Abstract
OBJECTIVE
Although screening for diabetes and prediabetes is recommended, it is not clear how best or whom to screen. We therefore compared the economics of screening according to baseline risk.
RESEARCH DESIGN AND METHODS
Five screening tests were performed in 1,573 adults without known diabetes—random plasma/capillary glucose, plasma/capillary glucose 1 h after 50-g oral glucose (any time, without previous fast, plasma glucose 1 h after a 50-g oral glucose challenge [GCTpl]/capillary glucose 1 h after a 50-g oral glucose challenge [GCTcap]), and A1C—and a definitive 75-g oral glucose tolerance test. Costs of screening included the following: costs of testing (screen plus oral glucose tolerance test, if screen is positive); costs for false-negative results; and costs of treatment of true-positive results with metformin, all over the course of 3 years. We compared costs for no screening, screening everyone for diabetes or high-risk prediabetes, and screening those with risk factors based on age, BMI, blood pressure, waist circumference, lipids, or family history of diabetes.
RESULTS
Compared with no screening, cost-savings would be obtained largely from screening those at higher risk, including those with BMI >35 kg/m2, systolic blood pressure ≥130 mmHg, or age >55 years, with differences of up to −46% of health system costs for screening for diabetes and −21% for screening for dysglycemia110, respectively (all P < 0.01). GCTpl would be the least expensive screening test for most high-risk groups for this population over the course of 3 years.
CONCLUSIONS
From a health economics perspective, screening for diabetes and high-risk prediabetes should target patients at higher risk, particularly those with BMI >35 kg/m2, systolic blood pressure ≥130 mmHg, or age >55 years, for whom screening can be most cost-saving. GCTpl is generally the least expensive test in high-risk groups and should be considered for routine use as an opportunistic screen in these groups.
Recommendations to screen for diabetes and prediabetes are prompted by the increase in prevalence of diabetes, its associated morbidity, mortality, and cost, and the availability of interventions to prevent diabetes and its complications. However, there is controversy regarding the target population and the screening test. The American Diabetes Association recommends screening all people 45 years and older or all people with a BMI ≥25 kg/m2 and an additional risk factor every 3 years (1) using A1C, fasting plasma glucose, or oral glucose tolerance tests; however, other studies have found that other screening protocols may be equally or more cost-effective (2).
Because patients prefer tests that do not require fasting (3), we previously evaluated costs associated with tests that can be used opportunistically, during outpatient visits, at any time of day, without the need for a fast, such as a glucose challenge test (plasma or capillary glucose 1 h after a 50-g oral glucose challenge [GCTpl or GCTcap], similar to screening for gestational diabetes), random plasma glucose (RPG) or random capillary glucose (RCG), or A1C (4). With the volunteer population of the Screening for Impaired Glucose Tolerance (SIGT) study, we found that all of the screening tests would be cost-saving compared with no screening for the detection and 3 years of treatment of dysglycemia110 (diabetes or prediabetes110, i.e., impaired glucose tolerance [IGT] and/or impaired fasting glucose [IFG] with fasting plasma glucose 110–125 mg/dL [6.1–6.9 mmol/L]) from a health system perspective and cost-neutral from a societal perspective. However, screening costs also could be impacted by factors other than the tests themselves, such as the population targeted for screening. In this study, we compared the health system costs associated with screening for diabetes or dysglycemia110 for groups with different risks of having these disorders.
RESEARCH DESIGN AND METHODS
The study was approved by the Emory University Institutional Review Board and used data from 1,573 adults in the SIGT study, described previously (5). Briefly, this study recruited participants without known diabetes between January 2005 and March 2008. The participants’ first visits were at different times of the day, without an overnight fast. RCG and RPG were measured, a 50-g glucose drink was given, and GCTcap and GCTpl glucose levels were measured 1 h later. At a second visit, A1C was measured and a 75-g oral glucose tolerance test (OGTT) was begun before 11:00 a.m., after an overnight fast.
Case definitions
Diabetes included fasting glucose ≥126 mg/dL (7 mmol/L) or 2-h OGTT glucose ≥200 mg/dL (11.1 mmol/L); A1C ≥6.5% (48 mmol/mol) was included in sensitivity analyses. Prediabetes110 was targeted based on glucose levels that confer increased mortality (6,7). Our definition of prediabetes110 included the following: IFG110, which is fasting glucose 110–125 mg/dL (6.1–6.9 mmol/L) and 2-h OGTT glucose <140 mg/dL (7.8 mmol/L); IGT, which is fasting glucose <110 mg/dL and 2-h OGTT glucose 140–199 mg/dL (7.8–11.1 mmol/L); and IFG110 with IGT (IFG plus IGT), which is fasting glucose 110–125 mg/dL (6.1–6.9 mmol/L) and 2-h OGTT glucose 140–199 mg/dL (7.8–11.1 mmol/L). Dysglycemia110 included both prediabetes110 and diabetes.
