Abstract
OBJECTIVE
Factors associated with increasing maternal triglyceride concentrations in late pregnancy include gestational age, obesity, preeclampsia, and altered glucose metabolism. In a subgroup of women in the Metformin in Gestational Diabetes (MiG) trial, maternal plasma triglycerides increased more between enrollment (30 weeks) and 36 weeks in those treated with metformin compared with insulin. The aim of this study was to explain this finding by examining factors potentially related to triglycerides in these women.
RESEARCH DESIGN AND METHODS
Of the 733 women randomized to metformin or insulin in the MiG trial, 432 (219 metformin and 213 insulin) had fasting plasma triglycerides measured at enrollment and at 36 weeks. Factors associated with maternal triglycerides were assessed using general linear modeling.
RESULTS
Mean plasma triglyceride concentrations were 2.43 (95% CI 2.35–2.51) mmol/L at enrollment. Triglycerides were higher at 36 weeks in women randomized to metformin (2.94 [2.80–3.08] mmol/L; +23.13% [18.72–27.53%]) than insulin (2.65 [2.54–2.77] mmol/L, P = 0.002; +14.36% [10.91–17.82%], P = 0.002). At 36 weeks, triglycerides were associated with HbA1c (P = 0.03), ethnicity (P = 0.001), and treatment allocation (P = 0.005). In insulin-treated women, 36-week triglycerides were associated with 36-week HbA1c (P = 0.02), and in metformin-treated women, they were related to ethnicity.
CONCLUSIONS
At 36 weeks, maternal triglycerides were related to glucose control in women treated with insulin and ethnicity in women treated with metformin. Whether there are ethnicity-related dietary changes or differences in metformin response that alter the relationship between glucose control and triglycerides requires further study.
Maternal metabolism in late pregnancy is catabolic, with increasing insulin resistance, decreased adipose tissue lipoprotein lipase (LPL) activity, and increased lipolysis (1). These processes combine to ensure the availability of maternal fuels such as glucose, fatty acids, and ketone bodies for fetal use (1). It is recognized that gestational age, maternal obesity (2), and preeclampsia (3) are associated with increases in lipids during pregnancy. Gestational diabetes mellitus (GDM) is also associated with abnormalities in maternal lipid metabolism (4–6), which may contribute to the elevated fat mass seen at birth in infants of women with GDM (7–10).
Maternal glucose control and the pharmacological therapies used for treatment of GDM have the potential to influence these changes in maternal lipids (11). Insulin suppresses adipose tissue lipolysis and might be expected to reduce circulating triglycerides (12). Metformin reduces insulin resistance, but it has also been suggested to influence lipid metabolism (13), independent of glycemic control. In type 2 diabetes, metformin treatment is associated with a reduction in plasma triglyceride, total cholesterol, LDL cholesterol (13), and VLDL cholesterol concentrations (14). Metformin treatment in type 2 diabetes is also associated with increases in LPL mass level and LDL cholesterol particle size (15) and with a reduction in the release of free fatty acids from adipose tissue (16).
We have recently examined maternal lipids in the Metformin in Gestational Diabetes (MiG) trial and found that maternal fasting plasma triglycerides and measures of glucose control at 36 weeks were the strongest predictors of customized birth weight >90th percentile (17). Interestingly, triglycerides increased more from randomization to 36 weeks' gestation in women allocated to metformin than in those allocated to treatment with insulin, but there was no difference in customized birth weights or other neonatal anthropometry measures between the groups; there were also no differences in cord blood triglycerides (17). The aim of this study was to examine the known and putative determinants of maternal triglyceride concentrations and determine whether the difference seen in maternal plasma triglycerides at 36 weeks was due to treatment or other factors that may have differed between treatment groups.
RESEARCH DESIGN AND METHODS
Data were obtained from the MiG trial dataset (18). This was a prospective, open-label, randomized, controlled trial at multiple sites in New Zealand and Australia. MiG compared the use of metformin with insulin treatment for the control of GDM in 733 women diagnosed with GDM, who were unable to achieve adequate glucose control after lifestyle intervention. GDM was diagnosed using standard Australasian Diabetes in Pregnancy Society criteria (19). Women were not on either metformin or insulin at enrollment and were subsequently randomized to primary pharmacological treatment with one of these agents. Supplemental insulin was used as required in the metformin group to achieve predefined glucose goals, as previously described (18). The trial was approved by the institutional review boards at each participating site, and all subjects gave written informed consent (18).
A total of 432 participants in the MiG study with available measurements of fasting plasma triglycerides at enrollment and at 36 weeks' gestation were eligible for inclusion in the current study (18). Seventeen women (insulin, n = 8; metformin, n = 9) were excluded from these analyses as their ethnicity was unclear or their ethnic category contained <10 individuals. Inclusion or exclusion of these women, through assigning them all to the category of “other ethnicity” did not change the analyses presented here.
Laboratory analyses
Fasting blood samples were collected from each woman after an overnight fast at baseline (20–33 weeks' gestation) and at 36 weeks' gestation. All blood was collected in EDTA and plain tubes and was sent for processing within 10 min of collection or stored on ice for processing within 90 min. Aliquots were stored at −80°C for subsequent analysis.
