Abstract
OBJECTIVE
To establish minimal clinically important difference (MCID) scores representing the smallest detectable change in quality of life (QOL), using the Pediatric Quality of Life Inventory (PedsQL) Generic Core and Diabetes Module among youth with diabetes and their parents, and to identify demographic and clinical correlates of QOL change over 1 year.
RESEARCH DESIGN AND METHODS
Participants in the SEARCH for Diabetes in Youth Study aged >5 years and parents of youth aged <18 years completed PedsQL surveys at their initial and 12-month study visits. MCIDs for each PedsQL module were calculated using one standard error of measurement. Demographic and clinical characteristics associated with QOL change were identified through multiple linear and logistic regression analyses.
RESULTS
The sample comprised 5,004 youth (mean age, 12.5 ± 4.7 years; mean diabetes duration, 3.4 ± 3.7 years). Of 100 possible points, PedsQL total score MCIDs for youth with type 1 and type 2 diabetes, respectively, were Generic Core, 4.88, 6.27 (parent) and 4.72, 5.41 (youth); Diabetes Module, 4.54, 6.06 (parent) and 5.27, 5.96 (youth). Among 1,402 youth with a follow-up visit, lower baseline QOL, male sex, private insurance, having type 1 diabetes, longer diabetes duration, and better glycemic control predicted improvements in youth- and parent-reported PedsQL total scores over 1 year. Clinically meaningful (≥1 MCID) improvements in total score for at least one PedsQL module were predicted by private insurance, lower BMI, and lower A1C at baseline.
CONCLUSIONS
These diabetes-specific reference points to interpret clinically meaningful change in PedsQL scores can be used in clinical care and research for youth with type 1 and type 2 diabetes.
Quality of life (QOL) is an important health outcome representing subjective well-being in domains directly and indirectly related to a chronic illness and its management (1,2), including physical symptoms, disease management and barriers, disease-related worries, and social, academic, and emotional functioning (3). Clinical trials research frequently uses QOL as a clinically relevant patient-reported outcome (PRO) (2,4). In pediatric diabetes, the importance of QOL and its associations with diabetes management and control have gained increasing attention (5). Impairments in general and health-related QOL have demonstrated links with suboptimal outcomes, including poor glycemic control (6–10).
Clinicians in pediatric diabetes clinics may observe fluctuations in their patients’ mood, behavior, and QOL and may be concerned how these fluctuations will affect their patients’ overall health and well-being. Likewise, clinical trials researchers who measure QOL as a key PRO must speculate about the degree to which observed changes in participants’ scores are meaningful in interpreting their trial results. Unlike other screening instruments (e.g., for depression), QOL assessments do not have clinical cutoffs indicating clinically meaningful values. As such, there is a need for a reference point for clinicians and researchers to interpret the clinical significance of changes in youths’ QOL over time.
The Pediatric Quality of Life Inventory (PedsQL) is a commonly used, psychometrically sound measure of general (Generic Core) and diabetes-specific (Diabetes Module) health-related QOL (3). The content of the Diabetes Module reflects the substantial medical and technological advances in diabetes care that have occurred during the past 2 decades (3). For clinicians who routinely administer the PedsQL to track patient QOL and clinical trials researchers who evaluate the effect of treatment on QOL, it is important to establish the amount of change in PedsQL scores that youth with diabetes and their parents perceive as meaningful.
Clinically meaningful change in QOL occurs when an individual with a chronic condition perceives an improvement or worsening in his or her subjective well-being. The minimal clinically important difference (MCID) of a QOL measure is a numerical value indicating the smallest clinically meaningful amount of QOL change that can be detected (11,12). To date, no MCIDs have been calculated for the PedsQL Diabetes Module. MCIDs for the PedsQL Generic Core have also not been calculated for youth with diabetes or their parents (10). Given the complexity of diabetes management, it is unclear whether the established PedsQL Generic Core MCID scores from other disease populations (13) can be applied to youth with diabetes.
The primary aim of this study was to calculate diabetes-specific MCIDs for the PedsQL parent- and youth-reports on the Generic Core and Diabetes Module for type 1 and type 2 diabetes. A secondary aim was to assess demographic and clinical correlates and predictors of change in QOL meeting the MCID threshold during a 12-month follow-up period for participants with longitudinal data.
RESEARCH DESIGN AND METHODS
SEARCH for Diabetes in Youth (SEARCH) is a multicenter observational study of youth diagnosed with diabetes before age 20 years. Participants were recruited from four geographically defined populations in Ohio, Washington, South Carolina, and Colorado, health plan enrollees in Hawaii and California, and Indian Health Service beneficiaries from four American Indian populations. All registered patients were invited by mail, telephone, or at a clinic visit to complete a brief initial survey, and all survey completers whose diabetes was not secondary to other conditions were invited by telephone or at a clinic visit to complete a baseline research visit.
Participants whose diabetes was incident in 2002–2005 who completed the baseline study visit were invited for follow-up visits at 12, 24, and 60 months after their baseline study visit. During these visits, participants provided informed consent or, for those younger than 18 years old, assent and completed questionnaires. For metabolically stable participants (i.e., no episode of diabetic ketoacidosis during the previous month), fasting blood samples were drawn after a minimum 8-h overnight fast. The study was approved by the institutional review board(s) at each study site.
