Abstract
OBJECTIVE
To assess whether intermittent real-time continuous glucose monitoring (CGM) improves glycemic control and pregnancy outcome in unselected women with pregestational diabetes.
RESEARCH DESIGN AND METHODS
A total of 123 women with type 1 diabetes and 31 women with type 2 diabetes were randomized to use real-time CGM for 6 days at 8, 12, 21, 27, and 33 weeks in addition to routine care, including self-monitored plasma glucose seven times daily, or routine care only. To optimize glycemic control, real-time CGM readings were evaluated by a diabetes caregiver. HbA1c, self-monitored plasma glucose, severe hypoglycemia, and pregnancy outcomes were recorded, with large-for-gestational-age infants as the primary outcome.
RESULTS
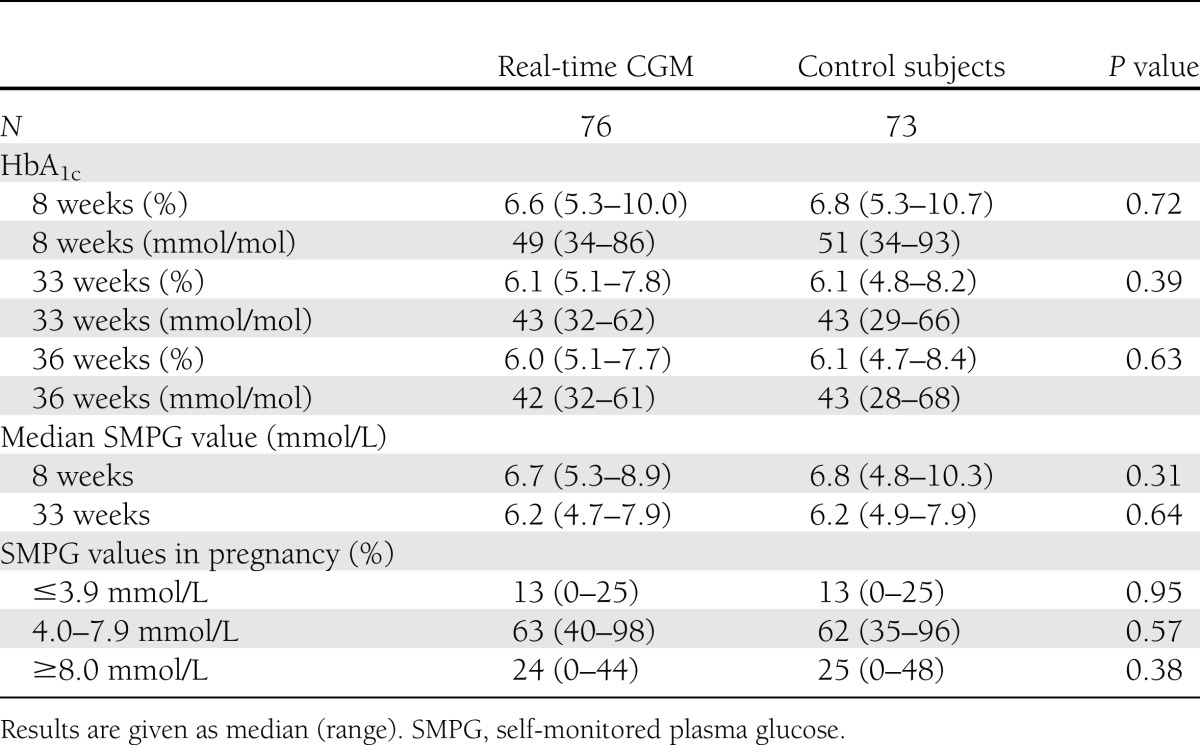
Women assigned to real-time CGM (n = 79) had baseline HbA1c similar to that of women in the control arm (n = 75) (median 6.6 [range 5.3–10.0] vs. 6.8% [5.3–10.7]; P = 0.67) (49 [34–86] vs. 51 mmol/mol [34–93]). Forty-nine (64%) women used real-time CGM per protocol. At 33 weeks, HbA1c (6.1 [5.1–7.8] vs. 6.1% [4.8–8.2]; P = 0.39) (43 [32–62] vs. 43 mmol/mol [29–66]) and self-monitored plasma glucose (6.2 [4.7–7.9] vs. 6.2 mmol/L [4.9–7.9]; P = 0.64) were comparable regardless of real-time CGM use, and a similar fraction of women had experienced severe hypoglycemia (16 vs. 16%; P = 0.91). The prevalence of large-for-gestational-age infants (45 vs. 34%; P = 0.19) and other perinatal outcomes were comparable between the arms.
CONCLUSIONS
In this randomized trial, intermittent use of real-time CGM in pregnancy, in addition to self-monitored plasma glucose seven times daily, did not improve glycemic control or pregnancy outcome in women with pregestational diabetes.
Pregnancy in women with pregestational diabetes is still associated with adverse perinatal outcomes largely attributed to maternal hyperglycemia, including large-for-gestational-age infants, preterm delivery, and perinatal morbidity (1–4). Large-for-gestational-age infants to mothers with diabetes are at increased risk for birth trauma, transient tachypnea, and neonatal hypoglycemia (5), and maternal diabetes in pregnancy is associated with later-life morbidity in the offspring (6). The major barrier in the strive for strict maternal glycemic control is the risk of severe hypoglycemia (1), occurring up to five times more frequently in early pregnancy than in the period prior to pregnancy in women with type 1 diabetes (7).