Plasma glucose and triglycerides were measured by colorimetric enzymatic analysis on a Hitachi 912 automated metabolic analyzer, using commercially available kits (Roche Diagnostics GmbH, Mannheim, Germany). Glycated hemoglobin (HbA1c) was measured as previously described, in local laboratories by methods yielding results that were consistent with those of the Diabetes Control and Complications Trial (18).
Statistical analysis
Data were analyzed using SPSS (SPSS Statistics, version 19; IBM, Chicago, IL), using α = 0.05 (two-sided) to determine significance. The smoking variable reflects a history of smoking during pregnancy at entry into the study only. A missing values analysis was undertaken. All variables included had <10% missing data. For pairwise comparisons, missing data were dealt with by pairwise deletion, and for the regression, missing data were dealt with by listwise deletion. Variables showing significant differences at enrollment were analyzed as percentage change from enrollment to 36 weeks' gestation rather than the absolute value at 36 weeks. Continuous variables were described using means and 95% CIs. When not normally distributed, data were logarithmically transformed (log[x]) for analysis, and geometric means and CIs were reported. Paired Student t tests were used to compare triglycerides at enrollment and 36 weeks within treatment groups. Fisher least significant differences test was used when post hoc analysis was required. Categorical variables were summarized using frequencies and percentages and analyzed using Pearson χ2 test. Univariate and multivariable general linear models were used to explore the associations with maternal triglycerides at enrollment and 36 weeks. At both time points, triglycerides required log transformation to provide an appropriately normally distributed outcome variable. Although enrollment triglycerides were significantly correlated with triglycerides at 36 weeks' gestation in univariate modeling, they were not included in the multivariate model because of a high degree of collinearity. The initial multivariate models included those variables that had P < 0.4 in univariate regression. The model was refined by backward elimination, reducing the variables included to those with P < 0.05. Regression output is reported as geometric mean (categorical variables) or geometric regression coefficient (continuous variables) and 95% CI as appropriate. Treatment allocation and outcomes were examined further to determine the association of treatment with triglyceride concentrations, with the regression model at 36 weeks being stratified by treatment allocation (intention to treat). Regression analysis at 36 weeks' gestation was also performed by treatment received.
RESULTS
Participant characteristics
Of the 733 women in the MiG trial, 432 women (219 metformin and 213 insulin) were included in this analysis. Of the women allocated metformin, 97 (44.3%) were prescribed supplementary insulin. Women who were excluded because they had not performed the fasting blood samples at both time points were heavier and younger and had higher enrollment glucose and HbA1c (but not triglycerides) than those who were included (data not shown). Additionally, the excluded group had a higher percentage of