Abstract
OBJECTIVE
Glucagon like peptide-1 (GLP-1) has been suggested as a major factor for the improved glucose tolerance ensuing after Roux-en-Y gastric bypass (RYGBP) surgery. We examined the effect of blocking endogenous GLP-1 action on glucose tolerance in subjects with sustained remission of type 2 diabetes mellitus (T2DM) present before RYGBP.
RESEARCH DESIGN AND METHODS
Blood glucose, insulin, C-peptide, glucagon, GLP-1, and glucose-dependent insulinotropic peptide levels were measured after a meal challenge with either exendin-(9–39) (a GLP-1r antagonist) or saline infusion in eight subjects with sustained remission of T2DM after RYGBP and seven healthy controls.
RESULTS
Infusion of exendin-(9–39) resulted in marginal deterioration of the 2-h plasma glucose after meal intake in RYGBP subjects [saline 78.4 ± 15.1 mg/dL compared with exendin-(9–39) 116.5 ± 22.3 mg/dL; P < 0.001]. Furthermore, glucose response to meal intake was similarly enlarged in the two study groups [percent change in the area under the curve of glucose exendin-(9–39) infusion versus saline infusion: controls 10.84 ± 8.8% versus RYGBP 9.94 ± 8.4%; P = 0.884]. In the RYGBP group, the blockade of the enlarged GLP-1 response to meal intake resulted in reduced insulin (P = 0.001) and C-peptide (P < 0.001), but no change in glucagon (P = 0.258) responses.
CONCLUSIONS
The limited deterioration of glucose tolerance on blockade of GLP-1 action in our study suggests the resolution of T2DM after RYGBP may be explained by mechanisms beyond enhancement of GLP-1 action.
The beneficial effect of Roux-en-Y gastric bypass (RYGBP) surgery on glycemic control in morbidly obese subjects with type 2 diabetes mellitus (T2DM) is well established (1,2). However, the precise mechanisms mediating T2DM remission after RYGBP are not yet clear (3–5). Although it traditionally has been asserted that bariatric operations are associated with improvement of glucose tolerance merely by caloric restriction and weight loss, several lines of evidence support weight-independent mechanisms are involved (6–11). An enhanced postsurgical glucagon-like peptide-1 (GLP-1) secretion, inducing a normalized or exaggerated insulin secretion after meal intake, has been hypothesized to play a major role in the improved glucose tolerance after RYGBP (3). Association studies have demonstrated larger improvements of glucose tolerance early after RYGBP being associated with a larger GLP-1 response to nutrient intake as compared with other surgical or nonsurgical interventions resulting in equivalent weight loss (7–9). Likewise, an exaggerated GLP-1 response has been reported up to 10 years after RYGB in subjects with sustained T2DM remission, suggesting a key role of GLP-1 in maintaining normal glucose tolerance in the long term after this type of surgery (12). However, because association does not prove causation, these data do not definitely prove GLP-1 plays a critical role in T2DM remission after RYGBP.
Understanding the role of endogenous GLP-1 in metabolic physiology has been greatly enhanced by the availability of a potent GLP-1 receptor antagonist, exendin-(9–39). Exendin-(9–39) blockade of GLP-1 action in healthy volunteers results in a significant enlargement of postprandial glucose excursions (13–17). Moreover, using hyperglycemic clamp technique in combination with a mixed meal test, Salehi et al. (18) demonstrated that blocking GLP-1 action results in a larger decrease in the insulin secretion rate in RYGBP-operated subjects (−33%) as compared with nonoperated controls (−16%). This study clearly supports GLP-1 as an important determinant of insulin secretion after RYGBP. However, the use of hyperglycemic clamp limited the ability of the study to investigate the relative importance of GLP-1 secretion on glucose tolerance. Furthermore, because only one-third of the study participants presented with T2DM before surgery, the study also was limited in establishing the role of GLP-1 secretion in the remission of T2DM. Of note, in Goto-Kakizaki rats (a nonobese rat model of T2DM) administration of exendin-(9–39) has been shown to totally reverse the improved glucose tolerance resulting from duodeno-jejunal exclusion surgery (an experimental metabolic surgery similar to RYGBP) (19).
Against this background, the main aim of our study was to examine the effect of endogenous GLP-1 blockade by exendin-(9–39) on glucose tolerance in subjects who had undergone RYGBP and with T2DM antedating surgery that had remitted after the surgical procedure. As secondary aims, we evaluated the effect of exendin-(9–39) on the insulin, C-peptide, glucagon, GLP-1, and glucose-dependent insulinotropic peptide (GIP) responses to meal intake. We evaluated individuals during the long-term after surgery to avoid the potential confounding effect of intense caloric restriction or rapid weight loss or both on glucose tolerance.
