Abstract
OBJECTIVE
To assess the prognostic role of multidetector computed tomography coronary angiography (MDCT-CA) in patients with diabetes with suspected coronary artery disease (CAD). Use of MDCT-CA is increasing in patients with suspected CAD. However, data supporting its prognostic value in patients with diabetes are limited.
RESEARCH DESIGN AND METHODS
Between January 2006 and September 2007, 429 consecutive diabetic patients were prospectively studied with MDCT-CA for detecting the presence and assessing the extent of CAD (disease extension and coronary plaque scores). Patients were classified according to the presence of normal coronary arteries and nonobstructive (<50%) and obstructive (≥50%) coronary lesions. The composite rates of hard cardiac events (cardiac death, nonfatal myocardial infarction, unstable angina) and all cardiac events (including revascularization) were the end points of the study.
RESULTS
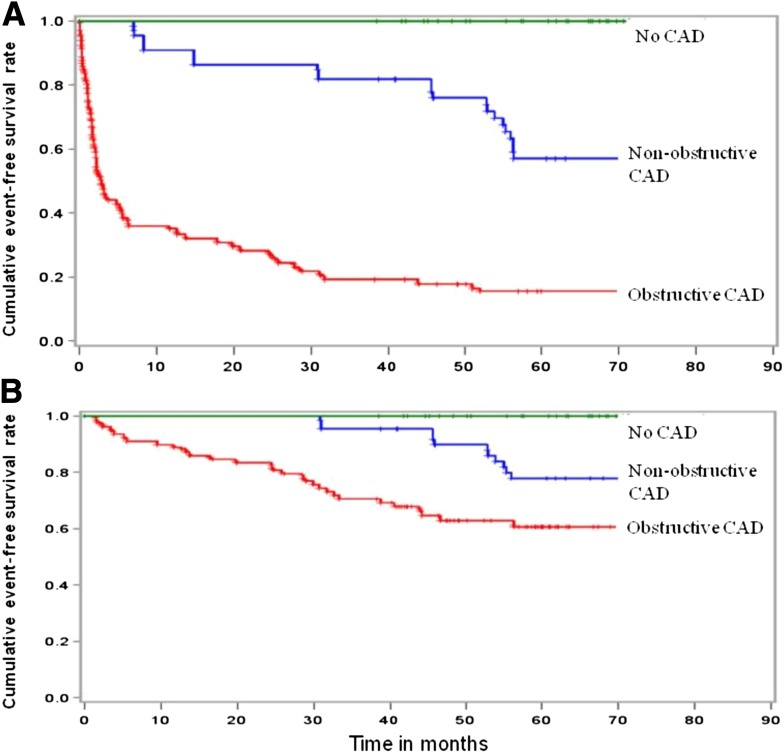
Twenty-four patients were excluded because MDCT-CA data were not able to be interpreted. Of the remaining 405 patients, clinical follow-up (mean 62 ± 9 months) was obtained in 390 (98%). Multivariate analysis showed that predictors of hard and all events were obstructive CAD, three-vessel CAD, and left main coronary artery (LMCA) disease. Cumulative event-free survival was 100% for hard and all events in patients with normal coronary arteries, 78% for hard events and 56% for all events in patients with nonobstructive CAD, and 60% for hard events and 16% for all events in patients with obstructive CAD. Three-vessel CAD and LMCA disease were associated with a higher rate of hard cardiac events.
CONCLUSIONS
MDCT-CA provides long-term prognostic information for patients with diabetes with suspected CAD, showing excellent prognosis when there is no evidence of atherosclerosis and allowing risk stratification when CAD is present.
Diabetes is associated with premature atherosclerosis (1) and increased risk of coronary artery disease (CAD), which is the most common cause of death in patients with diabetes (1). However, a diagnosis of CAD may be missed or delayed in patients with diabetes because the typical symptoms may be absent or elusive even in the presence of multivessel disease. Therefore, early detection of CAD in patients with diabetes is a major clinical need for the prevention of both fatal and nonfatal cardiac events. Unfortunately, cardiac stress imaging tests have a limited negative predictive value in patients with diabetes (2). Multidetector computed tomography coronary angiography (MDCT-CA) is a reliable imaging modality with high diagnostic performance for the detection of obstructive coronary lesions in patients with suspected CAD (3). Moreover, recent studies demonstrated that detection of CAD by MDCT-CA may predict cardiac events in patients with suspected CAD (4–7). The CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter) Registry, a large international multicenter study, strengthened the prognostic value of MDCT-CA still further and showed that patient risk for all-cause mortality differs with age and sex (8). However, data supporting the prognostic utility of MDCT-CA in patients with diabetes stem from only two studies, demonstrating that MDCT-CA is a predictor of major adverse cardiac events, yet with a limited midterm (20 and 33 months) follow-up (9,10). Thus, the aim of this study was to evaluate the long-term prognostic role of MDCT-CA in a population of patients with diabetes without known cardiac disease and in whom CAD was suspected.