Real-time continuous glucose monitoring (CGM) measures interstitial glucose in an ongoing fashion and offers the possibility of hyper- and hypoglycemic alarms. Studies of nonpregnant patients with type 1 diabetes indicate that real-time CGM lowers HbA1c (8–19) and may reduce the tendency to biochemical hypoglycemia (9). Pregnant women with diabetes may also profit from real-time CGM, but experience is still limited (20–26). A randomized controlled trial evaluating intermittent use of a previous CGM system (not real-time) on top of routine pregnancy care reported improved glycemic control and a reduced risk of large-for-gestational-age infants in the intervention arm (27). Against this background, it is tempting to suggest that women with pregestational diabetes would benefit even more from the use of real-time CGM in pregnancy.
In this investigator-driven trial, we therefore aimed to assess whether intermittent real-time CGM, as part of routine pregnancy care, could improve maternal glycemic control and pregnancy outcome in an unselected cohort of women with pregestational type 1 or type 2 diabetes.
RESEARCH DESIGN AND METHODS
Patients
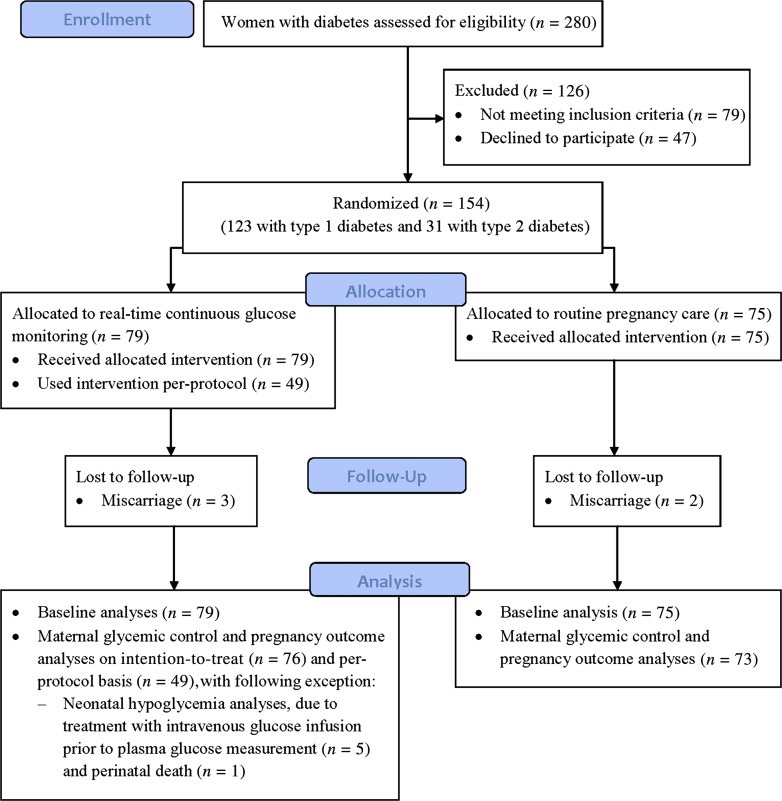
During the study period of 15 February 2009 to 15 February 2011, all Danish-speaking pregnant women with pregestational diabetes referred to the Center for Pregnant Women with Diabetes, Rigshospitalet, before 14 completed gestational weeks with one living intrauterine fetus (n = 222), were offered participation in the study (Fig. 1). Patients were referred from the Capital Region of Denmark and Region Zealand, covering 2.4 million inhabitants. Exclusion criteria were present use of real-time CGM (n = 7), severe mental or psychiatric barriers (n = 4), diabetic nephropathy (n = 3), or severe concurrent comorbidity (one with severe psoriasis and two with previous gastric bypass surgery). If a woman had more than one pregnancy in the study period (n = 4), the woman was only offered inclusion at first referral. Among eligible patients, a total of 123 (79%) women with type 1 diabetes and 31 (67%) women with type 2 diabetes were accepted to take part in the study, of whom 79 (51%) women were randomized to intermittent use of real-time CGM in pregnancy in addition to routine pregnancy care (see below). The major reason for rejecting participation was the possibility of real-time CGM allocation.
Figure 1.
Progression of women through the trial. (A high-quality color representation of this figure is available in the online issue.)
The research protocol was approved by the Danish National Committee on Biomedical Research Ethics and the Danish Data Protection Agency. All eligible women were invited at first pregnancy visit. They were offered time to think it through, often with their partner, before accepting participation. Most women gave written informed consent, were randomized, and had allocated intervention initiated later the same day and at the latest before 14 completed weeks. Many women had been informed about the running of the trial beforehand by their local diabetologists, who had all received written information. Information about medications and pregnancy complications was drawn from the hospital maternity records.
Management of diabetes in pregnancy
All women in both study arms followed the routine pregnancy care program for pregestational diabetes with antenatal visits at our clinic at 8, 12, 21, 27, and 33 gestational weeks. Self-monitored plasma glucose measurements were recommended seven times daily (before and 1.5 h after each main meal and at bedtime), and diet and insulin doses were adjusted by the women themselves every third day and in collaboration with an experienced diabetologist every second week. Treatment goals for self-monitored plasma glucose values were 4.0–6.0 mmol/L preprandially, 4.0–8.0 mmol/L 1.5 h postprandially, and 6.0–8.0 mmol/L prebedtime. At single-standing preprandial self-monitored plasma glucose values >8.0 and >10.0 mmol/L, supplementary rapid-acting insulin was recommended (1–2 and 2–4 insulin units in the first half of pregnancy and thereafter 2–4 and 4–6 insulin units, respectively). At self-monitored plasma glucose values <4.0 mmol/L and/or mild hypoglycemia (see below), supplementary carbohydrate intake of ∼20 g was recommended. For HbA1c, the aim was <5.6% (38 mmol/mol) after 20 weeks (28). HbA1c was measured on a DCA 2000 analyzer by a latex immunoagglutination inhibition method (DCA 2000; Bayer, Mishawaka, IN). Blood pressure was measured as formerly described (25), and if >135/85 mmHg, treatment was initiated (29). Elevated urine albumin excretion was defined as albumin-to-creatinine ratio ≥30 mg/mmol in a random urine sample (25). Diabetic retinopathy was diagnosed with retinal photos at first pregnancy visit (30). Obstetrical ultrasound scanning was performed at all five routine visits and when indicated (7). At first pregnancy visit, all women had a dietitian appointment for individual dietary planning following national guidelines for diabetes diet. Carbohydrate counting was mainly used in women on insulin pumps, and specific recording of dietary data was not performed. Women with BMI <30 kg/m2 were advised to gain 10–15 kg in pregnancy, whereas women with BMI >30 kg/m2 were advised to stay weight-neutral in the first half of pregnancy and thereafter to limit weight gain to 5 kg. All women visited our clinic and/or their local diabetes clinic at ∼2-week intervals throughout pregnancy, at which time HbA1c, blood pressure, weight, and urine examination were registered.
