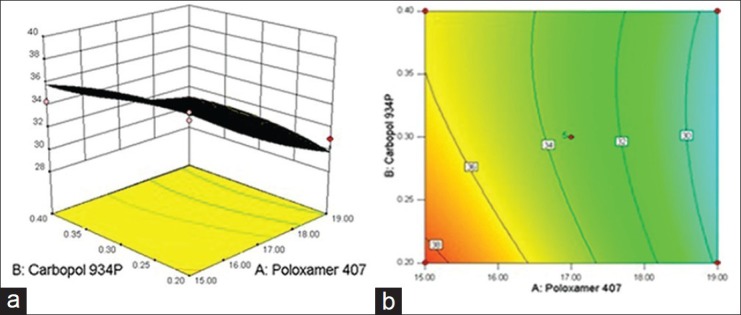
The relationship between formulation variables (X1 and X2) and Y2 was further elucidated using response surface and contour plot. The effects of X1 and X2 on Y2 are shown in Figures 7a and b. As the concentration of X1 (Poloxamer 407) increases, there is increase in the spreadability of in situ gel, and as the effect of concentration of X2 (Carbopol 934P) increases, spreadability of in situ gel decreases.
Figure 7.
(a) Response surface plot showing effect of spreadability of variables [Poloxamer 407 (X1) and Carbopol 934P (X2)] (b) the corresponding Contour plot
Results of the spreadability testing are shown in Table 4. All the prepared in situ gels using different polymers concentrations were spreadable. Data in Table 4 revealed that increasing the concentration of any of the gelling agents was always associated with a decrease in the spreadability.[34] One of the criteria for a gel to meet the ideal qualities is that it should possess good spreadability. It is the term expressed to denote the extent of area, to which gel readily spreads on application site. Lesser the time taken for separation of two slides, better the spreadability.[35] The spreadability of all prepared in situ gel formulations were found be in range of 17.21-25.48 gm·cm/sec.
Effect of formulation variable on CPR at 2 hr (Y3)
Concerning Y3, the results of multiple linear regression analysis showed that both the co-efficients b1 and b2 bear a negative sign [Table 5]. The negative of both X1 and X2 co-efficient indicates that as the concentration of X1 (Poloxamer 407) and X2 (Carbopol 934P) increases; there is decrease in the cumulative percentage drug release. The fitted equation relating the response Y3 to the transformed factor is shown in following equation,
![]()
The Y3 for all batches F1 to F13 shows good correlation co-efficient of 0.852097. Variable X1 has P value 0.001251 (P < 0.05), and variable X2 has P value 0.036108 (P < 0.05). Variables, which have P value less than 0.05, significantly affect the release profile.
The relationship between formulation variables (X1 and X2) and Y3 was further elucidated using response surface and contour plot. The effects of X1 and X2 on Y3 are shown in Figures 8a and b. This finding was probably due to the increased strength of the formed gel structure. It is worthwhile that the drug diffusion is controlled by the penetration of liquid through the gel structure and retard release of drug into the medium.[36]
Figure 8.
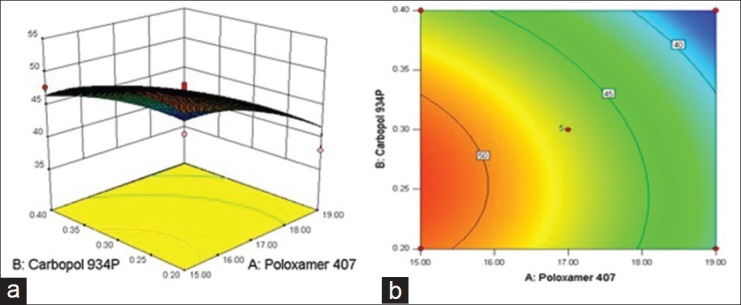
(a) Response surface plot showing effect of CPR at 2 h of variables [Poloxamer 407 (X1) and Carbopol 934P (X2)] (b) the corresponding Contour plot
Effect of formulation variable on t50% (Y4)
Concerning Y4, the results of multiple linear regression analysis showed that both the co-efficients b1 and b2 bear a positive sign [Table 5]. The positive of both X1 and X2 co-efficient indicates that as the concentration of X1 (Poloxamer 407) and X2 (Carbopol 934P) increases, there is increase in the time for the drug release from formulation. The fitted equation relating the response Y4 to the transformed factor is shown in following equation,
![]()
The Y4 for all batches F1 to F13 shows good correlation co-efficient of 0.848547. Variable X1 has P value 0.00125 (P < 0.05), and variable X2 has P value 0.042157 (P < 0.05). Variables, which have P value less than 0.05, significantly affect the release profile. The relationship between formulation variables (X1 and X2) and Y4 was further elucidated using response surface and contour plot. The effects of X1 and X2 on Y4 are shown in Figures 9a and b. A strong influence of both independence variables (Poloxamer 407 and Carbopol 934P) was observed on in vitro drug release (t50%). The highest t50% value (258.23 ± 0.57) was observed with Batch 7 [Poloxamer 407 (19% w/v) and Carbopol 934P (0.4% w/v)]. It is possible that at higher polymers concentration, drug is trapped in smaller polymer cells, and it is structured by its close proximity to the polymer molecules. So, increasing the amount of the polymer in the formulations increased the time for the drug to leave the formulation and delayed release of drug into the medium.[36]
Figure 9.
