Abstract
Currently patients with diabetes comprise up to 25–30% of the census of adult wards and critical care units in our hospitals. Although evidence suggests that avoidance of hyperglycemia (>180 mg/dL) and hypoglycemia (<70 mg/dL) is beneficial for positive outcomes in the hospitalized patient, much of this evidence remains controversial and at times somewhat contradictory. We have recently formed a consortium for Planning Research in Inpatient Diabetes (PRIDE) with the goal of promoting clinical research in the area of management of hyperglycemia and diabetes in the hospital. In this article, we outline eight aspects of inpatient glucose management in which randomized clinical trials are needed. We refer to four as system-based issues and four as patient-based issues. We urge further progress in the science of inpatient diabetes management. We hope this call to action is supported by the American Diabetes Association, The Endocrine Society, the American Association of Clinical Endocrinologists, the American Heart Association, the European Association for the Study of Diabetes, the International Diabetes Federation, and the Society of Hospital Medicine. Appropriate federal research funding in this area will help ensure high-quality investigations, the results of which will advance the field. Future clinical trials will allow practitioners to develop optimal approaches for the management of hyperglycemia in the hospitalized patient and lessen the economic and human burden of poor glycemic control and its associated complications and comorbidities in the inpatient setting.
Over the past decade, there has been increasing interest in glycemic management of hospitalized patients. There is now broad consensus that both hyperglycemia and hypoglycemia in hospitalized patients are associated with adverse outcomes, including mortality. There is less agreement, however, as to whether these associations actually reflect the effects of the quality of glucose management or are merely underlying paraphenomena of the severity of acute illness. Even more controversial is the actual potential impact of glycemic control during these hospitalizations that are often relatively brief, the specific glucose ranges that should be targeted, and the methods by which clinicians might achieve these.
In the 1960s, research on the benefits of glucose-insulin-potassium infusion during acute myocardial infarction began, but this line of inquiry was not focused on glucose control per se (1). Interest in the general field of glycemic management in the inpatient setting began in the mid 1990s (2). The next 10 years were marked by both prospective observational trials and randomized clinical trials (RCTs), the majority of which seemed to indicate that “lower is better”: hospital complications, length of stay, cost, and even mortality could be dramatically decreased in a variety of critical care settings if mean glucose concentrations were reduced, usually with intravenous insulin, toward or within the euglycemic range (3,4). Some results, however, seemed too good to be true, especially in the context of such short hospital stays. This skepticism led to confirmatory trials, most conducted using a multicenter design. These could not confirm the initial positive findings from single-center investigations (5–7). There was resulting confusion as to how these results might shape clinical practice. Several consensus documents have emerged, each endorsing a more moderate approach to the management of glycemia in the hospitalized patient (8–11). Notably, all have called for more research in this area so that we can better understand the impact of both hyperglycemia and hypoglycemia on inpatient outcomes and better delineate evidence-based standards for hospital practice.
To date, most investigations have been funded through local resources or industry, as agencies appear reluctant to commit financial support for research in inpatient glycemic management. However, greater efforts devoted to the study of diabetes in the hospital setting would have broad implications for our health care system (12). In addition to funding, the nascent discipline of inpatient glucose management will benefit from standardized nomenclature, consistent and meaningful metrics, and transparent study designs and analytical methods allowing for comparison of study outcomes.
