Abstract
OBJECTIVE
Although diabetes increases the risk of cardiovascular disease (CVD) and mortality, the dose-response relationship between fasting glucose levels below those diagnostic of diabetes with cardiovascular events has not been well characterized.
RESEARCH DESIGN AND METHODS
A prospective cohort study of more than one million Koreans was conducted with a mean follow-up of 16 years. A total of 1,197,384 Korean adults with no specific medical conditions diagnosed were classified by baseline fasting serum glucose level. Associations of fasting glucose level with CVD incidence and mortality, stroke incidence and mortality, and all-cause mortality were analyzed using multivariate proportional hazards regression.
RESULTS
The relationships between fasting glucose levels and CVD risks generally followed J-shape curves, with lowest risk in the glucose range of 85–99 mg/dL. As fasting glucose levels increased to >100 mg/dL, risks for CVD, ischemic heart disease, myocardial infarction, and thrombotic stroke progressively increased, but risk for hemorrhagic stroke did not. Fasting glucose levels <70 mg/dL were associated with increased risk of all stroke (hazard ratio 1.06, 95% CI 1.01–1.11) in men and (hazard ratio 1.11, 1.05–1.17) in women.
CONCLUSIONS
Both low glucose level and impaired fasting glucose should be considered as predictors of risk for stroke and coronary heart disease. The fasting glucose level associated with the lowest cardiovascular risk may be in a narrow range.
Diabetes is a well-established risk factor for cardiovascular disease (CVD) and all-cause mortality (1–3). Impaired fasting glucose (IFG), defined by the American Diabetes Association as having a fasting plasma glucose level of 100–125 mg/dL (5.6–7.0 mmol/L) or a 2-h value on the oral glucose tolerance test of 140–199 mg/dL (7.8–11.1 mmol/L) (4) was associated with CVD risk in several studies (1,5–7). The evidence is inconsistent, however, and the clinical relevance of IFG as a predictor of CVD is still unclear (8–11). In addition, the shape of the dose-response relationship between CVD risk and fasting glucose level has not been well characterized across the full range of fasting blood glucose values.
It is unclear whether there is an optimum fasting glucose level associated with the lowest level of CVD risk (12,13), or whether risk increases at very low fasting glucose levels (14). Several studies have shown J-shape or U-shape relationships between fasting glucose levels and mortality (3,5,14,15).
The Korean Cancer Prevention Study (16,17) (KCPS) is a cohort study of >1.3 million Korean adults designed to evaluate major risk factors for chronic diseases and mortality. The large sample size of this cohort facilitated detailed characterization of the dose-response relationship of fasting glucose level with the incidence of clinical CVD end points.
RESEARCH DESIGN AND METHODS
Study population
The KCPS is a prospective cohort study of Korean government employees, public and private school teachers, and their dependents who were insured by the Korean Medical Insurance Corporation, the former National Health Insurance Corporation (16,17). The cohort includes 1,329,525 Koreans (846,907 men and 482,618 women) aged 30–95 years; 784,870 (59%) subjects were enrolled in 1992, 367,903 (27.7%) were enrolled in 1993, 98,417 (7.4%) were enrolled in 1994, and 78,335 (5.9%) were enrolled in 1995. Follow-up began on 1 January 1993, so 904 people enrolled in 1992 and who died in that year were excluded. To avoid confounding of the association between fasting serum glucose and the risk of death by preexisting disease, 88,420 who reported having CVD, liver disease, cancer, respiratory disease, and diabetes diagnosed at or before the initial study visit were excluded. In addition, 42,817 people with missing information on fasting serum glucose, total cholesterol, systolic blood pressure, and alcohol intake and those with extremely low BMI (<16.0 kg/m2), or those with exceptionally short stature (<130 cm) were also excluded. The final sample included 1,197,384 participants (761,955 men and 435,429 women).
Data collection
People insured by the Korean Medical Insurance Corporation were required to have biennial medical examinations conducted at designated hospitals or clinics nationwide by medical staff following guidelines provided by the Korean Medical Insurance Corporation. Participants were asked to provide their medical history and to respond to a lifestyle questionnaire that included items on smoking, alcohol drinking, and performance of regular exercise. The examination included height, weight, and blood pressure measurements, urinalysis, blood cell count, and routine blood chemistries after overnight fasting.