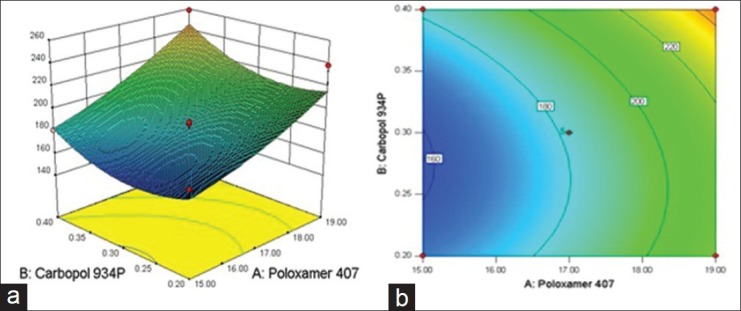
(a) Response surface plot showing effect of t50% of variables [Poloxamer 407 (X1) and Carbopol 934P (X2)] (b) the corresponding Contour plot
Analysis of variance
The R2 value for gelation temperature (Y1), spreadability (Y2), CPR at 2 h (Y3), and t50% (Y4) are 0.910009, 0.749198, 0.852097, and 0.848547, respectively, indicating good correlation between dependent and independent variables [Table 6]. The reduced models were developed for response variables by omitting the insignificant terms with P > 0.05. For all selected dependent variables, co-efficients b3, b4, b5 were found to be insignificant, as P values were more than 0.05 and hence removed from the full model.
Table 6.
Calculation of testing the model in portions
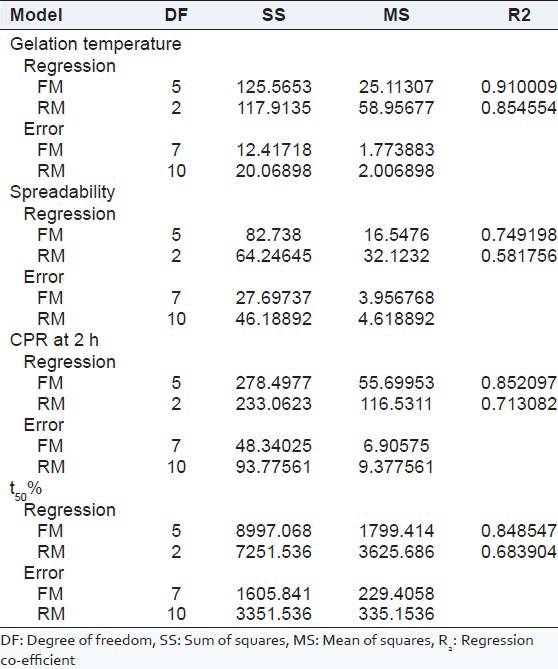
Table 6 shows the results of analysis of variance (ANOVA) performed to justify the removal of insignificant factors. The high values of correlation co-efficients for gelation temperature, spreadability, CPR at 2 h, and t50% indicate a good fit. The calculated values of F for gelation temperature, spreadability, CPR at 2 h, and t50% was found to be 1.43, 1.55, 2.19, and 1.91, respectively, which were less than F critical value (4.35). These observations suggest no significant difference between the full and reduced model.