In this article, we outline eight aspects of inpatient glucose management in which RCTs and/or rigorously designed observational studies are needed. We refer to four as system-based issues and four as patient-based issues. Our goal was to identify existing research gaps and clinical care challenges in inpatient glucose management and to suggest future directions for each. These are summarized in Table 1.
Table 1.
Key issues in inpatient glucose management, suggested solutions, and areas in which future research is needed
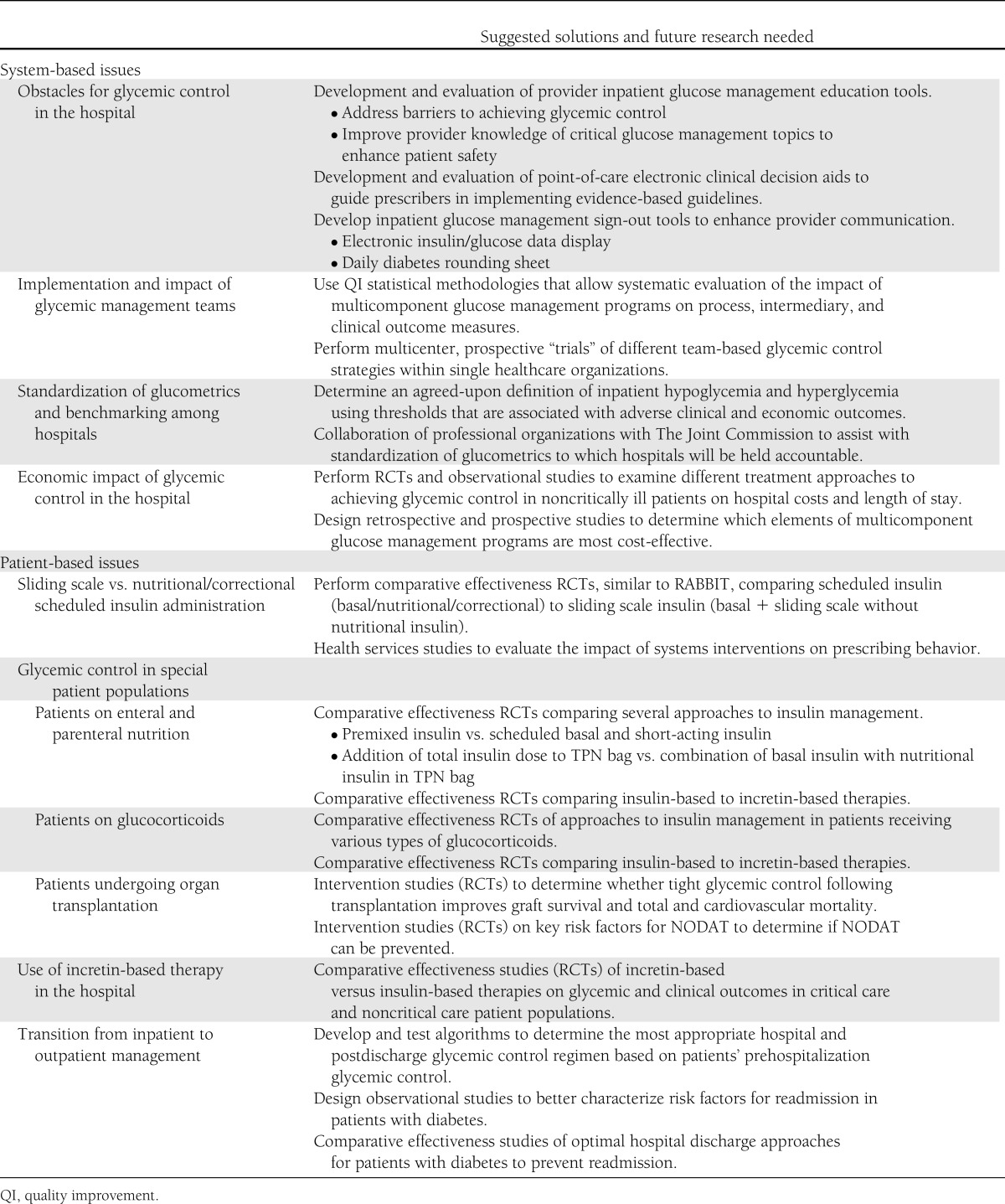
System-based issues
Obstacles for glycemic control in the hospital
Despite growing evidence supporting the importance of glycemic control in the hospital setting (13–15), numerous obstacles stand in the way of its achievement. Major factors include unanticipated changes in nutrition; medication changes and the use of medications associated with increased insulin resistance such as glucocorticoids, often in variable and changing doses; physiologic stress responses to illness; comorbid events such as acute or worsening renal insufficiency which may heighten the risk for hypoglycemia; and multiple system/organizational barriers, including the lack of communication and/or diabetes management knowledge deficits among providers and care givers.
Many patients experience changes in nutritional status during the course of a hospitalization, switching from oral intake to temporary nil per os status, and occasionally to the initiation of enteral or parenteral feeding. Each of these changes requires distinct, individualized, and specific insulin regimens (16). In addition, patients admitted to the hospital may have inadequate outpatient glycemic control, or need to be transitioned from oral antihyperglycemic agents to insulin. Stress can come from many sources including procedures, surgeries, infection, and pain, adding to the numerous factors that can unexpectedly raise or lower glucose values in the hospital setting.
Furthermore, communication is always a challenge in the inpatient setting. Good communication and coordination, as well as agreement between the primary team and the consulting diabetes specialist, are essential for appropriate insulin adjustments and anticipation of major changes in a patient’s status. In addition, synchrony between physicians and other providers including nurses, nurse practitioners, dietitians, pharmacists, as well as staffing issues required for efficient coordination of care (orders, timing, dietary requirements, and effective protocol implementation) must be emphasized in order to lessen the risk of errors. Clear communication with the patient, devoting particular attention to those with hypoglycemic unawareness, can be especially helpful in preventing untoward outcomes, particularly in the transition to discharge.
Finally, system issues often present further obstacles to glycemic control in the hospital setting. Coordinating insulin dosing with meals is often problematic. Hospital staff may have varying degrees of knowledge regarding proper management of both hyper- and hypoglycemia, including a lack of awareness of consensus- or evidence-based practices. The behaviors and beliefs of physicians and other providers are often difficult to change because of clinical inertia. This is best demonstrated by the continued use of “sliding-scale insulin” as a substitute for scheduled insulin with correction doses. Insufficient understanding of the importance of inpatient glycemic control and a fear of the risk of inducing hypoglycemia may influence various care practices.
Suggested future directions.
To address and overcome these obstacles we recommend development and evaluation of 1) provider education tools to improve knowledge of and address barriers to achieving glycemic control, 2) clinical decision aids at the point of care to guide prescribers in implementing evidence-based guidelines, and 3) inpatient glucose management sign-out tools to enhance provider communication. These interventions should target physicians, midlevel care providers, nurses, pharmacists, and dietitians. Studies evaluating the interventions should assess their impact on processes of care (insulin prescribing practices), intermediary outcomes (hyperglycemia and hypoglycemia), clinical outcomes (in-hospital mortality, nosocomial infections), and economic outcomes (length of stay, hospital admission costs, readmissions) (Fig. 1).
Figure 1.
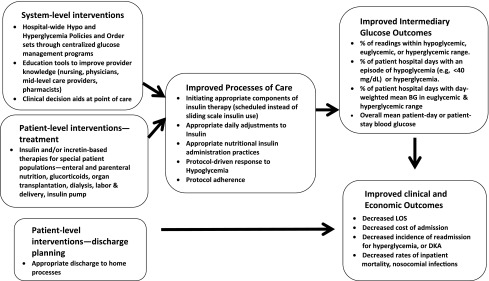
Diagram of a conceptual model for pathways to quality inpatient management of hyperglycemia and diabetes, adapted from Munoz et al. (18).