The follow-up period was up to 18.0 years. Incident events were determined from diagnoses on the discharge summaries of inpatient hospital records. In Korea, certified medical chart recorders review and abstract the medical chart and assign discharge diagnoses in a standardized form using the International Classification of Diseases, 10th revision (World Health Organization 1992). Vital status and cause of death were determined from computerized searches of death certificate data from the Korean National Statistical Office.
Study outcomes were defined as hospitalization or mortality attributable to ischemic heart disease (International Classification of Diseases, 10th revision, codes I20–I25), stroke (codes I60–I69), and atherosclerotic CVD, which included ischemic heart disease (codes I20–I25), stroke (codes I60–I69), hypertensive heart disease (codes I10–I15), other forms of heart disease likely related to atherosclerosis (codes I44–I52), disease of arteries (codes I70–I74), and other sudden death with cause unknown (code R96). Cardiovascular outcomes included hemorrhagic stroke (I60–I62) and ischemic stroke (I63–I66). For those who had more than one event during follow-up, we used only the first event for this analysis. An ischemic heart disease event validation study was conducted in collaboration with the Korean Society of Cardiology through the formation of the Event Validation Committee (July 2008–May 2009). For 673 participants with an ischemic heart disease event, review of individual hospital records showed that 73% of myocardial infarction diagnoses were valid (18). Another validation study reported that 83% of stroke diagnoses were valid (19).
Statistical analysis
Proportional hazards models were used to evaluate the association between baseline fasting serum glucose levels and atherosclerotic CVD. The assumption of proportional hazards was tested and met. Fasting serum glucose levels were categorized as <70, 70–84, 85–99, 100–109, 110–125, 126–139, and ≥140 mg/dL. We used the group with fasting glucose level of 85–99 mg/dL as the reference category. All analyses were conducted separately for men and women and were adjusted for the following covariates: age at enrollment; BMI; systolic blood pressure; total cholesterol; alcohol intake (five categories based on grams consumed per day: 0, 1–24 g, 25–49 g, 50–99 g, and ≥100 g); participation in regular physical activity (yes or no) and smoking status (never smoker, former smoker, or current smoker); and the number of cigarettes smoked daily among current smokers (1–9, 10–19, and ≥20). For more detailed analyses of the dose-response trends, we also used restricted quadratic spline models with knots at fasting glucose plasma levels of 70, 85, 100, 110, 126, and 140 mg/dL. All analyses were performed with SAS statistical software 9.2.
RESULTS
On enrollment, the average age of study participants was 45.0 years for men and 49.4 years for women, and the average BMI was 23.2 kg/m2 for both sexes. The average fasting glucose values were 91.1 mg/dL and 94.4 mg/dL in men and women, respectively. The percentages of study participants with fasting serum glucose <100, 100–125, and ≥126 mg/dL were 76.8, 19.3, and 3.9% in men and 82.0, 14.8, and 3.2% in women. Among men, 60.2% were current smokers and only 20.8% were never smokers; among women, the corresponding figures were 4.1 and 93.9%, respectively.
In the cross-sectional baseline data, men and women with higher fasting glucose were more likely to be older, to have a higher BMI, and to have higher systolic and diastolic blood pressure and total cholesterol levels (Supplementary Tables 1 and 2). Participants in higher fasting glucose categories were also more likely to report that they were physically active. In men, the prevalence of current smoking was inversely, but weakly, associated with fasting glucose level, but positively and also weakly positively associated with glucose level in women.