RESEARCH DESIGN AND METHODS
Subjects
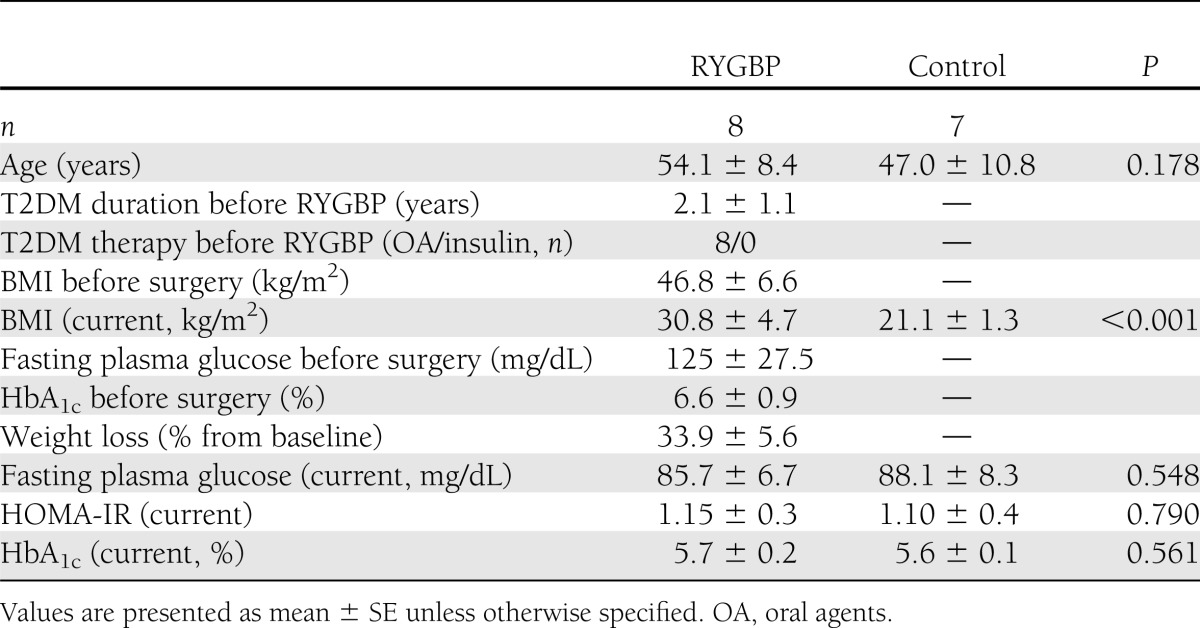
Eight Caucasian women who had undergone a standardized laparoscopic RYGBP (20) and seven Caucasian, age-matched, normal-weight, healthy controls participated in our study (Table 1). Eligibility criteria for the RYGBP group included the following: history of T2DM with duration >6 months and using pharmacological treatment before surgery; complete remission of T2DM at the time of evaluation; and postsurgical follow-up period ≥24 months. T2DM remission was defined as fasting plasma glucose <100 mg/dL plus glycated hemoglobin (HbA1c) <6.0% in the absence of active pharmacological therapy and lasting at least 1 year (21). Normal glucose tolerance in the control group was established based on a fasting plasma glucose and HbA1c in the normal range. All study participants had stable body weight for at least 1 month before the studies. The study was approved by the Hospital Ethics Committee and written informed consent was obtained from all the participants.
Table 1.
Characteristics of the study subjects
Experimental procedures
Subjects attended the research facility at 8:30 a.m. after an overnight fast on two occasions, in random order, and separated by at least 72 h. On admission, a canula was inserted into a forearm for blood sample collection and another one was inserted in the opposite forearm for infusion of synthetic exendin-(9–39) (Clinalfa Basic, Bachem, Germany) or saline. After withdrawal of baseline blood samples (time −30 min), subjects received either an intravenous bolus of synthetic exendin-(9–39) (7,500 pmol/kg) in 1 min followed by continuous infusion (750 pmol/kg/min) for the remainder of the study or saline (up to 120 min). At 30 min, subjects ingested a standardized liquid meal (SLM; 250 mL, 398 kcal, 50% carbohydrates, 35% fat, 15% protein; Isosource Energy; Novartis, Switzerland) over 5 min. The SLM was well tolerated by all study participants. Subjects were maintained in the recumbent position with the backside of the bed inclined at 30 degrees throughout the test.
Venous samples were obtained every 10 min (from time −30 to time 120 min) for the measurement of plasma glucose, insulin, and C-peptide. Glucagon, GLP-1, and GIP were assessed in blood samples obtained at baseline, every 10 min from 0 to 40 min, and every 20 min thereafter. Samples for the determination of glucose and insulin were collected in tubes containing heparin. Blood samples for the measurement of C-peptide, glucagon, GLP-1, and GIP were obtained in chilled EDTA tubes containing 500 units of aprotinin per milliliter of blood. Plasma samples were centrifuged immediately at +4°C and stored at −80°C until assayed.
The area under the curve (AUC) for glucose, insulin, C-peptide, glucagon, and total GLP-1 after the ingestion of the SLM were calculated using the trapezoidal method. The insulinogenic index was calculated as the increment of insulin (mU/L) or C-peptide (ng/mL) between time 0 and time 30 divided by the change in plasma glucose (mg/dL) in the same time frame. Insulin sensitivity was estimated from the homeostasis model assessment of insulin resistance (HOMA-IR) according to the formula: HOMA-IR = [insulin (mU/L) ⋅ glucose (mmol/L) / 22.5].
Assays
Plasma glucose and insulin levels were measured respectively using a glucose oxidase method (Bayer Diagnostics, Munich, Germany) and a monoclonal immunoradiometric assay (Medgenix Diagnostics, Fleunes, Belgium) as previously described (22,23). Plasma C-peptide was measured by RIA (Millipore, Billerica, MA). Human plasma total GLP-1 and glucagon were measured by radioimmunoassays (Glucagon-Like Peptide [Total] RIA Kit, and Glucagon RIA Kit; Millipore), and GIP was measured with an ELISA (Human GIP [total] ELISA; Millipore) as previously reported (22,23). The glucagon assay uses an antibody that is specific for pancreatic glucagon, with <0.1% cross-reactivity to oxytomodulin and no cross-reactivity with the exendin-(9–39) used in our study (data not shown).
Statistical analysis
Data are expressed as mean (SD) unless specified otherwise. The sample size of our study was powered (β error <0.1) to detect an enlargement of the glucose excursion after the SLM of 75.0% in the RYGBP group with an α error <0.05. Unpublished observations from our group have shown a 75.4% larger AUC0–120 of glucose after the same SLM used in this study when subjects with T2DM before RYGBP but with either sustained remission or T2DM relapse were compared. Normality of study variables was assessed with Kolmogorov-Smirnov normality test. All variables followed Gaussian distribution (P > 0.05); consequently, parametric test were used in statistical analysis. Parameters obtained in studies with exendin-(9–39) or saline were compared within each group using paired t test. Differences between groups were assessed with t test for independent samples. Statistical analysis was performed using SPSS 17.0. Statistical significance was set at P < 0.05.