Cost perspectives
Costs were expressed in the equivalent of 2007 United States dollars. Cost components have been described in detail previously (4). Cost components and base-case cost assessment also are shown in Supplementary Table 1. Health system costs were costs that would be incurred in a United States health care system with the government-funded Medicare program as the primary health insurer. Included were direct medical costs associated with testing, direct medical costs of false-negative results, and direct medical costs for treatment of true-positive results (including cases of prediabetes110 and diabetes) with generic metformin. Two cost scenarios were considered, health system costs of screening and treatment for dysglycemia110 and health system costs for screening and treatment of diabetes only. All cost scenarios were calculated for a 3-year period of time.
Costs of testing
The direct medical costs of testing included 2007 Medicare costs for the laboratory tests, the cost of the glucose challenge test glucose drink, as well as staff costs based on 2007 United States Bureau of Labor Services wages. We assumed that blood draws for GCTpl, RPG, and OGTT assessments would be obtained on-site, and that medical staff would measure RCG and GCTcap. Because we assumed that initial screening would be opportunistic during a visit, the visit time was not included. We used 70% specificity cut-offs to define positive screen results for each test to detect diabetes or dysglycemia110, using the OGTT data, to determine these cut-offs. For our cost analyses, we assumed that those participants with a positive screen result would have a subsequent confirmatory OGTT with fasting and postchallenge glucose cut-offs for prediabetes110 and diabetes as defined.
Costs of false-positive results
The cost of a false-positive result for each screen included the cost of the follow-up OGTT test as per protocol.
Costs of false-negative results
For the base-case analyses, we evaluated the cost of a false negative result—undetected prediabetes110 or diabetes—as 10% of the projected incremental 3-year medical costs for that condition, assuming that these incremental costs could be decreased by appropriate management, as they were in the Diabetes Prevention Program (DPP) (8,9). We assumed that the cost of a false-negative result would include the 3-year direct medical cost of diabetes, prediabetes110, or prediabetes110 that progressed to diabetes. We based the direct medical costs for diabetes on Medical Expenditure Panel Survey costs from 2000 to 2004, which was $4,174 per year in 2005 United States dollars for a 50-year-old with new-onset diabetes (10). We calculated the costs for prediabetes based on the cost of patients with IFG110 in Kaiser Permanente Northwest who had incremental direct medical costs of $1,316 per year (11), although IGT might incur higher costs (12).
Costs of true-positive results
We based the direct medical costs for a true positive result (prediabetes110 or diabetes) on 3-year costs for the DPP metformin group. This group’s incremental costs for laboratory tests, physician visits, and follow-up were $703 (9). We assumed that all true-positive results would be treated with metformin, and we substituted recent pharmacy-based generic costs for metformin 850 mg twice per day. The direct medical cost incurred outside of the study for the DPP metformin versus placebo arms was used as the other component for the health system cost of true-positive results (9); the direct medical cost outside the study was −$329 for the metformin versus placebo arms.
Risk group stratification
We analyzed costs for subgroups of our study population based on the presence of the following risk characteristics: age <40, age 40–55, and age >55 years; BMI <25, 25–35, >35 kg/m2; waist circumference with cut-offs of <88 cm in men and <102 cm in women (low-risk) or ≥88 cm in men and ≥102 cm in women (high-risk); triglycerides <150 mg/dL (1.695 mmol/L; low-risk) or ≥150 mg/dL (1.695 mmol/L; high-risk); HDL ≥40 mg/dL (1.036 mmol/L) in men and ≥50 mg/dL (1.295 mmol/L) in women (low-risk) or <40 mg/dL (1.036 mmol/L) in men and <50 mg/dL (1.295 mmol/L) in women (high-risk); presence or absence of a family history of diabetes in first-degree relatives; and presence or absence of elevated blood pressure, defined as systolic blood pressure <130 (low-risk) or ≥130 mmHg (high-risk). For each characteristic, prevalence of diabetes and prevalence of dysglycemia110 were calculated in this population.
Sensitivity analyses
We examined the costs when we included A1C results of ≥6.5% (48 mmol/mol) in the definition of diabetes. We examined the following alternatives for the cost components to determine whether our findings were robust: substituting Veterans Affairs system costs for testing and treatment with metformin to provide a single-payer perspective; using lower costs for false-negative results, assuming 5% of projected incremental 3-year medical costs, similar to the outside medical care costs of participants in the DPP (8); and using costs for treatment with lifestyle modification rather than metformin, assuming group intervention costs and costs for other lifestyle changes as described in the DPP protocol (13).
We also calculated the societal costs of each of our screening scenarios. We have presented the results of the societal costs in the Supplementary Data. Societal costs included the additional nonmedical costs of testing, indirect (lost labor productivity) costs of false-negative results, and direct nonmedical and indirect costs of true-positive results. The direct nonmedical costs of testing reflected excess time spent by the patient. Indirect costs for false-negative results (absenteeism, reduced productivity at work and for those who did not work) were derived from the American Diabetes Association 2007 economic assessment and were attributed only to those with diabetes or whose prediabetes progressed to diabetes during the 3 years (14).
Statistical analyses
Costs of screening and treatment were expressed as mean ± SEM for the five types of screens for each risk group. We compared overall high-risk versus low-risk group costs as average costs for the seven higher-risk or highest-risk versus lower-risk or lowest-risk groups. We used nonparametric Mann-Whitney tests for statistical comparisons of cost variables using SAS 9.1.3 (SAS Institute, Cary NC) (15).