Polynesian women (93 [34.3%] vs. 66 [15.3%]), fewer Caucasian women (120 [44.3%] vs. 237 [54.9%]), and lower rates of tertiary education (111 [37.4%] vs. 212 [49.3%]). There were no differences in the rates of smoking during pregnancy, diagnosis of preeclampsia, or treatment allocation. The maternal characteristics of the women included in this study are shown in Table 1 and Supplementary Table 1, and maternal characteristics stratified by treatment received (i.e., metformin, metformin and insulin, and insulin alone) are shown in Supplementary Table 2. Maternal triglyceride concentrations increased from enrollment to 36 weeks while measures of glucose control improved.
Table 1.
Maternal characteristics at enrollment and at 36 weeks' gestation, stratified by treatment allocation
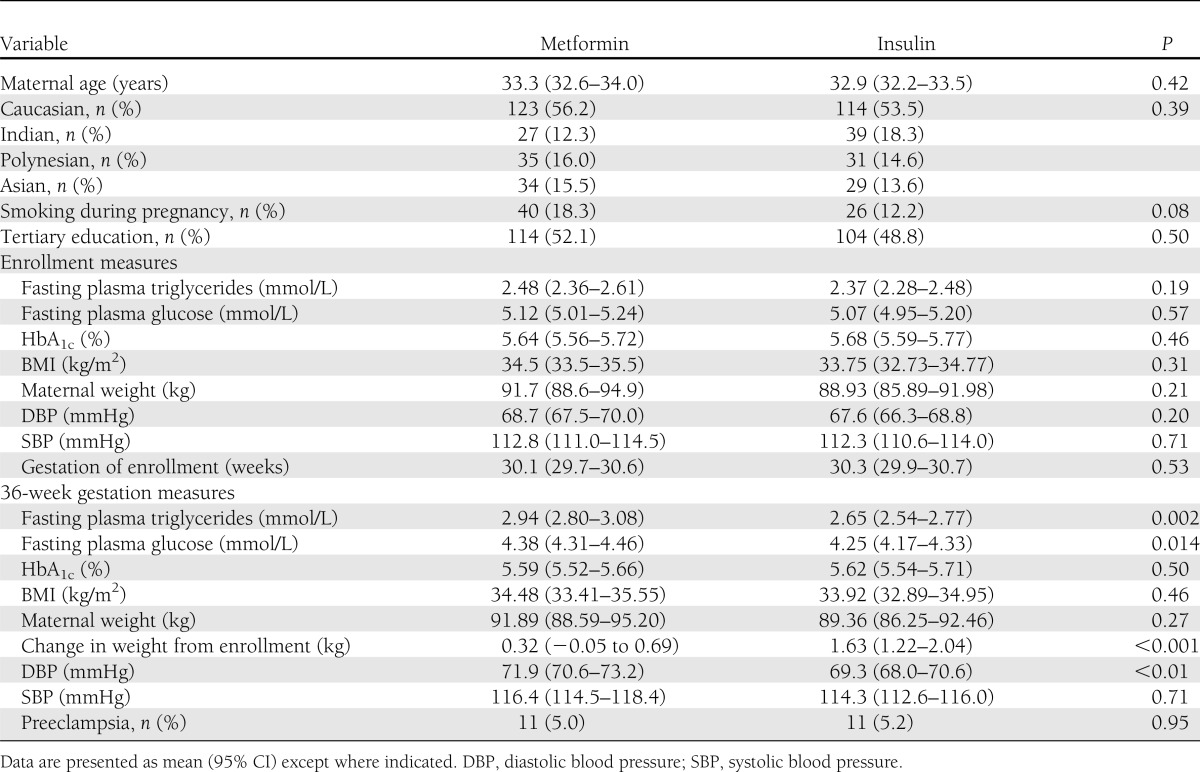
There were no significant differences in enrollment characteristics between the treatment groups (Table 1). Gestation at enrollment did not differ significantly between the two groups. At 36 weeks' gestation, triglyceride concentrations were higher in the metformin arm (2.94 [95% CI 2.80–3.08] mmol/L) than the insulin arm (2.65 [2.54–2.77] mmol/L, P = 0.002). Women treated with metformin had a higher fasting plasma glucose at 36 weeks (4.38 [4.31–4.46] mmol/L vs. 4.25 [4.17–4.33] mmol/L, P = 0.014) and gained less weight from enrollment to 36 weeks' gestation (0.32 [95% CI −0.05 to 0.69] kg vs. 1.63 [1.22–2.04] kg, P < 0.001) compared with women treated with insulin. There was no significant difference in plasma HbA1c concentrations, BMI, systolic blood pressure, or rates of preeclampsia and smoking between the two treatment groups at 36 weeks.
Women were also divided into three groups based on the treatment received: treatment with metformin alone, metformin with insulin, and insulin alone. There was no significant difference in triglycerides at enrollment. At 36 weeks' gestation, plasma triglycerides in these three groups were significantly different (P = 0.007): metformin alone (2.97 [95% CI 2.79–3.17] mmol/L), metformin with insulin (2.90 [2.69–3.12] mmol/L), and insulin alone (2.65 [2.54–2.77]), with a significant mean difference between both metformin groups and the insulin group but not between the two groups receiving metformin. Fasting glucose and HbA1c were both significantly different between these three groups at enrollment and at 36 weeks' gestation, with the group on metformin with insulin having higher concentrations than the other two groups at both time points (Supplementary Table 2).
Associations with maternal triglycerides at enrollment
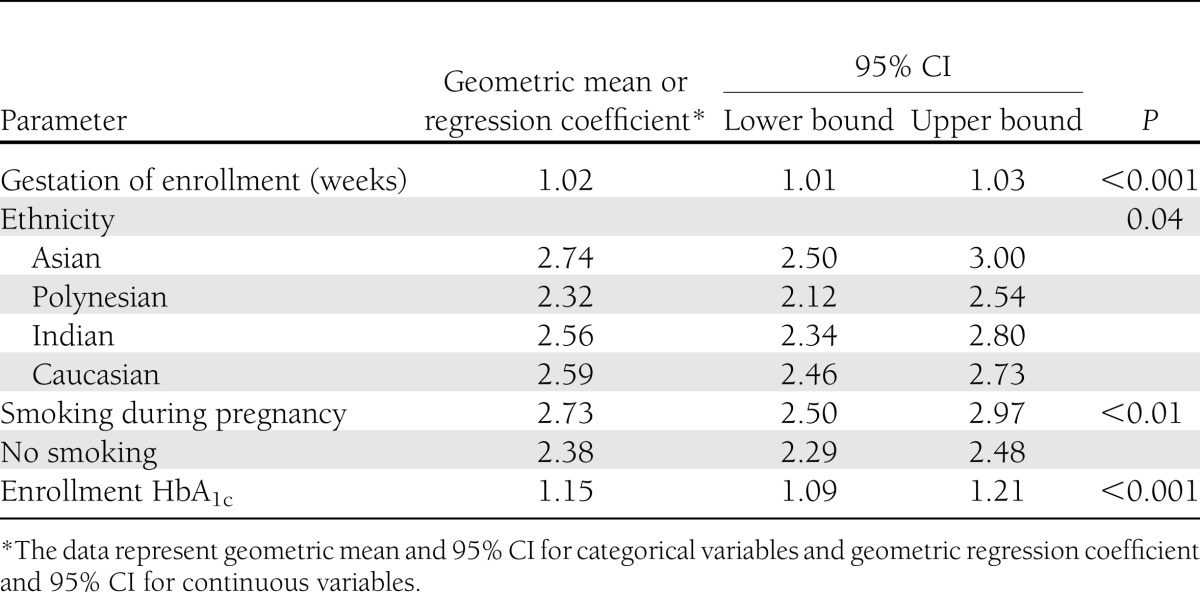
When the data were analyzed overall, by univariate analysis (Supplementary Table 3), significant associations with maternal plasma triglyceride concentrations at enrollment were gestational age (P < 0.001), enrollment HbA1c (P < 0.001), plasma glucose (P = 0.01), smoking (P = 0.002), and diastolic blood pressure (P = 0.05). Maternal age, weight, BMI, education, and systolic blood pressure were not significantly associated with the triglyceride level at enrollment. When data were analyzed using multivariate analysis (Table 2), gestational age, HbA1c at enrollment, ethnicity, and smoking remained as significant factors.