RESULTS
The clinical characteristics of study participants are shown in Table 1.
Glucose response to an SLM
The effects of exendin-(9–39) on the glucose response to SLM in subjects in the RYGBP and control groups are shown in Fig. 1 and Table 2. Before exendin-(9–39) or saline infusion (time −30 min), plasma glucose was not significantly different either within or between study groups. Exendin-(9–39) infusion resulted in a slight, albeit significant, increase in plasma glucose before meal ingestion (time 0 min) in both groups (RYGBP: P = 0.001; control: P = 0.053) relative to before the initiation of the test.
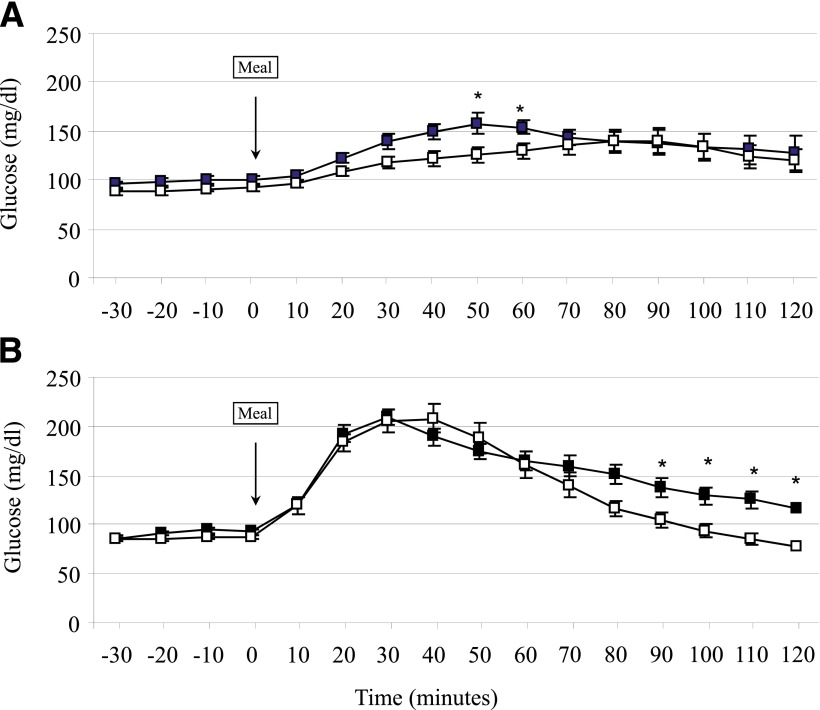
Figure 1.
Blood glucose response to a standardized meal test with saline infusion (open squares) or exendin-(9–39) (black squares) in control (A) and RYGBP (B) subjects. Data are presented as mean ± SE. *P < 0.05 relative to the saline condition.
Table 2.
Effect of SLM ingestion on plasma glucose and hormonal secretion in studies with or without exendin-(9–39)
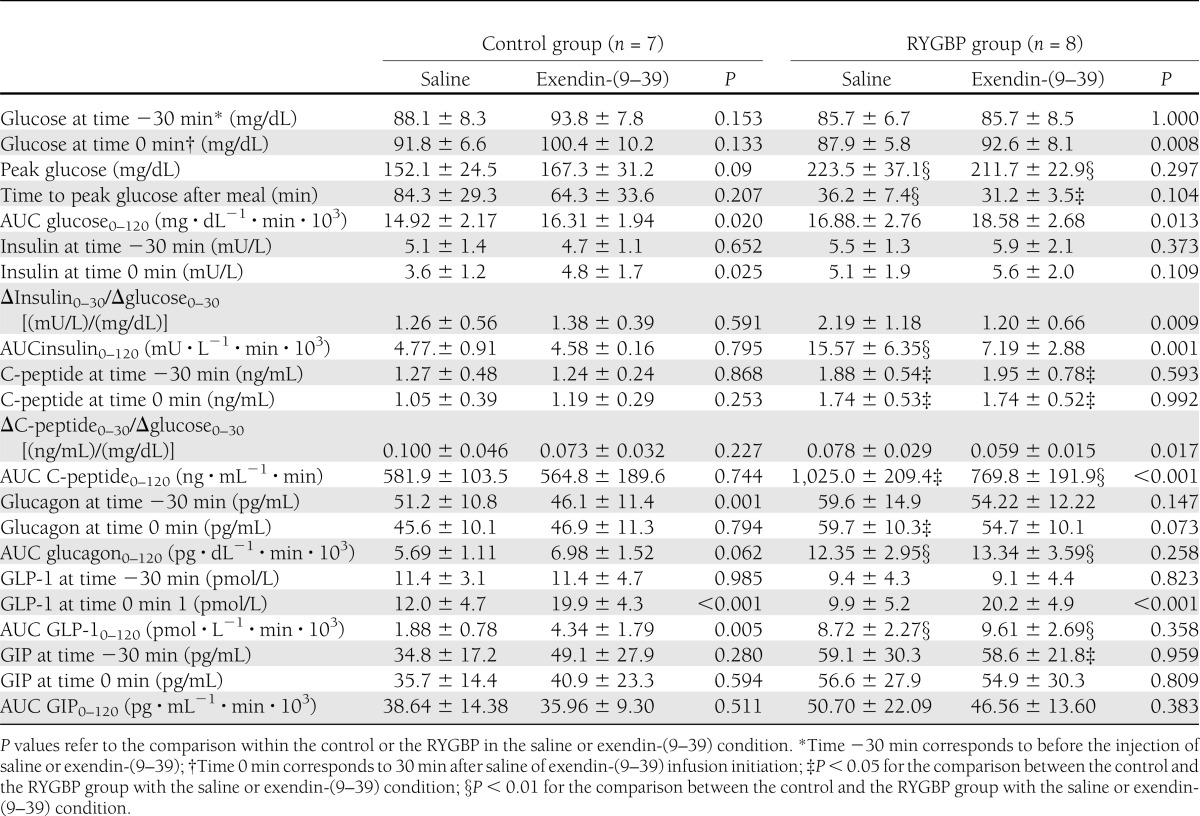
As shown in Table 2, infusion of exendin-(9–39) resulted in a significant enlargement of the AUC of glucose after meal intake (AUC glucose0–120) in both study groups (RYGBP: P = 0.013; control: P = 0.020). A similar relative increase of the AUC glucose0–120 in the exendin-(9–39) day relative to the saline day was found when the two groups were compared (RYGBP: 9.94 ± 8.4%; control: 10.84 ± 8.8%; P = 0.884). In the RYGBP group, the 2-h plasma glucose after SLM intake was larger after exendin-(9–39) infusion (116.5 ± 22.3 mg/dL) as compared with that in the saline study (78.4 ± 15.1 mg/dL; P < 0.001). However, the 2-h plasma glucose was <200 mg/dL in all study participants in the RYGBP group. Two subjects in the RYGBP group presented 2-h plasma glucose between 140 and 200 mg/dL (respectively, 141 mg/dL and 143 mg/dL).