RESULTS
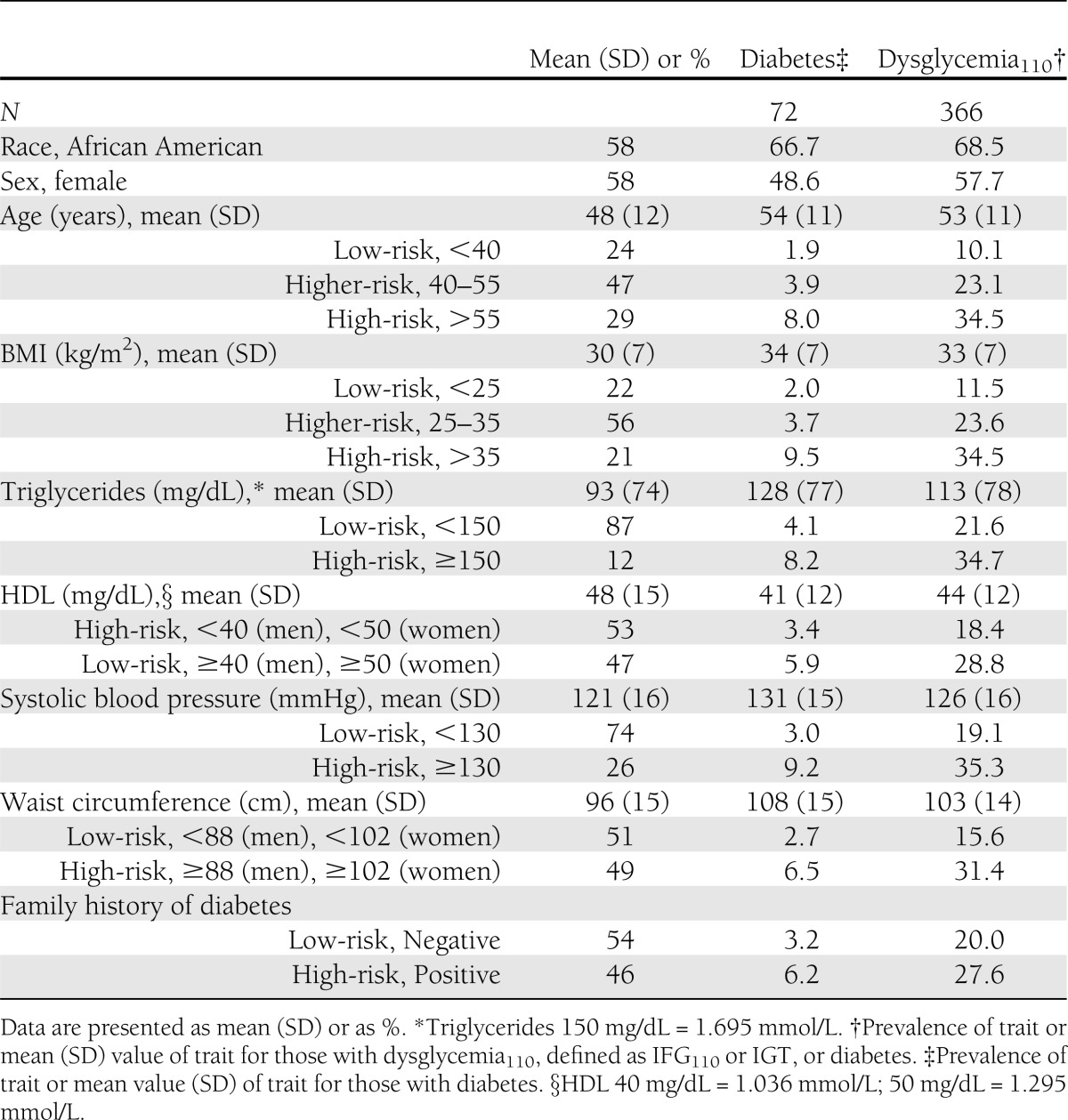
Baseline characteristics of participants have been reported previously and are shown in Table 1; 366 (23%) had dysglycemia110 and 72 (5%) had diabetes. Fifty-eight percent of the study population was African American. More than 75% of participants were 45 years of age or older or had BMI ≥25 kg/m2. However, only 12% had a triglyceride level ≥150 mg/dL (1.695 mmol/L), 26% had systolic blood pressure ≥130 mmHg, and 46% had a family history of diabetes. The higher-risk groups were more likely to have diabetes or dysglycemia110; the latter was present in 35% of participants with age >55 years, BMI >35 kg/m2, or systolic blood pressure ≥130 mmHg compared with 10–19% in the corresponding lower-risk groups.
Table 1.
