Abstract
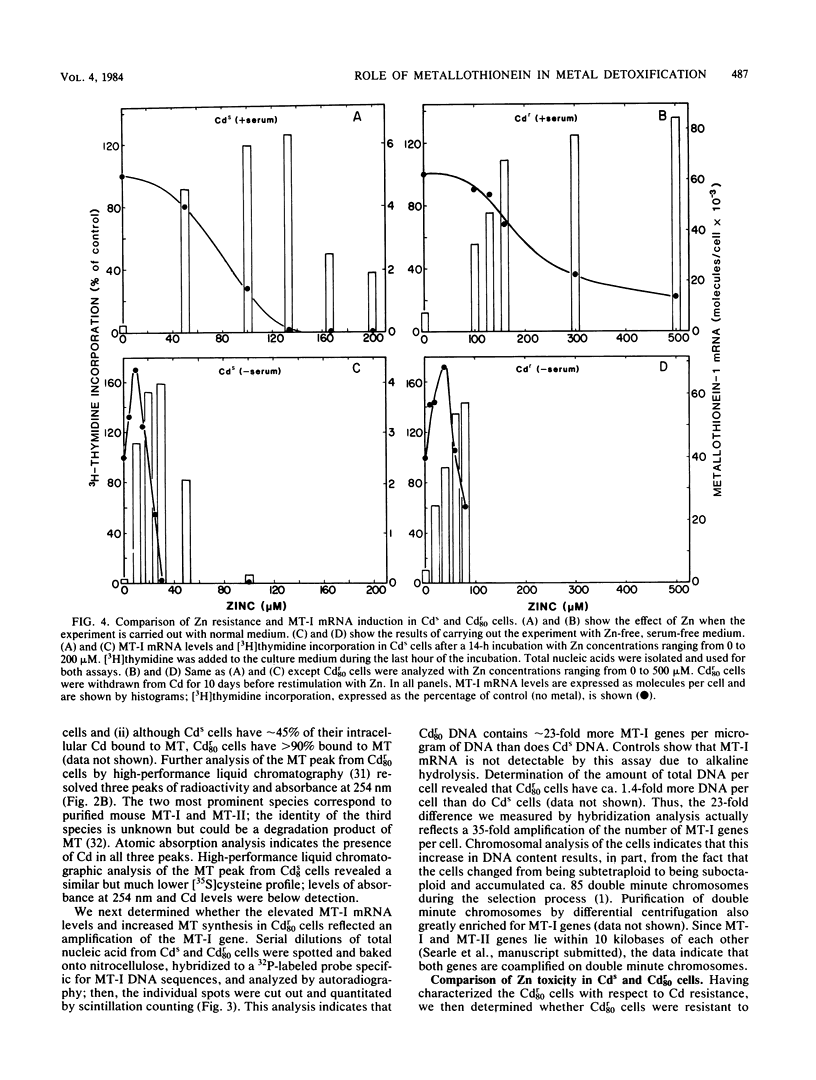
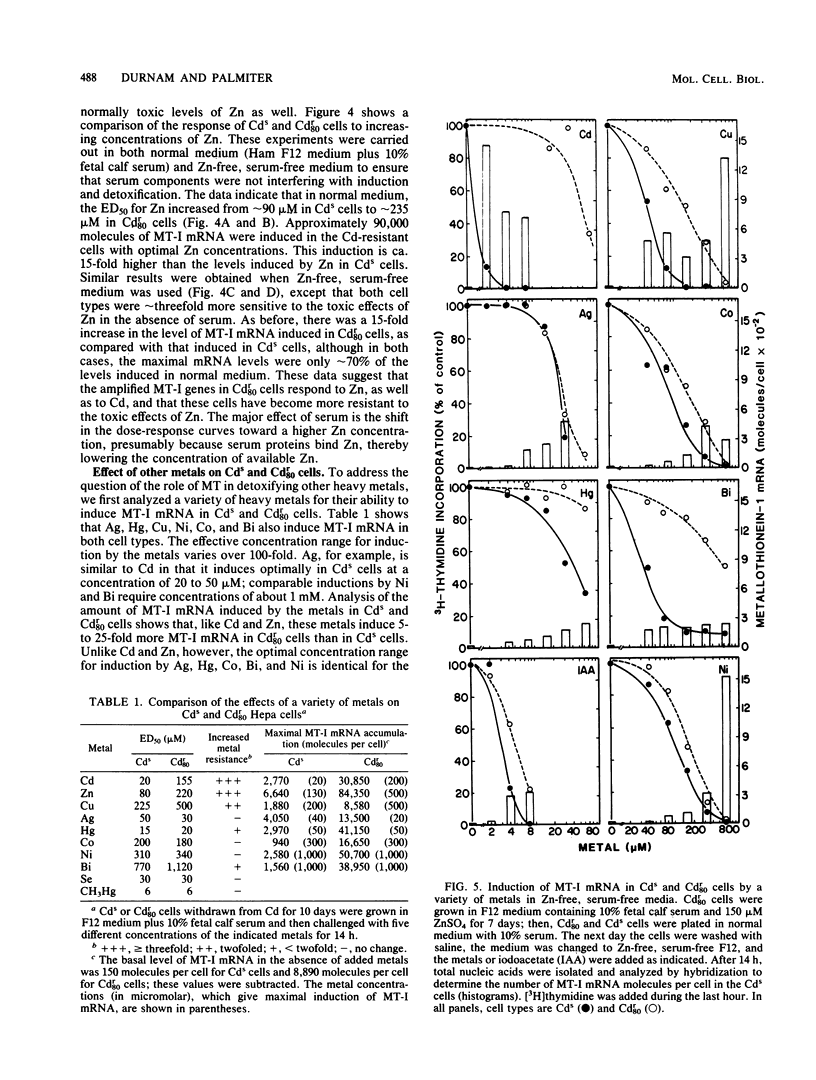
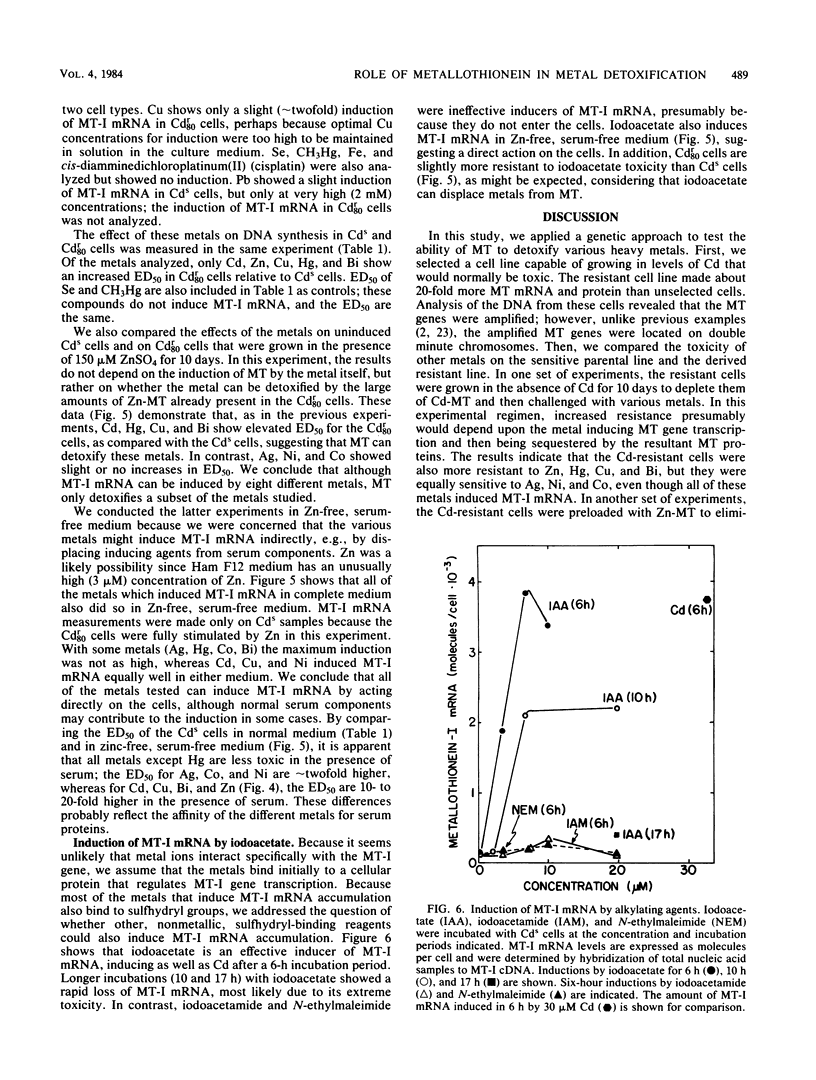
A mouse hepatocyte cell line selected for growth in 80 microM CdSO4 (Cdr80 cells) was used to test the role of metallothioneins in heavy metal detoxification. The cadmium-resistant (Cdr80) cells have double minute chromosomes carrying amplified copies of the metallothionein-I gene and accumulate ca. 20-fold more metallothionein-I mRNA than unselected cadmium-sensitive (Cds) cells after optimal Cd stimulation. As a consequence, the amount of Cd which inhibits DNA synthesis by 50% is ca. 7.5-fold higher in Cdr80 cells than in Cds cells. Cds and Cdr80 cells were compared in terms of their resistance to other heavy metals. The results indicate that although Zn, Cu, Hg, Ag, Co, Ni, and Bi induce metallothionein-I mRNA accumulation in both Cdr80 and Cds cells, the Cdr80 cells show increased resistance to only a subset of these metals (Zn, Cu, Hg, and Bi). This suggests that not all metals which induce metallothionein mRNA are detoxified by metallothionein and argues against autoregulation of metallothionein genes. Metallothionein-I mRNA is also induced by iodoacetate, suggesting that the regulatory molecule has sensitive sulfhydryl groups.