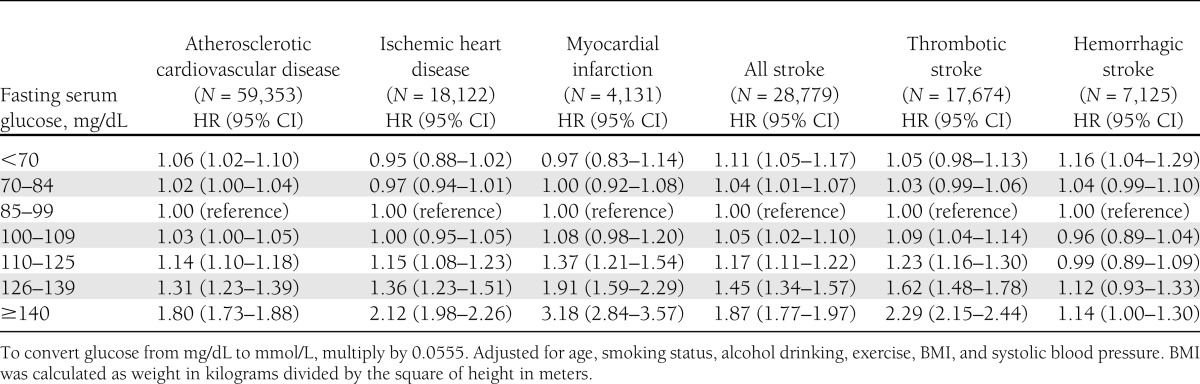
We separately assessed the association of fasting serum glucose for risk of various CVD outcomes in men and women, finding similar patterns of association by sex for fatal and nonfatal events combined (Tables 1 and 2). The risks for the various CVD outcomes were lowest in both sexes for those with serum glucose in the range of 70–109 mg/dL, and increased above and below this range. Fasting glucose levels <70 mg/dL were associated with slightly increased risk of atherosclerotic CVD in men (hazard ratio [HR] 1.04, 95% CI 1.01–1.08) and in women (HR 1.06, 1.02–1.10). Fasting glucose levels <70 mg/dL were associated with increased risk of all stroke in men (HR 1.06, 1.01–1.11) in women (HR 1.11, 1.05–1.17). For categories >100–109 mg/dL group, the HRs increased progressively with increasing serum glucose, except for hemorrhagic stroke. The results were similar after exclusion of the first 5 years of follow-up (data not shown).
Table 1.
Fasting serum glucose levels at enrollment and risk of cardiovascular diseases in male participants of the Korean Cancer Prevention Study 1993–2010
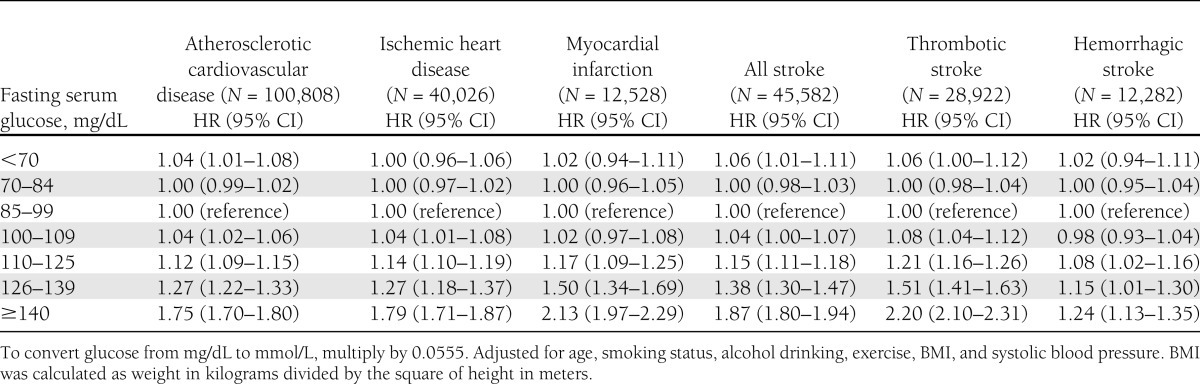
Table 2.
