Abstract
Significant data suggest that overt hyperglycemia, either observed with or without a prior diagnosis of diabetes, contributes to an increase in mortality and morbidity in hospitalized patients. In this regard, goal-directed insulin therapy has remained as the standard of care for achieving and maintaining glycemic control in hospitalized patients with critical and noncritical illness. As such, protocols to assist in management of hyperglycemia in the inpatient setting have become commonplace in hospital settings. Clearly, insulin is a known entity, has been in clinical use for almost a century, and is effective. However, there are limitations to its use. Based on the observed mechanisms of action and efficacy, there has been a great interest in using incretin-based therapy with glucagon-like peptide-1 (GLP-1) receptor agonists instead of, or complementary to, an insulin-based approach to improve glycemic control in hospitalized, severely ill diabetic patients. To provide an understanding of both sides of the argument, we provide a discussion of this topic as part of this two-part point-counterpoint narrative. In the point narrative preceding the counterpoint narrative below, Drs. Schwartz and DeFronzo provide an opinion that now is the time to consider GLP-1 receptor agonists as a logical consideration for inpatient glycemic control. In the counterpoint narrative provided below, Drs. Umpierrez and Korytkowski provide a defense of insulin in the inpatient setting as the unquestioned gold standard for glycemic management in hospitalized settings.
—William T. Cefalu, MD
Editor in Chief, Diabetes Care
Hyperglycemia is reported in one-third of general medicine and surgery patients with and without a known history of diabetes (1). The prevalence is even higher in intensive care unit (ICU) patients and following cardiac surgery, occurring in up to 80% of patients (2). While previously thought to be an epiphenomenon related to the acute underlying illness of the hospitalization itself, hyperglycemia is now recognized as a contributor to adverse outcomes in critical and noncritically ill patients, with higher mortality and disease-specific morbidity (3,4).
Protocols using insulin to maintain glycemia within a reasonable range reduces both mortality and morbidity (2,5,6). In critically ill patients in ICU settings, intravenously (IV) administered insulin is the preferred method of achieving recommended glycemic targets. The short half-life of IV insulin permits rapid dosing adjustments in response to alterations in insulin sensitivity observed during critical illness. For the majority of ICU patients, insulin infusion is started at a threshold of no higher than 10.0 mmol/L (180 mg/dL). Once IV insulin is started, glucose levels should be maintained between 6.1 and 10.0 mmol/L (110 and 180 mg/dL) (6). For patients in non-ICU settings, recent guidelines (6–8) recommend the use of subcutaneous (SC) insulin as the preferred therapy. Scheduled basal-bolus SC insulin therapy consisting of long- or intermediate-acting preparations in combination with short- or rapid-acting analogs has been proven to be safe and effective for glycemic management and to reduce hospital complications including wound infections, pneumonia, bacteremia, and acute renal and respiratory failure when compared with the use of sliding-scale insulin alone in patients with type 2 diabetes (5).
Improved glycemic control with insulin therapy is associated with amelioration of the hormonal and proinflammatory aberrations associated with stress hyperglycemia (9,10). These include reductions in counterregulatory hormones and proinflammatory transcription factors, and potentially the formation of reactive oxygen species (9). Insulin therapy induces vasodilatation by stimulating nitric oxide release and inducing expression of endothelial nitric oxide synthase (11). Insulin-mediated inhibition of lipolysis reduces circulating free fatty acid levels with improved insulin sensitivity, while inhibition of platelet aggregation reduces thrombosis (9). The major concern raised with the use of insulin therapy in the hospital is hypoglycemia, which has been observed primarily in studies targeting near normal glucose ranges of 80–110 mg/dL (6,12). This concern has prompted a search for alternative methods of inpatient glycemic management that are not known to cause hypoglycemia.