A distinct temporal pattern of the glucose response was found in the two study groups in each experimental condition. During saline studies, patients in the RYGBP group showed an earlier (P = 0.001) and higher glucose peak (P = 0.001) as compared with the control group. As a result, the AUC of glucose during the first hour after SLM ingestion (AUC glucose0–60) was larger in the RYGBP group (RYGBP: 10.28 ± 1.41 mg ⋅ dL−1 ⋅ min ⋅ 103; control: 6.82 ± 0.50 mg ⋅ dL−1 ⋅ min ⋅ 103; P < 0.001). In contrast, plasma glucose levels were lower from 90 min onwards in the RYGBP group as compared with controls (Fig. 1; P < 0.05 for the time points of 90, 100, 110, and 120 min). Nonetheless, no significant difference was found when the AUC of glucose during the second hour after ingestion of the SLM (AUC glucose60–120) was compared between the two groups on the saline day (RYGBP: 6.59 ± 1.57 mg ⋅ dL−1 ⋅ min ⋅ 103; control: 7.99 ± 1.67 mg ⋅ dL−1 ⋅ min ⋅ 103; P = 0.120). Only one subject in the surgical group presented with plasma glucose levels <60 mg/dL from 90 min onwards in the absence of symptoms on the day of saline infusion. The differences between the two study groups in the plasma glucose response to SLM on GLP-1 action blockade are illustrated in Fig. 1. As shown in Table 2, in the RYGBP group exendin-(9–39) infusion did not result in a significant change in peak glucose (P = 0.297), time to peak glucose (P = 0.104), or the AUC glucose0–60 (10.15 ± 1.15 103 ⋅ mg ⋅ dL−1 ⋅ min; P = 0.597) as compared with the saline study. Peak glucose (P = 0.090) and the AUC glucose0–60 (8.02 ± 0.92 mg ⋅ dL−1 ⋅ min; P = 0.056) were larger, albeit not significantly, after exendin-(9–39) as compared with saline infusion in the control group. Throughout the second hour after the SLM, exendin-(9–39) infusion resulted in higher plasma glucose levels (P < 0.05 for the time points of 90, 100, 110, and 120 min) and larger AUC glucose60–120 in the RYGBP group (8.42 ± 1.62 103 ⋅ mg ⋅ dL−1 ⋅ min; P = 0.003) but not in the control group as compared with the saline day.
Insulin response to an SLM
As shown in Table 2, fasting plasma insulin did not differ between the two study groups or within each study group before the initiation of the tests performed in the two experimental conditions. Fasting C-peptide levels did not differ between the saline and exendin-(9–39) studies within each group (RYGBP group: P = 0.593; control group: P = 0.868), although the surgical group presented a significantly higher fasting C-peptide as compared with the control group on both study days (Table 2). During fasting, GLP-1 action blockade had no effect on insulin or C-peptide plasma levels neither in the RYGBP group (insulin: P = 0.582; C-peptide: P = 0.130) nor in the control group (insulin: P = 0.963; C-peptide: P = 0.609).
In the saline condition, and despite comparable HOMA-IR (Table 1), the insulin (AUC insulin0–120, P = 0.001) and C-peptide (AUC C-peptide0–120, P = 0.001) responses ensuing after SLM ingestion were larger in the RYGBP group. Corresponding to the glucose curve, differences in the insulin response between the two study groups were accounted for differences during the first hour (AUC insulin0–60: 11.68 ± 4.33 mU ⋅ L−1 ⋅ min ⋅ 103 for RYGBP compared with 2.11 ± 1.57 mU ⋅ L−1 ⋅ min ⋅ 10−3 for control; P < 0.001; AUC C-peptide0–60: 422.5 ± 91.5 ng ⋅ mL−1 ⋅ min for RYGPB and 239.7 ± 54.7 ng ⋅ mL−1 ⋅ min for control; P < 0.001) but not during the second hour (AUC insulin60–120: RYGBP compared with controls, P = 0.205; AUC C-peptide60–120: RYGBP compared with controls, P = 0.055) after the SLM challenge.
In the RYGBP group, exendin-(9–39) infusion resulted in blunted insulin response to SLM (Fig. 2 and Table 2), with a mean reduction of the AUC0–120 of insulin and C-peptide of 52.1 ± 10.9% and 24.1 ± 8.7%, respectively. The insulin-calculated (P = 0.009) and C-peptide–calculated (P = 0.017) insulinogenic indices were reduced in the RYGBP group during exendin-(9–39) infusion. In the control group, exendin-(9–39) yielded no significant effect on insulin (ΔAUC insulin0–120: −12.2 ± 35.2%) or C-peptide (ΔAUC insulin0–120: −8.5 ± 22.6%) postprandial response (Fig. 2 and Table 2).
Figure 2.