These analyses include data from baseline visits of the 2001 (prevalent) cohort and from the baseline and 12-month follow-up visits of the 2002–2005 (incident) cohorts. Baseline visits were completed by 45.7% of the eligible cohort (5,294 visits/11,592 subjects). After excluding 290 participants (5.5%) without at least one PedsQL module completed by youth or parent, the final baseline sample included 5,004 participants (2,462 prevalent and 2,542 incident subjects). These data were used to calculate MCIDs. Of the 2002–2005 incident cohorts who completed the baseline and 12-month follow-up visits (n = 1,449, 57% of incident sample with baseline visits), 47 (3.2%) without at least one PedsQL module were also excluded, resulting in a final sample of 1,402 participants to identify predictors of QOL change over time.
Youth and their parents reported on youths’ generic and health-related QOL using the PedsQL Generic Core and Diabetes Module (3). All youth aged 5 years and older completed an age-appropriate self-report version of the two PedsQL modules. Items were read to the child or given to the child to read, depending on the child’s reading skills. In addition, parents completed a parent-proxy version of the measures for all youth aged younger than 18 years at the time of their visit. Thus, participants aged 18 years and older had one score for each module (self-report), participants aged between 5 and 17 years received two scores for each module (youth and parent report), and participants aged younger than 5 years had one score for each module (parent report only). Scores reported by youth and parents on each module were analyzed separately.
For all versions, items assess the degree of difficulty youth experience with different aspects of everyday living, including physical symptoms and emotional, social, and academic functioning (Generic Core), as well as treatment adherence and barriers, diabetes-related worries, and communication with others about diabetes (Diabetes Module). Item scores were transformed, with subscale and total scale scores ranging from 0 to 100. Higher scores reflect better QOL. The PedsQL Generic Core and Diabetes Module are psychometrically sound (3).
Demographic and medical information was collected by self-report or parent report and medical record review. Participants’ self-reported race and ethnicity were collected using standard census questions. Those who self-reported being of Hispanic ethnicity were categorized as Hispanic, regardless of race, and the remainder were categorized by self-reported race. If no or multiple races were endorsed, participants were categorized as “other/unknown race/ethnicity.” Health insurance was categorized as private, state/federally funded, other (including Indian Health Service, student health clinics, military, or other/unknown sources), or none based on self-report.
Clinical data, including physical measurement and fasting blood samples, were obtained at the baseline SEARCH study visit. Glycemic control was assessed via glycated hemoglobin (A1C) using high-performance liquid chromatography (Tosoh Bioscience, Montgomeryville, PA). BMI was calculated from height and weight measured at the initial study visit, and z-scores (zBMI) were calculated using Centers for Disease Control and Prevention algorithms (14,15). Diabetes type was determined by physician diagnosis, and comorbid medical diagnoses were based on self-report or parent report.
Data analysis
The mean (SD) and scale reliability (Cronbach α) values were calculated for each subscale and the total scores for the Generic Core and the Diabetes Module. MCIDs were calculated using a commonly used distribution-based method: one standard error of measurement (SEM) (12,16). The following equation is used to calculate the SEM: SEM = SD√ [1− α], where α is the total score or subscale reliability estimate. The SEM estimates the variation in scores due to the measurement precision in the scale and assumes that a change in scores smaller than the value of the SEM likely results from measurement error rather than a meaningful increase or decrease in the value of the construct being measured (in this case, QOL). A score change greater than or equal to the value of the SEM represents meaningful variation in the measured construct that is likely not due to measurement error. MCIDs for various QOL (17,18) measures, including the MCID for the PedsQL across a range of pediatric chronic conditions (13), have been calculated using this approach. This statistical methodology produces MCIDs that are expressed in the same units of measurement as the QOL score (19). The SEM and resulting MCIDs were calculated for the sample of participants and parents for the total and subscale scores of the Generic Core and Diabetes Module and were also established separately for each diabetes type. The result is a single value that represents the magnitude of change in scores on QOL measures that is detectable to the individual with the chronic condition. Thus, the MCID represents a clinically meaningful or important difference in QOL that can be referenced in clinical practice and research.
To examine predictors of change in QOL, two sets of regression analyses were conducted using data from the subset of participants diagnosed from 2002–2005 with a 12-month follow-up visit and complete data at both time points (total N = 1,402 [n range 1,072–1,094] across the two modules and two reporters). General linear regression models were constructed to determine the demographic and clinical characteristics that predicted the amount and direction of improvement or worsening in PedsQL change scores. PedsQL change scores were calculated as total score at the follow-up visit minus total score at the baseline visit. Predictors were demographic and clinical variables that have previously demonstrated associations with QOL, including age, sex, insurance coverage, race/ethnicity, diabetes type, diabetes duration, z-BMI, and A1C, all of which were assessed at the baseline visit, and models controlled for PedsQL scores at baseline.
Next, to determine the characteristics that predicted clinically meaningful improvement in QOL, participants’ PedsQL change scores for 1 year were categorized as positive (score increase ≥1 MCID), negative (score decrease ≥1 MCID), or no change. Binary logistic regression models were constructed to predict positive QOL change of at least 1 MCID compared with negative/no QOL change, controlling for all measured demographic and medical variables noted above, time from baseline to follow-up, and baseline PedsQL scores. Separate analyses were conducted for parent and child report of the Generic Core and Diabetes Module total scores.