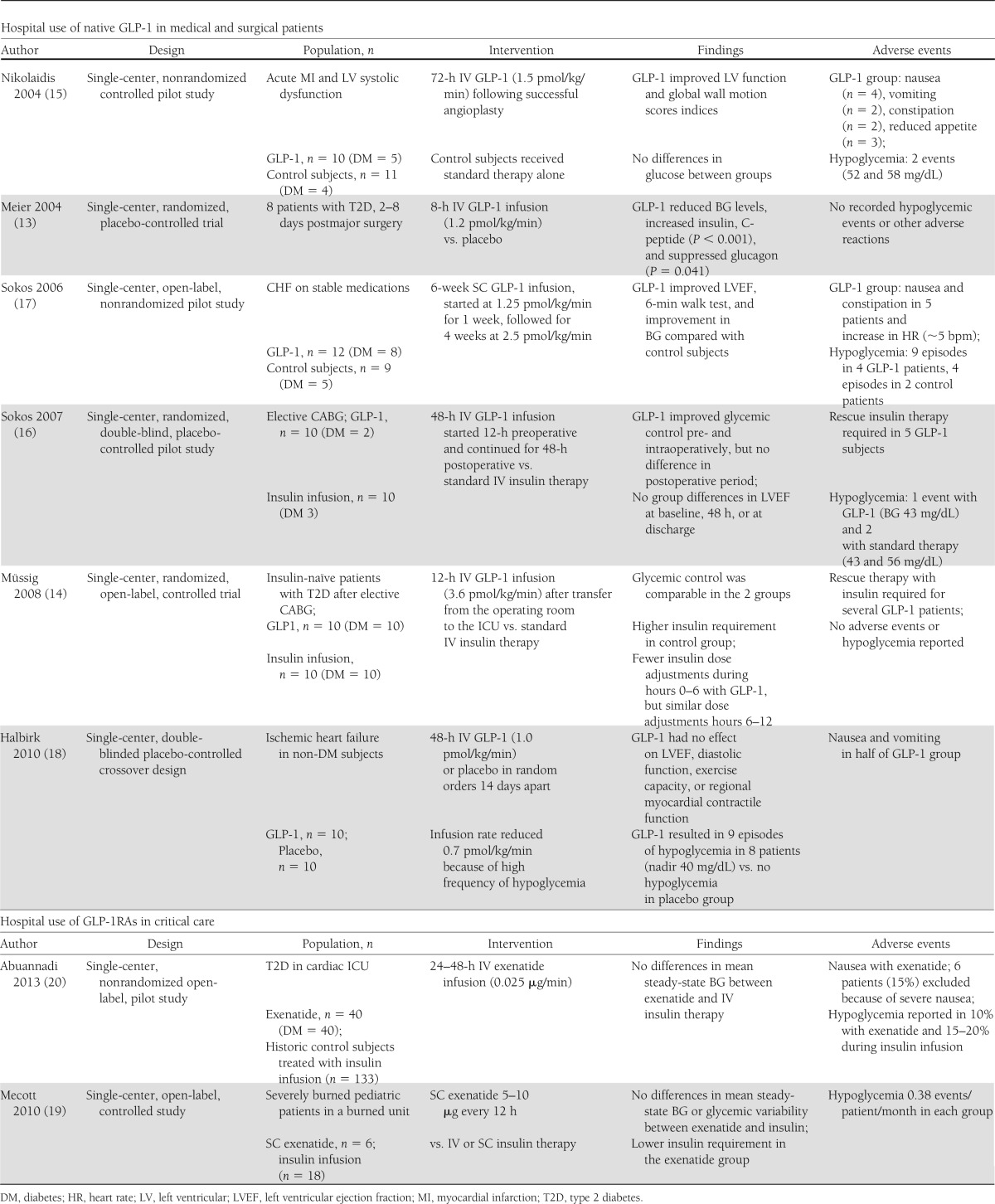
Hospital use of native glucagon-like peptide-1 and glucagon-like peptide-1 receptor agonist in medical and surgical patients
In contrast to the solid data supporting the use of insulin in hospitalized patients, there are only a handful of studies investigating the use of native glucagon-like peptide-1 (GLP-1) or GLP-1 receptor agonist (GLP-1RA) infusions in the inpatient setting. Most of these studies are uncontrolled, open-label, and of short-duration with small numbers of subjects with or without a history of diabetes (13–20) (Table 1). The majority of these studies were performed with the primary objective of investigating the safety and efficacy of these agents, with the hypothesis that GLP-1 infusions will achieve the desired glycemic targets without the risk for hypoglycemia. To date, none of these benefits have been demonstrated, and in many GLP-1–treated patients, rescue therapy with insulin was required to achieve and maintain the desired glycemic targets with no difference in the frequency of hypoglycemia when compared with insulin therapy. In addition, there was added risk for gastrointestinal side effects (nausea, vomiting, constipation), which occurred in up to 66% of subjects (15,16,18).