RESEARCH DESIGN AND METHODS
Patients and study protocol
The study population consisted of 539 consecutive patients with diabetes who presented to our outpatient clinic or were admitted to our hospital for cardiac evaluation (exercise electrocardiogram, stress echocardiography, or invasive coronary angiography) because of suspected CAD (new-onset chest pain, abnormal stress test, multiple cardiovascular risk factors including diabetes) between January 2006 and September 2007. In all, MDCT-CA was performed in addition to a standard clinical work-up. A total of 90 patients were excluded because of known CAD (42 patients, of whom 27 had previous myocardial infarction and 15 had previous coronary revascularization) or other known cardiovascular diseases (48 patients, of whom 10 had heart failure, 4 had congenital heart disease, 20 had valvular disease, 7 had cardiomyopathy, 7 had aortic aneurysm). Other exclusion criteria were contraindications to contrast agents (five patients), impaired renal function (creatinine clearance <60 mL/min) (eight patients), inability to sustain a 15-s breath hold (two patients), and arrhythmias (five patients). Thus, the study population consisted of 429 subjects. In all patients, a previous diagnosis of diabetes had been confirmed by a diabetologist using serial fasting plasma glucose evaluations. Sixty-nine patients were classified as having type 1 diabetes and 321 had type 2 diabetes. The study was approved by our institution’s scientific and ethical committees, and all patients gave written informed consent. A structured interview was performed and a clinical history was acquired; the following cardiac risk factors were assessed before MDCT-CA: diabetes (glucose level of ≥7 mmol/L or the need for medications) (11), hypercholesterolemia (cholesterol level ≥5 mmol/L or treatment with lipid-lowering drugs) (12), hypertension (blood pressure ≥140/90 mmHg or use of antihypertensive medications) (13), positive family history of CAD (presence of CAD in first-degree relatives younger than 55 years [men] or 65 years [women]), (14) and current smoking. Pretest probability of CAD was determined using the Diamond-Forrester method (15).
MDCT-CA scan protocol, image reconstruction, and patient preparation
Metoprolol was administered intravenously before MDCT-CA with a titration dose up to 20 mg in patients with a heart rate >65 beats per minute. In all patients, MDCT-CA was performed using a 64-slice scanner (VCT, GE Medical System, Milwaukee, WI; 64 × 0.625 mm collimation, 330-ms gantry rotation time, 100 kVp tube voltage, and 600 mA tube current). Dose modulation was attained with “electrocardiographic gating” for a maximum gantry delivery between 40 and 80% during the R-R interval. A bolus of 80 mL of high-concentration contrast (Iomeron 400 mg/mL, Bracco, Milan, Italy) was administered intravenously at 5 mL/s, followed by 50 mL of saline injected at same infusion rate. The scan was initiated according to the bolus-tracking technique. Image data sets were analyzed using multiplanar reconstruction on postprocessing workstations (CardioQ3 package, Advantage Workstation version 4.2, GE Healthcare, Milwaukee, WI). The effective dose for MDCT-CA was calculated as the product of the dose-length product multiplied by a conversion coefficient for the chest (k = 0.014 mSv/mGy · cm).
MDCT-CA data analysis
All MDCT-CA examinations were evaluated by two expert readers who were unaware of the patients’ clinical data. In the case of disagreement, a joint reading was performed and a consensus decision was reached. Coronary arteries were divided into 16 segments according to American Heart Association classification (16). Each segment was classified as interpretable or not. Patients were excluded when proximal or mid-segment or more than 3 segments were not interpretable (4). Then, interpretable segments were evaluated for the presence of atherosclerotic plaques. Coronary plaques were defined as structures >1 mm2 within artery lumen, adjacent to artery lumen, or both and clearly distinguishable from vessel lumen and surrounding pericardial tissue (4). One coronary plaque was assigned per coronary segment, even in the presence of multiple plaques. Plaque type was determined using the following classification: 1) noncalcified (plaques having a lower density compared with the contrast-enhanced vessel lumen); 2) calcified (high-density plaques); and 3) mixed (noncalcified and calcified components within a single plaque). If a segment contained calcified and noncalcified plaques, we classified the plaque as calcified. The number of segments with noncalcified, calcified, and mixed plaques was recorded. Vessel segments were graded on the basis of visually estimated obstruction of coronary lumen as normal, nonobstructive lesions (<50%) and obstructive lesions (≥50%). The number of segments with any obstructive plaque also was recorded. Patients were divided into three groups: normal (no coronary plaques), nonobstructive CAD (lesions <50%), and obstructive CAD (lesions ≥50%). Moreover, coronary arteries were analyzed using two evaluation methods: the presence of obstructive lesions in major epicardial vessels and coronary plaque scores (4). To this purpose, we first analyzed MDCT-CA scans, and the number of major epicardial vessels exhibiting ≥50% stenosis was recorded. Patients with obstructive CAD in diagonal or obtuse marginal branches were included in the left anterior descending and left circumflex artery obstructive CAD groups, respectively. In case of obstructive stenosis of the left main coronary artery (LMCA), patients were assigned to this category even if they had other diseased vessels. Second, we analyzed the extent of atherosclerotic burden using two coronary artery plaque scores (4): 1) the segment-involvement score (SIS), that is, the number of segments (minimum = 0; maximum = 16) with at least one plaque, irrespective of the degree of stenosis; and 2) the segment-stenosis score (SSS), that is, the overall extent of coronary artery plaque. With the latter score, each coronary segment was graded as having no to severe plaque (i.e., score from 0 to 3, with no plaque = 0, stenosis <50% = 1, 50–69% stenosis = 2, and ≥70% stenosis = 3), based on the extent of obstruction of coronary lumen diameter. The SSS of the 16 coronary segments were summed to yield a total score, ranging from 0 to 48. For both scores, a cut off of 5 was used to differentiate patients with a low or high probability of cardiac events (4).