RESULTS
Characteristics of participants
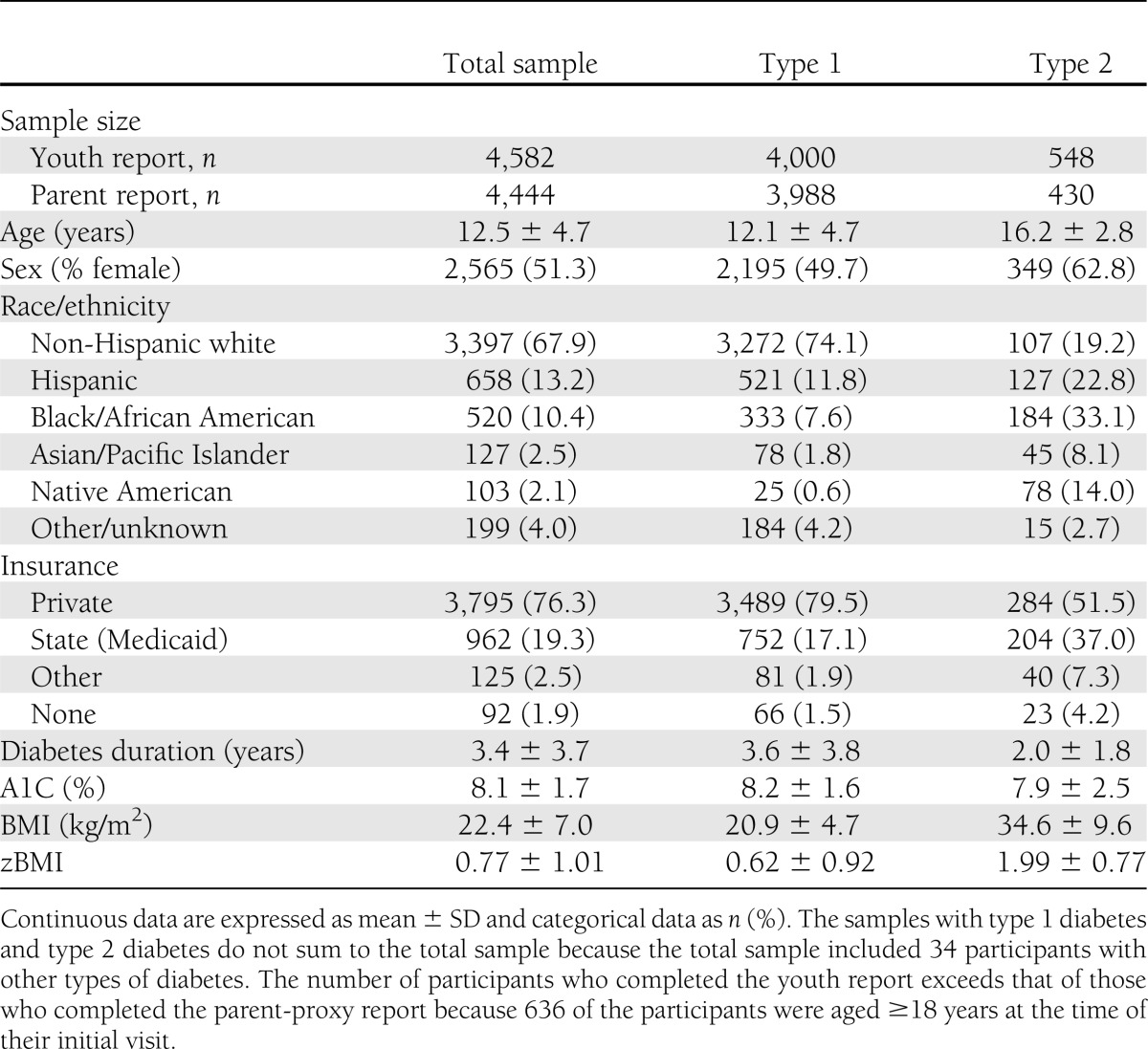
The study sample was composed of 5,004 children, adolescents, and young adults diagnosed with type 1 or type 2 diabetes in 2002 through 2005 (n = 4,393) or whose diabetes was prevalent in 2001 (n = 611). Youth were a mean age of 12.5 years (SD, 4.7; range, 2–22 years), 51.3% female, 67.9% non-Hispanic white, and 19.3% were publically insured (Medicaid/Medicare). The demographic and clinical characteristics of participants in the study sample stratified by diabetes type are reported in Table 1.
Table 1.