Table 1.
Hospital use of native GLP-1 in medical and surgical patients and GLP-1RAs in critical care
Surgical patients
In one small study of 20 subjects (5 with type 2 diabetes) undergoing coronary artery bypass grafting, glycemic control and left ventricular function was compared between 10 subjects (2 with type 2 diabetes) randomized to GLP-1 infusions initiated 12-h preoperatively at a dose of 1.5 pmol/kg/min and continued for 48-h postoperatively, and 10 subjects (3 with type 2 diabetes) assigned to standard insulin therapy (16). Standard therapy was not clearly defined but included IV insulin infusion, which was given to 5 subjects in each group. GLP-1 resulted in better glycemic control in the pre- and perioperative periods compared with insulin therapy, but there were no differences in postoperative blood glucose (BG) levels between treatment groups. In addition, there were no differences in insulin levels, hemodynamic parameters, or the number of hypoglycemic events during the study period. Half of the GLP-1 subjects required exogenous insulin infusions to achieve the desired level of glycemic control during the postoperative period. In a different study, 20 insulin-naïve patients with type 2 diabetes were randomly assigned to 12-h infusions of GLP-1 (3.6 pmol/kg/min) or insulin following elective coronary artery bypass grafting procedures (14). Rescue therapy with insulin was common for BG >7.77 mmol/L in the GLP-1 group. The insulin group received more insulin and required more dose adjustments in the first 6 h following surgery. Mean BG levels were similar in each group (7.9 ± 0.3 vs. 8.1 ± 0.1 mmol) without difference in hypoglycemia.
Cardiac patients
There are several studies conducted primarily to investigate the effect of GLP-1 infusions on left ventricular function in patients following acute myocardial infarction complicated by severe systolic dysfunction and in patients with congestive heart failure (CHF) (15). Each of these reports provided information on glycemic outcomes in addition to cardiovascular parameters; however, none of these studies included an insulin comparison. In one small nonrandomized pilot study, 10 patients (5 with type 2 diabetes) received a 72-h infusion of GLP-1 (1.2 pmol/kg/min) in addition to standard therapy alone, the latter of which was provided to 11 control subjects (4 with type 2 diabetes) (16). GLP-1 infusion was associated with reductions in glucose and insulin levels, improvements in left ventricular function, but some nondiabetic subjects experienced hypoglycemia, and gastrointestinal side effects (anorexia, nausea, vomiting, and constipation). Similarly, in an open-label nonrandomized study, 6-week infusions of GLP-1 (1.25–2.5 pmol/kg/min) added to standard therapy in 12 subjects with CHF, 8 with type 2 diabetes resulted in improved cardiovascular parameters and lower glucose levels compared with standard therapy alone; however, there was more hypoglycemia and gastrointestinal side effects in the GLP-1–treated group (17). In another double-blind crossover study, 20 patients without diabetes with New York Heart Association II and III heart failure and ischemic heart disease were randomized to receive 48-h infusions of GLP-1 or placebo in random order (18). GLP-1 infusions resulted in more hypoglycemia with 8 patients experiencing 9 episodes of hypoglycemia (glucose <3.5 mmol/L) compared with none with placebo. Five patients were unable to complete the study because of severe nausea and vomiting and were excluded from analysis. Contrary to the results in prior studies, there was no beneficial effect of GLP-1 on left ventricular ejection fraction, diastolic function, or myocardial contractile function.
