Abstract
OBJECTIVE
The landmark Diabetes Prevention Program (DPP) showed that lifestyle intervention can prevent or delay the onset of diabetes for those at risk. We evaluated a translational implementation of this intervention in a diverse set of American Indian and Alaska Native (AI/AN) communities.
RESEARCH DESIGN AND METHODS
The Special Diabetes Program for Indians Diabetes Prevention (SDPI-DP) demonstration project implemented the DPP lifestyle intervention among 36 health care programs serving 80 tribes. A total of 2,553 participants with prediabetes were recruited and started intervention by 31 July 2008. They were offered the 16-session Lifestyle Balance Curriculum and underwent a thorough clinical assessment for evaluation of their diabetes status and risk at baseline, soon after completing the curriculum (postcurriculum), and annually for up to 3 years. Diabetes incidence was estimated. Weight loss, changes in blood pressure and lipid levels, and lifestyle changes after intervention were also evaluated.
RESULTS
The completion rates of SDPI-DP were 74, 59, 42, and 33% for the postcurriculum and year 1, 2, and 3 assessments, respectively. The crude incidence of diabetes among SDPI-DP participants was 4.0% per year. Significant improvements in weight, blood pressure, and lipid levels were observed immediately after the intervention and annually thereafter for 3 years. Class attendance strongly correlated with diabetes incidence rate, weight loss, and change in systolic blood pressure.
CONCLUSIONS
Our findings demonstrate the feasibility and potential of translating the lifestyle intervention in diverse AI/AN communities. They have important implications for future dissemination and institutionalization of the intervention throughout the Native American health system.
Type 2 diabetes, a serious global public health problem, affects disadvantaged populations disproportionately, especially American Indians and Alaska Natives (AI/ANs) (1). In 2009, the age-adjusted prevalence of diabetes for adults eligible for Indian Health Service (IHS) was 16.1%—more than twice that of non-Hispanic white adults (1). Landmark clinical trials, such as the Diabetes Prevention Program (DPP), have showed that lifestyle interventions can prevent or delay the onset of diabetes for those at risk (2–5). In addition, DPP found no significant differences in the reduction of diabetes incidence by race/ethnicity, including in American Indians (4). While under well-controlled circumstances (e.g., clinical trials) lifestyle intervention may have equivalent efficacy across race/ethnicity groups, the effectiveness of implementing such programs in community-based settings among underserved populations remains underexplored. In particular, implementations of large-scale public health interventions in AI/AN communities are plagued by lack of resources, diverse health care settings, and the highly mobile population, all of which are challenges to the successful recruitment, retention, and effectiveness of translational efforts.
Translating the DPP intervention into real-world situations has occurred in other settings (6–16), such as urban medically underserved communities (7), faith-based settings (8), YMCAs (9), work sites (10), and primary care practices (11–13). However, most of these were small studies implemented in relatively uniform settings; particularly, none of them included a substantial number of AI/ANs—the U.S. population that suffers most from diabetes (1,17). Given the significant economic and sociocultural diversity of AI/AN communities, it is important to determine the feasibility and effectiveness of such an intervention in a large sample of this population.
Mandated and funded by Congress, the IHS implemented the Special Diabetes Program for Indians Diabetes Prevention (SDPI-DP) demonstration project and collected data that allowed an unprecedented investigation of the translational effectiveness of the DPP lifestyle intervention in preventing diabetes across 36 diverse programs, representing rural, reservation, and urban AI/AN communities. This article reports the primary and secondary outcomes of SDPI-DP participants after a follow-up of 3 years.
RESEARCH DESIGN AND METHODS
The SDPI-DP Program is a congressionally mandated demonstration project and is designed to reduce diabetes incidence among AI/ANs with prediabetes through implementation of the DPP lifestyle intervention. In 2004, 128 AI/AN local health care programs applied for funding to participate in SDPI demonstration projects: 36 received funding for SDPI-DP. Grantees represented a diverse mix of programs, serving 80 tribes in 18 states and 11 of the 12 IHS administrative areas. These programs included six IHS hospitals/clinics and 30 tribal or IHS-contracted health care programs administered by tribes. Embracing the principles of community-based participatory research (18), SDPI-DP was a collaborative effort. This partnership started with a planning year wherein bimonthly meetings oriented grant programs to the project, provided technical assistance, and provided opportunities to jointly develop required activities and their evaluation. This process allowed grant programs to effectively market their efforts to local health program/organizations, tribal leaders, and community stakeholders and to complete extensive local approval processes.
The collaborative process led to a common set of activities adopted by a diverse group of grant programs. Briefly, the participating programs were required to implement the 16-session Lifestyle Balance Curriculum drawn from the DPP (4) and to participate in the evaluation of the effectiveness of their prevention activities. The inclusion of a control group was deemed unethical owing to strong evidence supporting the efficacy of the lifestyle intervention approach in preventing diabetes (2–5). Rather, the goal of SDPI-DP was to pursue a comprehensive public health evaluation of the translation of a proven intervention in diverse AI/AN communities.