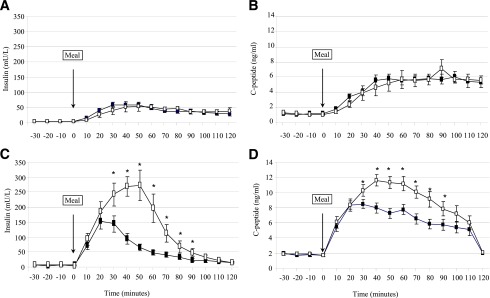
Insulin and C-peptide response to a standardized meal test with saline infusion (open squares) or exendin-(9–39) (black squares) in control (A, B) and RYGBP (C, D) subjects. Data are presented as mean ± SE. *P < 0.05 relative to the saline condition.
Glucagon, GLP-1, and GIP response to an SLM
As shown in Table 2, no differences were found in baseline plasma glucagon levels between the 2 study days in the RYGBP group. Unexpectedly, slightly but statistically elevated fasting glucagon levels were found in the control group before saline infusion as compared with previous to exendin-(9–39) infusion (P = 0.001). As shown in Fig. 3, in the saline infusion condition the SLM challenge resulted in slight decrease in glucagon plasma concentration (incremental AUC glucagon0–120: −0.31 ± 1.17 (pg ⋅ dL−1 ⋅ min ⋅ 103). This physiological response was blunted by exendin-(9–39) infusion (incremental AUC glucagon0–120: 0.99 ± 1.52 pg ⋅ dL−1 ⋅ min ⋅ 103; P = 0.062). In contrast, a paradoxical increase in the glucagon response was observed in the RYGBP group during the saline and exendin-(9–39) conditions [incremental AUC glucagon0–120: 6.35 ± 2.96 pg ⋅ dL−1 ⋅ min ⋅ 103 for saline and 7.34 ± 3.60 pg ⋅ dL−1 ⋅ min ⋅ 103 for exendin-(9–39)]. Despite glucose tolerance being similar, the AUC glucagon0–120 was larger in the surgical group in both study days (Table 2).
Figure 3.
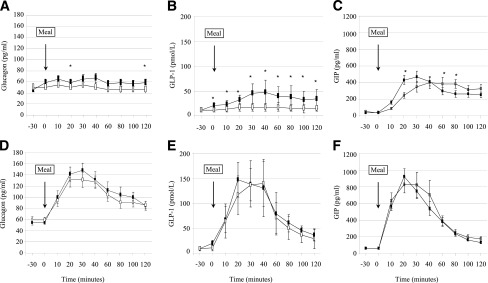
Glucagon, GLP-1, and GIP response to a standardized meal test with saline infusion (open squares) or exendin-(9–39) (black squares) in control (A–C) and RYGBP (D–F) subjects. Data are presented as mean ± SE. *P < 0.05 relative to the saline condition.
Fasting GLP-1 plasma levels did not differ between or within the two study groups in both experimental conditions (Table 2). As expected, GLP-1 response to SLM was larger in the RYGBP group as compared with the control group on the saline day (P < 0.001) (Fig. 3). Exendin-(9–39) resulted in a significant increase in the GLP-1 response to SLM intake in the control group (P = 0.005) but not in the RYGBP group (P = 0.358). Nonetheless, in that experimental condition the GLP-1 response to the meal challenge also was larger in the RYGBP group (P = 0.001).
The GIP response to meal intake is shown in Table 2 and Fig. 3. In control subjects, exendin-(9–39) infusion resulted in an earlier GIP response but no change in the AUC0–120 as compared with the saline condition. The early (AUC0–60) response of GIP was larger (P = 0.014) in the RYGBP group as compared with the control group, but it was not changed on infusion of exendin-(9–39).
CONCLUSIONS
Our data show that in the long-term after RYGBP, blocking the action of GLP-1 with exendin-(9–39) results in limited deterioration of the glucose response to a mixed meal in subjects with remission of T2DM antedating surgery. Thus, our data suggest that the dramatic increase in GLP-1 secretion observed during the long-term after RYGBP surgery is not the key determinant of the resolution of T2DM after this type of surgery.
Previous studies implicating GLP-1 as mechanism for the improvement in glucose tolerance after RYGBP have relied on association. RYGBP has been associated with a large GLP-1 response to meal intake that parallels diabetes remission (7), or that is larger than that observed after other surgical techniques (9) or dietary interventions (8) associated with smaller ameliorations of glucose tolerance. Furthermore, it has been reported that per oral feeding versus feeding through a gastrostomy catheter inserted in the gastric remnant in RYGBP-operated patients is associated with an exaggerated GLP-1 response along with better glucose tolerance (24). However, because association studies are not well suited to prove causality, to assess the degree to which increased GLP-1 release in RYGBP improved glucose tolerance we performed a mixed-meal tolerance test in the presence or absence of exendin-(9–39). If antagonizing GLP-1 receptors had been critical for the maintenance of a normal glucose tolerance in our RYGBP-operated cohort, then meal ingestion would have resulted in plasma glucose levels in the diabetic range or, alternatively, in a much larger deterioration of the glucose response to SLM as compared with normal-weight healthy controls. The 2-h plasma glucose after the SLM in our cohort was much lower than the threshold of 200 mg/dL used for the diagnosis of diabetes, and only two out the eight RYGBP patients presented with plasma glucose slightly higher than 140 mg/dL, which defines normal glucose tolerance. Moreover, deterioration of glucose excursions after meal ingestion in the surgical group was comparable with that in controls. Using hyperglycemic clamp combined with SLM, Salehi et al. (18) evaluated the effects of GLP-1 action blockade on insulin secretion in a cohort of subjects who had undergone RYGBP. At variance with our series, only three out the nine study participants presented with T2DM before surgery. Noteworthy, the glucose infusion rate after meal ingestion had to be decreased ∼13% with exendin-(9–39) infusion as compared with saline infusion to maintain the glucose clamped at the predefined target level. On the other hand, Deane et al. (13) reported a 10% deterioration of glucose tolerance associated with exendin-(9–39) infusion after the ingestion of a solid meal in a series of eight healthy subjects. Thus, the data reported herein concord with those in previous studies in humans using different experimental approaches.