Twenty-seven (22%) women with type 1 diabetes were on insulin pump therapy (mainly initiated before pregnancy) with rapid-acting insulin analogs. Most of these women used insulin pumps that could be connected to the prescribed real-time CGM system. The majority were using the bolus calculator, but they did not have the possibility of low glucose suspend. Women with type 1 diabetes on multiple daily injections were treated with rapid- and long-acting insulin analogs (n = 66), rapid-acting insulin analogs with intermediate NPH insulin (n = 19), human short-acting and intermediate NPH insulin (n = 9), or human short-acting insulin and long-acting insulin analog (n = 2). All but one of 31 women with type 2 diabetes received insulin therapy in pregnancy with insulin aspart mix (n = 14), intermediate NPH insulin combined with rapid-acting insulin analog or human short-acting insulin (n = 12), or solely intermediate NPH insulin (n = 3) or rapid-acting insulin analog (n = 1). Patients were recommended to administer rapid-acting insulin shortly before meals throughout pregnancy. During labor and delivery, intravenous glucose infusion was given, and if indicated, insulin was administered subcutaneously. Self-monitored plasma glucose measurements were performed hourly, aiming for plasma glucose levels of 4.0–7.0 mmol/L.
For study purpose, participants were asked to perform eight daily self-monitored plasma glucose measurements for 6 days, including measurements at 3 a.m., at study visits at 8, 12, 21, 27, and 33 weeks. Registration of self-monitored plasma glucose values for study purpose was handled as previously described (7,25). All women were offered free use of a blood glucose meter with corresponding test strips (Contour; Bayer, Wakimachi, Japan). Results on HbA1c and insulin doses were obtained at all five study visits and shortly before delivery at median 36 (range 29–39) weeks. Mild hypoglycemia was defined as events familiar to the patient as hypoglycemia and managed by the patient, whereas severe hypoglycemia was defined as self-reported events with symptoms of hypoglycemia requiring help from another person to actively administer oral carbohydrate or injection of glucose or glucagon in order to restore normal blood glucose level (7). Information on mild and severe hypoglycemic events was obtained in detailed questionnaires and structured interviews at all study visits and shortly after delivery, as previously described (7).
Other medications
During the study period, 30 women received antihypertensive treatment, mainly methyldopa (n = 27). Eight women were treated with antidepressive medicine—six of them with selective serotonin reuptake inhibitors. Thyroid dysfunction was treated in 32 women with levothyroxine (n = 29), thiamazole (n = 2), or propylthiouracil (n = 1), resulting in normal thyroid function in all women.
Randomization
A computer-generated randomization program was used and treatment allocation was properly concealed using automated telephone allocation service (Paravox) provided by an independent organization. Participants were stratified according to type of diabetes. Trained research personnel (A.L.S. or project nurse) provided the women with their arm allocation.
Intervention
Participants in the intervention arm were offered intermittent real-time CGM (Guardian Real-time Continuous Glucose Monitoring System with the Sof-Sensor; Medtronic Minimed, Northridge, CA) for 6 days at the first pregnancy visit at 8 weeks and at 12, 21, 27 and 33 weeks, on top of routine pregnancy care. The women were encouraged to use real-time CGM continuously, especially in cases of hypoglycemia unawareness (7,24). Real-time CGM was free of charge regardless of number of monitoring periods. A small number of women were only willing to use real-time CGM for 3 days per monitoring period, which was accepted. Blinded real-time CGM was not performed in the control arm.
At the first pregnancy visit, women were allocated to intervention in which they were educated in sensor insertion and the maintenance of the system on a one-to-one basis for 1 to 2 h by a trained diabetes caregiver (A.L.S. or one of two project nurses) (25). They were instructed to continue performing self-monitored plasma glucose measurements as recommended and to verify the accuracy of real-time CGM glucose values with self-monitored plasma glucose measurement before making management decisions. At real-time CGM alarms with subsequent plasma glucose <4.0 mmol/L, the women were advised to supplement carbohydrate intake. Real-time CGM alarms for hyperglycemia were tackled on an individual basis, including physical exercise like walking or supplementary rapid-acting insulin. All women received a scheduled phone call the day after initial sensor insertion. Patients were encouraged to contact the clinic at any time and were offered visits on weekdays other than those planned if convenient. The majority of the women preferred to have the sensor inserted in the abdominal skin, but in late pregnancy, during labor or caesarean section, some patients chose to insert the sensor in the upper arm. At enrollment, all women were advised to set the hypoglycemic alarm (mainly at 3.5 mmol/L) (25). In order to limit excessive alarms, it was in general recommended that patients deactivate the alarm for hyperglycemia, but the alarm was sometimes set at the patient’s request (mainly at 10.0 mmol/L) (25). Throughout pregnancy, alarm limits were flexible, and patients were supported in individual alarm settings. Shortly after each monitoring period, downloaded real-time CGM data were printed out, with hard copies given to both the participants and health professionals. Each real-time CGM reading was discussed with a diabetes caregiver (mainly A.L.S., H.U.A., and E.R.M.) using locally developed guidelines on how to interpret real-time CGM readings in pregnancy (Supplementary Fig. 1). The primary focus was glycemic trends during nighttime with emphasis on the prevention of hypoglycemia. Thereafter, hypoglycemia and pre- and postprandial glucose values during the daytime were evaluated, aiming for glucose values between 4.0 and 8.0 mmol/L. Therapeutic adjustments in diet, exercise, and insulin doses were primarily based on self-monitored plasma glucose values, in combination with real-time CGM data. The prescribed device was the only real-time CGM system available at the Danish market during study preparation.