Follow-up
Follow-up, either a clinical visit or telephone interview, was performed by researchers who were blinded to MDCT-CA data. Our hospital records and outsourced clinical documents were screened for clinical events to confirm the information obtained. To avoid missing cardiac events, correspondence with treating physicians was done for all events. All events were reviewed by a blinded clinical event committee comprising two cardiologists. The mean follow-up was 62 ± 9 months. Outcome measures were a composite of hard cardiac events (cardiac death, nonfatal myocardial infarction, and unstable angina requiring hospitalization) and all cardiac events (cardiac death, nonfatal myocardial infarction, unstable angina requiring hospitalization, and revascularization). All deaths were reviewed and classified as cardiac (death caused by acute myocardial infarction, ventricular arrhythmias, or refractory heart failure) or noncardiac. All revascularizations were classified as early (elective revascularization within 60 days after MDCT-CA) or late. Only late revascularizations were considered as cardiac events, whereas patients with elective early revascularization were excluded from the analysis. The diagnosis of nonfatal myocardial infarction was based on the presence of typical chest pain, elevated cardiac enzymes, and typical electrocardiographic changes (17). Unstable angina was defined as acute chest pain with or without the presence of electrocardiographic abnormalities and no cardiac enzyme elevation (18).
Statistical analysis
Statistical analysis was performed using SAS (version 9.1.3, SAS Institute Inc., Cary, NC) and SPSS software (version 13.0, SPSS Inc., Chicago, IL). Statistical significance was defined as P < 0.05. Continuous variables are presented as mean ± SD, and discrete variables as absolute numbers and percentages. To compare patient characteristics and MDCT-CA data, χ2 or Fisher exact tests were used for categorical variables and the Student t test was used for continuous variables. When not normally distributed, continuous variables were expressed as median (25th to 75th percentile range) and compared using the nonparametric Mann-Whitney U test. To identify the association between MDCT-CA variables and outcomes, Cox regression analysis was used. First, univariate analysis of clinical characteristics and MDCT-CA variables was performed to identify potential predictors. Hazard ratios (HRs) were calculated with 95% CIs as an estimate of the risk associated with a particular variable. To determine independent predictors of the composite end points, multivariate analysis of MDCT-CA variables with P ≤ 0.05 in univariate analysis was performed, which was corrected for