SDPI-DP grant programs identified potential participants mainly through community events such as health fairs but also recruited participants from local clinics or by provider referral. Eligibility criteria were being AI/AN (based on eligibility to receive IHS services), being at least 18 years of age, no previous diagnosis of diabetes, and having either impaired fasting glucose (IFG) (i.e., a fasting blood glucose [FBG] level of 100–125 mg/dL and an oral glucose tolerance test [OGTT] result <200 mg/dL) or impaired glucose tolerance (IGT) (i.e., an OGTT result of 140–199 mg/dL 2 h after a 75-g oral glucose load and an FBG level <126 mg/dL). Four exclusion criteria were used: 1) a previous diagnosis of diabetes, 2) pregnancy, 3) end-stage renal disease (ESRD) on dialysis, and 4) any condition that would affect successful participation based on provider judgment (e.g., active alcohol or substance abuse or cancer diagnosis that prohibited participation by provider judgment). The SDPI-DP eligibility criteria were very similar to those for the American Indian participants of the original DPP program, except that the DPP required the American Indian participants to be at least 25 years old, have a BMI of ≥24 kg/m2, and have IGT (with or without IFG).
Enrollment began in January 2006 and is ongoing. Although the grant programs were not required to report the number or reasons for excluding individuals from enrolling, 23 grantee sites submitted relatively complete data for us to roughly estimate the percentages of participants excluded based on different criteria. On average, ~65% of the screened individuals were excluded owing to normal blood glucose, 29% were excluded because of a previous diagnosis of diabetes, 2% were excluded owing to pregnancy, 0.04% were excluded for end-stage renal disease, and 4% were excluded for other reasons based on the judgment of the provider. The analyses here included baseline and annual data for up to 3 years from 2,553 participants who completed the baseline assessment and started intervention by 31 July 2008. The SDPI-DP protocol was approved by the institutional review board of the University of Colorado Denver and the National IHS Institutional Review Board. When required, grantees obtained approval from other entities overseeing research in their programs (e.g., tribal review boards). All participants provided written informed consent and Health Insurance Portability and Accountability Act authorization.
Intervention
As in the DPP lifestyle intervention arm (19), the primary goal of the intervention was to achieve and maintain a weight reduction of at least 7% of initial body weight through a healthy diet and increased physical activity. Grantees used the 16-lesson DPP curriculum covering diet, exercise, and behavior modification to help participants achieve this goal. Adaptation for local culture and situation was allowed provided that the same basic information was presented and adaptation was documented. Many grantees drew upon their local culture to translate educational concepts and curriculum into tribal languages and incorporated, for instance, talking circles, indigenous foods, or drumming into intervention sessions.
The curriculum was delivered in group settings within 16–24 weeks after baseline assessment and typically was taught by the program dietitian and/or health educator. It was supplemented by monthly individual lifestyle coaching sessions to individualize goals and plan and to identify and solve barriers to participation. Participants were encouraged to use a Keeping Track booklet to monitor their fat and calorie intake and weekly physical activity. If used, booklets were reviewed by lifestyle coaches who gave feedback to the participants during the monthly lifestyle coaching sessions. Approximately one-half of the lifestyle coaches were health educators or dietitians. Others were nurses, nursing students, nurse or medical assistants, exercise specialist, or lay health workers from various professional backgrounds.
Outcome measures
At baseline, within a month of completing the last lifestyle class (usually 4–6 months after baseline, hereafter called the postcurriculum assessment), and annually after baseline for up to 3 years, participants underwent a comprehensive clinical assessment to evaluate their diabetes risk and incidence. At the same time, each participant completed a questionnaire including questions regarding sociodemographics, health-related behavior, and a range of psychosocial factors. Every participant underwent an additional FBG test midway between annual assessments to assess possible diabetes conversion.
The primary outcome was incidence of diabetes, diagnosed by an annual OGTT or a semiannual FBG test conducted in local or regional laboratories, according to the 2004 criteria of the American Diabetes Association: an FBG ≥126 mg/dL or a 2-h test result ≥200 mg/dL after a 75-g oral glucose load. In addition to the semiannual measurements, FBG was measured if symptoms suggestive of diabetes developed. The diagnosis required confirmation by a second test, usually within 6 weeks of the first test. If diabetes was diagnosed, the participant was informed and referred to his or her doctor for treatment. All data collection for that participant was discontinued. Secondary outcomes included weight loss, blood pressure, lipid profile, and physical activity. At each clinical assessment, body weight, height, and BMI were measured with participants wearing light clothing and no shoes; blood pressure was assessed by a grantee staff member. Laboratory assays of FBG, OGTT, HDL cholesterol (HDL-C), LDL cholesterol (LDL-C), and triglyceride were conducted after 9–12 h of fasting in local or regional laboratories following standardized protocols. Additionally, the average minutes of physical activity per week in the previous month for each participant were recorded by the program staff at each clinical assessment.
Statistical analysis
Baseline characteristics were compared between completers and noncompleters of each clinical assessment using χ2 tests for categorical variables and two-sample t tests for continuous variables. Product-limit curve was used to assess the primary outcome of the intervention (cumulative incidence of diabetes). Proportional hazards regression models were used to estimate the hazard ratio of diabetes between SDPI-DP subgroups after controlling for baseline demographic characteristics (age and sex) and clinical diabetes risk factors (FBG, OGTT 2-h result, BMI, HDL-C, systolic blood pressure, triglyceride, and family history of diabetes).