Baseline demographic and clinical characteristics of 5,004 study participants, by diabetes type: SEARCH for Diabetes in Youth Study, 2001–2005
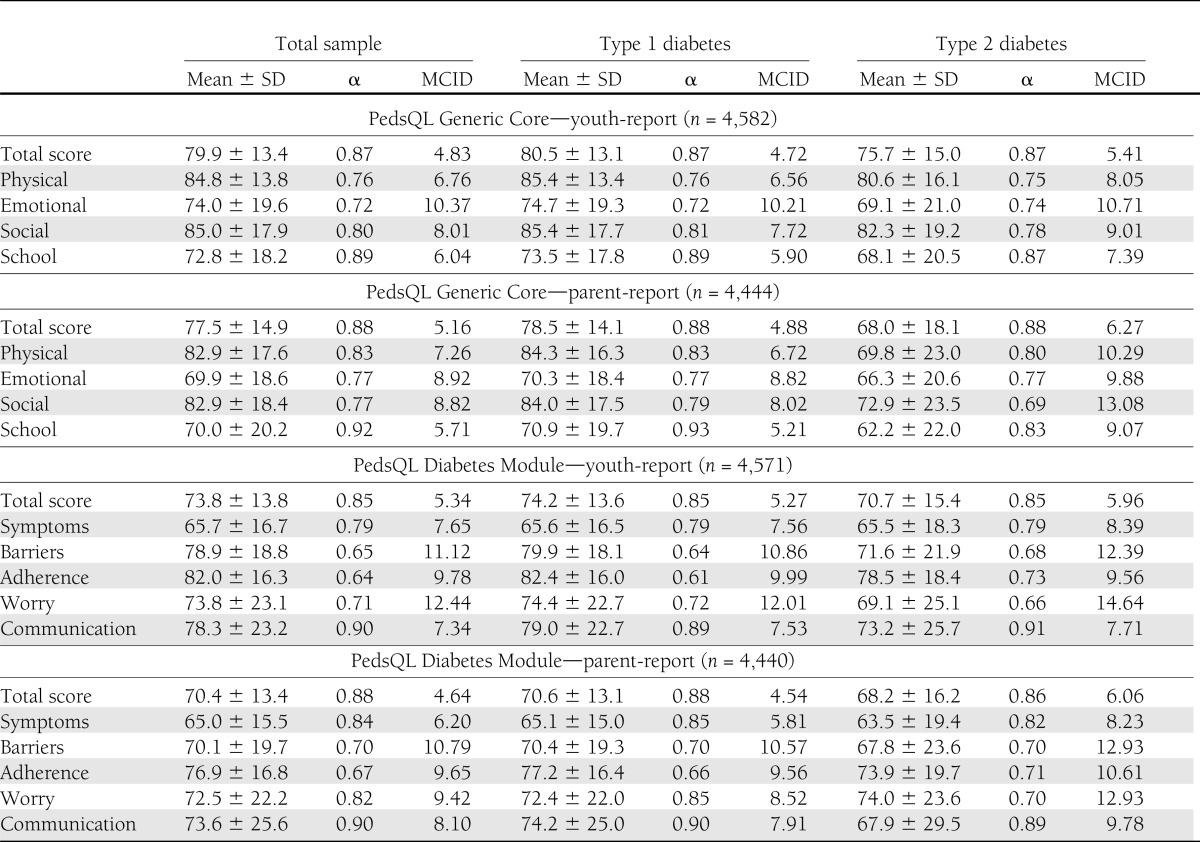
The internal consistency of the PedsQL total scores for the sample was excellent for the Generic Core (youth report: α = 0.87, parent report: α = 0.88) and Diabetes Module (youth report: α = 0.85, parent report: α = 0.88). Subscale α values were more variable (Table 2). In most cases, the youth- and parent-reported total and subscale scores were consistently higher for type 1 diabetes than for type 2 diabetes, although the SDs were quite large for both groups.
Table 2.
MCIDs for the PedsQL Generic Core and Diabetes Module at the baseline study visit, by reporter (youth/parent) and diabetes type: SEARCH for Diabetes in Youth Study, 2001–2005
MCIDs
The PedsQL was completed by 4,041 participant–parent pairs for participants aged between 5 and 17 years, 551 participants aged 18 years or older, and 412 parents of participants aged younger than 5 years. The MCID for the Generic Core total score was 4.83 for youth-report and 5.16 for parent-report (Table 2). For youth with type 1 diabetes, the Generic Core MCIDs were 4.72 for youth-report and 4.88 for parent-report. The Generic Core MCIDs were higher for those with type 2 diabetes compared the MCIDs for youth with type 1 diabetes; MCIDs among youth with type 2 diabetes were 5.41 for youth-report and 6.27 for parent-report. The MCID for the Diabetes Module total score was 5.34 for youth-report and 4.64 for parent-report. The Diabetes Module MCIDs for participants with type 1 diabetes were 5.27 for youth-report and 4.54 for parent-report. As with the Generic Core MCIDs, the Diabetes Module MCIDs were higher among youth with type 2 diabetes: 5.96 for youth-report and 6.06 for parent-report. Subscale MCIDs for the Generic Core and Diabetes Module are presented in Table 2.
Predictors of change in QOL scores during 1 year
Longitudinal analyses were conducted with data from 1,402 youth with at least one parent- or youth-reported score on the Generic Core or Diabetes Module at the 12-month follow-up visit (n range 1,072–1,984 across the two modules and two reporters; Table 3). This subset comprises 55% of the 2002–2005 incident cohorts that were included in the baseline visit sample for this study. The 12-month follow-up visits occurred on average 14.6 months (SD 3.1 [range 6.0–28.1]) after the initial visit. The youth in the longitudinal analysis were younger and had shorter disease duration (due to the exclusion of the participants whose diabetes was prevalent in 2001), and lower zBMI (all P < 0.05) than youth in the larger cross-sectional sample used to calculate MCIDs at baseline.