baseline characteristics (male sex, age, hypercholesterolemia, hypertension, family history of CAD, smoking). Moreover, another multivariate analysis of MDCT-CA variables stratified by age (<65 vs. ≥65 years) and by sex was performed. Cumulative event-free survival rates as a function over time were obtained using the Kaplan-Meier method. Hard and all cardiac event-free survival curves were compared using the log-rank test.
RESULTS
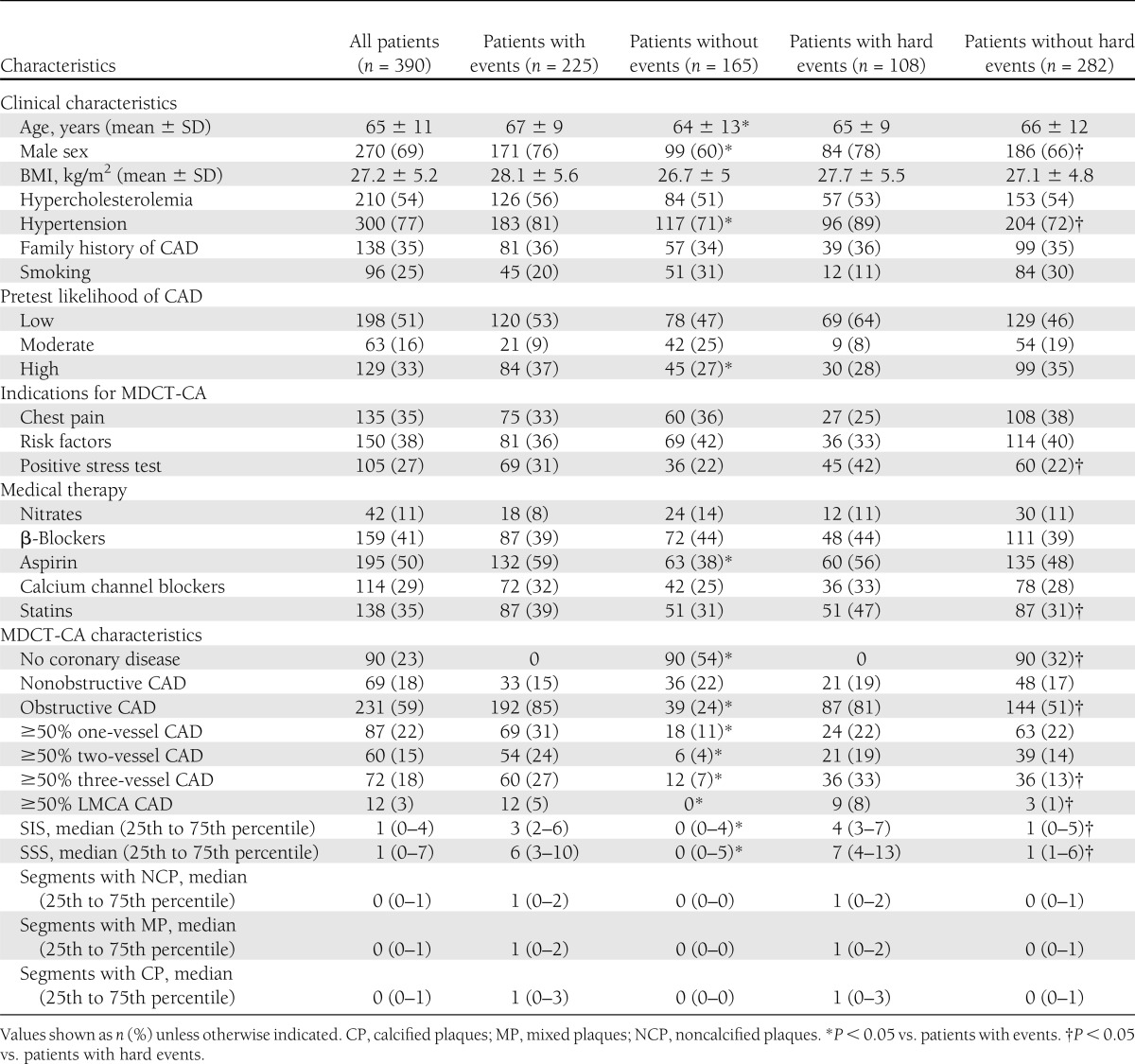
Of the 429 patients prospectively enrolled, 24 were excluded because MDCT-CA images were not interpretable. Of the remaining 405 patients, 15 were lost to follow-up (3 with normal coronary arteries, 3 with nonobstructive CAD, and 9 with obstructive CAD), whereas 390 (98%) had a complete follow-up (mean 62 ± 9 months, up to 72 months). Among these 390 patients, indications for MDCT-CA were chest pain (35%), multiple cardiac risk factors including diabetes (38%), and equivocal or abnormal stress test (27%). Mean pretest probability of CAD was 46 ± 28%. Blood glucose levels were controlled by diet in 40 patients, whereas oral antidiabetic medication and insulin were used in 281 and 69 patients, respectively. Patients lost to follow-up had no significant difference in clinical characteristics or MDCT-CA results. We recorded 279 events, of which 117 were hard events (9 cardiac deaths, 66 nonfatal myocardial infarctions, and 42 cases of unstable angina) and 142 late revascularizations. Twenty patients with early elective revascularizations were excluded from the survival analysis. Prevalence of male sex and hypertension were significantly higher in patients with events than in those without events (Table 1).
Table 1.
Clinical characteristics of the study population, MDCT-CA results, and patient clinical outcomes
MDCT-CA results
The mean effective dose of MDCT-CA examinations was 8.7 ± 3.5 mSv. Table 1 shows MDCT-CA results and patient outcomes. SIS, SSS, number of segments with obstructive plaques, and prevalence of obstructive CAD, three-vessel disease (VD), and LMCA disease were significantly higher in patients with events than in patients without events.
Univariate predictors of events
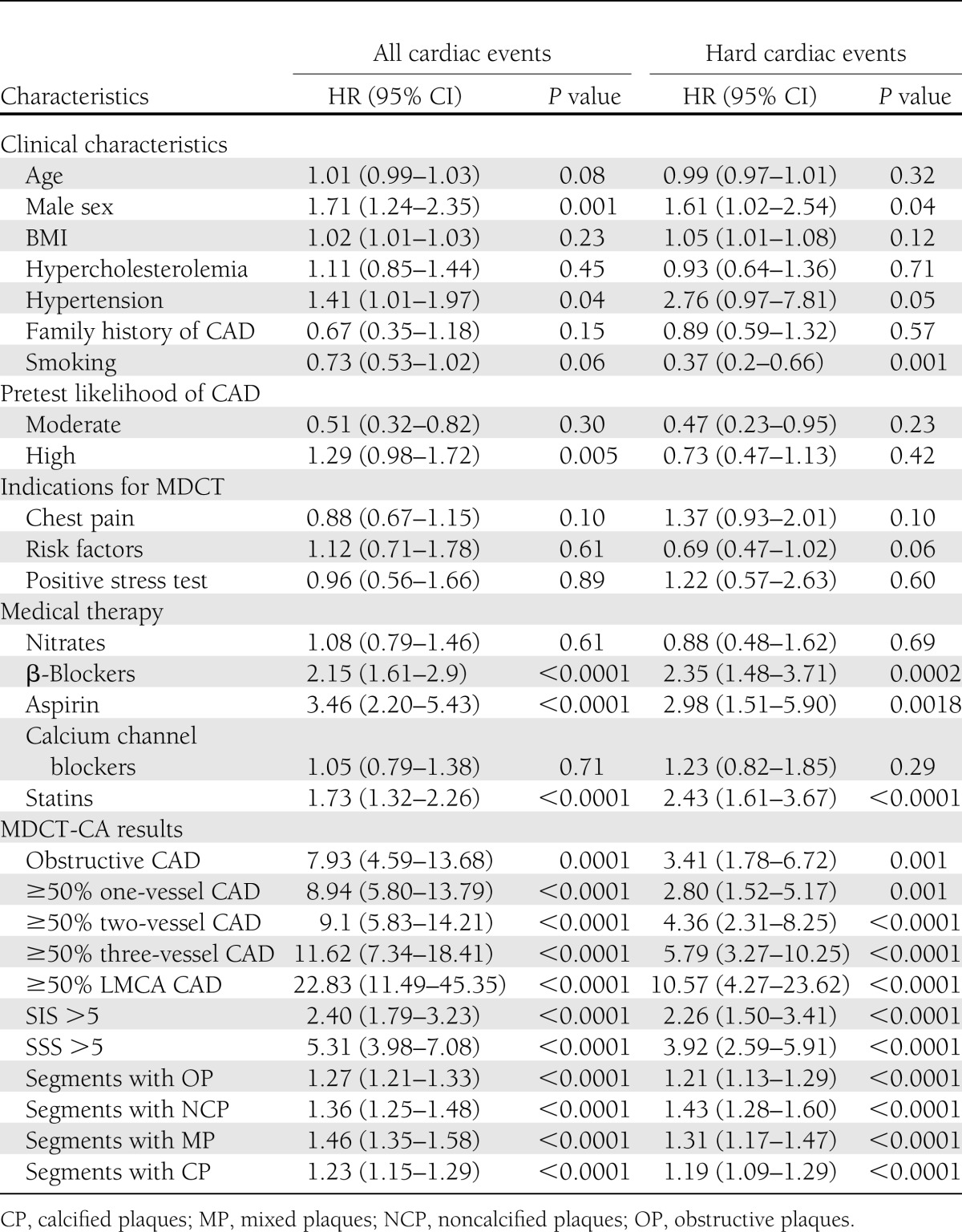
Univariate clinical predictors of events were male sex, hypertension, high pretest likelihood of CAD, and medical therapy with β-blockers, aspirin, and statins (Table 2). Univariate MDCT-CA predictors of events also are reported in Table 2. Regarding obstructive CAD, HR was 3.41 for hard events and 7.93 for all events. HRs were increased particularly in patients with three-VD (5.79 for hard events and 11.62 for all events) and LMCA disease (10.57 for hard events and 22.83 for all events).