Fasting serum glucose levels at enrollment and risk of cardiovascular diseases in female participants of the Korean Cancer Prevention Study 1993–2010
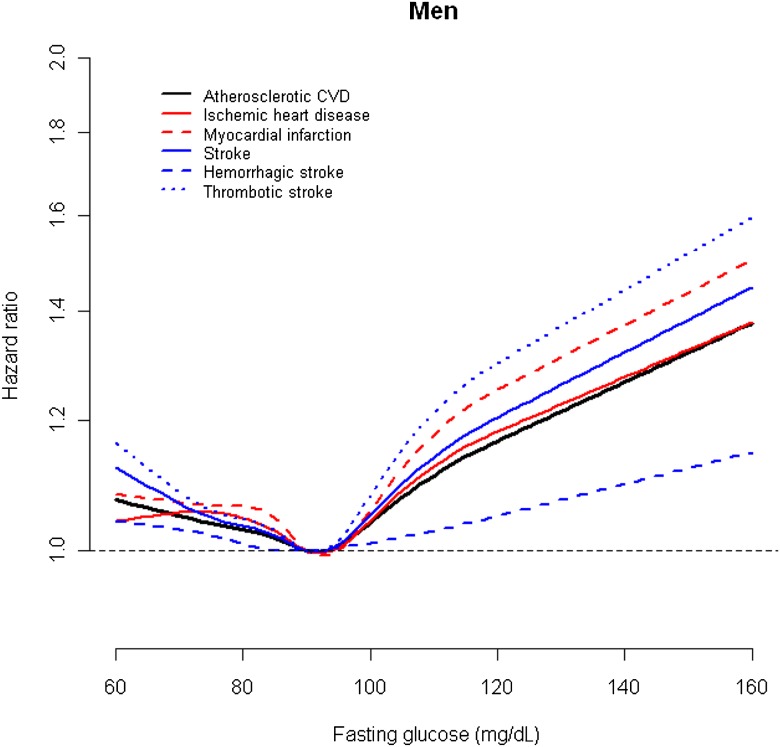
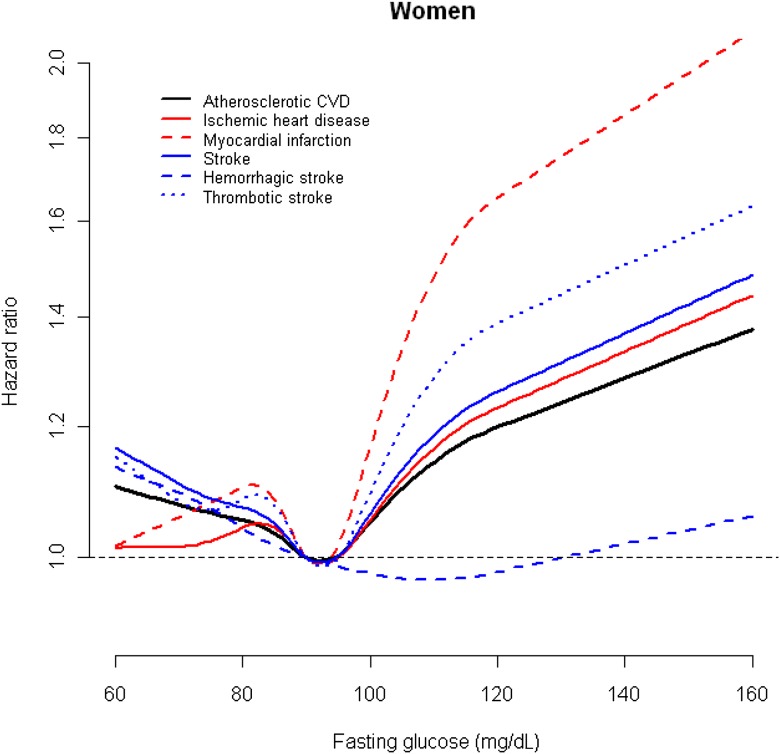
For cardiac outcomes and thrombotic stroke, the general patterns of association were quite comparable. For participants having a glucose level in the range of 110–125 mg/dL the HR was increased by 10–20%, and those in the highest category experienced a doubling or more of the risk of having a CVD event. The HRs were highest for thrombotic stroke and myocardial infarction. In restricted quadratic spline models, the risks of cardiac outcomes and thrombotic stroke were lowest at fasting serum glucose levels of ∼90 mg/dL and increased sharply above these levels in both men and women (Figs. 1 and 2).