Table 3.
Linear regression analysis of predictors of change in PedsQL total scores* between the baseline and 12-month follow-up visit: SEARCH for Diabetes in Youth Study, 2002–2005 incident cases
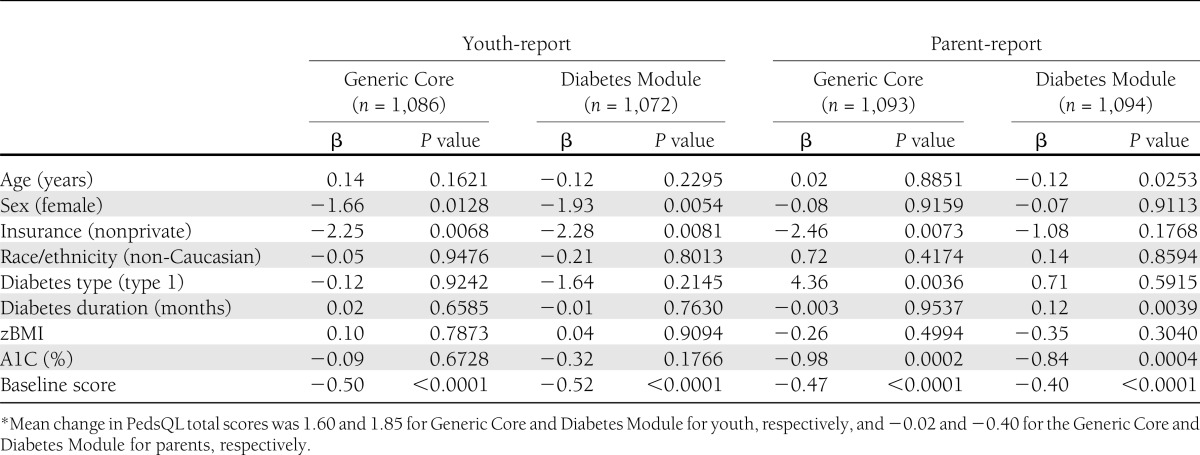
In the linear regression models, improvements in youth-reported Generic Core and Diabetes Module PedsQL change scores during 1 year were predicted by lower baseline QOL (β = −0.50 and β = −0.52, respectively; P < 0.0001), male sex (β = −1.66 and β = −1.93, respectively; P < 0.05), and being privately insured (β = −2.25 and β = −2.28, respectively; P < 0.01). For parent-reported scores, lower baseline QOL (β = −0.47, P < 0.0001), private insurance (β = −2.46, P < 0.01), diagnosis of type 1 diabetes (β = 4.36, P < 0.01), and lower baseline A1C (β = −0.98, P < 0.001) predicted greater improvements in Generic Core change scores. Lower baseline QOL (β = −0.40, P < 0.0001), lower baseline A1C (β = −0.84, P < 0.001), and longer duration of diabetes (β = 0.12, P < 0.01) predicted greater improvement in Diabetes Module change scores.
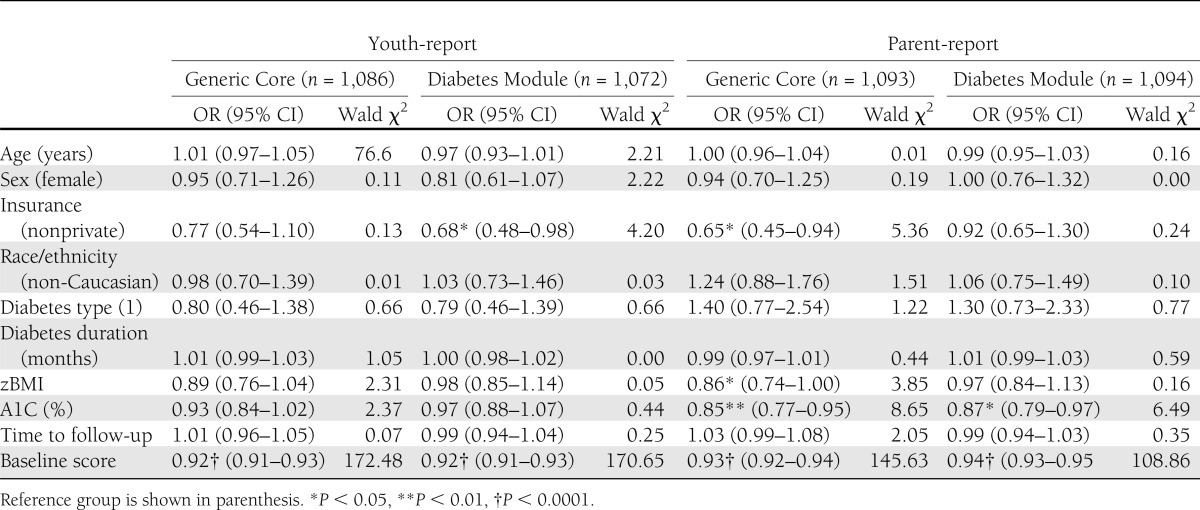
Across scales, reporters, and diabetes type, approximately one-third of the sample had PedsQL change scores that decreased by ≥1 MCID, one-third had no significant QOL change, and one-third had change scores that increased by ≥1 MCID. In the logistic regression models predicting a clinically meaningful improvement (≥1 MCID) in PedsQL change scores (Table 4), baseline QOL scores significantly predicted an improvement for both youth- and parent-reports on the Generic Core and Diabetes Module (OR range 0.92–0.94, all P < 0.0001). For youth-report, there were no additional predictors of clinically meaningful improvements on the Generic Core beyond baseline QOL scores, whereas clinically meaningful improvements in Diabetes Module scores were also predicted by being privately insured (OR 0.68 [95% CI 0.48–0.98], P < 0.05). For parent-report, beyond baseline QOL scores, clinically meaningful improvements on the Generic Core were also predicted by private insurance (OR 0.65 [0.45–0.94], P < 0.05), lower BMI (0.86 [0.74–1.00], P < 0.05), and lower A1C (0.85 [0.77–0.95], P < 0.01), whereas clinically meaningful improvements on the Diabetes Module were also predicted by lower A1C (0.87 [0.79–0.97], P < 0.05).