Pregnancy outcome parameters
The prevalence of large-for-gestational-age infants [i.e., infant birth weight ≥90th centile adjusted for sex and gestational age (31)] was predefined as the primary outcome of this study. To reflect neonatal morbidity, the prevalence of preterm delivery (<37 weeks of gestation) and/or severe neonatal hypoglycemia (2-h plasma glucose <2.5 mmol/L treated with intravenous glucose infusion) was selected as the secondary combined end point prior to study onset. Other pregnancy outcomes were defined as follows: miscarriage (before 22 weeks), preeclampsia [blood pressure ≥140/90 and proteinuria (29)], birth weight SD score (z-score), neonatal hypoglycemia (2-h plasma glucose <2.5 mmol/L), and major congenital malformation (i.e., abnormality requiring surgery and/or resulting in permanent injury).
Sample size
Based on the assumption that the prevalence of large-for-gestational-age infants was 50% in our study population (7) and that the use of real-time CGM could reduce it to 20%, and a type 1 error of 5% and a type 2 error of 20%, the number of patients needed in each arm was 45. We intended to analyze predefined primary and secondary outcome parameters of women receiving intervention versus control subjects in the entire study population, as well as in the subpopulation of women with type 1 diabetes. To secure sufficient numbers, a 2-year inclusion period was planned. The study population of 154 women (of whom 123 had type 1 diabetes) was therefore judged to have sufficient power to detect a possible effect of real-time CGM on the prevalence of large-for-gestational-age infants in the entire study population, as well as in women with type 1 diabetes alone.
Statistical analysis
We compared the characteristics of the women using the Fisher exact test or χ2 test when appropriate for categorical variables and t test or Mann–Whitney test when appropriate for continuous variables. Continuous variables were given as median (range), and categorical variables were given as numbers (percentage). A two-sided P value <0.05 was considered statistically significant. Analyses were done on an intention-to-treat basis, counting 154 subjects at baseline, and thereafter with the exclusion of women with miscarriages (n = 5). We primarily analyzed data as planned by comparing women allocated to real-time CGM with control subjects in the entire study population and in women with type 1 diabetes alone. We also analyzed the prevalence of severe hypoglycemia and main outcome parameters in women using real-time CGM per protocol, and despite limited numbers, we analyzed glycemic control and pregnancy outcomes in women with type 2 diabetes and the prevalence of large-for-gestational-age infants in women with type 1 diabetes on insulin pump therapy. Six infants were excluded from calculations on neonatal hypoglycemia because of intravenous glucose infusion prior to routine 2-h plasma glucose measurements (n = 5) or perinatal death (n = 1) (Fig. 1, Table 3, and Supplementary Table 3). Glucose data in Table 2 and Supplementary Table 1 were based on 25,546 self-monitored plasma glucose values, and glucose fractions (%) have been calculated for each individual participant after sorting glucose values into intervals (≤3.9, 4.0–7.9, and ≥8.0 mmol/L). Statistical analyses were performed using STATA 11.1 SE software (STATA Corp., College Station, TX).
Table 3.
Maternal and perinatal outcomes in 154 included women with pregestational diabetes
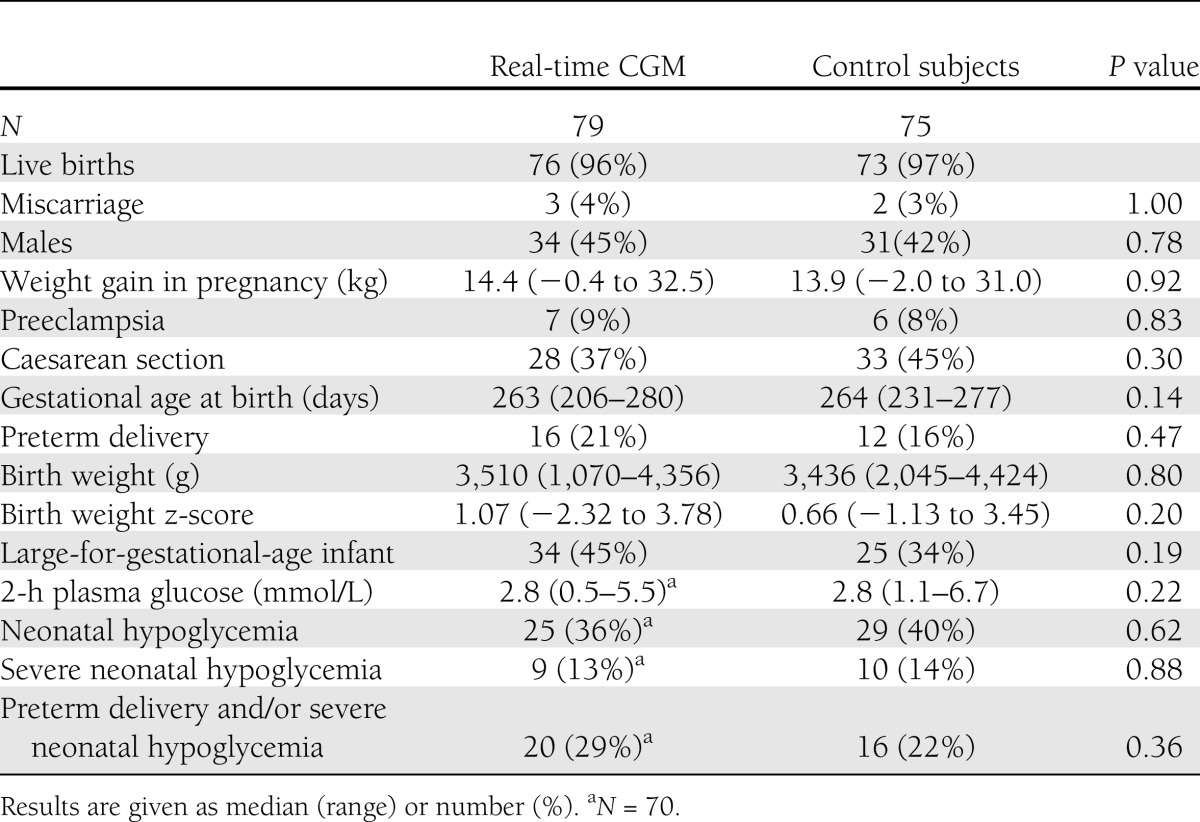
Table 2.