Table 2.
Associations with maternal triglycerides at enrollment, in multivariate analysis
Associations with maternal triglycerides at 36 weeks' gestation
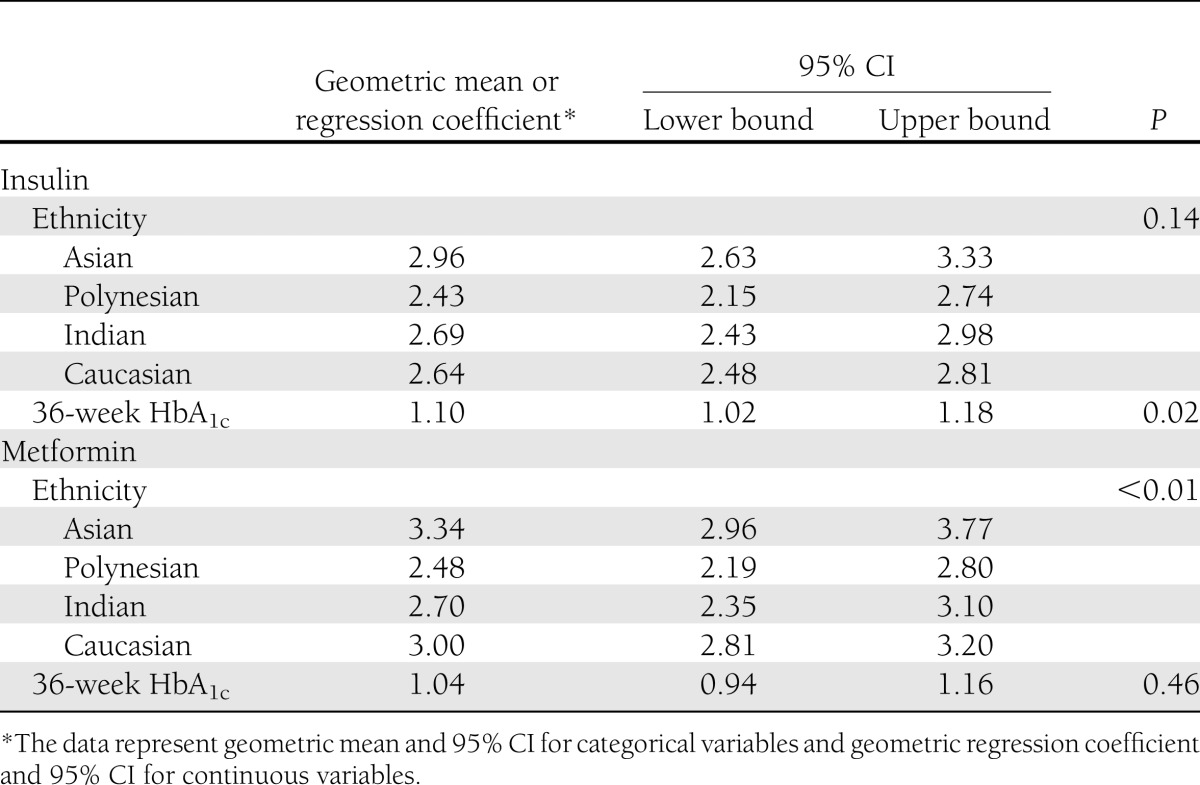
At 36 weeks' gestation, by univariate analysis (Supplementary Table 3), factors significantly associated with maternal plasma triglyceride concentrations were triglycerides at enrollment (P < 0.001), ethnicity (P = 0.001), and treatment allocation (P = 0.002). Maternal age, duration of treatment, 36-week BMI, preeclampsia, measures of 36-week glucose control, blood pressure, smoking, and educational status were not significantly associated with maternal plasma triglycerides. Supplementary Table 4 shows the significant univariate associations with maternal triglycerides when the analysis was stratified by treatment received. By multivariate analysis of the data overall, ethnicity (P = 0.001), treatment allocation (P = 0.005), and 36-week HbA1c (P = 0.03) remained as significant factors.
When the 36-week multivariate analysis was stratified by treatment allocation (Table 3), ethnicity (P = 0.005) was the only significant factor associated with triglycerides in women treated with metformin, and 36-week HbA1c (P = 0.02) was the only significant factor associated with triglycerides in women treated with insulin.
Table 3.
Associations with maternal triglycerides at 36 weeks' gestation, in multivariate analysis, stratified by treatment allocation
When the 36-week multivariate analysis (Supplementary Table 5) was stratified by treatment received, ethnicity (P = 0.02) remained significant for metformin alone but not for the group receiving both metformin and insulin or the one receiving insulin alone. Thirty-six-week HbA1c was only significant for the women receiving insulin alone (P = 0.02).