For secondary outcomes, linear mixed-effects models were used to obtain adjusted mean changes for each outcome at each assessment, with baseline age and sex, continuous time, and a change point at each postbaseline assessment included in the model. A spatial power covariance structure with time as the distance measure accounted for the time-series correlation between repeated outcomes; a random intercept at the participant level was included to model subject-level heterogeneity (20). Multiple linear regression models were used to investigate the relationship between changes in secondary outcomes at the postcurriculum assessment and class attendance after controlling for age, sex, and baseline level of each outcome.
RESULTS
Baseline characteristics and follow-up
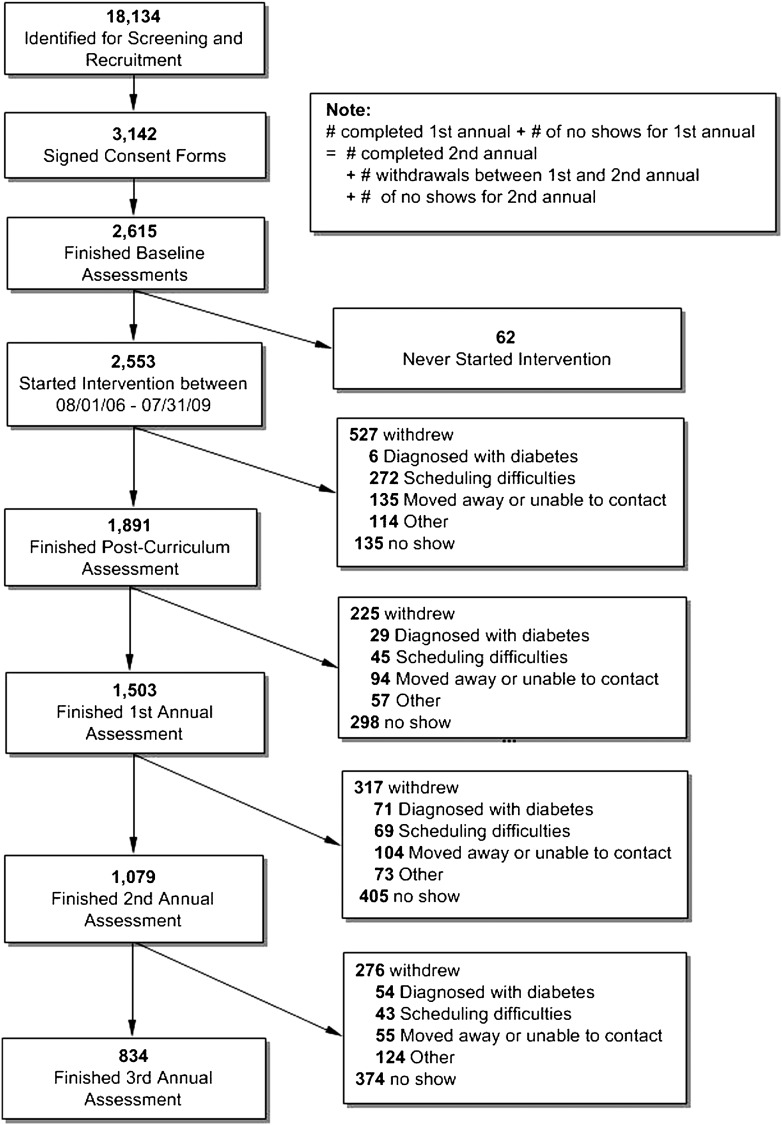
As shown in Fig. 1, SDPI-DP grant programs identified 18,134 individuals for screening and recruitment; 2,615 participants met the inclusion criteria, enrolled, and finished the baseline assessment. Of these, 2,553 started the intervention by 31 July 2008; 1,891 (74%) completed the postcurriculum assessment, and 1,503 (59%), 1,079 (42%), and 834 (33%) finished the first, second, and third annual assessment, respectively. On average, the SDPI-DP participants were followed for 2.0 years (range 1 day to 3 years) in the data reported here.
Figure 1.
SDPI-DP recruitment and retention flow chart.