Glycemic control during pregnancy in 149 women with live births
RESULTS
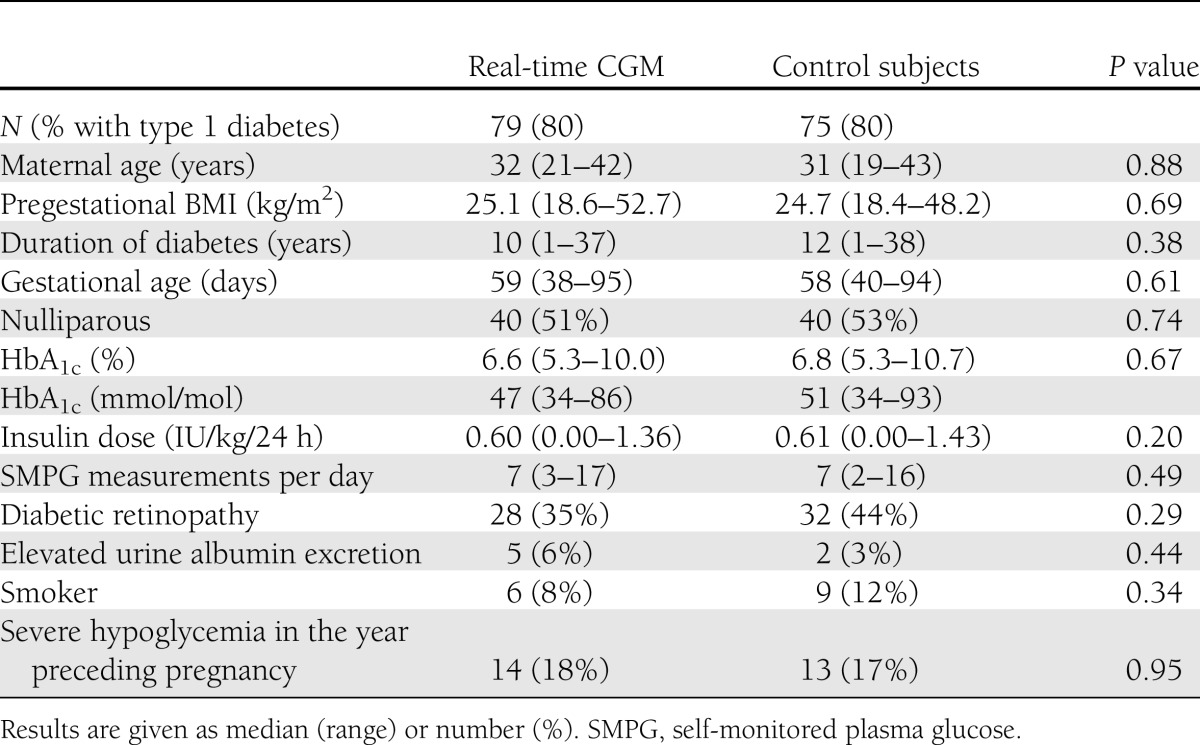
During the period of 15 February 2009 to 15 February 2011, 123 women with type 1 diabetes and 31 with type 2 diabetes, with a median age of 32 (range 19–43) years, baseline HbA1c of 6.7% (5.3–10.7) (50 [34–93] mmol/mol), pregestational BMI of 25.0 (18.4–52.7) kg/m2, and diabetes duration of 11 (1–38) years, were included in the trial. Baseline data were comparable between women randomized to real-time CGM (n = 79) and control subjects (n = 75), both in the entire population (Table 1), and when stratified according to type of diabetes (data not shown). Eligible women unwilling to participate (n = 47) were similar to included women with respect to type of diabetes, age, pregestational BMI, baseline HbA1c, and parity, but had slightly shorter duration of diabetes (data not shown). The included women were seen in our clinic at all five study visits at 8 (5–13), 12 (11–14), 21 (19–25), 27 (21–29), and 33 (32–35) weeks. Self-monitored plasma glucose measurements were documented seven times daily in relation to all study visits in both arms (Table 1 for baseline data). Real-time CGM was initiated at first pregnancy visit in all 79 women allocated to intervention and was generally well tolerated without severe side effects. Forty-nine (64%) women with either type 1 or type 2 diabetes used real-time CGM per protocol (i.e., at 8, 12, 21, 27, and 33 weeks or more). Near-continuous real-time CGM use (at least 60% of the time) was only chosen by five (7%) women.
Table 1.