We acknowledge that by not controlling for changes in blood glucose, our study is not optimal to disentangle the relative contributions of glucose and GLP-1 in the control of postprandial glycemia after RYGB (25). Nonetheless, comparison of the glucose and hormonal responses between the control and surgical groups in our study strongly suggests that RYGBP is associated with profound changes in the mechanisms involved in the control of postprandial glucose after the ingestion of the SLM. Under normal physiological conditions, insulin secretion, glucagon secretion, and gastric emptying are considered important in the control of glycemic excursions after meal intake (15,26). Noteworthy, it has been demonstrated that in addition to stimulating insulin release in a glucose-dependent manner, GLP-1 action results in the inhibition of glucagon secretion and delayed gastric gastric emptying (27). Thus, the comparison of the glucose and hormonal responses between our two study groups in the absence or presence of exendin-(9–39) may help us illustrate differences in these mechanisms under normal living conditions.
The earlier and higher peak glucose observed in the RYGBP group as compared with controls during the saline infusion condition in our study is compatible with the previously demonstrated increased rate of exogenous glucose absorption into the systemic circulation after this type of surgery (28). This finding most likely reflects a rapid emptying rate of the liquid meal from the gastric remnant after RYGBP (29,30). Furthermore, the lack of an effect of exendin-(9–39) infusion on the time to peak glucose and the peak glucose concentration after meal intake despite profound effects on the insulin response further supports the importance of the rate of glucose absorption as determinant of plasma glucose concentration in the early period after the ingestion of a liquid meal in RYGBP operated subjects (28). In contrast, exendin-(9–39) infusion resulted in a tendency toward larger peak glucose and larger early glucose curve in our control subjects consistent with accelerated gastric emptying as a result of the absence of the physiological GLP-1 action on gastric motility. Unfortunately, we did not measure gastric emptying in our study cohort. The relevance of gastric emptying in the control of postprandial glucose excursions in healthy subjects recently has been challenged (15). However, pharmacological deceleration of gastric emptying has been associated with smaller postprandial glucose excursions in the presence of decreased insulin secretion (31). Conversely, studies have shown that blockade of GLP-1 action in healthy volunteers is associated with parallel acceleration of gastric emptying and larger glucose excursions occurring during the first 60 min after meal ingestion (13,16).
Data on the insulin response after the SLM challenge in our study support the relevance of GLP-1 as determinant of insulin secretion after RYGBP (3,18). As previously shown, meal ingestion resulted in profound β-cell stimulation in the RYGBP group as compared with the control group (22,28,32). The enlarged insulin response during the saline condition could be accounted for by the discussed increased rate of glucose absorption and an enlarged secretion and potentiated insulinotropic action of GLP-1 as compared with the nonsurgical controls (27,33). Nonetheless, the early blunting of the insulin response, despite no significant change in plasma glucose throughout the first 90 min after meal challenge, emphasizes GLP-1 as determinant of the insulin response in the RYGBP group. Interestingly, the percent decreases in the AUC insulin (−52%) and C-peptide (−24%) in our surgical group are similar to those reported by Salehi et al. (18) (insulin −51%; C-peptide −32%) in RYGBP-operated subjects in an experimental setting consisting of a meal tolerance test combined with a hyperglycemic clamp. Admittedly, the β-cell function data reported herein refer to peripheral insulin concentration. Thus, because insulin clearance was not taken into account, true insulin secretion rate could not be calculated (18). However, in the study by Salehi et al. (18), the effect of exendin-(9–39) on the estimated insulin secretion rate (−33%) was similar to that derived from insulin and C-peptide concentrations. Importantly, as discussed, the blunted insulin response secondary to GLP-1 action blockade did not affect the early (0–60 min) glucose response to meal intake. Furthermore, despite profound inhibition, reduced insulin response resulted in limited deterioration of glucose tolerance at 120 min of meal ingestion. Thus, it could be argued that the insulin response pattern observed in the RYGBP subjects is a result of the deregulation of the normal delivery of nutrients into the gut (26,27). The increased insulin response results in limited impact on the ensuing glucose tolerance and may rather result in increased risk of hypoglycemia late after meal ingestion (18,32). At variance to our finding in the RYGBP group, and as previously reported, we failed to find a significant change in the insulin response to meal intake in the control group on blockade of GLP-1 action (15). A lower contribution of enteral factors to insulin secretion in control subjects in comparison with RYGBP-operated subjects was demonstrated by Salehi et al. (18). The lack of a descent in the insulin response in the control group in our study could be accounted for, at least in part, by the increased plasma glucose concentration.
The enlarged glucagon response in the exendin-(9–39) condition observed in our control group is concordant with the glucanostatic role of GLP-1 (27). In contrast, in the RYGBP group, meal ingestion resulted in marked elevation of plasma glucagon, despite exaggerated GLP-1 response and in the absence of hypoglycemia throughout the meal test. A paradoxical glucagon response after meal intake in RYGBP patients previously has been reported both during the short-term and the long-term after surgery after nutrient challenge with different composition, and in subjects with or without a history of T2DM preceding the surgical procedure (8,18,23,33,34). The mechanisms underlying the lack of suppression of glucagon after RYGBP are unclear. It has been shown that RYGBP surgery does not alter the inhibitory effects of hyperglycemia on the α-cells (18). Alternatively, it has been proposed that changes in other gut hormones such as GIP with glucanotropic action could be involved (23). Of note, Nicolaus et al. (15) recently have demonstrated that GLP-1 inhibition of postprandial glucagon secretion is a significant contributor to normal glucose homeostasis after a mixed oral meal in healthy volunteers. Thus, our data clearly show the correction of the hyperglucagonemia that has been shown to contribute importantly to diabetic hyperglycemia (35) is not the mechanism by which glucose intolerance is corrected after RYGBP.