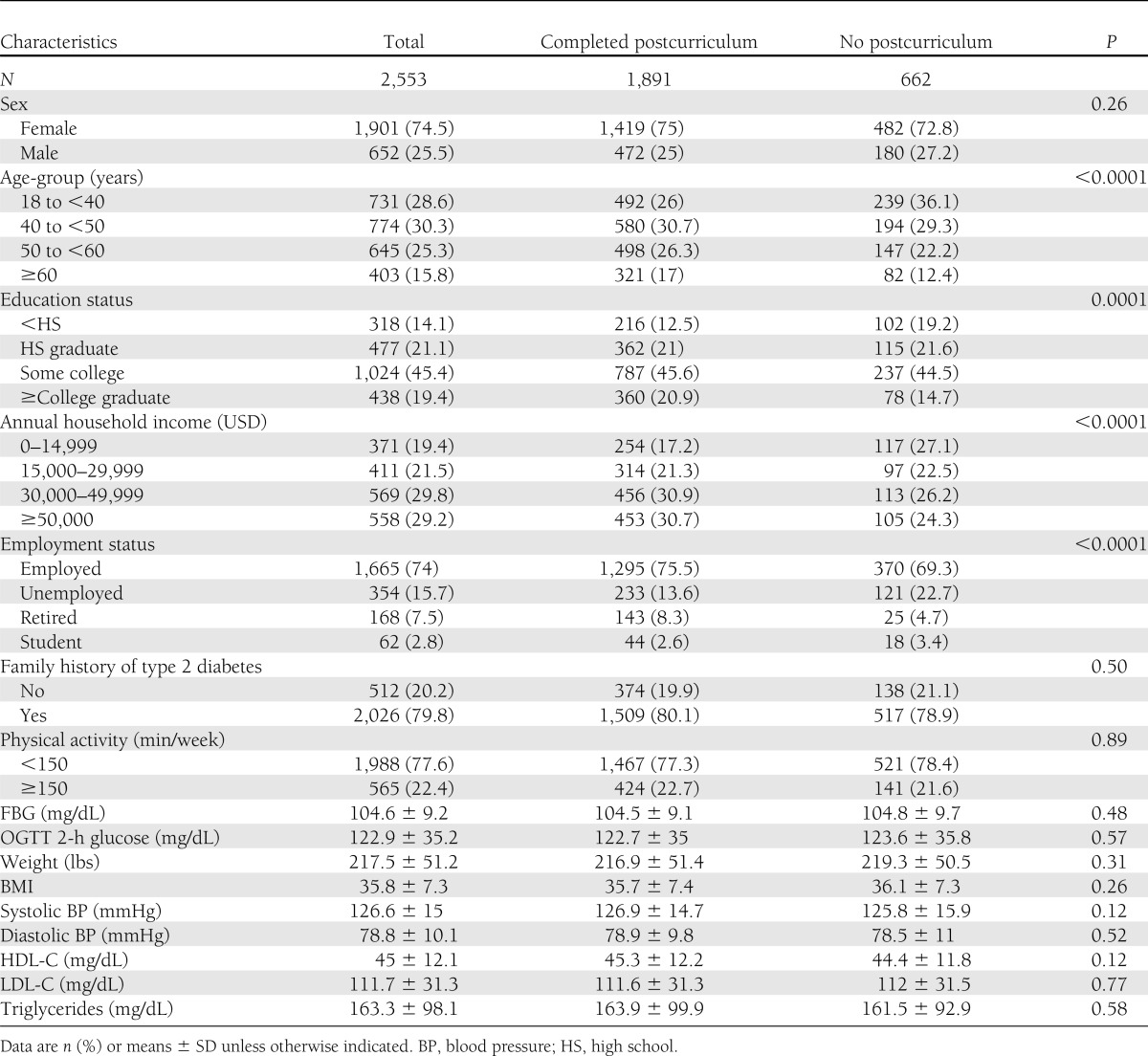
Table 1 compares the baseline characteristics of the SDPI-DP participants who completed and did not complete the postcurriculum assessment. Three-fourths of SDPI-DP participants were female, with an average age of 46.6 years and average BMI of 35.8 at baseline. Compared with the noncompleters, those who completed the postcurriculum were significantly older, were more educated, had higher income, and were more likely to be employed or retired (versus unemployed) at baseline. The results comparing the completers and noncompleters for the annual assessments were similar except that the completers for the year-2 and -3 assessments had lower baseline weight and FBG level than the noncompleters at those time points (Supplementary Table 1).
Table 1.
Baseline characteristics of SDPI-DP participants
Primary outcome
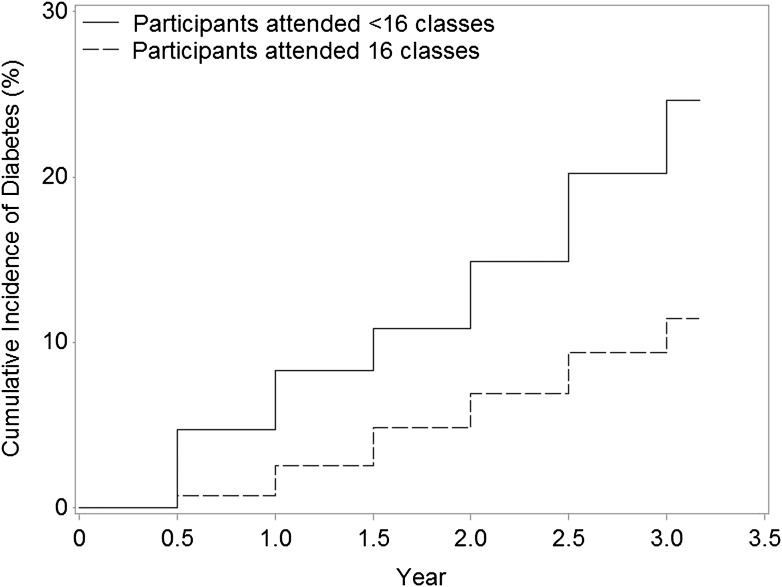
The crude incidence of diabetes among all SDPI-DP participants was 4.0% per year. As shown in Fig. 2, the cumulative diabetes incidence among SDPI-DP participants who attended all 16 classes was significantly lower than that of those who attended ≤15 classes (P < 0.0001). Specifically, the crude incidence of diabetes was ~3.5% each year among those who finished all 16 classes, while the rate more than doubled (7.5% each year) among the participants who did not finish all of the classes.
Figure 2.
Cumulative incidence of diabetes in SDPI-DP by DPP class attendance.