Baseline clinical data in 154 included pregnant women with pregestational diabetes
Glycemic control
HbA1c, self-monitored plasma glucose values, and plasma glucose profiles of biochemical hypo- or hyperglycemia were similar throughout pregnancy in women using real-time CGM versus control subjects for the entire study population (Table 2), and for the subpopulation of women with type 1 diabetes alone (Supplementary Table 1). The women experienced 4 (0–14) mild hypoglycemic events per week, with no difference between the arms. The percentage of women experiencing severe hypoglycemia at least once after inclusion was also similar (16 vs. 16%; P = 0.91). Of 38 (63%) women with type 1 diabetes using real-time CGM per protocol, 4 (11%) experienced severe hypoglycemia during the intervention period compared with 11 (19%) among 59 control subjects (P = 0.28). Nineteen (16%) women with type 1 diabetes experienced 59 severe hypoglycemic events, and 5 (17%) women with type 2 diabetes experienced 15 severe hypoglycemic events during study participation, with no difference between the arms (data not shown). Five women in the intervention arm experienced in total seven episodes of severe hypoglycemia despite ongoing and technically successful real-time CGM. Insulin dosages were similar between women in the intervention versus control arm, both as absolute values and as percentages of pregestational dose at all study visits (Table 1 for baseline data and Supplementary Table 2 for the entire pregnancy according to type diabetes).
Pregnancy complications and outcome
The 154 pregnancies resulted in five miscarriages and 149 live births. The predefined primary outcome, large-for-gestational-age infants, occurred to a similar extent in both arms (45 vs. 34%; P = 0.19), and no difference was found between the predefined secondary outcome: the prevalence of preterm delivery and/or severe neonatal hypoglycemia (29 vs. 22%; P = 0.36) (Table 3). Other maternal and perinatal parameters were also comparable between the arms. Even in the subpopulation of women with type 1 diabetes, similar results were found regardless of real-time CGM allocation (Supplementary Table 3).
For 49 (64%) women with either type 1 or type 2 diabetes using real-time CGM per protocol, the figures for large-for-gestational-age infants and preterm delivery and/or severe neonatal hypoglycemia compared with control subjects (n = 73) were 49 versus 34% (P = 0.10) and 24 versus 22% (P = 0.75), respectively. For women with type 1 diabetes on insulin pumps (n = 26), the prevalence of large-for-gestational-age infants was 55 versus 20% (P = 0.10) between the real-time CGM and control arms.
Major congenital malformations occurred in two infants of women with type 1 diabetes (one ventricular septal defect combined with coarctation of the aorta and one congenitally corrected transposition of the great arteries). One case of perinatal death occurred shortly after delivery in an infant of a woman with type 2 diabetes due to severe shoulder dystocia.
CONCLUSIONS
In this randomized trial, intermittent use of real-time CGM in pregnancy, in addition to self-monitored plasma glucose measurements seven times daily, did not improve glycemic control or pregnancy outcome in women with pregestational diabetes. These findings are disappointing in the light of real-time CGM efficiency in nonpregnant patients (16). However, participants in trials demonstrating an effect on HbA1c in both nonpregnant (8,11,12,17–19) and pregnant (27) populations had higher baseline HbA1c compared our cohort of women. The negative findings of this trial are in line with a study on well-controlled nonpregnant patients, in whom HbA1c was not further improved by real-time CGM (9). Recent data indicate the importance of frequent real-time CGM use (16). Our study was designed with intermittent real-time CGM use in relation to five routine antenatal visits at our clinic, but the women were encouraged to use real-time CGM continuously and offered much support and flexibility in scheduling to attain frequent real-time CGM use. Still, only very few women were willing to use real-time CGM continuously. Our aim was to improve our routine pregnancy care and therefore to intervene on an unselected cohort of women. The inclusion rate of 77% is comparable to that of Murphy et al. (27), whereas studies on nonpregnant patients with improved HbA1c due to real-time CGM mainly cover selected and highly motivated patients, willing to use real-time CGM continuously (10,12,17–19).
In this trial, sufficient numbers of women were included, and proper allocation with equal distribution of women with type 1 diabetes was secured with computer-generated randomization provided by an independent organization. Study arms were comparable at baseline, both in the entire cohort and when analyzed according to type diabetes. We believe that our study design was robust, despite it not being blinded, and broadly comparable to that of Murphy et al. (27), suggesting a beneficial effect of intermittent CGM in pregnancy.
Real-time CGM had been used for several years in our clinic, and we have previously published case reports suggesting beneficial effects of real-time CGM in pregnancy (24,26). Therefore, we had expected high compliance to real-time CGM in this trial. However, the relatively low number of per-protocol users has also been observed in the nonpregnant population (17,18), and the average use of CGM was comparable to the former study on pregnant women (27). We have previously published the results of a questionnaire on patient satisfaction with real-time CGM in early pregnancy based on the present population of women (25). This survey demonstrated that real-time CGM inaccuracy compared with self-monitored plasma glucose values, technical challenges, and skin irritation limit compliance (25), which is in line with previous studies of nonpregnant (12,32–35) and pregnant (27) patients. During the first period of real-time CGM, our women experienced up to 12 alarms per 24 h, of which one-third disturbed their sleep (25). Future development of the real-time CGM systems, leading to improved accuracy in the hypoglycemic range and fewer alarms, may improve compliance.
The main barrier in obtaining strict maternal glycemic control is the high risk of severe hypoglycemia, occurring in up to 45% of pregnant women with type 1 diabetes (7). A reduction of severe hypoglycemic events due to real-time CGM has been suggested in a study of nonpregnant patients (11) and in our case study of pregnant women with hypoglycemia unawareness (26). Therefore, elimination of nocturnal hypoglycemic episodes was emphasized in our guidelines on the interpretation of real-time CGM readings in pregnancy. This may have lead to slightly higher nocturnal glucose levels and could at least partly explain the tendency toward higher birth weight z-score in the intervention arm. The self-monitored plasma glucose levels, which were mainly performed during daytime, and the number of women experiencing at least one severe hypoglycemic event after inclusion were similar in spite of real-time CGM allocation. However, among women with type 1 diabetes using real-time CGM per protocol, there was a tendency toward fewer women with severe hypoglycemia compared with women in the control arm, but numbers were low. Surprisingly, we could document severe hypoglycemic events during ongoing real-time CGM without hypoglycemic alarms to warn the patient. This again calls for improved real-time CGM accuracy in the hypoglycemic range and future studies on continuous real-time CGM in pregnant women at high risk of severe hypoglycemia. Whether a sum of imbalances including tendencies toward higher pregestational BMI, fewer smokers, and higher weight gain in pregnancy in women in the intervention arm could have affected birth weight z-score in this trial remains speculative.