Figure 1.
HRs for cardiovascular outcomes by fasting serum glucose levels in male participants of the KCPS. To convert glucose from mg/dL to mmol/L, multiply by 0.0555.
Figure 2.
HRs for cardiovascular outcomes by fasting serum glucose levels in female participants of the KCPS. To convert glucose from mg/dL to mmol/L, multiply by 0.0555.
We repeated the analyses for fatal and nonfatal events separately (Supplementary Tables 3–6). For men, the results for the fatal and nonfatal events were generally comparable. For women, the risks associated with low blood glucose were greater for fatal compared with nonfatal events.
We also assessed potential effect modification of the risk for all CVD outcomes by other risk factors, age, and sex. In general, we found little indication of substantial effect modification (Supplementary Table 7). There were significant overall interactions for hypertension (present versus absent) and age (younger than 55 years versus 55 years or older), but the differences in HRs were small. For both men and women, the HRs changed in a similar pattern with fasting glucose concentration across the two strata of age.
CONCLUSIONS
In a large cohort of Korean men and women, we found that fasting glucose level was associated with higher risk for major CVD outcomes, increasing from a level of ∼90 mg/dL after controlling for other risk factors. The dose-response curves showed progressive increments in the HRs from this value at both higher and lower levels; the increased risk was greatest for stroke. The patterns of association were similar in men and women, but the associations were stronger in women.
Substantial evidence supports the biological plausibility of this finding. Experimental studies show that abnormal glucose metabolism impairs normal endothelial function, accelerates atherosclerotic plaque formation, and contributes to plaque rupture and thrombosis. Epidemiological studies provide complementary evidence. In the Rotterdam Study, among elderly participants with a fasting blood glucose <110 mg/dL and without diabetes, those with higher blood glucose levels had higher levels of arterial stiffness (20). The CATHAY study found that higher levels of glycemia (102–124 mg/dL) were associated with arterial endothelial dysfunction and intima-media thickening (21). In a biomarker study in Italy, a number of CVD biomarkers showed positive dose-response relationships with fasting glucose across three strata: <100; 100–109; and 110–125 mg/dL (22). Our study adds to the increasing evidence that IFG is an independent risk factor for incident CVD, including ischemic heart disease and stroke (7). In addition, the effects of other CVD risk factors may be enhanced by abnormal glucose metabolism (23–25).
In 1997, the American Diabetes Association Expert Committee introduced the IFG category, defined at the time as fasting plasma glucose level of 110 to 125 mg/mL or a 2-h value on the oral glucose tolerance test of 140–199 mg/dL (26). IFG is not a clinical entity in itself but is a risk category for development of diabetes and CVD (10). In 2003, the American Diabetes Association Expert Committee lowered the cut-point for IFG to 100 mg/dL to align the proportion of the population that would be included in the category using fasting glucose and the oral glucose tolerance test, and to indicate that glucose levels <110 mg/dL were predictive of diabetes development (26,27). Although previous studies also have identified an increased risk of CVD associated with IFG (100–125 mg/dL) (3,5,28,29), the size of KCPS provides a much more precise characterization of the up-turn in risk in relation to blood glucose level, placing it at ∼90 mg/dL. As a consequence, our findings support lowering the current American Diabetes Association IFG cut-off of 100 mg/dL, and they also open the possibility that low fasting glucose levels may identify people at increased risk for CVD.