Table 4.
Logistic regression analysis to identify predictors of ≥1 MCID improvement on PedsQL total score between the baseline and 12-month follow-up study visits: SEARCH for Diabetes in Youth Study, 2002–2005 incident cases
CONCLUSIONS
The MCIDs for the PedsQL Generic Core total score established in this study were consistent with published Generic Core total score MCIDs in other chronic conditions (4.36 for youth-report and 4.50 for parent-report) (13), ranging between 4 and 6 points across reporter and diabetes type. The values for most of the Generic Core subscales in the current sample of youth with type 1 and type 2 diabetes were higher than the PedsQL Generic Core subscale MCIDs for youth with other chronic conditions, which are physical health, 6.66, 6.92; emotional functioning, 8.94, 7.79; social functioning, 8.36, 8.98; and school functioning, 9.12, 9.67, for youth- and parent-report, respectively (13). The only exception was the School Functioning subscale, which was lower for both youth- and parent-reports.
The higher MCIDs for most Generic Core subscales in this sample compared with other conditions suggests that across various domains of functioning, youth with diabetes seem to require more improvement or deterioration in QOL to perceive a clinically meaningful difference in their subjective experience than do youth with other chronic conditions. Given the complexity of the diabetes treatment regimen, a relatively large amount of change in QOL (5–10 points) may be needed for youth or their parents to perceive any meaningful differences in everyday functioning or activities. This pattern was especially pronounced in youth with type 2 diabetes, who had the highest MCID thresholds for clinically meaningful QOL change. Youth with type 2 diabetes report poorer QOL and encounter multiple physical complaints and social and emotional difficulties (9,20). In addition, given the higher zBMI scores among youth with type 2 diabetes compared with type 1 diabetes, there may be a cumulative effect of obesity and type 2 diabetes on some individuals’ QOL (21). As such, greater change in QOL may be necessary for youth with type 2 diabetes to perceive an improvement or deterioration in their subjective well-being.
For the Diabetes Module, the total score MCIDs were similar to the Generic Core total score MCIDs. Although there is no direct point of comparison for the subscale MCIDs, it is notable that those of the Diabetes Module are substantially higher than the subscale MCIDs for the Generic Core. In particular, the treatment barriers, treatment adherence, and worries subscale MCIDs tended to be at or above 10 points. The higher MCIDs for these subscales reflect their lower Cronbach’s α values. Although these subscales have not been supported by factor analyses conducted on responses from youth with type 1 diabetes (7,10), subscale MCIDs are reported here for comparison purposes and to illustrate mean score and α similarities between parents of youth with type 1 and type 2 diabetes, which had not previously been reported by the SEARCH study.
For clinicians and clinical trials researchers, fluctuations in QOL that do not meet the MCID threshold may not be noticeable by patients or their families yet should be monitored to determine whether more substantial challenges arise and require intervention. The relatively higher MCIDs for youth with diabetes than for youth with other chronic conditions may translate to a need for interventions that explicitly target various components of general and diabetes-specific QOL to achieve a change of sufficient magnitude for patient and parent perception of improvement. Interventions to improve QOL may benefit from a direct, targeted focus on barriers and facilitators of diabetes management and control. For example, given previous evidence of strong associations between lower QOL and more depressive symptoms (10), QOL interventions that address diabetes-specific emotional concerns may be particularly beneficial. These data support published and ongoing intervention studies that demonstrate benefits of providing psychological support and skills training to youth with diabetes (12,23).
In addition, efforts to improve QOL among youth with type 2 diabetes may achieve greater QOL change by addressing diabetes and also weight-related domains of everyday functioning. Associations between PedsQL scores and A1C suggest that interventions that address and improve QOL may ultimately translate to clinical improvements in glycemic control. One hypothesis is that patients who perceive better QOL may encounter fewer barriers to diabetes management and may be more motivated to adhere to diabetes treatment recommendations such as self-monitoring. Adaptation of established psychological interventions for youth with type 1 diabetes to meet the unique needs of youth with type 2 diabetes is needed (23).