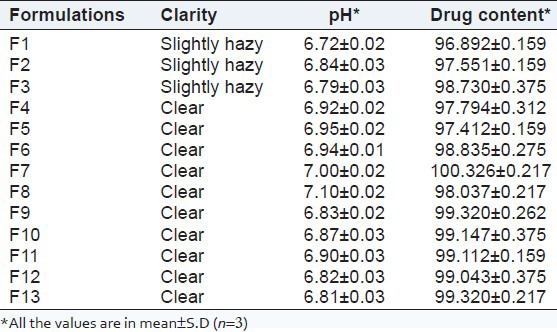
Clarity and pH
The clarity of first three formulations (F1-F3) was slightly hazy, and all other formulations (F4-F13) were found to be clear. The pH value of all prepared in situ gel formulations (F1-F13) were found in range 6.72 to 7.10 [Table 7]. The pH values of all prepared formulations were within the limit of neutral pH; this indicates formulations can be used without any irritation in the oral cavity.
Table 7.
Result of clarity and pH
Drug content
Drug content was one of a significant requirement for any type of dosage form. Amount of the drug present in the formulation should not deviate beyond certain specified limits from the labeled amount. All formulations were found to having drug content in the range of 96.89-100.32%, representing homogenous drug distribution throughout gel shown in Table 7.
Viscosity
One essential necessity for a periodontal in situ gel was viscosity of the formulation. As indicated, a formulation suitable for application to the periodontal pocket should ideally have a low viscosity when apply and after instillation, should have a high viscosity in order to stay at the application site. The viscosity of the optimized in situ gel formulation measured at 37°C was found to be 30596.33 ± 1.52 cps.
Differential scanning calorimetry study
Drug excipients compatibility study was carried out by Shimadzu DSC-60 in dry N2 atmosphere (flow rate 20 mL/min), and temperature scanning rate was 10°C/min up to 300°C. About 2 mg of each sample was weighed using closed aluminum pans. Thermogram of pure drug (Chlorhexidine HCl) is shown in Figure 10. Melting transition of Chlorhexidine HCl was observed from 266.63°C (Onset) to 275.46°C (Endset). Sharp melting transition of Chlorhexidine HCl was observed at 272.08°C. In the optimized formulation drug and excipients had two melting endotherm was observed first from 36.72°C (Onset) to 181.09°C (Endset), and second was observed at 265.69°C (Onset) to 276.4209°C (Endset). Sharp melting transition of Chlorhexidine HCl in optimized formulation was observed at 271.06°C, which is shown in Figure 10. There was no much difference in the melting point of the drug in all the thermogram, it was concluded that the drug is in the same state, even in the optimized formulation without interacting with polymers. Co-operation of thermogram of pure drug and optimized formulation is shown in Figure 10.
Figure 10.
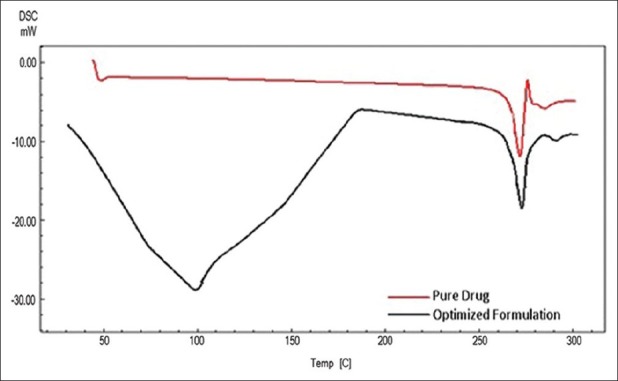
DSC thermogram of pure drug and optimized formulation
In vitro drug release
The effect of polymer concentration on in vitro drug release from in situ gels was shown in Figure 11. The cumulative percent of Chlorhexidine HCl released as a function of time is shown in Figure 11. The combination of Carbopol 934P with Poloxamer 407 decreased the release rate for Chlorhexidine HCl from gel. The results indicated that combination of bioadhesive agents with Poloxamer 407 could prolong drug action time in periodontal cavity due to the increase of viscosity with addition of Carbopol 934P. The mechanism for resistant barrier of single Poloxamer 407 gels to drug release may be due to reduction in the number and dimension of water channels and the increase in the number and size of micelles within the gel structure. The shorter intermicellar distance leads to greater numbers of cross-links between neighboring micelles, which result in higher viscosity and lower rate of drug release.[37]
Figure 11.