Implementation and impact of glycemic management teams
In 2004, the American College of Endocrinology published a position statement outlining the rationale for inpatient glycemic control (17). Following this early consensus conference, numerous successful campaigns were launched to inspire and produce champions in the burgeoning field of inpatient diabetes management. In 2009, the American College of Endocrinology partnered with the American Diabetes Association and released a call to action that outlined strategies for successful implementation of inpatient glucose management programs (8). Although clinical studies evaluating insulin use in the hospital setting have continued to emerge since then, rigorous scientific studies examining the risks and benefits of institutional programs with multilevel interventions have lagged behind. The optimal study should evaluate valid process (e.g., protocol adherence and prescribing practices), intermediary (e.g., glycemic control), clinical (e.g., nosocomial infections), and economic (e.g., length of stay) outcomes, as well as institutional acceptance and cost-effectiveness. Additionally, the expertise of glycemic management teams may be particularly important when managing patients treated with continuous subcutaneous insulin infusion or other special populations, such as those receiving enteral and parenteral nutrition, glucocorticoids, and transplant medications (see below). In this era of accountable care, high-quality research to identify the most effective glycemic management program characteristics and components, and the information systems required to maintain them, is absolutely imperative, since 30–50% of adult inpatients have diabetes and/or hyperglycemia during their hospital stay.
Suggested future directions.
Given ethical considerations in not exposing all inpatients to the same quality of care and logistical issues, RCTs in this area are challenging. However, several groups have described quality improvement statistical methodologies that allow systematic evaluation of a program’s impact on outcomes using process control charts (18). These methodologies should be used to 1) systematically evaluate the impact of multicomponent glucose management programs on process, intermediary, and clinical outcomes before and after the interventions, and (2) evaluate institutional interventions through regularly scheduled cycles of performance improvement (e.g., Plan-Do-Study-Act) allowing real-time alternations to make interventions most effective. Finally, multicenter prospective “trials” are needed to examine the impact of different team-based glycemic control strategies at single healthcare organizations (e.g., physician-led team vs. midlevel care provider-led team) on process, intermediary, and clinical outcomes.
Standardization of glucometrics and benchmarking among hospitals
Over the past decade, a growing body of knowledge has emerged regarding the importance of the management of hyperglycemia in hospitalized patients, both in intensive care units (ICUs) and in noncritical care settings (19). As a result of these data, clinical guidelines have been published, and The Joint Commission has even established an “Advanced Certification in Inpatient Diabetes” program. Glucose data collection and analysis are the foundation of all of these efforts. Unfortunately, there is a surprising lack of standardization that hampers benchmarking between various hospitals and direct comparison of different protocols.
Point-of-care glucose values from noncritical care units are used for most analyses, whereas for critically ill patients, both hospital laboratory plasma glucose values and point-of-care testing are often used. Even when the types of glucose data are homogeneous, adverse outcomes do not have agreed-upon definitions. For example, hypoglycemia can vary from “glucose level <70 mg/dL,” which encompasses the physiological levels of 60–70 mg/dL, to glucose levels <60 mg/dL. “Severe” hypoglycemia is frequently described as glucose values <40 mg/dL, without adequate data supporting these particular glucose thresholds.
Glucometrics efforts by Yale, the University Hospitals Consortium, and the Society of Hospital Medicine (20–23) are important steps in the direction of standardization, but the definition of optimal glucose control still differs among hospitals and does not always reflect the glycemic targets published by the American Association of Clinical Endocrinologists, the American Diabetes Association, and The Endocrine Society.
Suggested future directions.
Since RCTs are not ethical, rigorously designed retrospective and prospective observational studies are needed to determine hypoglycemia and hyperglycemia thresholds that are associated with adverse clinical (e.g., mortality, nosocomial infections) and economic outcomes (e.g., length of stay, hospital charges). These data can assist in achieving consensus universal definitions of glucose control or malglycemia (24). It is also important to achieve consensus on the most appropriate glucometric model for describing hyperglycemia (e.g., population, patient-day, or patient-stay model) based upon which model is most strongly associated with clinical and economic outcomes. Finally, professional organizations that develop inpatient glycemic guidelines need to collaborate with The Joint Commission to assist in standardizing glycemic definitions across hospitals.
Economic impact of glycemic control in the hospital
The potential economic impact of glycemic inpatient management initiatives needs to be established further. Available evidence shows that in the critically ill patient, intensive insulin therapy (IIT) results in a reduction in ICU length of stay and hospital costs. Van den Berghe et al. (25) estimated a reduction in ICU length of stay of 2 days with an associated reduction in costs of €2,680 (∼$3,410) per admission in patients who were treated with IIT. Krinsley et al. (26) also reported a reduction in ICU length of stay, but a more modest 0.3 days, with a cost savings of $1,580 per patient after implementing IIT. Finally, Sadhu et al. (27) showed an impressive reduction in ICU length of stay of 1.8 days with a savings in total hospital costs of $7,580 per patient in patients receiving IIT. Despite these encouraging data, further studies are needed in other patient populations. This is particularly pressing in the noncritically ill, which comprise the majority of hospitalized patients.
Suggested future directions.
RCTs examining different treatment approaches to achieving glycemic control in noncritically ill patients should also assess the impact of the interventions on hospital costs and length of stay. Multilevel modeling approaches applied to retrospectively and prospectively collected data should be used to examine the impact of hospital-wide glucose management programs on economic outcomes and to determine which elements of multicomponents programs are most cost-effective.