There are only two small uncontrolled pilot studies investigating the use of GLP-1RA for glycemic management in critically ill patients (19,20). One open-label study compared the efficacy and safety of SC administration of exenatide at doses of 5–10 μg every 12 h (n = 6) with standard intensive insulin therapy (n = 18) in severely burned pediatric patients without diabetes. Similar levels of glycemic control were achieved in both groups (130 ± 28 vs. 138 ± 25 mg/dL) (19); however, the dose of administered insulin was significantly lower in the exenatide group (22 ± 14 vs. 76 ± 11 unit/patients/day, P = 0.01). Three patients receiving exenatide required rescue therapy with SC insulin to maintain glycemic control. The number of BG determinations was identical, as was the incidence hypoglycemia (0.38 events/patient/month). There were no reported gastrointestinal side effects.
In another pilot nonrandomized, uncontrolled, open-label study evaluating the safety and efficacy of IV exenatide in 40 cardiac ICU patients, 75% with type 2 diabetes (20), subjects received an initial 30-min bolus of 0.05 µg/min followed by 0.025 µg/min for 24–48 h. Exenatide infusions resulted in similar mean steady-state BG and hypoglycemic events when compared with historic control subjects treated with IV insulin infusions targeting BG 90–119 mg/dL (n = 94) or 100–140 mg/dL (n = 39). The mean steady-state BG in the group treated with exenatide (139 ± 41 mg/dL) was similar to that achieved with IV insulin therapy (115 ± 36 mg/dL and 147 ± 52 mg/dL, respectively). Hypoglycemia (BG <70 mg/dL) was reported in 10% of patients receiving exenatide compared with 21 and 15% in those treated with IV insulin (P = 0.27). A total of 8 patients (20%) experienced nausea because of exenatide, and 6 patients (15%) requested early termination because of severe nausea.
Safety concerns of GLP-1 and GLP-1RA therapies in the hospital setting
Treatment with GLP-1 and GLP-1RA is associated with a high incidence of gastrointestinal side effects, as was observed in the majority of reported studies (Table 1). Nausea and vomiting can be potentially dangerous in hospitalized patients with altered sensorium, who are maintained in a supine position, or who receive sedating medications, all of which increase the risk for aspiration pneumonia. In addition, the risk for pancreatitis, although rarely reported with the GLP-1 therapy, cautions against the use of these agents in patients with abdominal pain or postsurgical ileus.
The observed increase in heart rate of 2–5 bpm reported in clinical trials with GLP-1 was also reported in several of the inpatient studies (17,18). The mechanism underlying the increase in heart rate has not yet been clarified, but in at least in one report was attributed to possible undetected hypoglycemia (18). Although preliminary cardiovascular safety analyses of GLP-1RA demonstrate trends toward reduced cardiovascular events (21,22), long-term studies are needed to determine the clinical relevance of these chronotropic effects, particularly in critically ill patients.
Current practice guidelines recommend against inpatient use of oral antidiabetic drugs in part because of the absence of efficacy studies as well as safety concerns (6–8). A major limitation to inpatient use of oral antidiabetic agents relates to the delay in and unpredictable onset of action, which prevents rapid attainment of glycemic control or dose adjustments to meet the changing needs of the acutely ill patient. The low risk of hypoglycemia and good tolerability of dipeptidyl peptidase 4 (DPP-4) inhibitors however make them attractive considerations for use in hospitalized patients. At this time however no randomized clinical trial studies have reported on the use of these agents in the hospital setting. A concern with the use of DPP-4 inhibitors is the increase in frequency of upper respiratory infections observed in some preclinical trials (23). There are no reports of an increase in serious infections, but this observation raises concern for the use of these medications in inpatients with compromised immune systems, such as those undergoing solid organ transplantation.
Conclusions
Since approval of exenatide and sitagliptin in 2005–2006, the use of GLP-1RA and DPP-4 inhibitors has been widely incorporated into clinical practice and treatment guidelines from leading diabetes organizations for use in outpatient settings. The question is whether or not these agents are safe for use in the inpatient population. It is important to emphasize that these are new medications for which even the long-term safety of their outpatient use remains to be established. It is therefore premature to make recommendations for their routine use in the inpatient population.
Insulin therapy remains the standard of care for achieving and maintaining glycemic control in hospitalized patients with critical and noncritical illness. Insulin is a known entity that has now been in clinical use for almost a century. Hypoglycemia, the major complication of insulin therapy, is avoidable in the majority of cases with appropriate use of either intravenous or basal-bolus insulin regimens with regular monitoring of bedside BG levels and modification of insulin dosage in response to changes in clinical and nutritional status (1,7,8).