Secondary outcomes
SDPI-DP participants had significant weight loss at each clinical assessment after baseline (Table 2). On average, they lost 9.6 lbs immediately after completing the lifestyle intervention classes, representing a 4.4% weight loss. The average weight loss attenuated over the three annual visits to 5.6, 3.1, and 2.4 lbs, respectively, but all were significantly different from 0. Over one-fifth (22.5%) of the SDPI-DP participants who completed the postcurriculum assessment achieved the 7% weight loss goal by the end of the Lifestyle Balance classes; 17.5% met this goal 3 years after the intervention began.
Table 2.
Secondary outcomes among SDPI-DP participants at each of the assessments
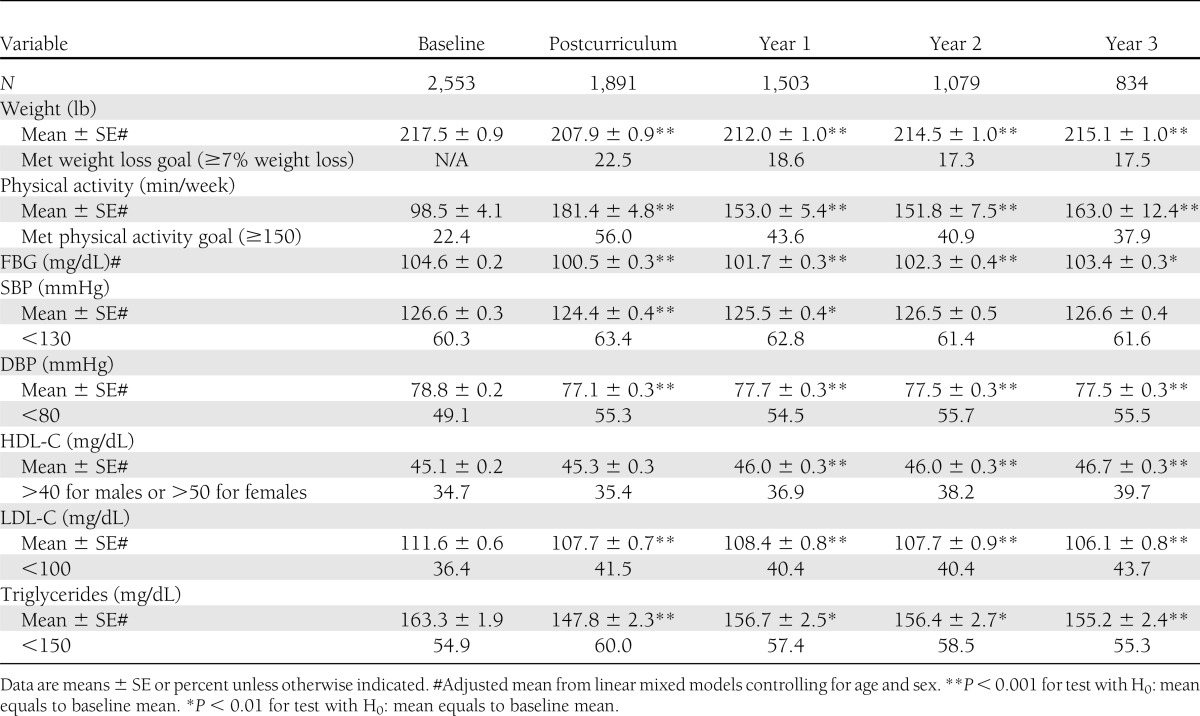
Table 2 also illustrates that SDPI-DP participants reported more exercise each week after intervention. On average, SDPI-DP participants reported 181 min physical activity/week immediately after the last intervention class (vs. 99 min/week at baseline). Physical activity levels decreased to ~150–160 min/week at the annual assessments, but all were significantly higher than the baseline level. The percentage achieving the physical activity goal (150 min/week) increased from 22% at baseline to 56% at the postcurriculum assessment and was ≥38% at the annual assessments.
In terms of laboratory testing values, FBG levels decreased significantly among SDPI-DP participants from baseline to all clinical assessments after the lifestyle intervention. On average, fasting blood glucose levels decreased by ~4 mg/dL from baseline to postcurriculum assessment. Both systolic and diastolic blood pressure of SDPI-DP participants decreased significantly at the postcurriculum and first annual assessments. Diastolic blood pressure also reduced significantly at the second and third annual assessments. A significant increase in average HDL-C levels was observed at all annual assessments but not immediately after completion of the lifestyle classes. Further, SDPI-DP participants had significant reductions in LDL-C and triglyceride levels at all postbaseline assessments.
Program participation
As of 31 July 2009, two-thirds (68%) of the SDPI-DP participants had attended all 16 Lifestyle Balance classes, 17% had attended 8–15 classes, and 15% had attended ≤7 classes. On average, each participant attended 13 classes and each class was attended by approximately four participants. During the implementation of the DPP curriculum, 84% of the SDPI-DP participants used the Keeping Track booklet to monitor weekly physical activity and 92% of them used the Keeping Track booklet to monitor fat and calorie intake. After completion of the Lifestyle Balance classes, each participant attended an average of six lifestyle coaching visits in the first year of the project. After age, sex, and baseline clinical diabetes risk factors were controlled for, participants who attended all 16 classes had a significantly lower risk of diabetes (hazard ratio 0.46 [95% CI 0.32 – 0.66]) than did participants who attended ≤15 classes. Moreover, those who attended all 16 classes had a significantly larger reduction in fasting blood glucose level, weight, and systolic blood pressure at the postcurriculum assessment (Supplementary Table 2).