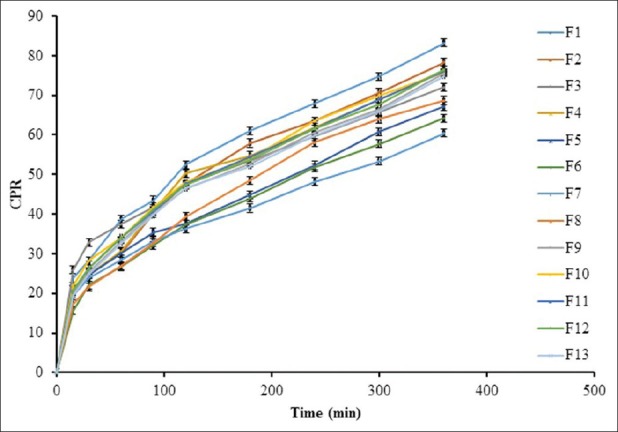
In vitro drug release profile of Chlorhexidine HCl
Optimization of the formulation using the desirability function
The desirability function was used for optimization of the formulation. During optimization of formulations, the responses have to be combined in order to produce a product of desired characteristics. The application of the desirability function combines all the responses in one measurement and gives the possibility of predicting optimum levels for the independent variables.[18]
The combination of the responses in one desirability function requires the calculation of the individual functions. A suitable in situ gel should have an optimum gelation temperature, maximum spreadability, minimum % drug release, and maximum time require to 50% of drug release (t50%). The individual desirability for each response was calculated using the following methods.[18,38] In this particular study, there were no specific requirements for gelation temperature of the optimum formulation. Therefore, the range of values of the produced formulations was selected. As moderate gelation temperature was desired, the formulations that have its value within the range of 25-37°C have a desirability of 1, while the formulations that have values outside this range have a desirability of 0. These can be described by the following equations:

Where d1 = the individual desirability of the gelation temperature.
The spreadability values were maximized in the optimization procedure, as suitable in situ gel should have high spreadability. The desirability functions of these responses were calculated using the following equation:
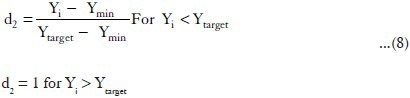
Where d2 = the individual desirability of Spreadability.
The CPR at 2 h value was minimized in the optimization procedure, as suitable in situ gel should have low CPR at 2 h. The desirability functions of this response were calculated using the following equation:
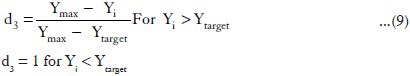
d3 = the individual desirability of % drug release at 2 h (CPR at 2 h).
The t50% values were maximized in the optimization procedure, as suitable in situ gel should have high t50%. The desirability functions of these responses were calculated using the following equation:
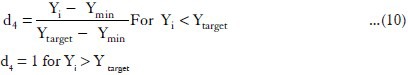
Where d4 = the individual desirability of t50%.
The overall desirability values were calculated from the individual values by using the following equation:[39]
![]()
Formulation of optimized formulation of in situ gel
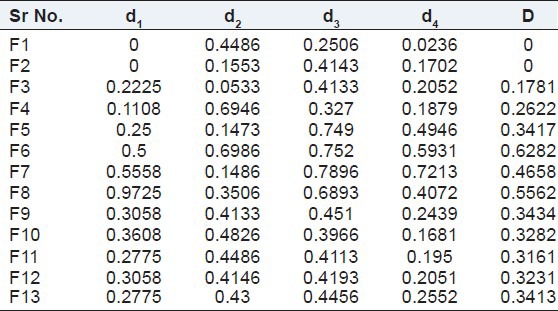
From the desirability function was utilized to find out the best batch out of 13 batches. Batch F6 showed the highest overall desirability of 0.6282 [Table 8]. Therefore, this batch was considered to be the best batch, and the values of independent variables of this batch were considered to be opti mum values for the preparation of in situ gel. The final optimized formulation was prepared using 19% w/v Poloxamer 407 and 0.2% w/v Carbopol 934P.
Table 8.