Table 2.
Clinical characteristics and MDCT-CA results and univariate predictors of events
Multivariate predictors of events
Significant independent predictors of hard events were ≥50% three-VD (HR 5.21 [95% CI 1.33–20.33]; P = 0.01), ≥50% LMCA disease (5.35 [1.39–20.52]; P = 0.01), and the number of segments with noncalcified (1.84 [1.49–2.27]; P < 0.0001), mixed (1.39 [1.12–1.72]; P = 0.003), and calcified plaques (1.62 [1.33–1.96], P < 0.0001). Significant independent predictors of all events were ≥50% one-VD (3.94 [1.49–10.45]; P = 0.006), ≥50% two-VD (4.82 [2.17–10.73]; P = 0.0001), ≥50% three-VD (7.93 [4.56–13.79]; P < 0.0001), ≥50% LMCA disease (7.92 [2.62–23.88]; P = 0.005), and number of segments with mixed (1.40 [1.22–1.61]; P < 0.0001) and calcified (1.18 [1.04–1.35]; P = 0.01) plaques. When stratified by age (<65 vs. ≥65 years), younger patients experienced a higher HR for hard events for obstructive CAD (10.13 [4.26–24.07]; P < 0.0001 vs. 2.96 [1.45–5.99]; P = 0.003). When stratified by sex, women experienced a higher HR for obstructive CAD for hard events (9.12 [4.11–21.12]; P < 0.0001 vs. 2.78 [1.65–4.71]; P = 0.0001) and for all events (17.19 [5.36–55.1]; P < 0.0001 vs. 4.04 [2.26–7.26]; P < 0.0001).
Survival analysis
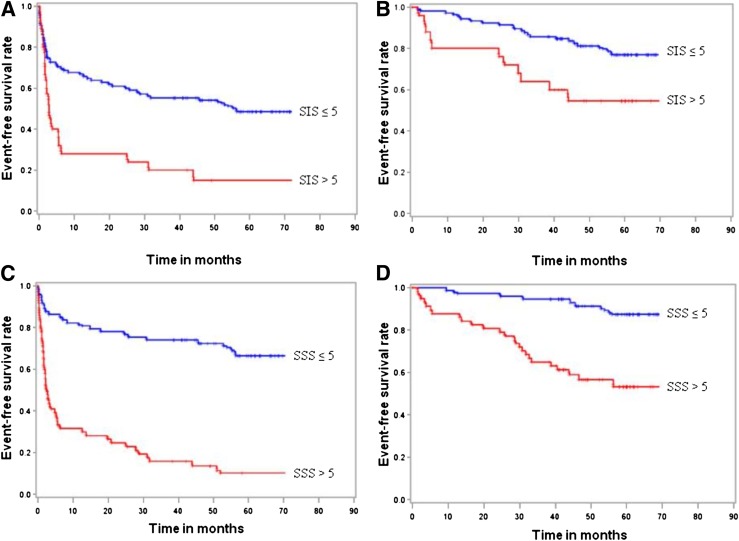
Kaplan-Meier survival curves about patients with normal coronary arteries, nonobstructive CAD, and obstructive CAD and about coronary plaque scores are shown in Figs. 1 and 2, respectively. No events occurred in patients with normal coronary arteries. On the contrary, the 62-month cumulative hard and all event-free survival rates were 78% and 56%, respectively, in patients with nonobstructive CAD and 60% and 16%, respectively, in those with obstructive CAD (log-rank P = 0.0001) (Fig. 1). The relationship between atherosclerotic burden, expressed as SIS and SSS, and event-free survival rate is reported in Fig. 2. Regarding all events, cumulative event-free survival was 50% with SIS ≤5, 16% with SIS >5, 67% with SSS ≤5, and 11% with SSS >5 (log-rank P = 0.0001). Regarding hard events, cumulative event-free survival was 77% with SIS ≤5, 54% with SIS >5, 88% with SSS ≤5, and 54% with SSS >5 (log-rank P = 0.0001). Moreover, we evaluated the relationship between CAD extension, expressed as the number of major epicardial vessels exhibiting ≥50% stenosis, and event-free survival rate. Regarding all events, cumulative event-free survival was 20% with one-VD, 12% with two-VD, 18% with three-VD, and 0% with LMCA disease (log-rank P = 0.0001). Excluding revascularization procedures, cumulative event-free survival was 73% with one-VD, 62% with two-VD, 50% with three-VD, and 25% with LMCA disease (log-rank P = 0.0001).