Full text
PDF
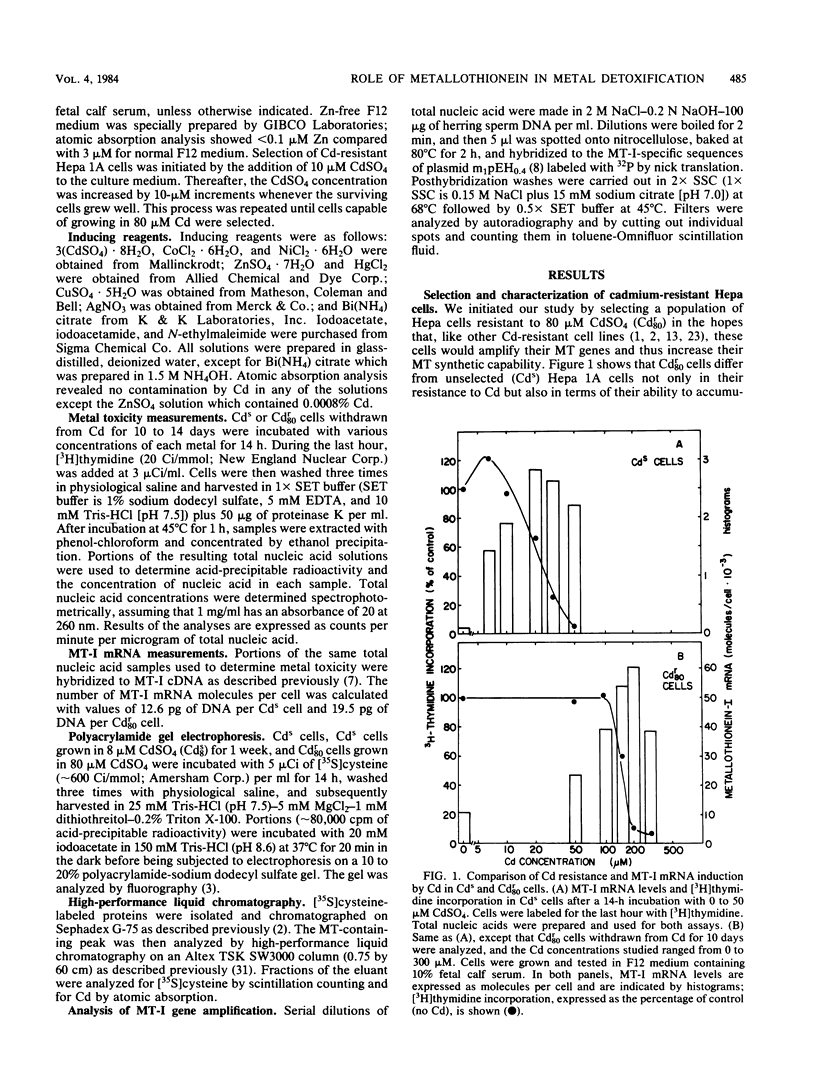
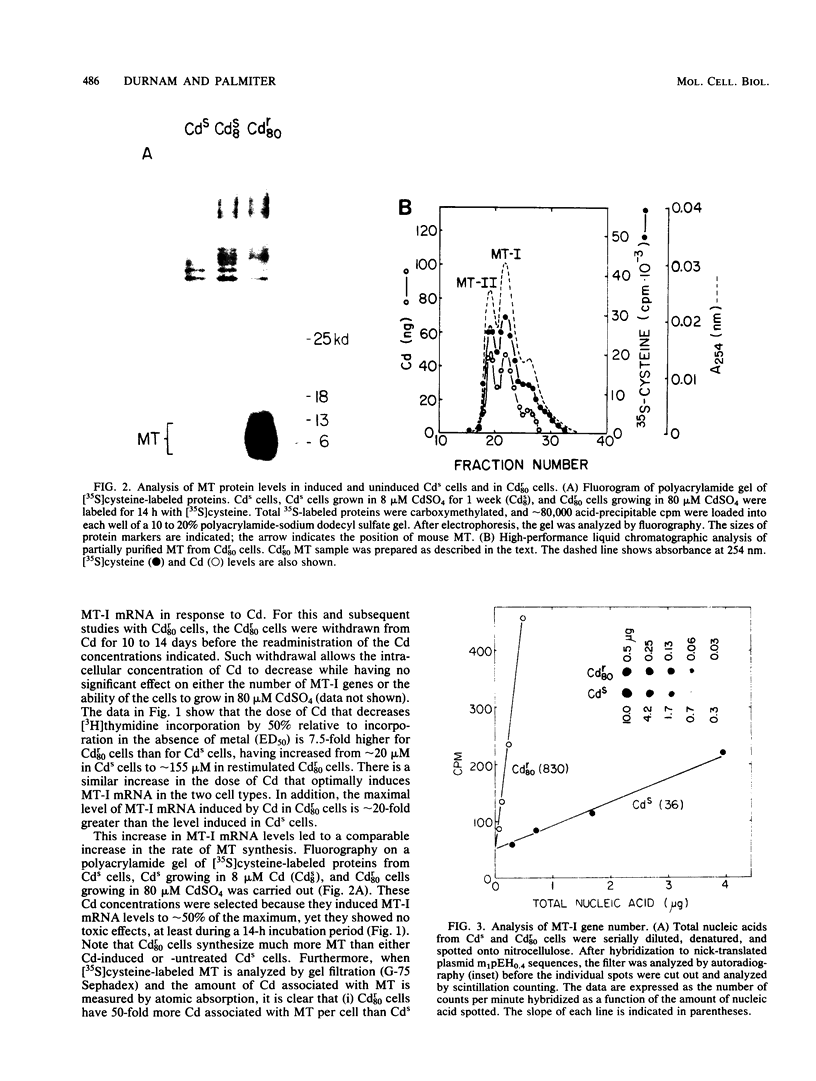


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beach L. R., Palmiter R. D. Amplification of the metallothionein-I gene in cadmium-resistant mouse cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2110–2114. doi: 10.1073/pnas.78.4.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Cherian M. G., Goyer R. A. Methallothioneins and their role in the metabolism and toxicity of metals. Life Sci. 1978 Jul 3;23(1):1–9. doi: 10.1016/0024-3205(78)90317-x. [DOI] [PubMed] [Google Scholar]
- Darlington G. J., Bernard H. P., Ruddle F. H. Human serum albumin phenotype activation in mouse hepatoma--human leukocyte cell hybrids. Science. 1974 Sep 6;185(4154):859–862. doi: 10.1126/science.185.4154.859. [DOI] [PubMed] [Google Scholar]
- Durnam D. M., Palmiter R. D. A practical approach for quantitating specific mRNAs by solution hybridization. Anal Biochem. 1983 Jun;131(2):385–393. doi: 10.1016/0003-2697(83)90188-4. [DOI] [PubMed] [Google Scholar]
- Durnam D. M., Palmiter R. D. Transcriptional regulation of the mouse metallothionein-I gene by heavy metals. J Biol Chem. 1981 Jun 10;256(11):5712–5716. [PubMed] [Google Scholar]
- Durnam D. M., Perrin F., Gannon F., Palmiter R. D. Isolation and characterization of the mouse metallothionein-I gene. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6511–6515. doi: 10.1073/pnas.77.11.6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton D. L., Stacey N. H., Wong K. L., Klaassen C. D. Dose-response effects of various metal ions on rat liver metallothionein, glutathione, heme oxygenase, and cytochrome P-450. Toxicol Appl Pharmacol. 1980 Sep 15;55(2):393–402. doi: 10.1016/0041-008x(80)90101-5. [DOI] [PubMed] [Google Scholar]
- Evans R. M., Patierno S. R., Wang D. S., Cantoni O., Costa M. Growth inhibition and metallothionein induction in cadmium-resistant cells by essential and non-essential metals. Mol Pharmacol. 1983 Jul;24(1):77–83. [PubMed] [Google Scholar]