Effect of maternal ethnicity
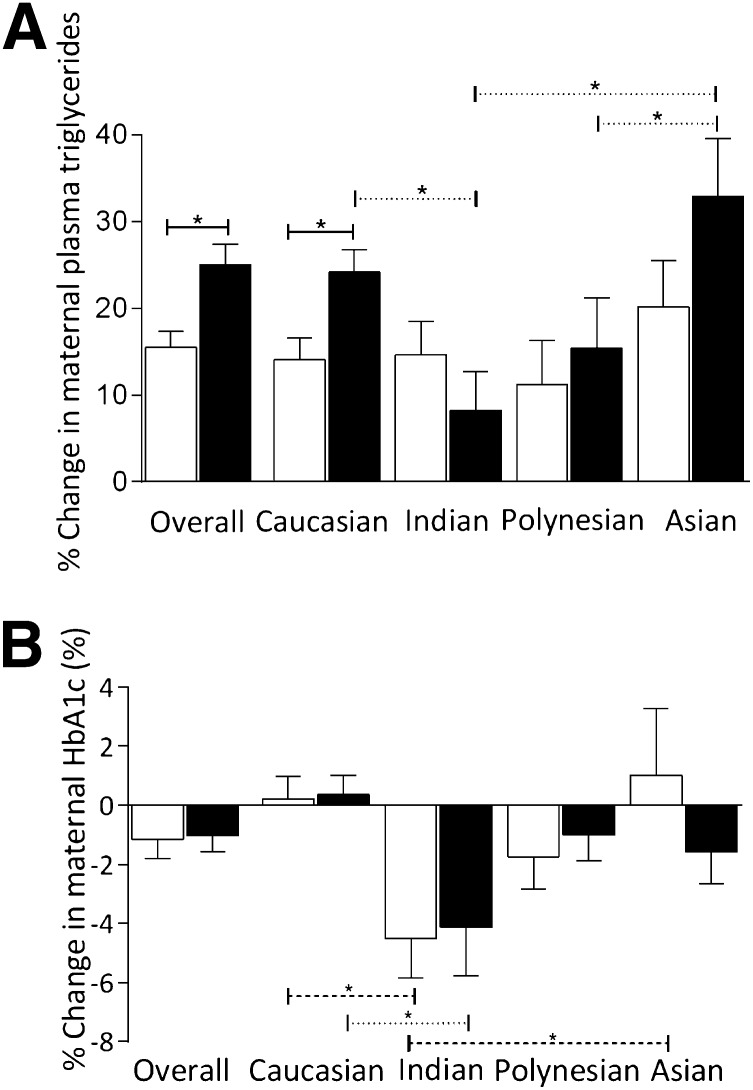
There was a complex relationship between ethnicity, percent change in triglycerides, and HbA1c (Fig. 1). At enrollment, there were no differences in ethnic composition between the metformin and insulin groups. Fasting glucose and HbA1c (but not triglycerides) differed between ethnic groups. The changes seen in maternal triglycerides and HbA1c between enrollment and 36 weeks were related to both treatment and ethnicity (Fig. 1A and B). With insulin treatment, there was no difference in percent change in triglycerides between ethnicities.
Figure 1.
Percent change in maternal triglycerides (mmol/L) (A) and maternal HbA1c (%) (B) from baseline to 36 weeks' gestation, stratified by ethnicity. White bars, insulin; black bars, metformin. Solid line compares between metformin and insulin, dashed line compares between ethnicities in women allocated insulin, and dotted line compares between ethnicities within women allocated metformin. *Significant at P < 0.05.
Effect of maternal smoking
Women who smoked during pregnancy had higher enrollment triglyceride concentrations (smoking, 2.75 [95% CI 2.51–3.02] mmol/L; nonsmoking, 2.37 [2.29–2.46] mmol/L; P = 0.002), but this difference was not significant at 36 weeks (smoking, 2.91 [2.67–3.17] mmol/L; nonsmoking, 2.78 [2.68–2.87] mmol/L; P = 0.30). The rates of smoking during pregnancy did not differ between ethnicities or by treatment allocation (data not shown).
CONCLUSIONS
In this group of women with GDM, who required medication for glucose control at a mean of 30 weeks' gestation, maternal plasma triglycerides at enrollment were associated with gestational age, measures of glucose control, ethnicity, and smoking. At 36 weeks' gestation, maternal triglyceride concentration was associated with ethnicity, glucose control, and treatment allocation. In women whose GDM was managed with insulin, 36-week triglycerides were most strongly associated with measures of glucose control, whereas in women whose GDM was managed with metformin, 36-week triglycerides were most strongly associated with ethnicity.
We found that plasma triglyceride concentrations in women treated with metformin were higher than in those treated with insulin at 36 weeks' gestation. The plasma triglyceride concentrations found in this study irrespective of the treatment arm are similar to or lower than those reported in women with treated GDM (2–3.0 mmol/L, managed with diet or insulin therapy) during the late second (8–10,20) and late third trimester (8,20,21). They are also similar to the concentrations reported in normal pregnancy in the third trimester (∼2–2.8 mmol/L) (20,22,23). Since elevated glucose concentrations are associated with increased triglyceride concentrations, the triglyceride concentrations in this study suggest that the level of glucose control achieved with treatment in the women in this study may have exerted a beneficial effect on triglyceride elevations.