Baseline characteristics of 1,573 participants from the SIGT study
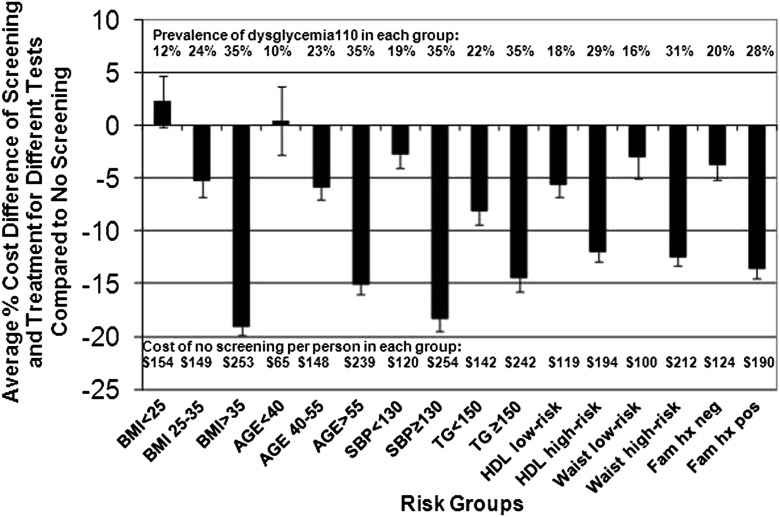
The health system cost components and overall assessment for screening (and 3 years of treatment) for dysglycemia110 are shown in Supplementary Table 1. These health system costs and the differences in health system costs for screening versus no screening are shown by risk group for each screening test in Table 2 and Fig. 1; in these analyses, “negative cost differences” for screening versus no screening indicate projected net “cost-savings.” The relative cost-savings of screening versus no screening would be greater (more “negative”) for the higher-risk groups for every risk group assessed (P < 0.01 for each higher-risk or highest-risk versus lower-risk or lowest-risk group), with average cost-savings of −19% for the highest BMI and −18% for the elevated blood pressure groups (both P < 0.01 compared with lowest-risk and lower-risk groups). Among the different tests, screening of the higher-risk groups was generally least expensive when GCTpl was used for screening, with cost-savings of −21% for the highest BMI and elevated blood pressure groups. Screening lower-risk groups would result in less cost-savings, or a net increase in costs, and in those groups RPG or RCG tended to be the least expensive tests.
Table 2.
Health system costs for screening and treatment of dysglycemia110 of 1,573 participants by risk group and percent cost difference for screening and treatment compared with no screening*
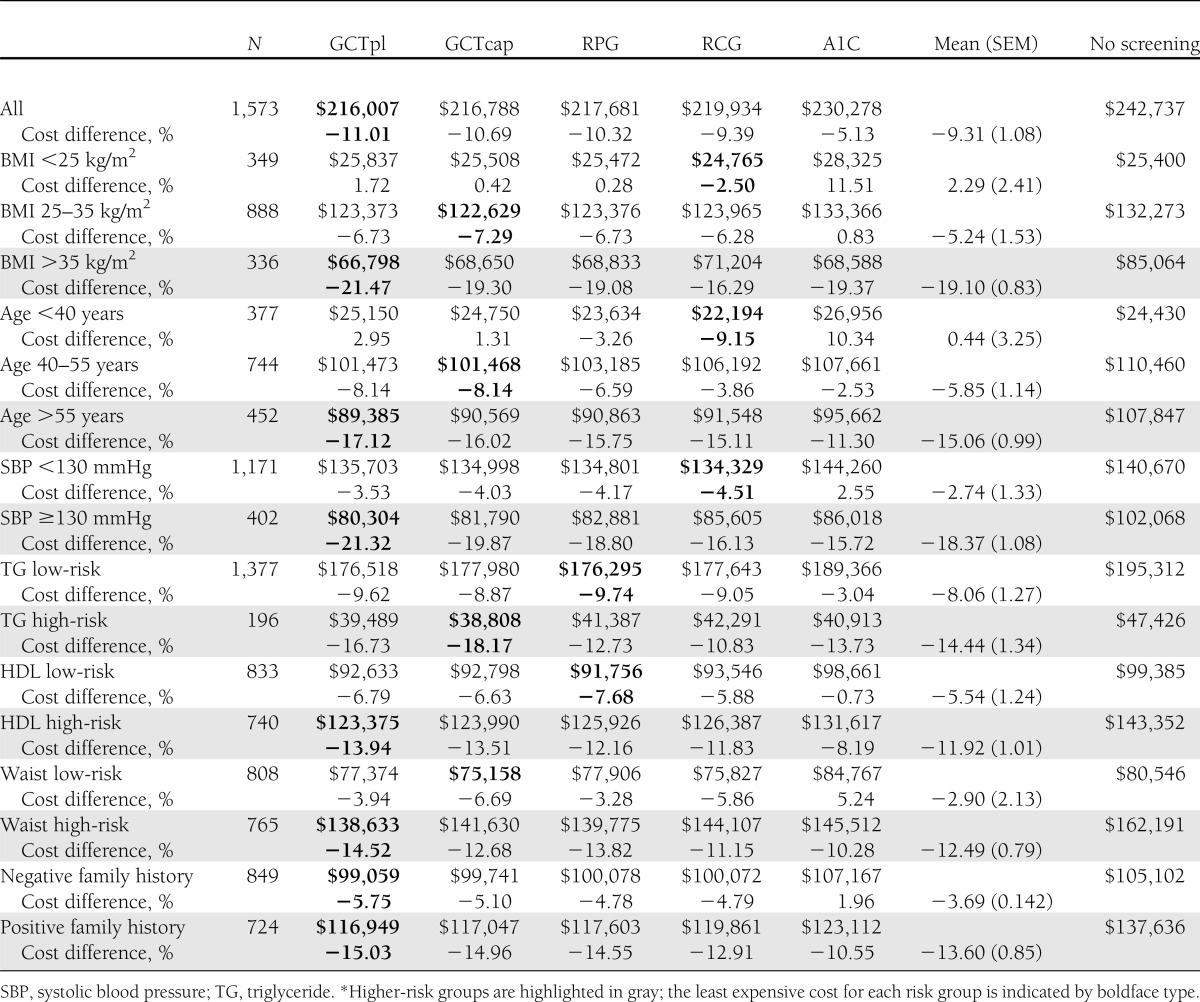
Figure 1.