Longitudinal findings demonstrate that a number of demographic and clinical variables predicted increases in PedsQL change scores over 1 year. The association between lower PedsQL scores at baseline and larger change scores at 12 months likely reflects that participants with poorer initial QOL had more opportunity for improvement. The associations of sex, insurance coverage, diabetes type, diabetes duration, and A1C at baseline with 12-month QOL improvements are consistent with previous studies examining changes in QOL after clinical or medical intervention (24,25) and help to identify patients who may experience QOL fluctuations over time. Given different predictors of parent- and youth-reported QOL change, interventions will need to be tailored to individuals’ unique experiences to influence each of their perceptions of QOL. Because parent-reported change scores were predicted by BMI and A1C, efforts to improve physical well-being and glycemic control may be particularly influential on parental perceptions of their children’s QOL. However, the finding that children’s QOL change scores were predicted by sex and insurance status suggests that girls and those with public insurance may be most likely to benefit from additional QOL monitoring. Through this monitoring, it will be necessary to engage youth in discussion to identify and address the factors that are affecting their QOL ratings.
The identification of PedsQL MCIDs also allowed us to determine the characteristics that predict who is most likely to experience clinically meaningful improvements in QOL. Private insurance coverage, lower BMI, and lower A1C at the baseline study visit predicted change that exceeded the threshold of perceived improvement in various QOL domains. Beyond the auto-correlational effects of baseline QOL, these variables may represent protective factors, such as greater access to resources or better health status, that help individuals rebound from periods of lower QOL and avoid long-lasting problems that may ultimately affect diabetes management and control.
The interpretation of this study’s findings should consider the response rates and limitations of the PedsQL modules and their analyses. As previously reported, the baseline visit response rate was lower than expected; older youth, African American youth, and those with type 2 diabetes were less likely to complete a visit (26). The PedsQL Diabetes Module was not developed or normed for youth with type 2 diabetes (3), so the results of the PedsQL Diabetes Module for youth with type 2 diabetes and their parents may have limited utility. Concerns about psychometric properties of the Diabetes Module subscales among youth with type 1 diabetes have been reported (7,10) suggesting that use of total scores, and the associated MCIDs, is more appropriate than subscale scores. In addition, MCIDs were calculated for all PedsQL subscales for consistency, but the lower α values for some of these subscales resulted in larger MCIDs, which should be used with caution. Finally, although we used the same approach to calculate MCIDs as used with the Generic Core for youth with other chronic conditions (13,18,19) and evidence demonstrates similarities between MCIDs established using statistical methods (e.g., 1 SEM) and those calculated in other ways (17), alternative MCID calculations might have yielded different results. Because concurrent measures of QOL were not collected, alternative MCID calculations could not be conducted.
Nonetheless, the establishment of diabetes-specific MCIDs for the PedsQL Generic Core and Diabetes Module for parents and youth provide an important reference point for clinicians and researchers interested in the effect of treatments on patient QOL. Clinical trials researchers may use these MCIDs to evaluate the degree to which an intervention improves participants’ QOL in a clinically significant way. Longitudinal research is needed to determine whether clinically meaningful changes in perceived QOL are associated with clinically meaningful changes in indices of diabetes health status, such as glycemic control and risk for acute complications.
Acknowledgments
The SEARCH for Diabetes in Youth Study is funded by the Centers for Disease Control and Prevention (PA number 00097, DP-05-069, and DP-10-001) and supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Site contract numbers: Kaiser Permanente Southern California: U48/CCU919219, U01 DP-000246, and U18 DP-002714; University of Colorado Denver: U48/CCU819241-3, U01 DP-000247, and U18 DP-000247-06A1; Kuakini Medical Center: U58CCU919256 and U01 DP-000245; Children’s Hospital Medical Center (Cincinnati): U48/CCU519239, U01 DP-000248, and U18 DP-002709; University of North Carolina at Chapel Hill: U48/CCU419249, U01 DP-000254, and U18 DP-002708-01; University of Washington School of Medicine: U58/CCU019235-4, U01 DP-000244, and U18 DP-002710-01; Wake Forest University School of Medicine: U48/CCU919219, U01 DP-000250, and 200-2010-35171; General Clinical Research Centers at the South Carolina Clinical and Translational Research Institute at the Medical University of South Carolina: National Institutes of Health (NIH)/National Center for Research Resources (NCRR) UL1 RR-029882; Children’s Hospital and Regional Medical Center: M01 RR-00037; Colorado Pediatric General Clinical Research Center: M01 RR-00069; the Barbara Davis Center at the University of Colorado at Denver: Diabetes Endocrinology Research Centers NIH P30 DK-57516; and Institutional Clinical and Translational Science Award, NIH/NCRR at the University of Cincinnati: 1UL1 RR-026314-01.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases.
No potential conflicts of interest relevant to this article were reported.
M.E.H. wrote the manuscript. J.M.L. and K.K.H. researched data, contributed to discussion, and reviewed and edited the manuscript. A.C.M. contributed to the discussion and reviewed and edited the manuscript. A.A., L.M.D., A.T.M., and J.P.Y.-.F. researched data and reviewed and edited the manuscript. T.C. reviewed and edited the manuscript. A.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The SEARCH for Diabetes in Youth Study is indebted to the many youth and their families and health care providers whose participation made this study possible.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1708/-/DC1.
A complete list of the members of the SEARCH for Diabetes in Youth Study Group can be found in the Supplementary Data online.