CONCLUSIONS
SDPI-DP is the first large-scale, national demonstration project to evaluate the effectiveness of the DPP lifestyle intervention in a geographically diverse group of AI/ANs. It also is among the first diabetes prevention translational projects to report the long-term effectiveness of the intervention in community settings (21) and report diabetes incidence instead of surrogate end points (22,23). Our findings strongly support the feasibility of translating the intervention across a wide range of Native communities. With similar eligibility criteria, the crude diabetes incidence of SDPI-DP (4.0% per year) was close to that of the American Indians in the lifestyle intervention group of the DPP clinical trial (4.7% per year) and lower than that of the American Indians in the placebo group of DPP (12.9% per year). It also was lower than the crude incidence rate of diabetes among the participants with prediabetes (IFG and/or IGT) in the Strong Heart Study (SHS) (6.6% per year), a cardiovascular disease project conducted in 13 Native American communities/tribes in three geographic areas (24). Furthermore, it was lower than the rates of other diabetes prevention translational projects that reported diabetes incidence, which was 36% per year in the Help Educate to Eliminate Diabetes project (22) and >7.4% per year for participants with IFG and/or IGT in the Finnish National Diabetes Prevention Program (FIN-D2D) (19).
In addition to diabetes incidence, the participants achieved substantial improvements in multiple secondary outcomes. On average, they lost 9.6 lbs immediately after the 16 Lifestyle Balance classes, which was 4.4% of their average baseline weight. This amount of weight loss was lower than that among the lifestyle group of DPP (6.9% weight loss). However, significant differences in weight loss among different ethnic groups were observed in DPP, with American Indians less likely to meet the initial weight loss goal but with reduction in diabetes incidence similar to that of other ethnic groups (25). Furthermore, modest weight loss was observed in a number of other translational projects of the DPP or Finnish Diabetes Prevention Study, such as the FIN-D2D (2.2 lbs at 1-year follow-up) (23) and a church-based translational project (7.5 lbs postcurriculum) (8,10). These previously published studies had different eligibility criteria, targeted populations, and implementation settings, though, which makes their results not directly comparable with ours.
In line with their weight loss, SDPI-DP participants exercised more after the intervention. Likewise, they exhibited significant improvements in their blood pressure and lipid levels. In general, all of these results are consistent with those of DPP and compare favorably with other translational projects with relatively small sample sizes (7,9,11,26), demonstrating the potential of successfully implementing this preventive intervention in AI/AN communities across a diverse set of local health programs nested in different types of health organizations.
The SDPI-DP experience also revealed important challenges for future translational efforts of this kind. First, the skepticism of grantee staff about the importance and success of evaluation required considerable discussion. Most staff previously had not participated in a project of this scope or in a program evaluation as rigorous as expected here. To address this skepticism, grantees were encouraged to assist with designing the evaluation. They prioritized factors to determine the success of the project at programmatic and individual levels. They also provided guidance on evaluation designs that would be acceptable to tribal leaders and members.
As a translation attempt, the SDPI-DP did not emphasize or allocate ample resources to follow-up participants beyond the first year. Hence, although most grantees successfully recruited substantial numbers of participants, retention, especially in the long-term, was a daunting challenge. Busy, stressful lives and high mobility compromised some participants’ attendance at all 16 sessions or monthly meetings with lifestyle coaches. Indeed, the most common withdrawal reasons were scheduling difficulties and moving away/unable to contact. The higher likelihood for older and retired participants of staying in the program probably reflects fewer hurdles related to scheduling and mobility among this group. Given the importance of full attendance for maximizing the impact of the intervention as shown in Fig. 2, future translational initiatives clearly will need additional creative retention strategies.
For many participants who stayed in the program, it was challenging to sustain the intervention effects achieved immediately after the curriculum: improvements in most diabetes risk factors among SDPI-DP participants attenuated at annual visits. DPP and other lifestyle intervention studies also noted similar attenuation of intervention effects (27,28). It has been proven, though, that successful lifestyle changes have an impact beyond the intervention period, even after participants regain some weight (28,29).
Lastly, although the SDPI-DP program sought to deliver the lifestyle balance classes in groups of 8–12 participants, in reality this proved difficult; the average was only four participants per class. Since group-based lifestyle interventions are more cost-effective (30), balancing the need to accommodate varying schedules of different participants with maintaining an adequate class size is another important task for future translational research.
Several study limitations should be acknowledged. As a demonstration project intended to translate proven intervention methods in community settings, the study did not include a placebo group in the design. Although this is appropriate for translational projects (26,31), the lack of a control group compromises our ability to determine diabetes incidence among SDPI-DP participants who did not receive the lifestyle intervention. This underscores the need for historical references to assess the primary outcome: diabetes incidence. The DPP placebo group served as a relevant historical control since SDPI-DP followed the DPP model. However, these two projects were different with respect to eligibility criteria, baseline characteristics of the participants, and the implementation of the intervention method. Diabetes incidence among those with prediabetes from the SHS provides another relevant benchmark for comparative purposes. Yet, the baseline data of the SHS were collected more than two decades ago for a different study purpose and only included American Indian participants from a limited age range (45–74 years), residing in three geographic areas.