The average percent cost differences between health system costs for screening and treatment of dysglycemia110 by risk group compared with no screening. Shown are the average percent differences in cost for screening with the five different screens and management of dysglycemia110 compared with no screening for different risk groups. The 95% CIs are depicted by the upper or lower lines or both. For each risk group, the prevalence of dysglycemia110 is shown along the top of the chart, and the costs of no screening per person are shown along the bottom of the chart. The prevalence of dysglycemia110 and the cost of no screening per person increased with higher-risk characteristics among the risk groups. SBP, systolic blood pressure; TG, triglycerides; Fam hx, family history.
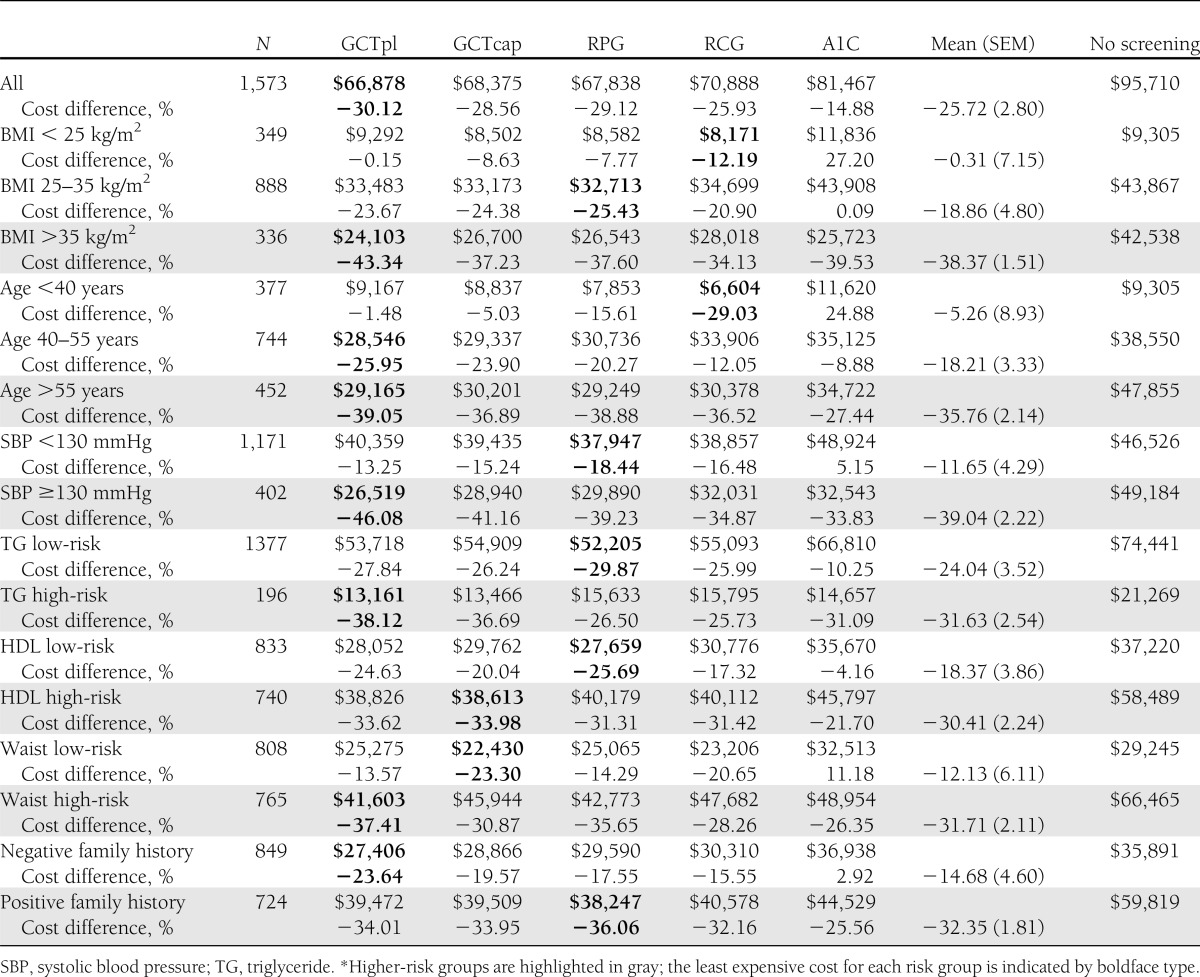
The health system costs for screening (and treatment) for diabetes are shown in Table 3, with the differences in costs for screening versus no screening in Table 3 and Supplementary Fig. 1. For all of these groups, screening would produce cost-savings compared with no screening, and cost-savings with targeting of diabetes would be greater than with dysglycemia110 (for all subjects, cost-savings of −26 vs. −9%; P < 0.01). As with dysglycemia110, cost-savings resulting from screening for diabetes would be greater with higher-risk and highest-risk groups compared with lower-risk and lowest-risk groups (−34 vs. −12%, over all groups; P < 0.01); screening those with elevated blood pressure, the highest BMI, and the oldest age groups would provide the greatest cost-savings (−39, −38, and −36%, respectively; all P < 0.01 versus the relevant lowest-risk group). GCTpl testing would provide the lowest costs for the higher-risk groups, with cost-savings of up to −46% for those with elevated blood pressure, and RCG or RPG would provide the lowest costs with the lowest-risk groups.