Patient-based issues
Sliding scale versus nutritional/correctional scheduled insulin administration
To the great chagrin of the majority of diabetologists (28,29), the use of sliding scale insulin administration persists, even at the most prestigious teaching hospitals (23,30). This reflexive behavior of ordering sliding scale may be harmful, comparing unfavorably to the more physiological scheduled insulin therapy. Several obstacles appear to exist in this seemingly perpetual and frustrating fight. The first is a lack of agreement on and understanding of what the term sliding scale actually means. Depending on the amount of food (or carbohydrates) to be consumed and the level of premeal glycemia, both components of scheduled bolus insulin, nutritional and correctional, may differ from one injection to another. This may create the deceptive appearance of a sliding scale regimen in the eyes of nonspecialists. It is our task to clearly articulate the differences between sliding scale insulin and scheduled insulin doses that include both nutritional and correctional (supplemental) insulin for our colleagues and the next generation of clinicians.
A significant challenge preventing the elimination of the sliding scale is the lack of clinical evidence for the superiority of basal-prandial correction therapy to the sliding scale approach during shorter hospital stays. The paucity of trials examining the potential impact of glycemic control during shorter stays raises reasonable questions about the appropriate approach in this setting. However, it may not be ethical to test sliding scale insulin therapy without scheduled insulin in type 1 diabetes.
An effective solution to the use of sliding scale insulin is the development and implementation of policies, protocols, and order sets for scheduled insulin administration in various clinical situations, as was demonstrated by the RABBIT (RAndomized Study of Basal-Bolus Insulin Therapy) studies (31,32). Unfortunately, these are not available in many hospital systems: institutional acceptance of protocols and order sets is far from universal. This leaves these key clinical decisions in the hands of practitioners, who may be reluctant to seek advice from a consultant but are unwilling or lack the expertise to implement proactive insulin strategies themselves.
Suggested future directions.
Additional well-designed comparative effectiveness RCTs, similar to the RABBIT studies, examining the effect of scheduled insulin (basal/nutritional/correctional) to sliding scale insulin (basal + correctional without nutritional insulin) on intermediary glycemic, clinical, and economic outcomes are needed to provide the evidence base for promoting changes in prescribing practices. In addition, health services studies are needed to evaluate the impact of systems interventions, such as implementation of policies, protocols, and order sets promoting scheduled insulin and provider education, on changing prescribing behavior.
Glycemic control in special clinical populations
A) Patients receiving enteral and parenteral nutrition.
Enteral and parenteral nutrition pose major challenges for glucose management in the hospital. These nutritional approaches frequently result in hyperglycemia, even in patients without a history of diabetes. The resultant hyperglycemia has been associated with poor outcomes (33,34). However, scheduled subcutaneous insulin may be difficult to implement safely because of frequent planned and unplanned interruptions and titration of the nutritional source. In the case of enteral nutrition, a small randomized clinical study demonstrated that subcutaneous basal insulin is more effective than correction dosing alone (35). Another study had suggested that the use of premixed 70/30 insulin twice or three times daily may be safer than the use of long-acting insulin in patients on continuous tube feeding (36). It is unclear whether approaches such as scheduled short-acting insulin may be equally safe and efficacious because studies of other potential approaches have not been published. In the case of total parenteral nutrition (TPN), no randomized controlled trials comparing various approaches to insulin therapy are available, although retrospective data suggest that the addition of insulin in the TPN bag provides good control with less hypoglycemia than the use of intravenous insulin or subcutaneous insulin alone (37).
Suggested future directions.
Comparative effectiveness RCTs are needed to compare several approaches to insulin management on intermediary glycemic, clinical, and economic outcomes for patients receiving continuous enteral nutrition and TPN. For either nutritional approach, a computerized intravenous insulin algorithm or noninsulin agents, such as incretin-based therapies, may provide safer or more effective alternatives, but very few data are available. Therefore, comparative effectiveness RCTs comparing the effect of insulin-based to incretin-based therapies on glycemic and clinical outcomes are needed for this population.
B) Patients receiving glucocorticoids.
Administration of glucocorticoids has a detrimental effect on glycemic control in patients with diabetes (38,39), presenting a significant challenge for both outpatient and inpatient management. The dose and frequency of steroid administration varies widely, and steroids may be tapered or stopped abruptly. Acute or short-term administration of methylprednisolone causes predominantly postprandial hyperglycemia that lasts 6 to 12 h. Prednisone and dexamethasone have even longer durations of action. Because NPH insulin has a duration of action of ∼8–12 h (40), it has been used at the time of methylprednisolone administration to counteract the hyperglycemic effect of this glucocorticoid (41). NPH insulin may be stopped as soon as methylprednisolone is discontinued, providing a safer option than having residual insulin action from high doses of long-acting insulin lasting for hours after discontinuation of steroids. However, no systematic research has been published in patients receiving either multiple daily doses of steroids or in those receiving dexamethasone.
Suggested future directions.
Comparative effectiveness RCTs of approaches to insulin management on glycemic, clinical, and economic outcomes in patients with steroid-induced hyperglycemia receiving various types of glucocorticoids are needed. Recently published communications suggest that either GLP-1 agonists or dipeptidyl peptidase-4 inhibitors may be useful in treating steroid-induced hyperglycemia (42). Thus, comparative effectiveness RCTs comparing the effect of insulin-based to incretin-based therapies on glycemic, clinical, and economic outcomes are needed in this population.