We acknowledge our study has several limitations. First, a larger BMI in the surgical group could have detrimentally influenced GLP-1 action as compared with lean nonsurgical controls (36). However, it has been proposed that the negative impact of a larger BMI on the incretin effect is accounted for by impaired insulin sensitivity, and HOMA-IR was comparable between our study groups (37). Second, although current bariatric surgery series mainly comprise females, further studies are needed to extrapolate our findings to the male gender. Third, our observation period of 120 min after meal intake resulted in glucose returning to baseline values in the RYGBP group but not in the control group. However, previous studies have not shown further amplification of the effect of exendin-(9–39) on the glucose and insulin responses to meal intake beyond the 120-min observation period in healthy controls (13,15,16). Thus, we deem unlikely that prolonging our observation period to 180 min would have changed our results appreciably. Although, it could be argued that exendin-(9–39) dosage was insufficient to induce a significant blockade of GLP-1 action, it previously has been shown that exendin-(9–39) at lower doses (600 pmol ⋅ kg−1 ⋅ min) blocked the effect of supraphysiological doses of GLP-1 on insulin and C-peptide response to a nutrient challenge by 92% and 86% (25). Noteworthy, because we elected to study subjects during the long-term after surgery, it could be argued that our data do not discard GLP-1 as a significant contributor to the rapid amelioration of glucose tolerance ensuing after RYGBP (3–5). Studies are warranted to further prove this hypothesis. However, we elected our study period to avoid the confounding effects of marked energy restriction and weight loss occurring early after surgery.
Finally, it is worth mentioning that, as previously reported by others, exendin-(9–39) infusion resulted in increased plasma glucose concentration before the ingestion of the meal challenge (38). Furthermore, exendin-(9–39) infusion resulted in enlarged GLP-1 response to a meal challenge in healthy controls. The mechanisms underlying these observations are poorly understood and warrant further studies. It has been suggested that basal levels of endogenous GLP-1, continuously secreted in the absence of nutrient ingestion, convey a physiologically important endocrine signal to islet GLP-1 receptor (38). However, effects of exendin-(9–39) beyond the blockade of GLP-1 receptor cannot be ruled out (39).
In summary, we have examined the effects of the blockade of GLP-1 action on the glucose response to an SLM in subjects with sustained remission of T2DM present before RYGBP. Our data show the blockade of GLP-1 receptor by means of exendin-(9–39) results in limited deterioration of the glucose response to meal intake. Hence, our data suggest the resolution of T2DM after RYGBP may be explained by mechanisms beyond enhancement of GLP-1 action.
Acknowledgments
This work was supported by a grant from the Fondo de Investigaciones Sanitarias (PI11/00892), Instituto de Salud Carlos III (Madrid, Spain), and European Funds for Regional Development (FEDER) from the European Union.
No potential conflicts of interest relevant to this article were reported.
A.J. and J.V. designed the study, analyzed the data, and wrote the manuscript. A.J. and J.V.-M. recruited subjects and supervised the studies. R.C. analyzed the data and reviewed and edited the manuscript. A.L. contributed to discussion and reviewed and edited the manuscript. J.V. is the guarantor of this work and, as such, had full access to all the data in the studies and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012;366:1577–1585 [DOI] [PubMed] [Google Scholar]
- 2.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012;366:1567–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dirksen C, Jørgensen NB, Bojsen-Møller KN, et al. Mechanisms of improved glycaemic control after Roux-en-Y gastric bypass. Diabetologia 2012;55:1890–1901 [DOI] [PubMed] [Google Scholar]
- 4.Rubino F, R’bibo SL, del Genio F, Mazumdar M, McGraw TE. Metabolic surgery: the role of the gastrointestinal tract in diabetes mellitus. Nat Rev Endocrinol 2010;6:102–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott WR, Batterham RL. Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: understanding weight loss and improvements in type 2 diabetes after bariatric surgery. Am J Physiol Regul Integr Comp Physiol 2011;301:R15–R27 [DOI] [PubMed] [Google Scholar]
- 6.Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 1995;222:339–350; discussion 350–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laferrère B, Heshka S, Wang K, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 2007;30:1709–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laferrère B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab 2008;93:2479–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pournaras DJ, Osborne A, Hawkins SC, et al. Remission of type 2 diabetes after gastric bypass and banding: mechanisms and 2 year outcomes. Ann Surg 2010;252:966–971 [DOI] [PubMed] [Google Scholar]
- 10.Torquati A, Lutfi R, Abumrad N, Richards WO. Is Roux-en-Y gastric bypass surgery the most effective treatment for type 2 diabetes mellitus in morbidly obese patients? J Gastrointest Surg 2005;9:1112–1116; discussion 1117–1118 [DOI] [PubMed] [Google Scholar]
- 11.Cohen RV, Pinheiro JC, Schiavon CA, Salles JE, Wajchenberg BL, Cummings DE. Effects of gastric bypass surgery in patients with type 2 diabetes and only mild obesity. Diabetes Care 2012;35:1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dar MS, Chapman WH, 3rd, Pender JR, et al. GLP-1 response to a mixed meal: what happens 10 years after Roux-en-Y gastric bypass (RYGB)? Obes Surg 2012;22:1077–1083 [DOI] [PubMed] [Google Scholar]