References
- 1.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care 2001;39:800–812 [DOI] [PubMed] [Google Scholar]
- 2.Wallander JL, Schmitt M, Koot HM. Quality of life measurement in children and adolescents: issues, instruments, and applications. J Clin Psychol 2001;57:571–585 [DOI] [PubMed] [Google Scholar]
- 3.Varni JW, Burwinkle TM, Jacobs JR, Gottschalk M, Kaufman F, Jones KL. The PedsQL in type 1 and type 2 diabetes. Diabetes Care 2003;26:631–637 [DOI] [PubMed] [Google Scholar]
- 4.Lohr KN, Zebrack BJ. Using patient-reported outcomes in clinical practice: challenges and opportunities. Qual Life Res 2009;18:99–107 [DOI] [PubMed] [Google Scholar]
- 5.Cameron FJ. The impact of diabetes on health-related quality of life in children and adolescents. Pediatr Diabetes 2003;4:132–136 [DOI] [PubMed] [Google Scholar]
- 6.Hoey H, Aanstoot HJ, Chiarelli F, et al. Good metabolic control is associated with better quality of life in 2,101 adolescents with type 1 diabetes. Diabetes Care 2001;24:1923–1928 [DOI] [PubMed] [Google Scholar]
- 7.Nansel TR, Weisberg-Benchell J, Wysocki T, Laffel L, Anderson B, Steering Committee of the Family Management of Diabetes Study Quality of life in children with Type 1 diabetes: a comparison of general and diabetes-specific measures and support for a unitary diabetes quality-of-life construct. Diabet Med 2008;25:1316–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guttmann-Bauman I, Flaherty BP, Strugger M, McEvoy RC. Metabolic control and quality-of-life self-assessment in adolescents with IDDM. Diabetes Care 1998;21:915–918 [DOI] [PubMed] [Google Scholar]
- 9.Naughton MJ, Ruggiero AM, Lawrence JM, et al. SEARCH for Diabetes in Youth Study Group Health-related quality of life of children and adolescents with type 1 or type 2 diabetes mellitus: SEARCH for Diabetes in Youth Study. Arch Pediatr Adolesc Med 2008;162:649–657 [DOI] [PubMed] [Google Scholar]
- 10.Lawrence JM, Yi-Frazier JP, Black MH, et al.; for the SEARCH for Diaebtes in Youth Study Group. Demographic and clinical correlates of diabetes-related quality of life among youth with type 1 diabetes. J Pediatr 2012;161:201-207.e2 [DOI] [PMC free article] [PubMed]
- 11.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials 1989;10:407–415 [DOI] [PubMed] [Google Scholar]
- 12.Crosby RD, Kolotkin RL, Williams GR. Defining clinically meaningful change in health-related quality of life. J Clin Epidemiol 2003;56:395–407 [DOI] [PubMed] [Google Scholar]
- 13.Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr 2003;3:329–341 [DOI] [PubMed] [Google Scholar]
- 14.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data 2000;314:1–27 [PubMed] [Google Scholar]
- 15.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11 2002;11:1–190 [PubMed] [Google Scholar]
- 16.Wyrwich KW, Wolinsky FD. Identifying meaningful intra-individual change standards for health-related quality of life measures. J Eval Clin Pract 2000;6:39–49 [DOI] [PubMed] [Google Scholar]
- 17.Modi AC, Zeller MH. The IWQOL-Kids(©): establishing minimal clinically important difference scores and test-retest reliability. Int J Pediatr Obes 2011;6:e94–e96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire-Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest 2009;135:1610–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wyrwich KW, Tierney WM, Wolinsky FD. Further evidence supporting an SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life. J Clin Epidemiol 1999;52:861–873 [DOI] [PubMed] [Google Scholar]
- 20.Anderson BJ, McKay SV. Psychosocial issues in youth with type 2 diabetes mellitus. Curr Diab Rep 2009;9:147–153 [DOI] [PubMed] [Google Scholar]
- 21.Zeller MH, Modi AC. Psychosocial factors related to obesity in children and adolescents. In Handbook of Child and Adolescent Obesity Jelalian E, Steele R, Eds. New York, Springer Science+Business Media, 2009, p. 25-32. [Google Scholar]
- 22.de Wit M, Delemarre-van de Waal HA, Bokma JA, et al. Monitoring and discussing health-related quality of life in adolescents with type 1 diabetes improve psychosocial well-being: a randomized controlled trial. Diabetes Care 2008;31:1521–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grey M. Coping skills training for youths with diabetes. Diabetes Spectr 2011;24:70–75 [Google Scholar]
- 24.de Wit M, Delemarre-van de Waal HA, Bokma JA, et al. Follow-up results on monitoring and discussing health-related quality of life in adolescent diabetes care: benefits do not sustain in routine practice. Pediatr Diabetes 2010;11:175–181 [DOI] [PubMed] [Google Scholar]
- 25.Hilliard ME, Goeke-Morey M, Cogen FR, Henderson C, Streisand R. Predictors of diabetes-related quality of life after transitioning to the insulin pump. J Pediatr Psychol 2009;34:137–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liese AD, Liu LL, Davis C, et al. Engaging pediatric and adolescent populations in epidemiologic research: The SEARCH for Diabetes in Youth Study experience. Contemp Clin Trials 2008;29:829–836 [DOI] [PMC free article] [PubMed] [Google Scholar]