C) Patients undergoing organ transplantation.
Hyperglycemia is a common problem in patients undergoing solid organ or hematopoietic stem cell transplantation. There is a significant prevalence of preexisting diabetes in patients undergoing transplantation as well as patients who develop new-onset diabetes after transplantation (NODAT) (43–45). In a large epidemiological study of kidney transplantation, the cumulative incidence of NODAT was 9% at 3 months and increased linearly to 24% at 36 months (46). Common risk factors for NODAT include hepatitis C infection and steroid and calcineurin/c inhibitor combination therapy.
In most studies, preexisting diabetes had no effect on renal graft survival but was associated with increased mortality, which was mostly cardiovascular (46,47). NODAT has been associated with renal graft failure and increased cardiovascular mortality (45,48). Whether hyperglycemia is causal or just a marker of graft rejection or cardiovascular mortality remains unknown.
Suggested future directions.
RCTs are needed to determine whether tight glycemic control following transplantation improves graft survival and total and cardiovascular mortality. Intervention studies on key risk factors for NODAT are also needed to determine whether the risk of NODAT can be reduced by 1) treating hepatitis C infection, 2) using steroid-free immunosuppression, 3) avoiding calcineurin/mammalian target of rapamycin inhibitor combination therapy, or 4) hyperglycemia management with insulin. A recent pilot study demonstrated that in kidney transplant patients without prior diabetes, short-term (3 weeks) tight glycemic control using insulin reduced the risk of development of NODAT at 12 months by 73% when compared with control subjects with less tight glucose control (49). The authors speculate that early insulin therapy is β-cell protective. This surprising finding needs to be replicated, and a larger multicenter trial is currently underway.
Use of incretin-based therapy in the hospital
Incretin-based therapies would be expected to control hyperglycemia in the hospital setting in patients with type 2 diabetes without risking hypoglycemia, when used in the absence of other hypoglycemic agents such as insulin. The rationale for predicting that incretin-based therapy might be safer than insulin therapy lies in its glucose-dependent insulin secretion, a marked benefit in reducing glycemic elevations from two stress hormones (glucagon and glucocorticoids), decreased glycemic variability, elimination of need for insulin, decreased requirement for bolus insulin even if basal insulin is needed, and potential beneficial effects on cardiovascular function (50). As alluded to in prior sections, the preliminary and mainly uncontrolled studies published thus far must be validated in well-designed RCTs (51).
Suggested future directions.
Comparative effectiveness RCTs are needed to examine the effect of incretin-based versus insulin-based therapies on glycemic, clinical, and economic outcomes in critical care and noncritical care patient populations.
Transition from inpatient to outpatient management
For a patient with diabetes being discharged to home from the hospital, there are both short- and long-term concerns. The immediate issue is whether or not the patient can safely return home. The long-term concern is how and when changes to preadmission medication regimens should be implemented at hospital discharge.
The distinct feature of diabetes transitional care is that the inpatient diabetes medical regimen is often completely different from what is used in an outpatient setting in the majority of patients (16). Current standards for inpatient diabetes management are based primarily on insulin therapy, regardless of whether they were treated with insulin or oral agents prior to admission (19). Conditions in the hospital may cause dramatic differences in glucose handling that may not return to baseline after discharge. This can be particularly challenging in patients on continuous subcutaneous insulin infusion who may need the devices temporarily removed or adjusted during certain procedures or due to mental status changes during a hospitalization.
The discrepancy in diabetes drug therapy between inpatient and outpatient care poses a significant threat to patient safety after patients are discharged. The doses of insulin recommended on discharge can be significantly higher or lower than those actually needed at home.
Furthermore, diabetes may contribute to the high readmission rates associated with certain conditions, such as cardiovascular disease. In addition, many patients with uncontrolled diabetes do not have their diabetes regimen adjusted properly both prior to or during the hospitalization, possibly due to apparent clinical inertia (52). The hospitalization per se and the design of a diabetes medication regimen for the patient upon hospital discharge may represent opportunities to modify previous outpatient diabetes care. How the level of prehospital control may guide therapy during hospitalization and after hospital discharge to prevent readmissions should also be a subject of intense investigation (53,54).
Suggested future directions.
Studies are needed to develop and test algorithms to determine the most appropriate hospital and postdischarge glycemic control regimen based upon patients’ prehospitalization glycemic control. Rigorously designed observational studies are needed to better characterize risk factors for readmission among diabetic patients because risk factors and causes specific to this population are not well characterized (55). Finally, comparative effectiveness studies are needed to determine optimal hospital discharge approaches for patients with diabetes, to maintain continuity of care and prevent readmissions.
Conclusion
The PRIDE group, a consortium for Planning Research in Inpatient Diabetes, has been formed to promote clinical research in the management of hyperglycemia and diabetes in the hospital. We urge further progress in the science of inpatient diabetes management. We hope this call to action is supported by the American Diabetes Association, The Endocrine Society, the American Association of Clinical Endocrinologists, the American Heart Association, the European Association for the Study of Diabetes, the International Diabetes Federation, and the Society of Hospital Medicine. Appropriate federal research funding in this area will help ensure high-quality investigations, the results of which will advance the field. Future clinical trials will allow practitioners to develop optimal approaches for the management of hyperglycemia in the hospitalized patient and lessen the economic and human burden of poor glycemic control and its associated complications and comorbidities in the inpatient setting.
Acknowledgments
B.D. has received research grants from Novo Nordisk and Sanofi. S.E.I. has consulted for Takeda, Merck, Boehringer Ingelheim, and Janssen.