The majority of the women with type 1 diabetes in this trial were on basal bolus therapy with multiple daily injections, but patients on insulin pumps may benefit more from real-time CGM (18). However, not even in the subset of women treated with insulin pumps in this study was a tendency toward a beneficial effect on large-for-gestational-age infants seen.
In conclusion, our data do not support intermittent real-time CGM on a routine basis in an unselected pregnant population of women with pregestational diabetes, already performing self-monitored plasma glucose measurements seven times per day. Careful introduction to real-time CGM and interpretation of the real-time CGM readings were offered, but otherwise, the two arms were treated similarly regarding diet, exercise, and insulin therapy. The negative finding of this trial may lead to intensified focus on dietary advice, including carbohydrate counting, stable day-to-day carbohydrate intake, especially in late gestation (36), and weight gain within the Institute of Medicine recommendations (37). This trial does not rule out that real-time CGM may be efficient in highly selected pregnant women, and whether introducing continuous real-time CGM prior to pregnancy can reduce the risk of severe hypoglycemia and enhance pregnancy outcome is yet to be examined.
Acknowledgments
A.L.S. received financial support from the European Foundation for the Study of Diabetes and LifeScan, Rigshospitalet’s Research Foundation, the Capital Region of Denmark, the Medical Faculty Foundation of Copenhagen University, Aase and Ejnar Danielsen Foundation, and Master Joiner Sophus Jacobsen and his wife Astrid Jacobsen’s Foundation. H.U.A. holds stock in Novo Nordisk. E.R.M. received financial support from the Novo Nordisk Foundation. Medtronic supplied the study with real-time CGM monitors and links, and glucose sensors were offered at a reduced price, but had no influence on study design, handling of data, or writing of the manuscript. No other potential conflicts of interest relevant to this article were reported.
A.L.S. was responsible for participant recruitment, introducing the women to real-time CGM, analyzing real-time CGM readings, collecting data, performing statistical analyses, writing the manuscript, and contributing to study design and manuscript revision. L.R. contributed to study design and manuscript revision. H.U.A. and E.R.M. analyzed real-time CGM readings and contributed to study design and manuscript revision. P.D. contributed to study design and manuscript revision. E.R.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 48th annual meeting of the European Association for the Study of Diabetes, Berlin, Germany, 1–5 October 2012 and at 44th Annual Meeting of the Diabetic Pregnancy Study Group (DPSG), Lille, France, 18–21 October 2012.
The authors thank David Dynnes Ørsted, Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark, for assistance with statistical analyses and project nurses Edna Stage and Charlotte Barfred, laboratory technician Karen Margrethe Larsen, nurse Birgitta Ellingsgaard, and medical secretary Susanne Graversen.
Footnotes
Clinical trial reg. no. NCT00994357, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-2360/-/DC1.
See accompanying commentary, p. 1818.
References
- 1.de Valk HW, Visser GH. Insulin during pregnancy, labour and delivery. Best Pract Res Clin Obstet Gynaecol 2011;25:65–76 [DOI] [PubMed] [Google Scholar]
- 2.Klemetti M, Nuutila M, Tikkanen M, Kari MA, Hiilesmaa V, Teramo K. Trends in maternal BMI, glycaemic control and perinatal outcome among type 1 diabetic pregnant women in 1989-2008. Diabetologia 2012;55:2327–2334 [DOI] [PubMed] [Google Scholar]
- 3.Mathiesen ER, Vaz JA. Insulin treatment in diabetic pregnancy. Diabetes Metab Res Rev 2008;24(Suppl. 2):S3–S20 [DOI] [PubMed] [Google Scholar]
- 4.Persson M, Pasupathy D, Hanson U, Norman M. Birth size distribution in 3,705 infants born to mothers with type 1 diabetes: a population-based study. Diabetes Care 2011;34:1145–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Persson M, Pasupathy D, Hanson U, Norman M. Disproportionate body composition and perinatal outcome in large-for-gestational-age infants to mothers with type 1 diabetes. BJOG 2012;119:565–572 [DOI] [PubMed] [Google Scholar]
- 6.Clausen TD, Mathiesen ER, Hansen T, et al. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care 2008;31:340–346 [DOI] [PubMed] [Google Scholar]
- 7.Nielsen LR, Pedersen-Bjergaard U, Thorsteinsson B, Johansen M, Damm P, Mathiesen ER. Hypoglycemia in pregnant women with type 1 diabetes: predictors and role of metabolic control. Diabetes Care 2008;31:9–14 [DOI] [PubMed] [Google Scholar]
- 8.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care 2010;33:17–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beck RW, Hirsch IB, Laffel L, et al. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care 2009;32:1378–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergenstal RM, Tamborlane WV, Ahmann A, et al. STAR 3 Study Group Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med 2010;363:311–320 [DOI] [PubMed] [Google Scholar]
- 11.Bode B, Beck RW, Xing D, et al. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group Sustained benefit of continuous glucose monitoring on A1C, glucose profiles, and hypoglycemia in adults with type 1 diabetes. Diabetes Care 2009;32:2047–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deiss D, Bolinder J, Riveline JP, et al. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care 2006;29:2730–2732 [DOI] [PubMed] [Google Scholar]