Since the SDPI-DP program by its very nature is not as rigorously controlled as a randomized clinical trial, the data had relatively high rates of loss to follow-up. Proportional hazard regression models and linear mixed models, which permit unbiased estimation for model parameters when the independent censoring or missing-at-random assumption is met, were used to address this problem (32). The missing-at-random assumption is difficult to evaluate, though. In particular, the retention analysis for the second and third annual assessments revealed that the noncompleters had significantly higher baseline weight and FBG level. This may imply missing not at random and potential “survivor bias” caused by the fact that only the outcomes of those who substantially improved after the intervention were recorded and analyzed. However, in a randomly selected sample from SDPI-DP participants whose baseline age and diabetes risk score were matched with the third-year noncompleters, the estimated diabetes incidence was only slightly above that of the entire SDPI-DP sample (4.9 vs. 4.0% per year), indicating the potential robustness of our results.
Despite these challenges and limitations, the SDPI-DP was highly successful in translating the DPP lifestyle intervention across an organizationally and geographically diverse array of AI/AN communities. The wide dissemination of these results in conjunction with the challenges as discussed above holds great promise for changing the trajectory of the diabetes epidemic among AI/ANs who suffer daunting health disparities attributable to this disease. Longer-term follow-up of the SDPI-DP participants, understanding site differences in program performance and intervention outcomes, and more thorough examination of factors related to successful attendance and retention represent important next steps scientifically as the IHS poises to implement this intervention across all AI/AN communities. Programmatically, the challenge shifts to disseminating the results, institutionalizing the intervention throughout the Native American health system, and continuing to document reductions in the substantial burden of diabetes on this population. The SDPI-DP is addressing this latter challenge by developing peer-to-peer consultation models, by disseminating tools that spring from the grantee programs’ experience, and by facilitating collaboration across the private, tribal, and federal entities that comprise the Native American health system.
Acknowledgments
Funding for this project was provided by the IHS (HHSI242200400049C to S.M.M.). Manuscript preparation was supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases (1P30DK092923 to S.M.M.). Grant programs participating in the SDPI-DP are as follows: Confederated Tribes of the Chehalis Reservation, the Cherokee Nation, Cheyenne River Sioux Tribe, the Chickasaw Nation, Coeur d’Alene Tribe, Colorado River Indian Tribes, Colville Confederated Tribes, Cow Creek Band of Umpqua Tribe, Fond du Lac Reservation, Gila River Health Care, Haskell Health Center, Ho-Chunk Nation, Indian Health Board of Minneapolis, Indian Health Center of Santa Clara Valley, Kenaitze Indian Tribe IRA, Lawton IHS Service Unit, Menominee Indian Tribe of Wisconsin, Mississippi Band of Choctaw Indians, Norton Sound Health Corporation, Pine Ridge IHS Service Unit, Pueblo of San Felipe, the Quinault Indian Nation, Rapid City IHS Diabetes Program, Red Lake Comprehensive Health Services, Rocky Boy Health Board, the Seneca Nation of Indians, Sonoma County Indian Health Project, South East Alaska Regional Health Consortium, Southcentral Foundation, Trenton Indian Service Area, Tuba City Regional Health Care Corporation, United American Indian Involvement, Inc., United Indian Health Services, Inc., Warm Springs Health and Wellness Center, Winnebago Tribe of Nebraska, and Zuni Pueblo.
No potential conflicts of interest relevant to this article were reported.
L.J. researched data, contributed to the discussion, and wrote, reviewed, and edited the manuscript. S.M.M. conceptualized and designed the project, contributed to the discussion, and reviewed and edited the manuscript. J.B. participated in the design of the project, contributed to the discussion, and reviewed and edited the manuscript. W.G.H. contributed to the discussion and reviewed and edited the manuscript. H.H. researched data and reviewed the manuscript. K.J.A. conceptualized the project, contributed to the discussion, and reviewed and edited the manuscript. Y.R. conceptualized and designed the project, contributed to the discussion, and reviewed and edited the manuscript. L.J. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors are grateful to the IHS as well as to the tribal and urban Native American health programs and participants involved in the SDPI-DP.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1250/-/DC1.
See accompanying commentary, p. 1820.