Individual and overall desirabilities for in situ gel
Comparison between observed and predicted results of checkpoint batches
In order to assess the reliability of the equations that describes the influence of the factors on the gelation temperature, spreadability, CPR at 2 h, and t50% of in situ gel. The experimental values and predicted values of each response are shown in Table 9. The % relative error between predicted values and experimental values of each response was calculated using the following equation:[38]
Table 9.
Comparison between observed and predicted results of checkpoint batches
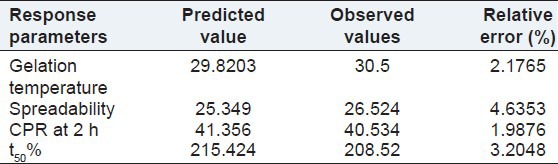
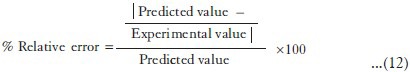
The % relative error obtained from checkpoint batch was in the range of 1.9876-4.6353. It can be seen that in all cases, there was a reasonable agreement of predicted values and experimental values, since low values of the relative error were found. This confirmed the role of a derived reduced polynomial equation, proved the validity of the model, and ascertained the effects of Poloxamer 407 and Carbopol 934P on dependent variables.
Stability studies
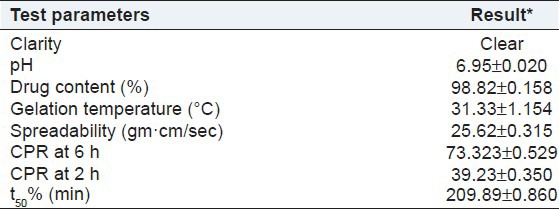
In situ gel formulation optimized from statistical design application and was selected for the stability studies. The accelerated stability studies were carried out according to ICH guidelines by storing the samples at 40 ± 2°C and 75 ± 5% RH for 1 month using stability chamber (Remi, India). The optimized formulation were evaluated for clarity, pH measurement, drug content, gelation temperature, spreadability, CPR at 6 h, CPR at 2 h, and t50%. The results after the stability period are given in Table 10. Results of the stability study show no remarkable change in the release profile, assay, and other evaluation parameters of the periodontal in situ gel after exposing to accelerated stability conditions.
Table 10.
Results of stability testing
CONCLUSION
Chlorhexidine hydrochloride, a broad-spectrum anti-microbial agent used in the treatment of periodontal disease, was successfully formulated as temperature-sensitive in situ gel by cold method using Poloxamer 407 and Carbopol 934P as gelling agent. The clarity, pH, and drug content of all formulations were found to be satisfactory. All the formulations showed sustained drug release for a period of 6 h. The desirability function was utilized in order to find out the best batch out of all 13 batches of the central composite design. Formulation F6 showed the highest overall desirability of 0.6282. Therefore, this formulation was considered to be the best formulation, and the values of independent variables of this formulation were considered to be optimum values for the preparation of in situ gel. The results of the stability study show no remarkable change in the release profile, assay, and other evaluation parameters of the periodontal in situ gel after exposing to accelerated stability conditions.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Vyas SP, Sihorkar V, Mishra V. Controlled and targeted drug delivery strategies towards intraperiodontal pocket diseases. J Clin Pharm Ther. 2000;25:21–42. doi: 10.1046/j.1365-2710.2000.00261.x. [DOI] [PubMed] [Google Scholar]
- 2.Haffajee AD, Socransky SS. Attachment level changes in destructive periodontal diseases. J ClinPeriodontol. 1986;13:461–75. doi: 10.1111/j.1600-051x.1986.tb01491.x. [DOI] [PubMed] [Google Scholar]
- 3.Iqbal Z, Jain N, Jain GK, Talegaonkar S, Ahuja A, Khar RK, et al. Dental therapeutic systems. Recent Pat Drug Deliv Formul. 2008;2:58–67. doi: 10.2174/187221108783331366. [DOI] [PubMed] [Google Scholar]