B.D. drafted the manuscript. All members of the writing group revised and edited the manuscript. J.G., S.H.G., and S.E.I. made extensive revisions. B.D. reviewed and finalized the manuscript.
Appendix
The PRIDE Writing Group in alphabetical order (all authors participated actively in writing and editing the manuscript): David Baldwin, Rush University Medical School (research grant from Novo Nordisk); Bruce W. Bode, Atlanta Diabetes Associates (stock ownership in Aseko; consulting for Medtronic, DexCom, Novo Nordisk, Sanofi, Halozyme; speaker for Medtronic, DexCom, Novo Nordisk, Eli Lilly, Sanofi, Bristol-Myers Squibb, Amylin, Merck, Insulet; research grants from Medtronic, DexCom, Novo Nordisk, Eli Lilly, Sanofi, Bristol-Myers Squibb, MannKind, Biodel, Halozyme, Macrogenetics, Merck, Abbott); Jeffrey B. Boord, Vanderbilt Heart and Vascular Institute; Susan S. Braithwaite, University of Illinois at Chicago; Enrico Cagliero, Massachusetts General Hospital, Harvard Medical School; Boris Draznin, University of Colorado School of Medicine (research grants from Novo Nordisk, Sanofi); Kathleen M. Dungan, Ohio State University School of Medicine (consulting for Eli Lilly, Pfizer, Diabetes Technology Management; speaker for Medikinetics; research grant from Novo Nordisk); Mercedes Falciglia, University of Cincinnati College of Medicine; M. Kathleen Figaro, Vanderbilt University School of Medicine; Janice Gilden, Rosalind Franklin University of Medicine and Science/Chicago Medical School and Captain James A. Lovell Federal Health Care Center; Sherita H. Golden, Johns Hopkins University School of Medicine; Irl B. Hirsch, University of Washington School of Medicine (consulting for Johnson & Johnson, Roche, Abbott; research grant from Sanofi); Silvio E. Inzucchi, Yale University School of Medicine, Yale-New Haven Hospital (consulting for Takeda, Merck, Boehringer Ingelheim, Janssen); David Klonoff, Mills-Peninsula Health Services, Diabetes Research Institute (consulting for Bayer, Insulet, Google, Roche, Sanofi; research grants from Biodel, Eli Lilly, MannKind, Medtronic, Novo Nordisk); Mary T. Korytkowski, University of Pittsburgh School of Medicine (consulting for Regeneron; research grant from Sanofi); Mikhail Kosiborod, St. Luke’s MidAmerica Heart Institute, University of Missouri Kansas City (consulting for Medtronic, Glumetrics, Gilead, Genentech, Hoffmann-La Roche, Sanofi, Boehringer Ingelheim, CardioMEMS; research grants from Medtronic, Glumetrics, Gilead, Genentech, Sanofi); Lillian F. Lien, Duke University Medical Center (consulting for Sanofi, Merck, and Eli Lilly); Michelle F. Magee, MedStar Health, Georgetown University School of Medicine; Umesh Masharani, University of California, San Francisco; Gregory Maynard, University of California, San Diego; Marie E. McDonnell, Boston University School of Medicine; Eti S. Moghissi, University of California Los Angeles; Neda Rasouli, University of Colorado School of Medicine; Daniel J. Rubin, Temple University School of Medicine; Robert J. Rushakoff, University of California, San Francisco (speaker for Novo Nordisk and Merck); Archana R. Sadhu, Weill Cornell Medical College; Stanley Schwartz, Main Line Health System, Emeritus, University of Pennsylvania (consulting for Santarus, Merck, Takeda, Janssen, Sanofi, Amylin; speaker for Santarus, Merck, Takeda, Janssen, Sanofi, Eli Lilly, Amylin, Bristol-Myers Squibb, AstraZeneca, Boehringer Ingelheim, Novo Nordisk); Jane Jeffrie Seley, New York-Presbyterian/Weill Cornell; Guillermo E. Umpierrez, Grady Health System, Emory University School of Medicine (research grants from Merck and Sanofi); Robert A. Vigersky, Walter Reed National Military Medical Center (research grant from DexCom Corporation); Cecilia C. Low Wang, University of Colorado School of Medicine; Deborah J. Wexler, Massachusetts General Hospital, Harvard Medical School.
Footnotes
*A complete list of the members of the PRIDE Writing Group can be found in the APPENDIX.
See accompanying articles, pp. 2107 and 2112.