The proposed reasons for using GLP-1 therapy for the management of hyperglycemia in hospitalized patients include a theoretical improvement in glucose control with low frequency of hypoglycemia, and less nursing time to monitor BG levels and adjust insulin doses. In the small numbers of patients studied to date, glycemic control with GLP-1 therapies has been shown to be superior to placebo, but not to insulin therapy (Table 1). In fact, almost 50% of subjects in some studies required rescue therapy with insulin in order to achieve and maintain glycemic control. The frequency of hypoglycemia was not reduced with GLP-1 therapies compared with insulin. In addition, in the studies investigating the frequency of BG monitoring and nurse time, no differences were found between insulin and GLP-1 therapies.
Novel drugs for the management of patients with diabetes and hyperglycemia are welcome when they are proven to be safe in improving glycemic control and in reducing cardiovascular and other complications. The preliminary experience with native GLP-1 is promising and has the potential to improve cardiac function in patients with heart failure and acute ischemic cardiovascular events (15–17). However, this requires further study in a larger number of patients. It is possible that these favorable results may extend to the use of GLP-1RA and DPP-4 inhibitors; however, until the safety and efficacy are addressed with large randomized controlled clinical trials, the routine use of these agents for inpatient glycemic control cannot be recommended.
History has taught us that despite the potential promising effects of some drugs, long-term and widespread use unmasks undesirable and in some cases life-threatening side effects resulting in the removal of these agents from clinical use. Recent examples from diabetes management occurred following the introduction of thiazolidinediones, a class of medications associated with effective glucose-lowering properties as well as potential cardiovascular benefits. However, troglitazone was removed from the market following reports of sometimes fatal idiopathic hepatic failure (24); and the use of rosiglitazone became highly restricted following reports of higher mortality because of ischemic cardiovascular events (25). More recent observational and clinical studies have shown an association between the use of thiazolidinediones and increased bone fractures (26). Until there are well-conducted published studies demonstrating both the efficacy and safety of using the potentially promising GLP-1RA or DPP-4 inhibitors in the inpatient setting, it is best to observe the sound principles of evidence-based medicine and adhere to the well-known saying from the 12th century: All that glitters is not gold.
Acknowledgments
G.E.U. is supported by the American Diabetes Association (7-03-CR-35) and the National Institutes of Health (UL1 RR025008) (Atlanta Clinical and Translational Science Institute).
G.E.U. has received research support from Sanofi and Merck Pharmaceuticals. M.K. has received research support from Sanofi. No other potential conflicts of interest relevant to this article were reported.
Footnotes
References
- 1.Swanson CM, Potter DJ, Kongable GL, Cook CB. An update on inpatient glycemic control in hospitals in the United States. Endocr Pract 2011;17:853–861 [DOI] [PubMed] [Google Scholar]
- 2.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med 2001;345:1359–1367 [DOI] [PubMed] [Google Scholar]
- 3.Falciglia M, Freyberg RW, Almenoff PL, D’Alessio DA, Render ML. Hyperglycemia-related mortality in critically ill patients varies with admission diagnosis. Crit Care Med 2009;37:3001–3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 2002;87:978–982 [DOI] [PubMed] [Google Scholar]
- 5.Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care 2011;34:256–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists. American Diabetes Association American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care 2009;32:1119–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schnipper JL, Magee M, Larsen K, Inzucchi SE, Maynard G, Society of Hospital Medicine Glycemic Control Task Force Society of Hospital Medicine Glycemic Control Task Force summary: practical recommendations for assessing the impact of glycemic control efforts. J Hosp Med 2008;3(Suppl.):66–75 [DOI] [PubMed] [Google Scholar]
