Inhibition of tumor necrosis factor (TNF)-α is an effective treatment for Crohn disease. It is recommended as a second-line therapy in steroid-dependent or -refractory disease, although first-line treatment may improve chances of intestinal mucosal healing (1). In the context of diabetes, TNF-α has been implicated in the development of insulin resistance during obesity (2) and in autoimmune destruction of pancreatic β-cells in an animal model of type 1 diabetes (3), and a recent study demonstrated some preservation of insulin secretion in children with new-onset type 1 diabetes upon TNF-α inhibition (4).
Here, we report a case of a 29-year-old Caucasian man who was diagnosed with type 1 diabetes at the age of 27 years on the basis of rapid onset of hyperglycemia, a lean BMI of 22.7 kg/m2, signs of β-cell autoimmunity (autoantibodies to GAD 476 IU/mL [normal value <10] and islet-cell autoantibodies 125 units/mL [normal value <1.5]), and a negative family history of diabetes. Upon treatment with insulin glargine and insulin lispro, A1C levels decreased from 9.4% (79 mmol/mol) to 6.1% (43 mmol/mol) within 6 months. In parallel to the diagnosis of type 1 diabetes, the patient reported intermittent feelings of abdominal fullness and subsequent diarrhea. Fecal calprotectin levels were found to be markedly elevated: up to 1,280.6 μg/g (normal value <50 μg/g stool), and Crohn disease was histologically confirmed 9 months after the diagnosis of type 1 diabetes.
To prevent worsening of diabetes upon steroid therapy and because of the putative role of TNF-α in β-cell demise, we decided to treat the patient with the TNF-α inhibitor infliximab. Infliximab (5 mg/kg body wt) was administered intravenously at weeks 0, 2, and 6 and every 8 weeks thereafter (1). After the first dose of infliximab, 9 months after diagnosing type 1 diabetes, remission of Crohn disease was achieved as reflected by normalization of bowel movements and by fecal calprotectin levels that were below the normal range (<50 μg/g stool) after the second dose of infliximab. High-sensitivity C-reactive protein levels decreased from 1.3 mg/L (normal value <10) to <0.3 mg/L after the first dose of infliximab was administered.
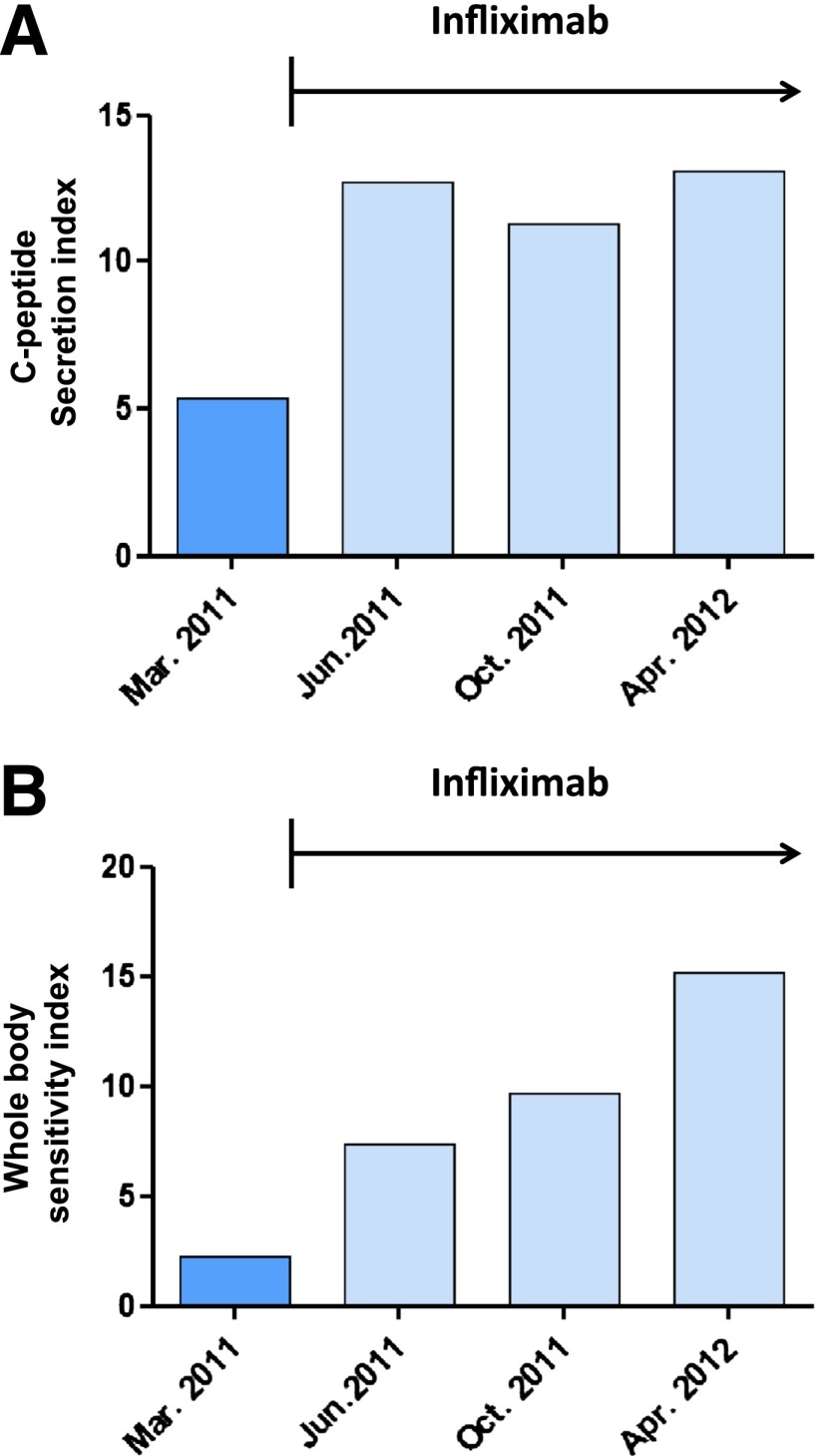
Remission of Crohn disease was paralleled by an immediate and sustained 2.4-fold increase in insulin secretion (Fig. 1A) and a progressive up to 6.9-fold decrease in insulin resistance (Fig. 1B) as derived from measurements of glucose and C-peptide assessed during mixed-meal tests that were performed right before and after the initiation of infliximab therapy every 3–4 months over a 1-year period. A1C remained ∼6.0% (42 mmol/mol), while insulin lispro requirements per 10 g carbohydrate dropped from 0.8 to 0.5 units after initiation of infliximab treatment.
Figure 1.