The fasting plasma glucose assessed concomitant with plasma triglycerides at 36 weeks was higher in the metformin treatment group than the insulin treatment group. In the multivariate analysis, however, plasma glucose did not predict triglyceride concentrations. Therefore, the higher fasting plasma glucose does not provide an explanation as to why triglyceride concentrations were higher in the metformin-treated women. Additionally, other factors known to increase triglyceride concentrations, namely obesity (2), smoking, and preeclampsia (3), were also similar between treatment arms. Metformin improves insulin resistance, and it has been suggested that it influences lipids directly. Insulin can directly affect adipose tissue by reducing lipolysis and increasing LPL activity (24), which could explain the lower triglycerides in the insulin arm compared with the metformin arm.
The subtle differences in plasma triglyceride levels between women allocated metformin and those allocated insulin could contribute to differences in overall fetal nutrition along a continuum of lipid exposure, as previously described for glucose exposure in the Hyperglycemia and Adverse Pregnancy Outcomes study (25). Elevated maternal triglycerides have been associated with multiple adverse outcomes for the mother and infant. However, no interventional trials directed at lowering maternal triglycerides have been reported. If it was demonstrated that lowering maternal triglycerides provides beneficial pregnancy outcomes, then the finding that metformin is associated with higher maternal triglycerides compared with insulin at 36 weeks' gestation may alter the choice of pharmacotherapy for women with GDM.
These analyses showed ethnic differences in the change in triglyceride levels in women on metformin but not in women on insulin. Maternal triglycerides at enrollment did not differ between ethnicities. The examination of ethnicity in the current analyses is a post hoc subgroup analysis and thus needs to be treated with caution. However, ethnic differences in the relationship between insulin resistance, body adipose stores (26), lipids (27,28), and adipokines (29,30) have been reported before, and ethnic differences in lipid and lipoprotein metabolism have also been described in pregnancy (31,32). It is also possible that there are ethnic variations in the metabolism of or pharmacological response to metformin (33–35). Alternatively, the findings of the current analyses may be due to systematic ethnic differences in diet or glucose and lipid metabolism and require confirmation and further exploration in future studies.
The strength of these analyses is that the data were collected as part of a prospective, randomized, controlled trial. Limitations include the use of a subgroup of women from the original trial who are different from those who were not included. Women who did not have blood tests taken at both time points could not be included in this study, and unsurprisingly, these women belong to a lower socioeconomic group. Another potential weakness is that the smoking status in women was documented at enrollment but not at 36 weeks, so there is a possibility that the rates at which women ceased or commenced smoking within the time frame of the study differed between the treatment groups. Given that this was a randomized trial, if that were true, we would have to propose that insulin or metformin had a direct effect on smoking behavior, which we consider unlikely. More detailed and consistent smoking status information would be important to document in future studies.
The other weakness of this study is a reduced number of observations in the subgroup analyses, increasing the chance for error. With this in mind, the analyses of ethnic differences, while intriguing, require confirmation in future studies. In particular, the issue of maternal diet requires further exploration. The examination of dietary information could account for the apparent differences in the relationship between ethnicity and treatment seen in this study. Although the details of the diet the women in this study were asked to follow are known, their actual diet is not. There may have been differences in diet related to the treatment, which would also account for less weight gain in women treated with metformin. Insulin is frequently associated with increased appetite (36), and women treated with insulin may not have restricted their dietary intake to the same degree as women taking metformin, who might have been trying to avoid supplemental insulin. Women taking insulin would have been able to increase their dose of insulin to maintain glucose control, despite eating more. Restrictions to dietary intake in women taking metformin, leading to prolonged fasting by the time of the morning blood sample could have produced increased maternal plasma triglycerides (37). This has not been reported in the setting of a hypocaloric diet in obese women with GDM (38), but increased free fatty acids and a trend to increased maternal fasting triglycerides have been reported with the short-term use of a low-carbohydrate diet in women with GDM (39). Future studies examining the impact of metformin and insulin on triglycerides should include detailed dietary assessment to adequately examine this issue.
In this cohort of metformin- and insulin-treated women in a randomized trial of women with GDM, maternal plasma triglyceride concentrations at 36 weeks were higher during treatment with metformin than treatment with insulin, despite a lower weight gain in women treated with metformin. The differences noted in triglyceride levels between treatments were small, and maternal triglyceride levels were within the reported normal range for pregnancy. Although fasting glucose measured at the same time was higher in women treated with metformin, this did not explain the difference in triglycerides. Glucose control was an important factor in triglyceride levels in women treated with insulin, whereas ethnicity was an important factor in women treated with metformin. These data demonstrate that we have an incomplete understanding about treating GDM and the interactions between glucose, lipids, diet, medication, and ethnicity.
Acknowledgments
The MiG trial was supported by grants from the Auckland Medical Research Foundation, the National Women’s Evelyn Bond Charitable Trust, the Health Research Council of New Zealand, and the National Health and Medical Research Council of Australia. H.L.B. was supported by a PHD scholarship from the National Health and Medical Research Council of Australia.