Figure 1.
Kaplan-Meier curves for all events (A) and hard events (B) in patients with normal coronary arteries, nonobstructive CAD, and obstructive CAD.
Figure 2.
Kaplan-Meier curves for all events (A) and hard events (B) in patients with SIS ≤5 and >5 and for all events (C) and hard events (D) in patients with SSS ≤5 and SSS >5.
CONCLUSIONS
In patients with diabetes, CAD is the major cause of morbidity, mortality, and medical costs (19). Indeed, management guidelines in Europe and the U.S. consider type 2 diabetes as a cardiovascular disease equivalent (19). Therefore, early CAD diagnosis in patients with diabetes is of the utmost importance in the attempt to prevent disease progression and clinical events. The American Diabetes Association recently convened an expert panel that reviewed the issue of CAD screening in patients with diabetes aimed at identifying high-risk patients in whom the outcome may be improved through more aggressive risk factor modification, medical surveillance, or revascularization (19). Unfortunately, the sensitivity of clinical risk assessment is limited in patients with diabetes, mainly because typical symptoms of ischemia often are absent and cardiac stress tests have limited negative predictive value (2). Indeed, the diagnostic accuracy of exercise electrocardiogram, as well as the specificity of stress echocardiography and nuclear perfusion imaging (20,21), are lower in patients with diabetes compared with nondiabetic patients (2). MDCT-CA currently is considered a reliable diagnostic method for the evaluation of patients with suspected CAD because of the high diagnostic performance in ruling out the disease and in detecting obstructive lesions (3). Several MDCT-CA studies demonstrated an increased prevalence of obstructive and nonobstructive CAD and fewer normal coronary arteries in patients with diabetes in comparison with nondiabetics (22,23). However, only two studies with midterm follow-up evaluated the prognostic value of this imaging modality in patients with diabetes (9,10). Compared with them, our study has a longer follow-up with a larger group of patients with diabetes who underwent MDCT-CA for suspected CAD. Moreover, patients with any type of known cardiac disease were excluded, making our study population significantly more homogeneous compared with the patients enrolled in previous studies. The main findings of our study are the following: 1) MDCT-CA is able to provide long-term prognostic information in patients with diabetes with suspected CAD and may predict hard cardiac events; 2) patients with diabetes without evidence of CAD seen on MDCT-CA have an excellent prognosis, with no cardiac events at 62-month follow-up. It is noteworthy that detection of obstructive CAD using MDCT-CA was a strong predictor of cardiac events in univariate analysis (HR 3.41 and 7.93 for hard and all events, respectively). Kaplan-Meier survival curves confirmed this finding, showing an event-free survival of 60% for hard events and 16% for all events in these patients. Moreover, MDCT-CA allowed prognostic grading according to one-VD, two-VD, three-VD, and LMCA disease classification. In both univariate and multivariate analysis, HRs for hard and all events were significantly increased in patients with three-VD and LMCA disease. Accordingly, survival free of hard events was reduced progressively from 73% in patients with one-VD to 25% in those with LMCA disease. Another major finding of this study pertains to patients with diabetes with nonobstructive CAD as seen on MDCT-CA. Among these patients, noninvasive testing is usually negative because this type of lesion rarely triggers myocardial ischemia, suggesting a prognosis similar to that found in patients with normal coronary arteries. However, our data indicate that these patients have a worse long-term outcome compared with patients with diabetes without CAD. Indeed, Kaplan-Meier survival curves showed an event-free survival of 78% for hard events and 56% for all events at 62-month follow-up. Thus, their risk of cardiac event is intermediate, between that found in patients with normal coronary arteries and patients with obstructive CAD. This finding requires some discussion. First of all, a recent study by van Velzen et al. (24) demonstrated that MDCT-CA has an accuracy of 100% in comparison with intravascular ultrasound in the detection of nonobstructive CAD. On the other hand, previous studies indicated that plaque composition also may be a predictor of adverse events. They demonstrated that vulnerable plaques may be present across the full spectrum of stenosis severity, suggesting that nonobstructive lesions may also contribute to cardiac events (25). In our study, the number of noncalcified plaques had higher HRs for hard events at univariate and multivariate analysis than the number of calcified and mixed plaques. Because nonobstructive plaques are more frequent than obstructive plaques, it is conceivable that moderate stenoses are more frequently associated with acute coronary occlusion (25). Another possible reason for the high prognostic value of nonobstructive CAD found in our study may be the long-term follow-up. In fact, we cannot rule out that some moderate stenoses at the time of MDCT-CA examination could have become obstructive over time. Nevertheless, early identification of nonobstructive CAD with MDCT-CA in patients with diabetes is clinically important because it may lead to a more aggressive strategy of cardiovascular risk factor control and modification of clinical follow-up. This is in agreement with the recommendations of the American Diabetes Association expert panel suggesting more aggressive risk factor modification and medical surveillance in high-risk patients with diabetes (19). Moreover, MDCT-CA also demonstrated the ability to stratify the prognosis of patients with diabetes according to their atherosclerotic burden. In agreement with our results, Hadamitzky et al. (9) were able to stratify cardiac events using the MDCT-CA atherosclerotic burden in patients with diabetes. Our study also demonstrates that MDCT-CA is able to predict events on the basis of atherosclerotic burden evaluated using coronary artery plaque scores. Indeed, event-free survival significantly decreased at 62-month follow-up, from 67% for SSS ≤5 to 11% for SSS >5, considering revascularizations, and from 88% for SSS ≤5 to 54% for SSS >5, excluding revascularizations. Another remarkable finding is that the atherosclerotic burden maintains a similar prognostic value using SIS.