- 4.Nagarwal RC, Srinatha A, Pandit JK. In situ forming formulation: Development, evaluation and optimization using 33 factorial design. AAPS Pharm Sci Tech. 2009;10:977–84. doi: 10.1208/s12249-009-9285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahajan H, Shaikh H, Gattani S, Nerkar P. In situ gelling system based on thiolatedgellan gum as new carrier for nasal administration of dimenhydrinate. Int J Pharm Sci Nanotech. 2009;2:544–50. [Google Scholar]
- 6.Krishna MK, Ravindran SK, Vivekanandan G, Navasivayam A, Thiagarajan R, Mohan R. Effect of a single episode of subgingival irrigation with tetracycline HCl or chlorhexidine: A clinical and microbiological study. J Indian Soc Periodontol. 2011;15:245–9. doi: 10.4103/0972-124X.85668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wannachaiyasit S, Phaechamud T. Development of chlorhexidine thermosensitive gels as a mouth antiseptic. J Miner Met Mater Soc. 2010;20:165–8. [Google Scholar]
- 8.Schwach-Abdellaouia K, Vivien-Castionib N, Gurny R. Local delivery of antimicrobial agents for the treatment of periodontal diseases. Eur J Pharm Biopharm. 2000;50:83–99. doi: 10.1016/s0939-6411(00)00086-2. [DOI] [PubMed] [Google Scholar]
- 9.Ruel-Gariépy E, Leroux JC. In situ-forming hydrogels: Review of temperature-sensitive systems. Eur J Pharm Biopharm. 2004;58:409–26. doi: 10.1016/j.ejpb.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Klouda L, Mikos AG. Thermoresponsive hydrogels in biomedical applications. Eur J Pharm Biopharm. 2008;68:34–45. doi: 10.1016/j.ejpb.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nirmal HB, Bakliwal SR, Pawar SP. In situ gel: New trends in controlled and sustained drug delivery system. Int J Pharm Tech Res. 2010;2:1398–408. [Google Scholar]
- 12.Jelvehgaria M, Rashidib MR, Samadia H. Mucoadhesive and drug release properties of benzocaine gel. Iranian J Pharm Sci Autumn. 2006;2:185–94. [Google Scholar]
- 13.Ji QX, Zhao QS, Deng J. A novel injectable chlorhexidinethermosensitive hydrogel for periodontal application: Preparation, antibacterial activity and toxicity evaluation. J Mater Sci Mater Med. 2010;21:2435–42. doi: 10.1007/s10856-010-4098-1. [DOI] [PubMed] [Google Scholar]
- 14.Jayaraj Kumar K, Jayachandran E, Srinivas GM, Giridhar B, Nair R, Jayakandan M. Formulation of thermoresponsive and buccal adhesive in situ gel for treatment of oral thrush containing itraconazole. J Pharm Sci Res. 2010;2:116–22. [Google Scholar]
- 15.Singh G, Pai RS, Devi VK. Response surface methodology and process optimization of sustained release pellets using taguchi orthogonal array design and central composite design. J Adv Pharm Technol Res. 2012;3:30–40. doi: 10.4103/2231-4040.93565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garala K, Patel J, Patel A, Dharamsi A. Enhanced encapsulation of metoprolol tartrate with carbon nanotubes as adsorbent. Appl Nanosci. 2011;1:219–230. [Google Scholar]
- 17.Harrington J. The desirability function. Ind Qual Control. 1965;21:494–8. [Google Scholar]
- 18.Derringer R, Suich R. Simultaneous optimization of several response variables. J Qual Technol. 1980;12:214–9. [Google Scholar]
- 19.Gupta H, Sharma A, Shrivastava B. Pluronic and chitosan based in situ gel system for periodontal application. Asian J Pharm. 2009:94–6. [Google Scholar]
- 20.Dabhi MR, Nagori SA, Gohel MC, Parikh RK, Sheth NR. Formulation development of smart gel periodontal drug delivery system for local delivery of chemotherapeutic agents with application of experimental design. Drug Deliv. 2010;17:520–31. doi: 10.3109/10717544.2010.490247. [DOI] [PubMed] [Google Scholar]
- 21.Nanjawade BK, Manvi FV, Manjappa AS. In situ forming hydrogels for sustained ophthalmic drug delivery. J Control Release. 2007;122:199–34. doi: 10.1016/j.jconrel.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Miller SC, Donovan MD. Effect of poloxamer 407 gel on the miotic activity of pilocarpine nitrate in rabbits. Int J Pharm. 1982;12:147–52. [Google Scholar]