References
- 1.Centers for Disease Control and Prevention. 2011 National Diabetes Fact Sheet [article online], 2011. Available from http://www.cdc.gov/diabetes/pibs/estimates11.htm Accessed 8 April 2011
- 2.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–544 [DOI] [PubMed] [Google Scholar]
- 3.Tuomilehto J, Lindström J, Eriksson JG, et al. Finnish Diabetes Prevention Study Group Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 4.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norris SL, Zhang X, Avenell A, et al. Long-term effectiveness of weight-loss interventions in adults with pre-diabetes: a review. Am J Prev Med 2005;28:126–139 [DOI] [PubMed] [Google Scholar]
- 6.Pagoto SL, Kantor L, Bodenlos JS, Gitkind M, Ma Y. Translating the diabetes prevention program into a hospital-based weight loss program. Health Psychol 2008;27(Suppl.):S91–S98 [DOI] [PubMed] [Google Scholar]
- 7.Seidel MC, Powell RO, Zgibor JC, Siminerio LM, Piatt GA. Translating the Diabetes Prevention Program into an urban medically underserved community: a nonrandomized prospective intervention study. Diabetes Care 2008;31:684–689 [DOI] [PubMed] [Google Scholar]
- 8.Boltri JM, Davis-Smith YM, Seale JP, Shellenberger S, Okosun IS, Cornelius ME. Diabetes prevention in a faith-based setting: results of translational research. J Public Health Manag Pract 2008;14:29–32 [DOI] [PubMed] [Google Scholar]
- 9.Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the Diabetes Prevention Program into the community. The DEPLOY Pilot Study. Am J Prev Med 2008;35:357–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aldana SG, Barlow M, Smith R, et al. The diabetes prevention program: a worksite experience. AAOHN J 2005;53:499–505 [PubMed] [Google Scholar]
- 11.Kramer MK, Kriska AM, Venditti EM, et al. Translating the Diabetes Prevention Program: a comprehensive model for prevention training and program delivery. Am J Prev Med 2009;37:505–511 [DOI] [PubMed] [Google Scholar]
- 12.Whittemore R, Melkus G, Wagner J, Dziura J, Northrup V, Grey M. Translating the diabetes prevention program to primary care: a pilot study. Nurs Res 2009;58:2–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McTigue KM, Conroy MB, Bigi L, Murphy C, McNeil M. Weight loss through living well: translating an effective lifestyle intervention into clinical practice. Diabetes Educ 2009;35:199–204, 208 [DOI] [PubMed] [Google Scholar]
- 14.Katula JA, Vitolins MZ, Rosenberger EL, et al. One-year results of a community-based translation of the Diabetes Prevention Program: Healthy-Living Partnerships to Prevent Diabetes (HELP PD) Project. Diabetes Care 2011;34:1451–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mau MK, Keawe’aimoku Kaholokula J, West MR, et al. Translating diabetes prevention into native Hawaiian and Pacific Islander communities: the PILI ’Ohana Pilot project. Prog Community Health Partnersh 2010;4:7–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanderwood KK, Hall TO, Harwell TS, Butcher MK, Helgerson SD, Montana Cardiovascular Disease and Diabetes Prevention Program Workgroup Implementing a state-based cardiovascular disease and diabetes prevention program. Diabetes Care 2010;33:2543–2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Indian Health Service. Diabetes in American Indians and Alaska Natives: Facts At-a-Glance Department of Health and Human Services, Washington, DC, 2008 [Google Scholar]
- 18.Israel BA, Schulz AJ, Parker EA, Becker AB. Review of community-based research: assessing partnership approaches to improve public health. Annu Rev Public Health 1998;19:173–202 [DOI] [PubMed] [Google Scholar]
- 19.Diabetes Prevention Program (DPP) Research Group The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care 2002;25:2165–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York, Springer-Verlag, 2000 [Google Scholar]
- 21.Absetz P, Oldenburg B, Hankonen N, et al. Type 2 diabetes prevention in the real world: three-year results of the GOAL lifestyle implementation trial. Diabetes Care 2009;32:1418–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parikh P, Simon EP, Fei K, Looker H, Goytia C, Horowitz CR. Results of a pilot diabetes prevention intervention in East Harlem, New York City: Project HEED. Am J Public Health 2010;100(Suppl. 1):S232–S239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saaristo T, Moilanen L, Korpi-Hyövälti E, et al. Lifestyle intervention for prevention of type 2 diabetes in primary health care: one-year follow-up of the Finnish National Diabetes Prevention Program (FIN-D2D). Diabetes Care 2010;33:2146–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, Shara NM, Calhoun D, Umans JG, Lee ET, Howard BV. Incidence rates and predictors of diabetes in those with prediabetes: the Strong Heart Study. Diabetes Metab Res Rev 2010;26:378–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wing RR, Hamman RF, Bray GA, et al. Diabetes Prevention Program Research Group Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes Res 2004;12:1426–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Absetz P, Valve R, Oldenburg B, et al. Type 2 diabetes prevention in the “real world”: one-year results of the GOAL Implementation Trial. Diabetes Care 2007;30:2465–2470 [DOI] [PubMed] [Google Scholar]
- 27.Knowler WC, Fowler SE, Hamman RF, et al. Diabetes Prevention Program Research Group 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindström J, Ilanne-Parikka P, Peltonen M, et al. Finnish Diabetes Prevention Study Group Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 2006;368:1673–1679 [DOI] [PubMed] [Google Scholar]
- 29.Li G, Zhang P, Wang J, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 2008;371:1783–1789 [DOI] [PubMed] [Google Scholar]
- 30.Ackermann RT, Marrero DG, Hicks KA, et al. An evaluation of cost sharing to finance a diet and physical activity intervention to prevent diabetes. Diabetes Care 2006;29:1237–1241 [DOI] [PubMed] [Google Scholar]
- 31.Garfield SA, Malozowski S, Chin MH, et al. Diabetes Mellitus Interagency Coordinating Committee (DIMCC) Translation Conference Working Group Considerations for diabetes translational research in real-world settings. Diabetes Care 2003;26:2670–2674 [DOI] [PubMed] [Google Scholar]
- 32.Fairclough DL. Design and Analysis of Quality of Life Studies in Clinical Trials 1st ed. Boca Raton, FL, Taylor and Francis, 2002 [Google Scholar]