Another important result of our study relates to patients with diabetes with normal coronary arteries at MDCT-CA. In agreement with a previous study that enrolled a smaller number of less homogeneous patients with diabetes and had shorter follow-up (10), our study found that the absence of CAD, found in 90 patients with diabetes, is associated with an event-free survival of 100% for both hard and all events at 62-month follow-up. The excellent outcome observed in this subset of patients is clinically relevant because it suggests that MDCT-CA, in contrast to other imaging modalities, can help to identify the truly low-risk patients with diabetes. Although nuclear stress imaging and stress echocardiography, the two stress imaging tests used most widely in clinical practice, demonstrated good prognostic value in the general population with suspected CAD (19), their performance was found to be lower in studies that evaluated patients with diabetes. The prognostic value of single-photon emission computed tomography imaging in patients with diabetes was assessed in seven large studies. Similar to what has been shown in nondiabetic patients, their results confirmed the higher event rate in the presence of an abnormal scan compared with a normal scan. However, the event rate was higher compared with the general population, even in the presence of a normal scan (19). Similar results were reported by another five large studies that evaluated the prognostic value of stress echocardiography in patients with diabetes (19). In one of these studies, Kamalesh et al. (2) demonstrated that patients with diabetes with a negative stress echocardiogram had a significantly higher incidence of events compared with nondiabetics (19). This finding was confirmed by Elhendy et al. (26), who found that patients with diabetes with normal exercise echocardiography had an increase in cardiac events (up to 7.6%) at 5-year follow-up, despite an event-free survival at 1 year. Interestingly, in our study, patients with diabetes with normal coronary arteries seen on MDCT-CA were event free at 5-year (mean 62 ± 9 months, up to 72 months) follow-up. Therefore, this imaging modality can be a useful tool to reassure patients with diabetes with suspected CAD regarding their outcome, with a warranty period of at least 5 years in the presence of a normal result. In conclusion, these results, if confirmed in further studies with larger patient populations, suggest that MDCT-CA could be used in patients with diabetes as a screening diagnostic modality to stratify the cardiovascular prognosis. We believe that this approach could be particularly useful in specific subsets of patients with diabetes with unknown CAD and equivocal or uninterpretable stress tests or in case of a discrepancy between clinical presentation and stress test results. Indeed, MDCT-CA already has demonstrated its utility in these clinical settings (27).
Moreover, the new generation of scanners has demonstrated the ability to evaluate coronary arteries with high diagnostic performance and a lower radiation exposure (<2 mSv) compared with that of the previous generation of scanners and traditional invasive coronary angiography (28).
When interpreting these data, some limitations should be considered. First, this is a relatively small, single-center study evaluating mainly Caucasian patients. Thus, the results may not necessarily reflect the patient population of other centers or countries, especially in terms of potential age-related differences in the study population. Second, we recognize that incomplete follow-up may result in underreporting of cardiac events, and we are not able to exclude the possibility that a few patients did not report some events. However, the percentage of patients with complete follow-up was remarkably high (98%). Third, MDCT-CA allowed the identification of patients with obstructive CAD, likely resulting in an increased revascularization rate, which constituted a large proportion of the composite all cardiac event end point. However, in our study, MDCT-CA was performed in addition to a standard diagnostic work-up. Moreover, the decision regarding revascularization was based on symptoms, the presence of ischemia on noninvasive testing, and invasive coronary angiography results; patients with early elective revascularizations were excluded from survival analysis and the presence of obstructive CAD at MDCT-CA was strongly associated with hard cardiac events. Fourth, although our study demonstrated an important prognostic role of MDCT-CA anatomical evaluation alone, assessment of the amount and distribution of regional ischemia documented by nuclear imaging would have been important to know the potential complementary prognostic value of MDCT-CA and nuclear stress tests in our patients. Fifth, although this study demonstrated that MDCT-CA is able to predict the prognosis of diabetic patients on the basis of the presence/extent of CAD and plaque type, coronary imaging by MDCT-CA is not, as in case of invasive angiography, able to predict which plaque may progress to destabilization and rupture, potentially causing a clinical event. Finally, in the subset of newly diagnosed type 2 patients with diabetes, a comparison between the prognostic value of MDCT-CA and that of the UK Prospective Diabetes Study Risk Engine would have been useful to demonstrate a potential additional value of MDCT-CA.
Acknowledgments
D.A. and G.P. have served on the speakers bureau for GE Healthcare. No other potential conflicts of interest relevant to this article were reported.