Table 3.
Health system costs for screening and treatment of diabetes of 1,573 participants by risk group and percent cost difference for screening and treatment compared with no screening*
The impact of screening on overall costs depends on the prevalence of the disease in the population, the cost of undetected disease (false-negative results), the cost of screening, and characteristics of different screening tests. With our study population and base-case assumptions, cost-savings for screening with GCTpl versus RCG would be achieved in screening for diabetes when the prevalence in the population is ≥4% and for dysglycemia110 when the prevalence is ≥20% (Supplementary Fig. 2A, B). A general equation describing these relationships is shown in Supplementary Table 2 and is illustrated for screening with GCTpl versus RCG in Supplementary Fig. 3. Greater cost-savings are achieved with GCTpl, a more accurate but more expensive test, either when cost of undetected disease is high or when prevalence of disease is high, and especially when both are high.
Testing costs per true-positive result also decrease as prevalence of the disease in the population increases. Supplementary Table 3 shows such costs for risk groups of BMI and age. In screening for dysglycemia110, average testing costs for the five screens for those with BMI <25 kg/m2 would be $203 ± 40 per true-positive result compared with $64 ± 7 for those with BMI >35 kg/m2 (P < 0.01). In screening for diabetes, average testing costs per true-positive result would be $836 ± 156 for those with BMI <25 kg/m2 compared with $185 ± 17 for those with BMI >35 kg/m2 (P < 0.01).
Sensitivity analyses
When we included A1C ≥6.5% (48 mmol/mol) in our definition of diabetes, we identified 10 additional participants with diabetes in this cohort; however, the total number of participants with dysglycemia110 did not change. Screening the higher-risk groups for diabetes continued to be significantly more cost-saving compared with no screening and compared with screening the equivalent low-risk group. For example, screening with GCTpl for those with an elevated blood pressure versus those without an elevated blood pressure resulted in cost-savings of −37.5 versus −2.5%, respectively (P < 0.01), both compared with no screening.
For both higher-risk and lower-risk groups, screening for dysglycemia110 or diabetes from a Veterans Affairs perspective would result in greater cost-savings compared with Medicare-based costs (Supplementary Tables 4 and 5) If false-negative result costs were only 5% of projected incremental medical costs over 3 years, then health system costs would be cost-neutral for screening for diabetes compared with no screening for some but not all of the higher-risk groups (Supplementary Table 6), but screening and treatment of dysglycemia110 would not produce cost-savings for any group (not shown). The health system costs with a lifestyle change intervention would be similar to costs with generic metformin, with cost-savings particularly for higher-risk groups (Supplementary Tables 7, 8). From a societal perspective, screening (and treatment with metformin) for dysglycemia110 (Supplementary Table 9) would result in cost-savings compared with no screening only in the highest-risk groups with substantial prevalence of disease, particularly for those with BMI >35 kg/m2 or elevated blood pressure, with ∼−11% savings compared with no screening. In contrast, societal costs for screening and treatment of diabetes (Supplementary Table 10) would produce broader cost-savings in both high-risk and lower-risk groups.
CONCLUSIONS
Our previous analyses demonstrated that screening the SIGT study population for either diabetes or dysglycemia110 would be cost-saving from a health system perspective over a 3-year horizon (4). We now show that the majority of cost-savings would come from screening individuals with higher risk, based on any one of the characteristics of age, BMI, triglycerides, HDL cholesterol, waist circumference, systolic blood pressure, or family history of diabetes. In most of the higher-risk groups, significant cost-savings would be achieved compared with no screening. However, the greatest cost-savings would be attained with screening of individuals with BMI >35 kg/m2 or systolic blood pressure ≥130 mmHg. Of the five screening tests considered, the GCTpl appears to be the least expensive screening test in most higher-risk groups.
Higher-risk groups would have higher costs associated with no screening. Because these groups have a higher prevalence of diabetes and dysglycemia110, failure to screen would result in more patients with missed diagnoses, with associated increases in downstream treatment costs. And as the costs of no screening increase, greater cost-savings can be obtained with a more accurate test, i.e., GCTpl compared with RCG (Supplementary Fig. 2A, B).