- 8.Umpierrez GE, Hellman R, Korytkowski MT, et al. Endocrine Society Management of hyperglycemia in hospitalized patients in non-critical care setting: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012;97:16–38 [DOI] [PubMed] [Google Scholar]
- 9.Chaudhuri A, Dandona P, Fonseca V. Cardiovascular benefits of exogenous insulin. J Clin Endocrinol Metab 2012;97:3079–3091 [DOI] [PubMed] [Google Scholar]
- 10.Stentz FB, Umpierrez GE, Cuervo R, Kitabchi AE. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes 2004;53:2079–2086 [DOI] [PubMed] [Google Scholar]
- 11.Aljada A, Ghanim H, Mohanty P, Kapur N, Dandona P. Insulin inhibits the pro-inflammatory transcription factor early growth response gene-1 (Egr)-1 expression in mononuclear cells (MNC) and reduces plasma tissue factor (TF) and plasminogen activator inhibitor-1 (PAI-1) concentrations. J Clin Endocrinol Metab 2002;87:1419–1422 [DOI] [PubMed] [Google Scholar]
- 12.Finfer S, Liu B, Chittock DR, et al. NICE-SUGAR Study Investigators Hypoglycemia and risk of death in critically ill patients. N Engl J Med 2012;367:1108–1118 [DOI] [PubMed] [Google Scholar]
- 13.Meier JJ, Weyhe D, Michaely M, et al. Intravenous glucagon-like peptide 1 normalizes blood glucose after major surgery in patients with type 2 diabetes. Crit Care Med 2004;32:848–851 [DOI] [PubMed] [Google Scholar]
- 14.Müssig K, Oncü A, Lindauer P, et al. Effects of intravenous glucagon-like peptide-1 on glucose control and hemodynamics after coronary artery bypass surgery in patients with type 2 diabetes. Am J Cardiol 2008;102:646–647 [DOI] [PubMed] [Google Scholar]
- 15.Nikolaidis LA, Mankad S, Sokos GG, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation 2004;109:962–965 [DOI] [PubMed] [Google Scholar]
- 16.Sokos GG, Bolukoglu H, German J, et al. Effect of glucagon-like peptide-1 (GLP-1) on glycemic control and left ventricular function in patients undergoing coronary artery bypass grafting. Am J Cardiol 2007;100:824–829 [DOI] [PubMed] [Google Scholar]
- 17.Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail 2006;12:694–699 [DOI] [PubMed] [Google Scholar]
- 18.Halbirk M, Nørrelund H, Møller N, et al. Cardiovascular and metabolic effects of 48-h glucagon-like peptide-1 infusion in compensated chronic patients with heart failure. Am J Physiol Heart Circ Physiol 2010;298:H1096–H1102 [DOI] [PubMed] [Google Scholar]
- 19.Mecott GA, Herndon DN, Kulp GA, et al. The use of exenatide in severely burned pediatric patients. Crit Care 2010;14:R153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abuannadi M, Kosiborod M, Riggs L, et al. Management of hyperglycemia with the administration of intravenous exenatide to patients in the cardiac intensive care unit. Endocr Pract 2013;19:81–90 [DOI] [PubMed] [Google Scholar]
- 21.Parks M, Rosebraugh C. Weighing risks and benefits of liraglutide—the FDA’s review of a new antidiabetic therapy. N Engl J Med 2010;362:774–777 [DOI] [PubMed] [Google Scholar]
- 22.Monami M, Cremasco F, Lamanna C, et al. Glucagon-like peptide-1 receptor agonists and cardiovascular events: a meta-analysis of randomized clinical trials. Exp Diabetes Res 2011;2011:215764 [DOI] [PMC free article] [PubMed]
- 23.Willemen MJ, Mantel-Teeuwisse AK, Straus SM, Meyboom RH, Egberts TC, Leufkens HG. Use of dipeptidyl peptidase-4 inhibitors and the reporting of infections: a disproportionality analysis in the World Health Organization VigiBase. Diabetes Care 2011;34:369–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee WM. Drug-induced hepatotoxicity. N Engl J Med 2003;349:474–485 [DOI] [PubMed] [Google Scholar]
- 25.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007;356:2457–2471 [DOI] [PubMed] [Google Scholar]
- 26.Dormuth CR, Carney G, Carleton B, Bassett K, Wright JM. Thiazolidinediones and fractures in men and women. Arch Intern Med 2009;169:1395–1402 [DOI] [PubMed] [Google Scholar]