D.A. and G.P. performed and reviewed all MDCT-CA. S.M., E.B., E.C., A.B., A.A., and A.F. contributed to the collection and analysis of MDCT-CA data. F.V. performed the statistical analyses. P.A. and P.M. represented the clinical event committee that reviewed cardiac events. G.B., A.L.B., C.F., and M.P. contributed to the conception of the study and drafted the manuscript. M.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.McGill HC, Jr, McMahan CA, Malcom GT, Oalmann MC, Strong JP, Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group Relation of glycohemoglobin and adiposity to atherosclerosis in youth. Arterioscler Thromb Vasc Biol 1995;15:431–440 [DOI] [PubMed] [Google Scholar]
- 2.Kamalesh M, Feigenbaum H, Sawada S. Assessing prognosis in patients with diabetes mellitus—the Achilles’ heel of cardiac stress imaging tests? [Review] Am J Cardiol 2007;99:1016–1019 [DOI] [PubMed] [Google Scholar]
- 3.Janne d’Othée B, Siebert U, Cury R, Jadvar H, Dunn EJ, Hoffmann U. A systematic review on diagnostic accuracy of CT-based detection of significant coronary artery disease. Eur J Radiol 2008;65:449–461 [DOI] [PubMed] [Google Scholar]
- 4.Min JK, Shaw LJ, Devereux RB, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol 2007;50:1161–1170 [DOI] [PubMed] [Google Scholar]
- 5.Carrigan TP, Nair D, Schoenhagen P, et al. Prognostic utility of 64-slice computed tomography in patients with suspected but no documented coronary artery disease. Eur Heart J 2009;30:362–371 [DOI] [PubMed] [Google Scholar]
- 6.Aldrovandi A, Maffei E, Palumbo A, et al. Prognostic value of computed tomography coronary angiography in patients with suspected coronary artery disease: a 24-month follow-up study. Eur Radiol 2009;19:1653–1660 [DOI] [PubMed] [Google Scholar]
- 7.Chow BJ, Wells GA, Chen L, et al. Prognostic value of 64-slice cardiac computed tomography severity of coronary artery disease, coronary atherosclerosis, and left ventricular ejection fraction. J Am Coll Cardiol 2010;55:1017–1028 [DOI] [PubMed] [Google Scholar]
- 8.Min JK, Dunning A, Lin FY, et al. CONFIRM Investigators Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol 2011;58:849–860 [DOI] [PubMed] [Google Scholar]
- 9.Hadamitzky M, Hein F, Meyer T, et al. Prognostic value of coronary computed tomographic angiography in diabetic patients without known coronary artery disease. Diabetes Care 2010;33:1358–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Werkhoven JM, Cademartiri F, Seitun S, et al. Diabetes: prognostic value of CT coronary angiography—comparison with a nondiabetic population. Radiology 2010;256:83–92 [DOI] [PubMed] [Google Scholar]
- 11.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 12.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001;285:2486–2497 [DOI] [PubMed] [Google Scholar]
- 13.European Society of Hypertension-European Society of Cardiology Guidelines Committee 2003 European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens 2003;21:1011–1053 [DOI] [PubMed] [Google Scholar]
- 14.Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O’Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol 2005;46:807–814 [DOI] [PubMed] [Google Scholar]
- 15.Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med 1979;300:1350–1358 [DOI] [PubMed] [Google Scholar]
- 16.Austen WG, Edwards JE, Frye RL, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation 1975;51(Suppl.):5–40 [DOI] [PubMed] [Google Scholar]
- 17.Myocardial infarction redefined—a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. Eur Heart J 2000;21:1502–1513 [DOI] [PubMed] [Google Scholar]
- 18.Bassand JP, Hamm CW, Ardissino D, et al. Task Force for Diagnosis and Treatment of Non-ST-Segment Elevation Acute Coronary Syndromes of European Society of Cardiology Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J 2007;28:1598–1660 [DOI] [PubMed] [Google Scholar]
- 19.Bax JJ, Inzucchi SE, Bonow RO, Schuijf JD, Freeman MR, Barrett EJ, Global Dialogue Group for the Evaluation of Cardiovascular Risk in Patients with Diabetes Cardiac imaging for risk stratification in diabetes. Diabetes Care 2007;30:1295–1304 [DOI] [PubMed] [Google Scholar]
- 20.Kang X, Berman DS, Lewin H, et al. Comparative ability of myocardial perfusion single-photon emission computed tomography to detect coronary artery disease in patients with and without diabetes mellitus. Am Heart J 1999;137:949–957 [DOI] [PubMed] [Google Scholar]
- 21.Hennessy TG, Codd MB, Kane G, McCarthy C, McCann HA, Sugrue DD. Evaluation of patients with diabetes mellitus for coronary artery disease using dobutamine stress echocardiography. Coron Artery Dis 1997;8:171–174 [DOI] [PubMed] [Google Scholar]
- 22.Wackers FJ. Diabetes and coronary artery disease: the role of stress myocardial perfusion imaging. Cleve Clin J Med 2005;72:21–25, 29–33 [DOI] [PubMed] [Google Scholar]
- 23.Pundziute G, Schuijf JD, Jukema JW, et al. Noninvasive assessment of plaque characteristics with multislice computed tomography coronary angiography in symptomatic diabetic patients. Diabetes Care 2007;30:1113–1119 [DOI] [PubMed] [Google Scholar]
- 24.van Velzen JE, Schuijf JD, de Graaf FR, et al. Diagnostic performance of non-invasive multidetector computed tomography coronary angiography to detect coronary artery disease using different endpoints: detection of significant stenosis vs. detection of atherosclerosis. Eur Heart J 2011;32:637–645 [DOI] [PubMed] [Google Scholar]
- 25.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation 1995;92:657–671 [DOI] [PubMed] [Google Scholar]
- 26.Elhendy A, Arruda AM, Mahoney DW, Pellikka PA. Prognostic stratification of diabetic patients by exercise echocardiography. J Am Coll Cardiol 2001;37:1551–1557 [DOI] [PubMed] [Google Scholar]
- 27.Pontone G, Andreini D, Ballerini G, Nobili E, Pepi M. Diagnostic work-up of unselected patients with suspected coronary artery disease: complementary role of multidetector computed tomography, symptoms and electrocardiogram stress test. Coron Artery Dis 2007;18:265–274 [DOI] [PubMed] [Google Scholar]
- 28.Andreini D, Pontone G, Mushtaq S, et al. Coronary in-stent restenosis: assessment with CT coronary angiography. Radiology 2012;265:410–417 [DOI] [PubMed] [Google Scholar]