References
- 1.Sodi-Pallares D, Testelli MR, Fishleder BL, et al. Effects of an intravenous infusion of a potassium-glucose-insulin solution on the electrocardiographic signs of myocardial infarction. A preliminary clinical report. Am J Cardiol 1962;9:166–181 [DOI] [PubMed] [Google Scholar]
- 2.Malmberg K, Rydén L, Efendic S, et al. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol 1995;26:57–65 [DOI] [PubMed] [Google Scholar]
- 3.Furnary AP, Zerr KJ, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg 1999;67:352–360; discussion 360–362 [DOI] [PubMed] [Google Scholar]
- 4.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med 2001;345:1359–1367 [DOI] [PubMed] [Google Scholar]
- 5.Brunkhorst FM, Engel C, Bloos F, et al. German Competence Network Sepsis (SepNet) Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008;358:125–139 [DOI] [PubMed] [Google Scholar]
- 6.Preiser JC, Devos P, Ruiz-Santana S, et al. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med 2009;35:1738–1748 [DOI] [PubMed] [Google Scholar]
- 7.Finfer S, Chittock DR, Su SY, et al. NICE-SUGAR Study Investigators Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009;360:1283–1297 [DOI] [PubMed] [Google Scholar]
- 8.Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists; American Diabetes Association American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care 2009;32:1119–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qaseem A, Humphrey LL, Chou R, Snow V, Shekelle P, Clinical Guidelines Committee of the American College of Physicians Use of intensive insulin therapy for the management of glycemic control in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2011;154:260–267 [DOI] [PubMed] [Google Scholar]
- 10.Umpierrez GE, Hellman R, Korytkowski MT, et al. Endocrine Society Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2012;97:16–38 [DOI] [PubMed] [Google Scholar]
- 11.Jacobi J, Bircher N, Krinsley J, et al. Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med 2012;40:3251–3276 [DOI] [PubMed] [Google Scholar]
- 12.Simmons D, Wenzel H. Diabetes inpatients: a case of lose, lose, lose. Is it time to use a ‘diabetes-attributable hospitalization cost’ to assess the impact of diabetes? Diabet Med 2011;28:1123–1130 [DOI] [PubMed] [Google Scholar]
- 13.Clement S, Braithwaite SS, Magee MF, et al. American Diabetes Association Diabetes in Hospitals Writing Committee Management of diabetes and hyperglycemia in hospitals. Diabetes Care 2004;27:553–591 [DOI] [PubMed] [Google Scholar]
- 14.Inzucchi SE, Bergenstal RM, Buse JB, et al. American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD) Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012;35:1364–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braithwaite SS, Magee M, Sharretts JM, Schnipper JL, Amin A, Maynard G, Society of Hospital Medicine Glycemic Control Task Force The case for supporting inpatient glycemic control programs now: the evidence and beyond. J Hosp Med 2008;3(Suppl):6–16 [DOI] [PubMed] [Google Scholar]
- 16.Barnard K, Batch B, Lien LF. Subcutaneous insulin: a guide for dosing regimens in the hospital. Glycemic Control in the Hospitalized Patient. 1st ed. Lien LF, Cox ME, Feinglos MN, Corsino L, Eds; New York, Springer, 2011, p. 7–16 [Google Scholar]
- 17.Garber AJ, Moghissi ES, Bransome ED, Jr, et al. American College of Endocrinology Task Force on Inpatient Diabetes Metabolic Control American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract 2004;10(Suppl. 2):4–9 [DOI] [PubMed] [Google Scholar]
- 18.Munoz M, Pronovost P, Dintzis J, et al. Implementing and evaluating a multicomponent inpatient diabetes management program: putting research into practice. Jt Comm J Qual Patient Saf 2012;38:195–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonnell ME, Umpierrez GE. Insulin therapy for the management of hyperglycemia in hospitalized patients. Endocrinol Metab Clin North Am 2012;41:175–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldberg PA, Bozzo JE, Thomas PG, et al. “Glucometrics”—assessing the quality of inpatient glucose management. Diabetes Technol Ther 2006;8:560–569 [DOI] [PubMed] [Google Scholar]
- 21.Kosiborod M, Inzucchi SE, Krumholz HM, et al. Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes-based measure of risk. Circulation 2008;117:1018–1027 [DOI] [PubMed] [Google Scholar]
- 22.Schnipper JL, Magee M, Larsen K, Inzucchi SE, Maynard G, Society of Hospital Medicine Glycemic Control Task Force Society of Hospital Medicine Glycemic Control Task Force summary: practical recommendations for assessing the impact of glycemic control efforts. J Hosp Med 2008;3(Suppl):66–75 [DOI] [PubMed] [Google Scholar]
- 23.Wexler DJ, Meigs JB, Cagliero E, Nathan DM, Grant RW. Prevalence of hyper- and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals. Diabetes Care 2007;30:367–369 [DOI] [PubMed] [Google Scholar]
- 24.Hammer MJ, Casper C, Gooley TA, O’Donnell PV, Boeckh M, Hirsch IB. The contribution of malglycemia to mortality among allogeneic hematopoietic cell transplant recipients. Biol Blood Marrow Transplant 2009;15:344–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van den Berghe G, Wouters PJ, Kesteloot K, Hilleman DE. Analysis of healthcare resource utilization with intensive insulin therapy in critically ill patients. Crit Care Med 2006;34:612–616 [DOI] [PubMed] [Google Scholar]
- 26.Krinsley JS, Jones RL. Cost analysis of intensive glycemic control in critically ill adult patients. Chest 2006;129:644–650 [DOI] [PubMed] [Google Scholar]
- 27.Sadhu AR, Ang AC, Ingram-Drake LA, Martinez DS, Hsueh WA, Ettner SL. Economic benefits of intensive insulin therapy in critically Ill patients: the targeted insulin therapy to improve hospital outcomes (TRIUMPH) project. Diabetes Care 2008;31:1556–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirsch IB. Sliding scale insulin—time to stop sliding. JAMA 2009;301:213–214 [DOI] [PubMed] [Google Scholar]