- 23.Badgujar SD, Sontakke MA, Narute DR, Karmarkar RR, Tupkar SV, Barhate SD. Formulation and evaluation of sumatriptan succinate nasal in situ gel using fulvic acid as novel permeation enhancer. Int J Pharm Res Dev. 2010;2:1–8. [Google Scholar]
- 24.Kumar P, Awasthi R, Kumar PR, Kumar M, Kumar MP. Mucoadhesive in situ gels of local anaesthetic for periodontia. Der Pharm Lettre. 2010;2:28–39. [Google Scholar]
- 25.Patel RP, Baria AH, Pandya NB, Tank HM. Formulation evaluation and optimization of stomach specific in situ gel of ranitidine hydrochloride. Int J Pharm Sci Nanotech. 2010;3:834–43. [Google Scholar]
- 26.Wamorkar V, Varma MM, Manjunath SY. Formulation and evaluation of stomach specific in situ gel of metoclopramide using natural, bio-degradable Polymers. Int J Res Pharm Biomed Sci. 2011;2:193–201. [Google Scholar]
- 27.Harish NM, Prabhu P, Charyulu RN, Gulzar MA, Subrahmanyam EV. Formulation and evaluation of in situ gel containing clotrimazole for oral candidiasis. Indian J Pharm Sci. 2009;71:421–7. doi: 10.4103/0250-474X.57291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar L, Verma R. In vitro evaluation of topical gel prepared using natural polymer. Int J Drug Del. 2010;2:58–63. [Google Scholar]
- 29.Scherlund M, Welin-Berger K, Brodin A, Malmsten M. Local anaesthetic block copolymer system undergoing phase transition on dilution with water. Eur J Pharm Sci. 2001;14:53–61. doi: 10.1016/s0928-0987(01)00145-2. [DOI] [PubMed] [Google Scholar]
- 30.Shams MS, Alam MI, Ali A, Sultana Y, Aqil M. Pharmacodynamics of a losartan transdermal system for the treatment of hypertension. Drug Dev Ind Pharm. 2010;36:385–92. doi: 10.3109/03639040903188471. [DOI] [PubMed] [Google Scholar]
- 31.Gui-Ling L, Mei L. Preparation and evaluation of ophthalmic thermosensitive in situ gels of penciclovir. J Chinese Pharm Sci. 2007;16:90–5. [Google Scholar]
- 32.Majithiya RJ, Ghosh PK, Umrethia ML, Murthy SR. Thermoreversible-mucoadhesive gel for nasal delivery of Sumatriptan. AAPS Pharm Sci Tech. 2006;7:67. doi: 10.1208/pt070367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonjari ID, Hosmani AH, Karmarkar AB, Godage AS, Kadam SB, Dhabale PN. Formulation and evaluation of in situ gelling thermoreversible mucoadhesive gel of fluconazole. Drug Discov Ther. 2009;3:6–9. [PubMed] [Google Scholar]
- 34.Abdel-Mottaleb MM, Mortada ND, Elshamy AA, Awad GA. Preparation and evaluation of fluconazolegels. Egypt J Biomed Sci. 2007;23:266–86. [Google Scholar]
- 35.Vijayalakshmi A, Tripura A, Ravichandiran V. Development and evaluation of anti-acne products from terminaliaarjuna bark. Int J Chem Tech Res. 2011;3:320–7. [Google Scholar]
- 36.Patel RP, Dadhani B, Ladani R, Baria AH, Patel J. Formulation, evaluation and optimization of stomach specific in situ gel of clarithromycin and metronidazole benzoate. Int J Drug Del. 2010;2:141–53. [Google Scholar]
- 37.Bhardwaj R, Blanchard J. Controlled release delivery system for the α-MSH analog melanotan-1 using poloxamer 407. J Pharm Sci. 1996;85:915–9. doi: 10.1021/js960097g. [DOI] [PubMed] [Google Scholar]
- 38.Paterakis PG, Korakianiti ES, Dallas PP, Rekkas DM. Evaluation and simultaneous optimization of some pellets characteristics using 3 (3) factorial design and the desirability function. Int J Pharm. 2002;248:51–60. doi: 10.1016/s0378-5173(02)00341-1. [DOI] [PubMed] [Google Scholar]
- 39.Mashru RC, Sutariya VB, Sankalia MG, Parikh PP. Development and evaluation of fast-dissolving film of salbutamol sulphate. Drug Dev Ind Pharm. 2005;31:25–34. doi: 10.1081/ddc-43947. [DOI] [PubMed] [Google Scholar]