- Gick G. G., McCarty K. S., Sr Amplification of the metallothionein-I gene in cadmium- and zinc-resistant Chinese hamster ovary cells. J Biol Chem. 1982 Aug 10;257(15):9049–9053. [PubMed] [Google Scholar]
- Gick G., McCarty K. S., Jr, McCarty K. S., Sr The role of metallothionein synthesis in cadmium- and zinc-resistant CHO-K1M cells. Exp Cell Res. 1981 Mar;132(1):23–30. doi: 10.1016/0014-4827(81)90078-1. [DOI] [PubMed] [Google Scholar]
- Hager L. J., Palmiter R. D. Transcriptional regulation of mouse liver metallothionein-I gene by glucocorticoids. Nature. 1981 May 28;291(5813):340–342. doi: 10.1038/291340a0. [DOI] [PubMed] [Google Scholar]
- Hildebrand C. E., Tobey R. A., Campbell E. W., Enger M. D. A cadmium-resistant variant of the Chinese hamster (CHO) cell with increased metallothionein induction capacity. Exp Cell Res. 1979 Dec;124(2):237–246. doi: 10.1016/0014-4827(79)90199-x. [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Kimura M. Different inducibility of metallothionein in various mammalian cells in vitro. Toxicol Lett. 1980 Apr;5(5):357–362. doi: 10.1016/0378-4274(80)90038-7. [DOI] [PubMed] [Google Scholar]
- Kotsonis F. N., Klaassen C. D. Increase in hepatic metallothionein in rats treated with alkylating agents. Toxicol Appl Pharmacol. 1979 Oct;51(1):19–27. doi: 10.1016/0041-008x(79)90004-8. [DOI] [PubMed] [Google Scholar]
- Mayo K. E., Palmiter R. D. Glucocorticoid regulation of the mouse metallothionein I gene is selectively lost following amplification of the gene. J Biol Chem. 1982 Mar 25;257(6):3061–3067. [PubMed] [Google Scholar]
- Oh S. H., Deagen J. T., Whanger P. D., Weswig P. H. Biological function of metallothionein. V. Its induction in rats by various stresses. Am J Physiol. 1978 Mar;234(3):E282–E285. doi: 10.1152/ajpendo.1978.234.3.E282. [DOI] [PubMed] [Google Scholar]