- 13.Deane AM, Nguyen NQ, Stevens JE, et al. Endogenous glucagon-like peptide-1 slows gastric emptying in healthy subjects, attenuating postprandial glycemia. J Clin Endocrinol Metab 2010;95:215–221 [DOI] [PubMed] [Google Scholar]
- 14.Edwards CM, Todd JF, Mahmoudi M, et al. Glucagon-like peptide 1 has a physiological role in the control of postprandial glucose in humans: studies with the antagonist exendin 9-39. Diabetes 1999;48:86–93 [DOI] [PubMed] [Google Scholar]
- 15.Nicolaus M, Brödl J, Linke R, Woerle HJ, Göke B, Schirra J. Endogenous GLP-1 regulates postprandial glycemia in humans: relative contributions of insulin, glucagon, and gastric emptying. J Clin Endocrinol Metab 2011;96:229–236 [DOI] [PubMed] [Google Scholar]
- 16.Schirra J, Nicolaus M, Roggel R, et al. Endogenous glucagon-like peptide 1 controls endocrine pancreatic secretion and antro-pyloro-duodenal motility in humans. Gut 2006;55:243–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Witte AB, Grybäck P, Jacobsson H, et al. Involvement of endogenous glucagon-like peptide-1 in regulation of gastric motility and pancreatic endocrine secretion. Scand J Gastroenterol 2011;46:428–435 [DOI] [PubMed] [Google Scholar]
- 18.Salehi M, Prigeon RL, D’Alessio DA. Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes 2011;60:2308–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kindel TL, Yoder SM, Seeley RJ, D’Alessio DA, Tso P. Duodenal-jejunal exclusion improves glucose tolerance in the diabetic, Goto-Kakizaki rat by a GLP-1 receptor-mediated mechanism. J Gastrointest Surg 2009;13:1762–1772 [DOI] [PubMed] [Google Scholar]
- 20.Morínigo R, Vidal J, Lacy AM, Delgado S, Casamitjana R, Gomis R. Circulating peptide YY, weight loss, and glucose homeostasis after gastric bypass surgery in morbidly obese subjects. Ann Surg 2008;247:270–275 [DOI] [PubMed] [Google Scholar]
- 21.Buse JB, Caprio S, Cefalu WT, et al. How do we define cure of diabetes? Diabetes Care 2009;32:2133–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vidal J, Nicolau J, Romero F, et al. Long-term effects of Roux-en-Y gastric bypass surgery on plasma glucagon-like peptide-1 and islet function in morbidly obese subjects. J Clin Endocrinol Metab 2009;94:884–891 [DOI] [PubMed] [Google Scholar]
- 23.Romero F, Nicolau J, Flores L, et al. Comparable early changes in gastrointestinal hormones after sleeve gastrectomy and Roux-En-Y gastric bypass surgery for morbidly obese type 2 diabetic subjects. Surg Endosc 2012;26:2231–2239 [DOI] [PubMed] [Google Scholar]
- 24.Dirksen C, Hansen DL, Madsbad S, et al. Postprandial diabetic glucose tolerance is normalized by gastric bypass feeding as opposed to gastric feeding and is associated with exaggerated GLP-1 secretion: a case report. Diabetes Care 2010;33:375–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salehi M, Vahl TP, D’Alessio DA. Regulation of islet hormone release and gastric emptying by endogenous glucagon-like peptide 1 after glucose ingestion. J Clin Endocrinol Metab 2008;93:4909–4916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horowitz M, Edelbroek MA, Wishart JM, Straathof JW. Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Diabetologia 1993;36:857–862 [DOI] [PubMed] [Google Scholar]
- 27.Aaboe K, Krarup T, Madsbad S, Holst JJ. GLP-1: physiological effects and potential therapeutic applications. Diabetes Obes Metab 2008;10:994–1003 [DOI] [PubMed] [Google Scholar]
- 28.Rodieux F, Giusti V, D’Alessio DA, Suter M, Tappy L. Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity (Silver Spring) 2008;16:298–305 [DOI] [PubMed] [Google Scholar]
- 29.Horowitz M, Collins PJ, Harding PE, Shearman DJ. Gastric emptying after gastric bypass. Int J Obes 1986;10:117–121 [PubMed] [Google Scholar]
- 30.Morínigo R, Moizé V, Musri M, et al. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab 2006;91:1735–1740 [DOI] [PubMed] [Google Scholar]
- 31.Woerle HJ, Albrecht M, Linke R, et al. Importance of changes in gastric emptying for postprandial plasma glucose fluxes in healthy humans. Am J Physiol Endocrinol Metab 2008;294:E103–E109 [DOI] [PubMed] [Google Scholar]
- 32.Goldfine AB, Mun EC, Devine E, et al. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab 2007;92:4678–4685 [DOI] [PubMed] [Google Scholar]
- 33.Vilsbøll T, Toft-Nielsen MB, Krarup T, Madsbad S, Dinesen B, Holst JJ. Evaluation of beta-cell secretory capacity using glucagon-like peptide 1. Diabetes Care 2000;23:807–812 [DOI] [PubMed] [Google Scholar]
- 34.Falkén Y, Hellström PM, Holst JJ, Näslund E. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J Clin Endocrinol Metab 2011;96:2227–2235 [DOI] [PubMed] [Google Scholar]
- 35.Dunning BE, Gerich JE. The role of alpha-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr Rev 2007;28:253–283 [DOI] [PubMed] [Google Scholar]
- 36.Muscelli E, Mari A, Casolaro A, et al. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes 2008;57:1340–1348 [DOI] [PubMed] [Google Scholar]
- 37.Knop FK, Aaboe K, Vilsbøll T, et al. Impaired incretin effect and fasting hyperglucagonaemia characterizing type 2 diabetic subjects are early signs of dysmetabolism in obesity. Diabetes Obes Metab 2012;14:500–510 [DOI] [PubMed] [Google Scholar]
- 38.Woerle HJ, Carneiro L, Derani A, Göke B, Schirra J. The role of endogenous incretin secretion as amplifier of glucose-stimulated insulin secretion in healthy subjects and patients with type 2 diabetes. Diabetes 2012;61:2349–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ban K, Kim KH, Cho CK, et al. Glucagon-like peptide (GLP)-1(9-36)amide-mediated cytoprotection is blocked by exendin(9-39) yet does not require the known GLP-1 receptor. Endocrinology 2010;151:1520–1531 [DOI] [PubMed] [Google Scholar]