- 29.Schnipper JL, Barsky EE, Shaykevich S, Fitzmaurice G, Pendergrass ML. Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital. J Hosp Med 2006;1:145–150 [DOI] [PubMed] [Google Scholar]
- 30.Boord JB, Greevy RA, Braithwaite SS, et al. Evaluation of hospital glycemic control at US academic medical centers. J Hosp Med 2009;4:35–44 [DOI] [PubMed] [Google Scholar]
- 31.Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care 2007;30:2181–2186 [DOI] [PubMed] [Google Scholar]
- 32.Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care 2011;34:256–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheung NW, Napier B, Zaccaria C, Fletcher JP. Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition. Diabetes Care 2005;28:2367–2371 [DOI] [PubMed] [Google Scholar]
- 34.Pasquel FJ, Spiegelman R, McCauley M, et al. Hyperglycemia during total parenteral nutrition: an important marker of poor outcome and mortality in hospitalized patients. Diabetes Care 2010;33:739–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korytkowski MT, Salata RJ, Koerbel GL, et al. Insulin therapy and glycemic control in hospitalized patients with diabetes during enteral nutrition therapy: a randomized controlled clinical trial. Diabetes Care 2009;32:594–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsia E, Seggelke SA, Gibbs J, Rasouli N, Draznin B. Comparison of 70/30 biphasic insulin with glargine/lispro regimen in non-critically ill diabetic patients on continuous enteral nutrition therapy. Nutr Clin Pract 2011;26:714–717 [DOI] [PubMed] [Google Scholar]
- 37.Baldwin D, Kinnare K, Draznin B, et al. Insulin treatment of hyperglycemia in hospitalized patients receiving total parenteral nutrition (TPN) (Abstract). Diabetes 2012;61(Suppl. 1):A1070 [Google Scholar]
- 38.Bevier WC, Zisser HC, Jovanovic L, et al. Use of continuous glucose monitoring to estimate insulin requirements in patients with type 1 diabetes mellitus during a short course of prednisone. J Diabetes Sci Tech 2008;2:578–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oyer DS, Shah A, Bettenhausen S. How to manage steroid diabetes in the patient with cancer. J Support Oncol 2006;4:479–483 [PubMed] [Google Scholar]
- 40.Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes 2000;49:2142–2148 [DOI] [PubMed] [Google Scholar]
- 41.Seggelke SA, Gibbs J, Draznin B. Pilot study of using neutral protamine Hagedorn insulin to counteract the effect of methylprednisolone in hospitalized patients with diabetes. J Hosp Med 2011;6:175–176 [DOI] [PubMed] [Google Scholar]
- 42.van Raalte DH, van Genugten RE, Linssen MM, Ouwens DM, Diamant M. Glucagon-like peptide-1 receptor agonist treatment prevents glucocorticoid-induced glucose intolerance and islet-cell dysfunction in humans. Diabetes Care 2011;34:412–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cosio FG, Pesavento TE, Osei K, Henry ML, Ferguson RM. Post-transplant diabetes mellitus: increasing incidence in renal allograft recipients transplanted in recent years. Kidney Int 2001;59:732–737 [DOI] [PubMed] [Google Scholar]
- 44.Foo SM, Wong HS, Morad Z. Risk factors and incidence of posttransplant diabetes mellitus in renal transplant recipients. Transplant Proc 2004;36:2139–2140 [DOI] [PubMed] [Google Scholar]
- 45.Schiel R, Heinrich S, Steiner T, Ott U, Stein G. Post-transplant diabetes mellitus: risk factors, frequency of transplant rejections, and long-term prognosis. Clin Exp Nephrol 2005;9:164–169 [DOI] [PubMed] [Google Scholar]
- 46.Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant 2003;3:178–185 [DOI] [PubMed] [Google Scholar]
- 47.Lufft V, Dannenberg B, Schlitt HJ, Pichlmayr R, Brunkhorst R. Cardiovascular morbidity and mortality in patients with diabetes mellitus type I after kidney transplantation: a case-control study. Clin Nephrol 2004;61:238–245 [DOI] [PubMed] [Google Scholar]
- 48.Cole EH, Johnston O, Rose CL, Gill JS. Impact of acute rejection and new-onset diabetes on long-term transplant graft and patient survival. Clin J Am Soc Nephrol 2008;3:814–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuo HT, Sampaio MS, Vincenti F, Bunnapradist S. Associations of pretransplant diabetes mellitus, new-onset diabetes after transplant, and acute rejection with transplant outcomes: an analysis of the Organ Procurement and Transplant Network/United Network for Organ Sharing (OPTN/UNOS) database. Am J Kidney Dis 2010;56:1127–1139 [DOI] [PubMed] [Google Scholar]
- 50.Schwartz S, Kohl BA. Type 2 diabetes mellitus and the cardiometabolic syndrome: impact of incretin-based therapies. Diabetes Metab Syndr Obes 2010;3:227–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwartz S, DeFronzo RA. Is incretin-based therapy ready for the care of hospitalized patients with type 2 diabetes? The time has come for GLP-1 receptor agonists! Diabetes Care 2013;36:2107–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Griffith ML, Boord JB, Eden SK, Matheny ME. Clinical inertia of discharge planning among patients with poorly controlled diabetes mellitus. J Clin Endocrinol Metab 2012;97:2019–2026 [DOI] [PubMed] [Google Scholar]
- 53.Stolker JM, Spertus JA, McGuire DK, et al. Relationship between glycosylated hemoglobin assessment and glucose therapy intensification in patients with diabetes hospitalized for acute myocardial infarction. Diabetes Care 2012;35:991–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu EQ, Zhou S, Yu A, et al. Outcomes associated with insulin therapy disruption after hospital discharge among patients with type 2 diabetes mellitus who had used insulin before and during hospitalization. Endocr Pract 2012;18:651–659 [DOI] [PubMed] [Google Scholar]
- 55.Robbins JM, Webb DA. Diagnosing diabetes and preventing rehospitalizations: the urban diabetes study. Med Care 2006;44:292–296 [DOI] [PMC free article] [PubMed] [Google Scholar]