Severe hypoglycemia in diabetes is known to increase risks of vascular events (30–32). Low fasting glucose levels in the nondiabetic population were associated with increased mortality in several studies (5,15). In our study, the lowest risk for CVD mortality occurred in the interval of 85–99 mg/dL, with a nadir at ∼90 mg/dL, suggesting that the range of fasting glucose levels associated with the lowest risk for CVD is narrow. In a prospective cohort study of >40,000 people, the lowest total mortality was associated with fasting plasma glucose of 79–109 mg/dL (15). In the study by Balkau et al. (33), the interval of lowest total mortality was 94–103 mg/dL, and in the DECODE study it was 81–90 mg/dL (34). The pooled analysis performed by the Emerging Risk Factors Collaboration described a nonlinear relationship between fasting glucose and coronary heart disease that had the same configuration as in the KCPS analysis (3). In the Atherosclerosis Risk in Communities (ARIC) study, Selvin et al. (35) found a J-shape relationship of glycated hemoglobin with risk for death attributable to coronary heart disease. Fasting blood glucose level <100 mg/dL was not associated with coronary heart disease death (35). Because different studies have used different categories for fasting glucose, further and perhaps pooled analyses of data from large studies should be performed using flexible models for dose-response analysis to confirm our findings of a narrow range of fasting glucose levels with lowest mortality.
The basis of the association between low fasting glucose levels and risk for CVD in people without diabetes is unclear. Wei et al. (15) hypothesized that long-term exposure to low fasting plasma glucose may serve as a risk factor for CVD mortality, perhaps through abnormal cardiac activity and thrombosis, particularly in patients with atherosclerosis. Tanne et al. (36) reported a J-shape relationship between fasting plasma glucose and incident ischemic cerebrovascular events in patients with preexisting atherothrombotic disease and suggested that hypoglycemia or rapid changes in plasma glucose may lead to elevations of counter-regulatory hormones, such as epinephrine and norepinephrine, and these increases induce vasoconstriction and platelet aggregation (36). In a pooled analysis of data from 97 cohorts involving people without vascular disease on enrollment, an increase in risk for vascular disease death was observed in those with blood glucose level <70 mg/dL on enrollment (7). The cohort of Tanne et al. (36) was comprised of patients with documented coronary heart disease. Because CVD risk factors and development of CVD are usually associated with metabolic abnormalities that increase fasting plasma glucose, additional studies need to be conducted to understand if low fasting plasma glucose levels are a consequence of impending disease (i.e., reverse causation) or if they have a role in precipitating acute CVD events.
Limitations of our findings need to be considered. First, single fasting glucose measurements are subject to substantial within-person variation and we did not perform 2-h glucose tolerance testing. Measurement error and between-study variability may have attenuated our results, adding to the identification of a narrow range of fasting glucose levels for optimal survival. Additionally, we measured serum glucose, not the currently recommended plasma glucose (37,38). However, because all measurements were of serum glucose, our findings should not be biased. Second, we lacked information on the evolution of fasting glucose levels and incidence of diabetes during follow-up, and we cannot identify if the increased risk associated with IFG depends on future evolution to diabetes or on other pathways. Third, we lacked data on other metabolic abnormalities associated with overweight and obesity and fat distribution. Fourth, the outcomes were obtained from admission records and death certificates. Because the outpatient records were not included, the incidence of CVD morbidity in our study may be lower than actual incidence.
With regard to generalizability of findings, the KCPS population differs from those of other investigations in several ways. Of course, participants were Asian and the population was also leaner than those in the other studies of blood glucose and CVD risk. The majority of males smoked, whereas only a few women smoked.
Fasting glucose is readily and widely measured for early detection and prevention of diabetes. Beyond risk for diabetes, it also conveys information about CVD risk. Additionally, the findings suggest that the optimal glucose level may be below the current cut-offs used to identify people at risk for CVD. Health care providers should recognize the J-shape relationship between fasting blood glucose and CVD risk in interpreting and communicating clinical data.
Acknowledgments
This study was funded by the National Cancer Institute of the National Institutes of Health, United States Department of Health and Human Services (1R03 CA94771-02), and was partially supported by Seoul City R&BD program (10526) and a grant from the National R&D Program for Cancer Control, Ministry for Health, Welfare, and Family Affairs (1220180), Republic of Korea.
No potential conflicts of interest relevant to this article were reported.