Recent studies have addressed the cost-effectiveness of different screening strategies. One review concluded that it would be very cost-effective to screen for diabetes using current American Diabetes Association guidelines among African Americans 45–54 years of age (16). Another study examined screening with fasting plasma glucose on the basis of age or blood pressure or both and found that it would be more cost-effective to screen at 30–45 years of age than at older age, and even more cost-effective to screen beginning at 30 years of age in people with hypertension (2). However, that study did not consider other risk factors or screening tests. Two other studies found that targeted screening for diabetes with fasting or RCG based on hypertension, and for prediabetes based on obesity, would be more cost-effective compared with universal screening (17,18), but those studies also did not consider other risk factors or other screening tests.
Whether screening should target prediabetes as well as diabetes also has been debated. Most analyses have found that it should be cost-effective to screen for and treat prediabetes to reduce both the risk of cardiovascular disease and progression to diabetes (18–20). Our study confirms that even over a 3-year time period, it should be cost-saving to screen for and treat dysglycemia110 (prediabetes110 as well as diabetes), particularly among higher-risk groups.
Our study has limitations. The study participants were volunteers, which might have resulted in selection bias from disproportionate participation of those at high risk. However, the prevalence of prediabetes and diabetes in the study population was similar to or lower than that in recent national surveys, such as NHANES 2005–2006 (21). Although we did gather information regarding risk of diabetes, information on history of gestational diabetes was not collected or used as a risk factor in our analyses. We also did not calculate costs associated with screening for either dysglycemia110 or diabetes with a fasting glucose alone. Whereas a fasting glucose may be considered an acceptable test for patients who do not mind fasting before a visit, many cases of postchallenge dysglycemia would be missed, leading to a higher number of false-negative results and higher overall costs.
Our analyses rely on estimates of testing, treatment, and false-negative result costs, which may not be applicable to every health system. Our estimates for the cost of false-negative results (that 10% of projected incremental 3-year medical costs of diabetes or dysglycemia110 could be reduced with detection and management) might be excessive. However, we project that cost-savings still could be achieved in higher-risk groups with a false-negative result cost of at least 5%, which was achieved in the tightly controlled environment of the DPP study (8). Some groups have found the cost of undiagnosed diabetes and prediabetes to be lower than the costs we used in our analyses, but it is still to be determined how much of these costs could be reduced with detection and management (22,23). We are not aware of other sources for the costs of false-negative results.
In sensitivity analyses addressing costs associated with lifestyle changes as treatment for dysglycemia110 or diabetes, we based our costs on group-based interventions designed by the DPP study. More recent studies have found that community-based interventions based on the DPP protocol could be performed with reduced costs. These studies generally have found that treatment with lifestyle interventions are cost-effective in the long-term (24–26) and possibly even are cost-saving in the short-term (26). Finally, our cost estimates projected a 3-year time period. Such a period might be relevant to employer-based health systems in which insurers are changed every few years, but lifetime cost analyses would be needed to determine if the cost-savings found in higher-risk groups are likely to be sustained.
Our findings show that screening and treatment for diabetes and dysglycemia110 should be cost-saving from a health system perspective in people with any single risk factor for these conditions. This likely is a large proportion of the United States adult population, because at least one such risk factor was present in 98% of our study subjects and in 96% of adult African American and white participants in NHANES 2005–2006. We also found that the greatest cost-savings likely would be attained with screening of individuals with BMI >35 kg/m2, systolic blood pressure ≥130 mmHg, or age >55 years. The GCTpl, previously shown to have the greatest accuracy for detection of diabetes or dysglycemia110, appears to be the least expensive screening test in most higher-risk groups and should be considered for use in clinical practice.
Acknowledgments
This work was supported in part by National Institutes of Health awards DK0-66204 (L.S.P.) and UL1-RR0-25008, VA award HSR&D IIR 07-138 (L.S.P., K.M.V.N., S.L.J.), and Cystic Fibrosis Foundation award PHILLI12A0 (L.S.P.).
No potential conflicts of interest relevant to this article were reported.
R.C. researched the data and wrote the manuscript. K.M.V.N. reviewed and edited the manuscript. J.L. reviewed and edited the manuscript. S.L.J. contributed to analysis. Q.L. contributed to analysis. M.Z. contributed to analysis. L.S.P. conceived the idea and wrote the manuscript. L.S.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010.
The authors thank Dr. Darin Olson of the Department of Medicine, Atlanta VA Medical Center, Decatur, Georgia, and Dr. Paul Kolm of Biostatistics, Christiana Healthcare, Newark, Delaware, for statistical assistance.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1752/-/DC1.