No potential conflicts of interest relevant to this article were reported.
H.L.B. performed the analyses, interpreted the data, wrote the manuscript, and gave final approval. M.D.N. and L.C. interpreted data, revised the manuscript, and gave final approval. L.J. and P.O. analyzed data, revised the manuscript, and gave final approval. K.L., K.L.G., M.J.D.B., S.C., J.A.O., W.M.H., and H.D.M. acquired data, revised the manuscript, and gave final approval. J.R. acquired and interpreted data, revised the paper, and gave final approval. J.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-2132/-/DC1.
References
- 1.Herrera E, Amusquivar E, López-Soldado I, Ortega H. Maternal lipid metabolism and placental lipid transfer. Horm Res 2006;65(Suppl. 3):59–64 [DOI] [PubMed] [Google Scholar]
- 2.Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J Clin Endocrinol Metab 2002;87:4231–4237 [DOI] [PubMed] [Google Scholar]
- 3.Ray JG, Diamond P, Singh G, Bell CM. Brief overview of maternal triglycerides as a risk factor for pre-eclampsia. BJOG 2006;113:379–386 [DOI] [PubMed] [Google Scholar]
- 4.Koukkou E, Watts GF, Lowy C. Serum lipid, lipoprotein and apolipoprotein changes in gestational diabetes mellitus: a cross-sectional and prospective study. J Clin Pathol 1996;49:634–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rizzo M, Berneis K, Altinova AE, et al. Atherogenic lipoprotein phenotype and LDL size and subclasses in women with gestational diabetes. Diabet Med 2008;25:1406–1411 [DOI] [PubMed] [Google Scholar]
- 6.Sobki SH, Al-Senaidy AM, Al-Shammari TA, Inam SS, Al-Gwiser AA, Bukhari SA. Impact of gestational diabetes on lipid profiling and indices of oxidative stress in maternal and cord plasma. Saudi Med J 2004;25:876–880 [PubMed] [Google Scholar]
- 7.Catalano PM, Thomas A, Huston-Presley L, Amini SB. Increased fetal adiposity: a very sensitive marker of abnormal in utero development. Am J Obstet Gynecol 2003;189:1698–1704 [DOI] [PubMed] [Google Scholar]
- 8.Schaefer-Graf UM, Graf K, Kulbacka I, et al. Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care 2008;31:1858–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Cianni G, Miccoli R, Volpe L, et al. Maternal triglyceride levels and newborn weight in pregnant women with normal glucose tolerance. Diabet Med 2005;22:21–25 [DOI] [PubMed] [Google Scholar]
- 10.Son GH, Kwon JY, Kim YH, Park YW. Maternal serum triglycerides as predictive factors for large-for-gestational age newborns in women with gestational diabetes mellitus. Acta Obstet Gynecol Scand 2010;89:700–704 [DOI] [PubMed] [Google Scholar]
- 11.Hue L, Taegtmeyer H. The Randle cycle revisited: a new head for an old hat. Am J Physiol Endocrinol Metab 2009;297:E578–E591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Semenkovich CF, Wims M, Noe L, Etienne J, Chan L. Insulin regulation of lipoprotein lipase activity in 3T3-L1 adipocytes is mediated at posttranscriptional and posttranslational levels. J Biol Chem 1989;264:9030–9038 [PubMed] [Google Scholar]
- 13.Wulffelé MG, Kooy A, de Zeeuw D, Stehouwer CD, Gansevoort RT. The effect of metformin on blood pressure, plasma cholesterol and triglycerides in type 2 diabetes mellitus: a systematic review. J Intern Med 2004;256:1–14 [DOI] [PubMed] [Google Scholar]
- 14.Reaven GM, Johnston P, Hollenbeck CB, et al. Combined metformin-sulfonylurea treatment of patients with noninsulin-dependent diabetes in fair to poor glycemic control. J Clin Endocrinol Metab 1992;74:1020–1026 [DOI] [PubMed] [Google Scholar]
- 15.Ohira M, Miyashita Y, Ebisuno M, et al. Effect of metformin on serum lipoprotein lipase mass levels and LDL particle size in type 2 diabetes mellitus patients. Diabetes Res Clin Pract 2007;78:34–41 [DOI] [PubMed] [Google Scholar]
- 16.Abbasi F, Carantoni M, Chen YD, Reaven GM. Further evidence for a central role of adipose tissue in the antihyperglycemic effect of metformin. Diabetes Care 1998;21:1301–1305 [DOI] [PubMed] [Google Scholar]
- 17.Barrett HL, Gatford KL, Houda CM, et al. Maternal and neonatal circulating markers of metabolic and cardiovascular risk in the Metformin in Gestational Diabetes (MiG) trial: responses to maternal metformin versus insulin treatment. Diabetes Care 2013;36:529–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowan JA, Hague WM, Gao W, Battin MR, Moore MP, MiG Trial Investigators Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med 2008;358:2003–2015 [DOI] [PubMed] [Google Scholar]
- 19.McElduff A, Cheung NW, McIntyre HD, et al. Australasian Diabetes in Pregnancy Society The Australasian Diabetes in Pregnancy Society consensus guidelines for the management of type 1 and type 2 diabetes in relation to pregnancy. Med J Aust 2005;183:373–377 [PubMed] [Google Scholar]