Response to infliximab therapy in a patient with Crohn disease and type 1 diabetes. A: C-peptide secretion index during mixed-meal test before and after initiation of infliximab {C-peptide secretion index = [(plasma C-peptide at 30 min of mixed-meal test) – (fasting plasma C-peptide)]/fasting plasma glucose}. B: Whole-body sensitivity index during mixed-meal test before and after start of infliximab therapy [whole-body sensitivity index = 10,000/√(fasting plasma glucose × fasting plasma C-peptide × mean mixed-meal test plasma glucose × mean mixed-meal test plasma C-peptide)]. Apr., April; Mar., March; Jun., June; Oct., October.
Several mechanisms may explain the observed effects of TNF-α antagonism on glucose metabolism. Since the treatment with infliximab was initiated 9 month after the diagnosis of diabetes, at a time when the patient had a stable requirement of insulin and A1C was ∼6%, a reversal due to “honeymoon” or glucotoxicity is unlikely. Thus, it is conceivable that insulin secretion may have improved as a direct result of the immunosuppressive effects on insulitis and enhanced insulin sensitivity may have alleviated the burden on the β-cells. Of note, it was not expected to see changes in insulin sensitivity in a patient with type 1 diabetes. However, in the case depicted herein, increased leakiness of the mucosal barrier for bacterial products or systemic inflammation due to Crohn disease may have promoted insulin resistance (5). Independently of the underlying mechanisms, we propose that TNF-α inhibitors be considered a first-line treatment in patients with both type 1 diabetes and Crohn disease.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
K.T. performed clinical tests, collected data, analyzed and interpreted data, and wrote the manuscript. P.H. collected data, performed clinical examinations, and edited the manuscript. C.B. collected data, performed clinical examinations, and edited the manuscript. M.Y.D. planned clinical tests, interpreted data, and wrote the manuscript. M.Y.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Patrizia Zala for excellent technical assistance.
References
- 1.Colombel JF, Sandborn WJ, Reinisch W, et al. SONIC Study Group Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010;362:1383–1395 [DOI] [PubMed] [Google Scholar]
- 2.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science 1993;259:87–91 [DOI] [PubMed] [Google Scholar]
- 3.Yang XD, Tisch R, Singer SM, et al. Effect of tumor necrosis factor alpha on insulin-dependent diabetes mellitus in NOD mice. I. The early development of autoimmunity and the diabetogenic process. J Exp Med 1994;180:995–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mastrandrea L, Yu J, Behrens T, et al. Etanercept treatment in children with new-onset type 1 diabetes: pilot randomized, placebo-controlled, double-blind study. Diabetes Care 2009;32:1244–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011;11:98–107 [DOI] [PubMed] [Google Scholar]