C.P. and E.G. helped to develop the concept, researched the data, and wrote the manuscript. J.A.L., D.-C.L., Y.J., D.K.S., E.-J.H., S.J.B., Y.D.Y., and S.H.J. contributed to discussion and reviewed the manuscript. S.H.J. analyzed data and edited the manuscript. J.M.S. developed the concept, discussed the analyses, and reviewed and edited the manuscript. S.H.J. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the staff of the Korean National Health Insurance Corporation. The authors thank Athena Foong of the Department of Preventive Medicine, University of Southern California, Los Angeles, California, for editorial assistance.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1577/-/DC1.
References
- 1.Barr ELM, Zimmet PZ, Welborn TA, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation 2007;116:151–157 [DOI] [PubMed] [Google Scholar]
- 2.Roglic G, Unwin N, Bennett PH, et al. The burden of mortality attributable to diabetes: realistic estimates for the year 2000. Diabetes Care 2005;28:2130–2135 [DOI] [PubMed] [Google Scholar]
- 3.Sarwar N, Gao P, Seshasai SR, et al. Emerging Risk Factors Collaboration Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies [erratum, Lancet 2010;376:958]. Lancet 2010;375:2215–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2004;27(Suppl. 1):S5–S10 [DOI] [PubMed] [Google Scholar]
- 5.Barr EL, Boyko EJ, Zimmet PZ, Wolfe R, Tonkin AM, Shaw JE. Continuous relationships between non-diabetic hyperglycaemia and both cardiovascular disease and all-cause mortality: the Australian Diabetes, Obesity, and Lifestyle (AusDiab) study. Diabetologia 2009;52:415–424 [DOI] [PubMed] [Google Scholar]
- 6.Levitan EB, Song Y, Ford ES, Liu S. Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? A meta-analysis of prospective studies. Arch Intern Med 2004;164:2147–2155 [DOI] [PubMed] [Google Scholar]
- 7.Seshasai SR, Kaptoge S, Thompson A, et al. Emerging Risk Factors Collaboration Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011;364:829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med 2002;19:708–723 [DOI] [PubMed] [Google Scholar]
- 9.Tominaga M, Eguchi H, Manaka H, Igarashi K, Kato T, Sekikawa A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care 1999;22:920–924 [DOI] [PubMed] [Google Scholar]
- 10.Pankow JS, Kwan DK, Duncan BB, et al. Cardiometabolic risk in impaired fasting glucose and impaired glucose tolerance: the Atherosclerosis Risk in Communities Study. Diabetes Care 2007;30:325–331 [DOI] [PubMed] [Google Scholar]
- 11.Sorkin JD, Muller DC, Fleg JL, Andres R. The relation of fasting and 2-h postchallenge plasma glucose concentrations to mortality: data from the Baltimore Longitudinal Study of Aging with a critical review of the literature. Diabetes Care 2005;28:2626–2632 [DOI] [PubMed] [Google Scholar]
- 12.Piché M-É, Arcand-Bossé J-F, Després J-P, Pérusse L, Lemieux S, Weisnagel SJ. What is a normal glucose value? Differences in indexes of plasma glucose homeostasis in subjects with normal fasting glucose. Diabetes Care 2004;27:2470–2477 [DOI] [PubMed] [Google Scholar]
- 13.Tirosh A, Shai I, Tekes-Manova D, et al. Israeli Diabetes Research Group Normal fasting plasma glucose levels and type 2 diabetes in young men. N Engl J Med 2005;353:1454–1462 [DOI] [PubMed] [Google Scholar]
- 14.Wändell PE, Theobald H. The association between blood glucose value and long-term mortality. Diabetes Metab 2005;31:588–594 [DOI] [PubMed] [Google Scholar]
- 15.Wei M, Gibbons LW, Mitchell TL, Kampert JB, Stern MP, Blair SN. Low fasting plasma glucose level as a predictor of cardiovascular disease and all-cause mortality. Circulation 2000;101:2047–2052 [DOI] [PubMed] [Google Scholar]
- 16.Jee SH, Sull JW, Park J, et al. Body-mass index and mortality in Korean men and women. N Engl J Med 2006;355:779–787 [DOI] [PubMed] [Google Scholar]
- 17.Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. JAMA 2005;293:194–202 [DOI] [PubMed] [Google Scholar]