References
- 1.American Diabetes Association. Standards of medical care in diabetes–2010. Diabetes Care 2010;33(Suppl. 1):S11-S61. [DOI] [PMC free article] [PubMed]
- 2.Kahn R, Alperin P, Eddy D, et al. Age at initiation and frequency of screening to detect type 2 diabetes: a cost-effectiveness analysis. Lancet 2010;375:1365–1374 [DOI] [PubMed] [Google Scholar]
- 3.Leiter LA, Barr A, Bélanger A, et al. Diabetes Screening in Canada (DIASCAN) Study Diabetes Screening in Canada (DIASCAN) Study: prevalence of undiagnosed diabetes and glucose intolerance in family physician offices. Diabetes Care 2001;24:1038–1043 [DOI] [PubMed] [Google Scholar]
- 4.Chatterjee R, Narayan KM, Lipscomb J, Phillips LS. Screening adults for pre-diabetes and diabetes may be cost-saving. Diabetes Care 2010;33:1484–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips LS, Ziemer DC, Kolm P, et al. Glucose challenge test screening for prediabetes and undiagnosed diabetes. Diabetologia 2009;52:1798–1807 [DOI] [PubMed] [Google Scholar]
- 6.Sorkin JD, Muller DC, Fleg JL, Andres R. The relation of fasting and 2-h postchallenge plasma glucose concentrations to mortality: data from the Baltimore Longitudinal Study of Aging with a critical review of the literature. Diabetes Care 2005;28:2626–2632 [DOI] [PubMed] [Google Scholar]
- 7.Kanaya AM, Herrington D, Vittinghoff E, et al. Impaired fasting glucose and cardiovascular outcomes in postmenopausal women with coronary artery disease. Ann Intern Med 2005;142:813–820 [DOI] [PubMed]
- 8.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernan WH, Brandle M, Zhang P, et al. Diabetes Prevention Program Research Group Costs associated with the primary prevention of type 2 diabetes mellitus in the diabetes prevention program. Diabetes Care 2003;26:36–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trogdon JG, Hylands T. Nationally representative medical costs of diabetes by time since diagnosis. Diabetes Care 2008;31:2307–2311 [DOI] [PMC free article] [PubMed]
- 11.Nichols GA, Brown JB. Higher medical care costs accompany impaired fasting glucose. Diabetes Care 2005;28:2223–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nichols GA, Bhakti A, Herman WH. Medical care costs one year after identification of hyperglycemia below the threshold for diabetes. Med Care 2008;46:287–292 [DOI] [PubMed]
- 13.Diabetes Prevention Program Research Group Within-trial cost-effectiveness of lifestyle intervention or metformin for the primary prevention of type 2 diabetes. Diabetes Care 2003;26:2518–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Diabetes Association Economic costs of diabetes in the U.S. In 2007. Diabetes Care 2008;31:596–615 [DOI] [PubMed] [Google Scholar]
- 15.Thompson SG, Barber JA. How should cost data in pragmatic randomised trials be analysed? BMJ 2000;320:1197–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li R, Zhang P, Barker LE, Chowdhury FM, Zhang X. Cost-effectiveness of interventions to prevent and control diabetes mellitus: a systematic review. Diabetes Care 2010;33:1872–1894 [DOI] [PMC free article] [PubMed]
- 17.Hoerger TJ, Harris R, Hicks KA, Donahue K, Sorensen S, Engelgau M. Screening for type 2 diabetes mellitus: a cost-effectiveness analysis. Ann Intern Med 2004;140:689–699 [DOI] [PubMed] [Google Scholar]
- 18.Hoerger TJ, Hicks KA, Sorensen SW, et al. Cost-effectiveness of screening for pre-diabetes among overweight and obese U.S. adults. Diabetes Care 2007;30:2874–2879 [DOI] [PubMed] [Google Scholar]
- 19.Gillies CL, Lambert PC, Abrams KR, et al. Different strategies for screening and prevention of type 2 diabetes in adults: cost effectiveness analysis. BMJ 2008;336:1180–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waugh N, Scotland G, McNamee P, et al. Screening for type 2 diabetes: literature review and economic modeling: Executive Summary. Health Technol Assess 2007. Availalble from http://eprints.whiterose.ac.uk/10716 Accessed June 2012 [DOI] [PubMed] [Google Scholar]
- 21.Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988-1994 and 2005-2006. Diabetes Care 2009;32:287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Dall TM, Chen Y, et al. Medical cost associated with prediabetes. popul. Health Manage 2009;12:157–163 [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Dall TM, Mann SE, et al. The economic costs of undiagnosed diabetes. Popul Health Manag 2009;12:95–101 [DOI] [PubMed] [Google Scholar]
- 24.Johansson P, Ostenson CG, Hilding AM, Andersson C, Rehnberg C, Tillgren P. A cost-effectiveness analysis of a community-based diabetes prevention program in Sweden. Int J Technol Assess Health Care 2009;25:350–358 [DOI] [PubMed] [Google Scholar]
- 25.Smith KJ, Hsu HE, Roberts MS, et al. Cost-effectiveness analysis of efforts to reduce risk of type 2 diabetes and cardiovascular disease in southwestern Pennsylvania, 2005-2007. Prev Chronic Dis 2010;7:A109. [PMC free article] [PubMed] [Google Scholar]
- 26.Eriksson MK, Hagberg L, Lindholm L, Malmgren-Olsson EB, Osterlind J, Eliasson M. Quality of life and cost-effectiveness of a 3-year trial of lifestyle intervention in primary health care. Arch Intern Med 2010;170:1470–1479 [DOI] [PubMed] [Google Scholar]