- 20.Schaefer-Graf UM, Meitzner K, Ortega-Senovilla H, et al. Differences in the implications of maternal lipids on fetal metabolism and growth between gestational diabetes mellitus and control pregnancies. Diabet Med 2011;28:1053–1059 [DOI] [PubMed] [Google Scholar]
- 21.Toescu V, Nuttall SL, Martin U, et al. Changes in plasma lipids and markers of oxidative stress in normal pregnancy and pregnancies complicated by diabetes. Clin Sci (Lond) 2004;106:93–98 [DOI] [PubMed] [Google Scholar]
- 22.Göbl CS, Handisurya A, Klein K, et al. Changes in serum lipid levels during pregnancy in type 1 and type 2 diabetic subjects. Diabetes Care 2010;33:2071–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsson A, Palm M, Hansson LO, Axelsson O. Reference values for clinical chemistry tests during normal pregnancy. BJOG 2008;115:874–881 [DOI] [PubMed] [Google Scholar]
- 24.Lass A, Zimmermann R, Oberer M, Zechner R. Lipolysis - a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog Lipid Res 2011;50:14–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metzger BE, Lowe LP, Dyer AR, et al. HAPO Study Cooperative Research Group Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002 [DOI] [PubMed] [Google Scholar]
- 26.Wulan SN, Westerterp KR, Plasqui G. Ethnic differences in body composition and the associated metabolic profile: a comparative study between Asians and Caucasians. Maturitas 2010;65:315–319 [DOI] [PubMed] [Google Scholar]
- 27.Sumner AE. Ethnic differences in triglyceride levels and high-density lipoprotein lead to underdiagnosis of the metabolic syndrome in black children and adults. J Pediatr 2009;155:e7–e11 [DOI] [PMC free article] [PubMed]
- 28.Gasevic D, Frohlich J, Mancini GB, Lear SA. The association between triglyceride to high-density-lipoprotein cholesterol ratio and insulin resistance in a multiethnic primary prevention cohort. Metabolism 2012;61:583–589 [DOI] [PubMed] [Google Scholar]
- 29.Shand B, Elder P, Scott R, Poa N, Frampton CM. Comparison of plasma adiponectin levels in New Zealand Maori and Caucasian individuals. N Z Med J 2007;120:U2606. [PubMed] [Google Scholar]
- 30.Gao H, Salim A, Lee J, Tai ES, van Dam RM. Can body fat distribution, adiponectin levels and inflammation explain differences in insulin resistance between ethnic Chinese, Malays and Asian Indians? Int J Obes (Lond) 2012;36:1086–1093 [DOI] [PubMed] [Google Scholar]
- 31.Retnakaran R, Hanley AJ, Connelly PW, Sermer M, Zinman B. Ethnicity modifies the effect of obesity on insulin resistance in pregnancy: a comparison of Asian, South Asian, and Caucasian women. J Clin Endocrinol Metab 2006;91:93–97 [DOI] [PubMed] [Google Scholar]
- 32.Schreuder YJ, Hutten BA, van Eijsden M, et al. Ethnic differences in maternal total cholesterol and triglyceride levels during pregnancy: the contribution of demographics, behavioural factors and clinical characteristics. Eur J Clin Nutr 2011;65:580–589 [DOI] [PubMed] [Google Scholar]
- 33.Ahlin G, Chen L, Lazorova L, et al. Genotype-dependent effects of inhibitors of the organic cation transporter, OCT1: predictions of metformin interactions. Pharmacogenomics J 2011;11:400–411 [DOI] [PubMed] [Google Scholar]
- 34.Chen L, Pawlikowski B, Schlessinger A, et al. Role of organic cation transporter 3 (SLC22A3) and its missense variants in the pharmacologic action of metformin. Pharmacogenet Genomics 2010;20:687–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song IS, Shin HJ, Shim EJ, et al. Genetic variants of the organic cation transporter 2 influence the disposition of metformin. Clin Pharmacol Ther 2008;84:559–562 [DOI] [PubMed] [Google Scholar]
- 36.Katsiki N, Mikhailidis DP, Gotzamani-Psarrakou A, Didangelos TP, Yovos JG, Karamitsos DT. Effects of improving glycemic control with insulin on leptin, adiponectin, ghrelin and neuropeptidey levels in patients with type 2 diabetes mellitus: a pilot study. Open Cardiovasc Med J 2011;5:136–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malhotra A, Scott PH, Scott J, Gee H, Wharton BA. Metabolic changes in Asian Muslim pregnant mothers observing the Ramadan fast in Britain. Br J Nutr 1989;61:663–672 [DOI] [PubMed] [Google Scholar]
- 38.Knopp RH, Magee MS, Raisys V, Benedetti T. Metabolic effects of hypocaloric diets in management of gestational diabetes. Diabetes 1991;40(Suppl. 2):165–171 [DOI] [PubMed] [Google Scholar]
- 39.Nolan CJ. Improved glucose tolerance in gestational diabetic women on a low fat, high unrefined carbohydrate diet. Aust N Z J Obstet Gynaecol 1984;24:174–177 [DOI] [PubMed] [Google Scholar]