- 18.Kimm H, Yun JE, Lee SH, Jang Y, Jee SH. Validity of the diagnosis of acute myocardial infarction in Korean national medical health insurance claims data: the Korean heart study (1). Korean Circ J 2012;42:10–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park JK, Kim KS, Kim CB, et al. The accuracy of ICD codes for cerebrovascular diseases in medical insurance claims. Korean J Prev Med 2000;33:76–82 [in Korean] [Google Scholar]
- 20.van Popele NM, Elizabeth Hak A, Mattace-Raso FU, et al. Impaired fasting glucose is associated with increased arterial stiffness in elderly people without diabetes mellitus: the Rotterdam Study. J Am Geriatr Soc 2006;54:397–404 [DOI] [PubMed] [Google Scholar]
- 21.Thomas GN, Chook P, Qiao M, et al. Deleterious impact of “high normal” glucose levels and other metabolic syndrome components on arterial endothelial function and intima-media thickness in apparently healthy Chinese subjects: the CATHAY study. Arterioscler Thromb Vasc Biol 2004;24:739–743 [DOI] [PubMed] [Google Scholar]
- 22.Andreozzi F, Succurro E, Mancuso MR, et al. Metabolic and cardiovascular risk factors in subjects with impaired fasting glucose: the 100 versus 110 mg/dL threshold. Diabetes Metab Res Rev 2007;23:547–550 [DOI] [PubMed] [Google Scholar]
- 23.Creager MA, Lüscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part I. Circulation 2003;108:1527–1532 [DOI] [PubMed] [Google Scholar]
- 24.Lüscher TF, Creager MA, Beckman JA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part II. Circulation 2003;108:1655–1661 [DOI] [PubMed] [Google Scholar]
- 25.Chait A, Bornfeldt KE. Diabetes and atherosclerosis: is there a role for hyperglycemia? J Lipid Res 2009;50(Suppl.):S335–S339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 27.Genuth S, Alberti KG, Bennett P, et al. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26:3160–3167 [DOI] [PubMed] [Google Scholar]
- 28.Gerstein HC, Pogue J, Mann JF, et al. HOPE investigators The relationship between dysglycaemia and cardiovascular and renal risk in diabetic and non-diabetic participants in the HOPE study: a prospective epidemiological analysis. Diabetologia 2005;48:1749–1755 [DOI] [PubMed] [Google Scholar]
- 29.Ford ES, Zhao G, Li C. Pre-diabetes and the risk for cardiovascular disease: a systematic review of the evidence. J Am Coll Cardiol 2010;55:1310–1317 [DOI] [PubMed] [Google Scholar]
- 30.Wright RJ, Frier BM. Vascular disease and diabetes: is hypoglycaemia an aggravating factor? Diabetes Metab Res Rev 2008;24:353–363 [DOI] [PubMed] [Google Scholar]
- 31.Zoungas S, Patel A, Chalmers J, et al. ADVANCE Collaborative Group Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010;363:1410–1418 [DOI] [PubMed] [Google Scholar]
- 32.Desouza CV, Bolli GB, Fonseca V. Hypoglycemia, diabetes, and cardiovascular events. Diabetes Care 2010;33:1389–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balkau B, Bertrais S, Ducimetiere P, Eschwege E. Is there a glycemic threshold for mortality risk? Diabetes Care 1999;22:696–699 [DOI] [PubMed] [Google Scholar]
- 34.DECODE Study Group, European Diabetes Epidemiology Group Is the current definition for diabetes relevant to mortality risk from all causes and cardiovascular and noncardiovascular diseases? Diabetes Care 2003;26:688–696 [DOI] [PubMed] [Google Scholar]
- 35.Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010;362:800–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanne D, Koren-Morag N, Goldbourt U. Fasting plasma glucose and risk of incident ischemic stroke or transient ischemic attacks: a prospective cohort study. Stroke 2004;35:2351–2355 [DOI] [PubMed] [Google Scholar]
- 37.WHO Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation. Geneva, WHO, 2006 [Google Scholar]
- 38.American Diabetes Association Standards of medical care in diabetes—2010. Diabetes Care 2010;33(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]