- 13.Hoeks LB, Greven WL, de Valk HW. Real-time continuous glucose monitoring system for treatment of diabetes: a systematic review. Diabet Med 2011;28:386–394 [DOI] [PubMed] [Google Scholar]
- 14.Ludvigsson J, Hanas R. Continuous subcutaneous glucose monitoring improved metabolic control in pediatric patients with type 1 diabetes: a controlled crossover study. Pediatrics 2003;111:933–938 [DOI] [PubMed] [Google Scholar]
- 15.O’Connell MA, Donath S, O’Neal DN, et al. Glycaemic impact of patient-led use of sensor-guided pump therapy in type 1 diabetes: a randomised controlled trial. Diabetologia 2009;52:1250–1257 [DOI] [PubMed] [Google Scholar]
- 16.Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ 2011;343:d3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raccah D, Sulmont V, Reznik Y, et al. Incremental value of continuous glucose monitoring when starting pump therapy in patients with poorly controlled type 1 diabetes: the RealTrend study. Diabetes Care 2009;32:2245–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riveline JP, Schaepelynck P, Chaillous L, et al. EVADIAC Sensor Study Group Assessment of patient-led or physician-driven continuous glucose monitoring in patients with poorly controlled type 1 diabetes using basal-bolus insulin regimens: a 1-year multicenter study. Diabetes Care 2012;35:965–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamborlane WV, Beck RW, Bode BW, et al. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008;359:1464–1476 [DOI] [PubMed] [Google Scholar]
- 20.Combs CA. Continuous glucose monitoring and insulin pump therapy for diabetes in pregnancy. J Matern Fetal Neonatal Med 2012;25:2025–2027 [DOI] [PubMed] [Google Scholar]
- 21.Kerssen A, de Valk HW, Visser GH. Forty-eight-hour first-trimester glucose profiles in women with type 1 diabetes mellitus: a report of three cases of congenital malformation. Prenat Diagn 2006;26:123–127 [DOI] [PubMed] [Google Scholar]
- 22.Murphy HR, Elleri D, Allen JM, et al. Closed-loop insulin delivery during pregnancy complicated by type 1 diabetes. Diabetes Care 2011;34:406–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrovski G, Dimitrovski C, Bogoev M, Milenkovic T, Ahmeti I, Bitovska I. Is there a difference in pregnancy and glycemic outcome in patients with type 1 diabetes on insulin pump with constant or intermittent glucose monitoring? A pilot study. Diabetes Technol Ther 2011;13:1109–1113 [DOI] [PubMed] [Google Scholar]
- 24.Secher AL, Schmidt S, Nørgaard K, Mathiesen ER. Continuous glucose monitoring-enabled insulin-pump therapy in diabetic pregnancy. Acta Obstet Gynecol Scand 2010;89:1233–1237 [DOI] [PubMed] [Google Scholar]
- 25.Secher AL, Madsen AB, Ringholm L, et al. Patient satisfaction and barriers to initiating real-time continuous glucose monitoring in early pregnancy in women with diabetes. Diabet Med 2012;29:272–277 [DOI] [PubMed] [Google Scholar]
- 26.Worm D, Nielsen LR, Mathiesen ER, Nørgaard K. Continuous glucose monitoring system with an alarm: a tool to reduce hypoglycemic episodes in pregnancy with diabetes. Diabetes Care 2006;29:2759–2760 [DOI] [PubMed] [Google Scholar]
- 27.Murphy HR, Rayman G, Lewis K, et al. Effectiveness of continuous glucose monitoring in pregnant women with diabetes: randomised clinical trial. BMJ 2008;337:a1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nielsen LR, Ekbom P, Damm P, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care 2004;27:1200–1201 [DOI] [PubMed] [Google Scholar]
- 29.Nielsen LR, Damm P, Mathiesen ER. Improved pregnancy outcome in type 1 diabetic women with microalbuminuria or diabetic nephropathy: effect of intensified antihypertensive therapy? Diabetes Care 2009;32:38–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vestgaard M, Ringholm L, Laugesen CS, Rasmussen KL, Damm P, Mathiesen ER. Pregnancy-induced sight-threatening diabetic retinopathy in women with Type 1 diabetes. Diabet Med 2010;27:431–435 [DOI] [PubMed] [Google Scholar]
- 31.Marsál K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr 1996;85:843–848 [DOI] [PubMed] [Google Scholar]
- 32.Beck RW, Buckingham B, Miller K, et al. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group Factors predictive of use and of benefit from continuous glucose monitoring in type 1 diabetes. Diabetes Care 2009;32:1947–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooke D, Hurel SJ, Casbard A, et al. Randomized controlled trial to assess the impact of continuous glucose monitoring on HbA(1c) in insulin-treated diabetes (MITRE Study). Diabet Med 2009;26:540–547 [DOI] [PubMed] [Google Scholar]
- 34.Ramchandani N, Arya S, Ten S, Bhandari S. Real-life utilization of real-time continuous glucose monitoring: the complete picture. J Diabetes Sci Tech 2011;5:860–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ritholz MD, Atakov-Castillo A, Beste M, et al. Psychosocial factors associated with use of continuous glucose monitoring. Diabet Med 2010;27:1060–1065 [DOI] [PubMed] [Google Scholar]
- 36.Murphy HR, Elleri D, Allen JM, et al. Pathophysiology of postprandial hyperglycaemia in women with type 1 diabetes during pregnancy. Diabetologia 2012;55:282–293 [DOI] [PubMed] [Google Scholar]
- 37.Institute of Medicine of the National Academies. Weight Gain During Pregnancy: Reexamining the Guidelines [Internet], 2009. Available from http://www.iom.edu/Reports/2009/Weight-Gain-During-Pregnancy-Reexamining-the-Guidelines.aspx Accessed 7 November 2012 [PubMed]