- Otvos J. D., Armitage I. M. Structure of the metal clusters in rabbit liver metallothionein. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7094–7098. doi: 10.1073/pnas.77.12.7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowski J. K., Szymańska J. A. Influence of certain metals on the level of metallothionein-like proteins in the liver and kidneys of rats. J Toxicol Environ Health. 1976 Jul;1(6):991–1002. doi: 10.1080/15287397609529402. [DOI] [PubMed] [Google Scholar]
- Richards M. P., Cousins R. J. Isolation of an intestinal metallothionein induced by parenteral zinc. Biochem Biophys Res Commun. 1977 Mar 21;75(2):286–294. doi: 10.1016/0006-291x(77)91041-5. [DOI] [PubMed] [Google Scholar]
- Rudd C. J., Herschman H. R. Metallothionein in a human cell line: the response of HeLa cells to cadmium and zinc. Toxicol Appl Pharmacol. 1979 Feb;47(2):273–278. doi: 10.1016/0041-008x(79)90321-1. [DOI] [PubMed] [Google Scholar]
- Rugstad H. E., Norseth T. Cadmium resistance and content of cadmium-binding protein in two enzyme-deficient mutants of mouse fibroblasts (L-cells). Biochem Pharmacol. 1978 Mar 1;27(5):647–650. doi: 10.1016/0006-2952(78)90499-9. [DOI] [PubMed] [Google Scholar]
- Suzuki K. T., Motomura T., Tsuchiya Y., Yamamura M. Separation of metallothioneins in rat liver, kidney, and spleen using SW and Sephadex columns. Anal Biochem. 1980 Sep 1;107(1):75–85. doi: 10.1016/0003-2697(80)90495-9. [DOI] [PubMed] [Google Scholar]
- Suzuki K. T., Yamamura M., Yamada Y. K., Shimizu F. Distribution of cadmium in heavily cadmium-accumulated rat liver cytosols: metallothionein and related cadmium-binding proteins. Toxicol Lett. 1981 Apr;8(1-2):105–114. doi: 10.1016/0378-4274(81)90145-4. [DOI] [PubMed] [Google Scholar]
- Winge D. R., Premakumar R., Rajagopalan K. V. Metal-induced formation of metallothionein in rat liver. Arch Biochem Biophys. 1975 Sep;170(1):242–252. doi: 10.1016/0003-9861(75)90115-0. [DOI] [PubMed] [Google Scholar]