Abstract
OBJECTIVE
To study the effects of high-protein versus high-carbohydrate diets on various metabolic end points (glucoregulation, oxidative stress [dichlorofluorescein], lipid peroxidation [malondialdehyde], proinflammatory cytokines [tumor necrosis factor-α and interleukin-6], adipokines, and resting energy expenditure [REE]) with high protein–low carbohydrate (HP) and high carbohydrate–low protein (HC) diets at baseline and after 6 months of dietary intervention.
RESEARCH DESIGN AND METHODS
We recruited obese, premenopausal women aged 20–50 years with no diabetes or prediabetes who were randomized to HC (55% carbohydrates, 30% fat, and 15% protein) or HP (40% carbohydrates, 30% fat, and 30% protein) diets for 6 months. The diets were provided in prepackaged food, which provided 500 kcal restrictions per day. The above metabolic end points were measured with HP and HC diet at baseline and after 6 months of dietary intervention.
RESULTS
After 6 months of the HP versus HC diet (12 in each group), the following changes were significantly different by Wilcoxon rank sum test for the following parameters: dichlorofluorescein (−0.8 vs. −0.3 µmol/L, P < 0.0001), malondialdehyde (−0.4 vs. −0.2 μmol/L, P = 0.0004), C-reactive protein (−2.1 vs. −0.8 mg/L, P = 0.0003), E-selectin (−8.6 vs. −3.7 ng/mL, P = 0.0007), adiponectin (1,284 vs. 504 ng/mL, P = 0.0011), tumor necrosis factor-α (−1.8 vs. −0.9 pg/mL, P < 0.0001), IL-6 (−1.3 vs. −0.4 pg/mL, P < 0.0001), free fatty acid (−0.12 vs. 0.16 mmol/L, P = 0.0002), REE (259 vs. 26 kcal, P < 0.0001), insulin sensitivity (4 vs. 0.9, P < 0.0001), and β-cell function (7.4 vs. 2.1, P < 0.0001).
CONCLUSIONS
To our knowledge, this is the first report on the significant advantages of a 6-month hypocaloric HP diet versus hypocaloric HC diet on markers of β-cell function, oxidative stress, lipid peroxidation, proinflammatory cytokines, and adipokines in normal, obese females without diabetes.
Obesity has reached epidemic proportions in the U.S., where more than one-third of U.S. adults (35.7%) are obese (1,2). Obesity is one of the highest risk factors for type 2 diabetes, heart disease, hypertension, and other metabolic diseases in women (3). Many diets have been recommended for weight loss, but there has been controversy regarding whether a low-carbohydrate or high-protein diet is more efficacious (4–7).
The use of high-protein diets for weight loss is based on a number of valid observations. Most studies suggest that high protein intake has the potential to suppress hunger and induce satiety (6,8,9). Different reasons have been postulated for this effect. Low glycemic index (GI) of proteins has been proposed as one such factor. A significant negative relationship is seen between protein content of foods and GI (10). Hyperglycemia after high-GI meals is followed 4–6 h later by a tendency for hypoglycemia with an earlier return of a sensation of hunger (11). Proteins also increase the thermic effect of feeding (12), mostly by increasing protein synthesis. Even though weight loss may not be different between isocaloric high-protein and high-carbohydrate diets, the diet composition can alter a number of other variables. Lipids are considered a primary risk factor for cardiovascular disease. The dietary composition can affect the plasma lipid profile and its metabolism. Some studies have evaluated the relationship of macronutrient composition and lipids (13,14) with greater decrease in triglycerides on a low-carbohydrate diet but no change in other lipid parameters (13). It has been shown that protein intake by itself induces insulin release. Protein is a much less potent secretagogue for insulin than is glucose in normal individuals. If they are given together, the insulin response has been found to be synergistic, and the effect on insulin secretion is only additive (15).
A hypocaloric high-protein diet may also help maintain lean body mass and positive nitrogen balance in comparison with a hypocaloric high-carbohydrate diet (16).
There are few studies comparing moderately high-carbohydrate diets with a variety of diets with different percentages of macronutrients in which cardiovascular risk factors have been evaluated for at least 6 months. Particularly lacking are studies of markers of oxidative stress, proinflammatory cytokines, and activation of the immune system between high-carbohydrate and high-protein diets.
Formulation of a diet with adequate lipid for improved taste and thus better adherence should follow the recommended 30% fat (consisting mostly of unsaturated fat) and adequate fiber recommended by the American Diabetes Association and Institute of Medicine (17,18). Some studies have suggested that calorie restriction is the primary factor for successful weight loss rather than macronutrients per se (6,19,20). Studies by Dandona and coworkers have demonstrated that hyperglycemia during glucose challenge (21), as well as elevation of free fatty acids (FFAs) by triglyceride infusion (Liposyn; Abbott Laboratories, Chicago, IL) (22) lead to activation of leukocytes and reactive oxygen species. In addition, dietary restriction with weight loss can modulate these parameters (23). We have also shown that hyperglycemia in vivo (24) and in vitro (25) activates T cells with de novo expression of growth factor receptors, reactive oxygen species, and inflammatory markers. A similar effect is also observed with saturated FFAs (26). Other studies suggest that obesity, type 2 diabetes, and hyperlipidemia are associated with proinflammatory states (27,28).
In our study on macronutrients, we investigated the effects of moderately high-protein diet versus moderately high-carbohydrate diet for 6 months in premenopausal women without diabetes with restriction of 500 kcal intake/day based on resting energy expenditure (REE). We hypothesized that a daily 500-kcal reduction in diet will result in similar amount of weight loss in both groups. We further hypothesized that high-protein diet compared with high-carbohydrate diet might provide greater advantage for various metabolic parameters such as β-cell function, cardiovascular risk factors, oxidative stress, and lipid peroxidation and, therefore, would be a more suitable diet for obese, normal females.
RESEARCH DESIGN AND METHODS
We randomized 32 premenopausal women age 20–50 years with a BMI >30 to <55 kg/m2. Inclusion criteria consisted of age, BMI, fasting glucose <110 mg/dL, and 2-h glucose level <170 mg/dL. Exclusion criteria consisted of proteinuria or elevated serum creatinine (>1.5 mg/dL), surgical or premature menopause, history of liver disease, abnormal liver function tests, diabetes, thyroid disease with abnormal thyrotropin, weight >350 lbs, triglycerides >400 mg/dL or LDL cholesterol >160 mg/dL, systolic blood pressure >145 mmHg or diastolic blood pressure >100 mmHg, use of medications known to affect lipid or glucose metabolism (niacin, steroids, and statins), pregnancy or the desire to become pregnant in the next 6 months, weight loss of >5% of body weight in the last 6 months, or history of cancer undergoing active treatment. After subjects met the above criteria, they were asked to keep a food diary for a week. Nonadherent subjects were excluded from the study.
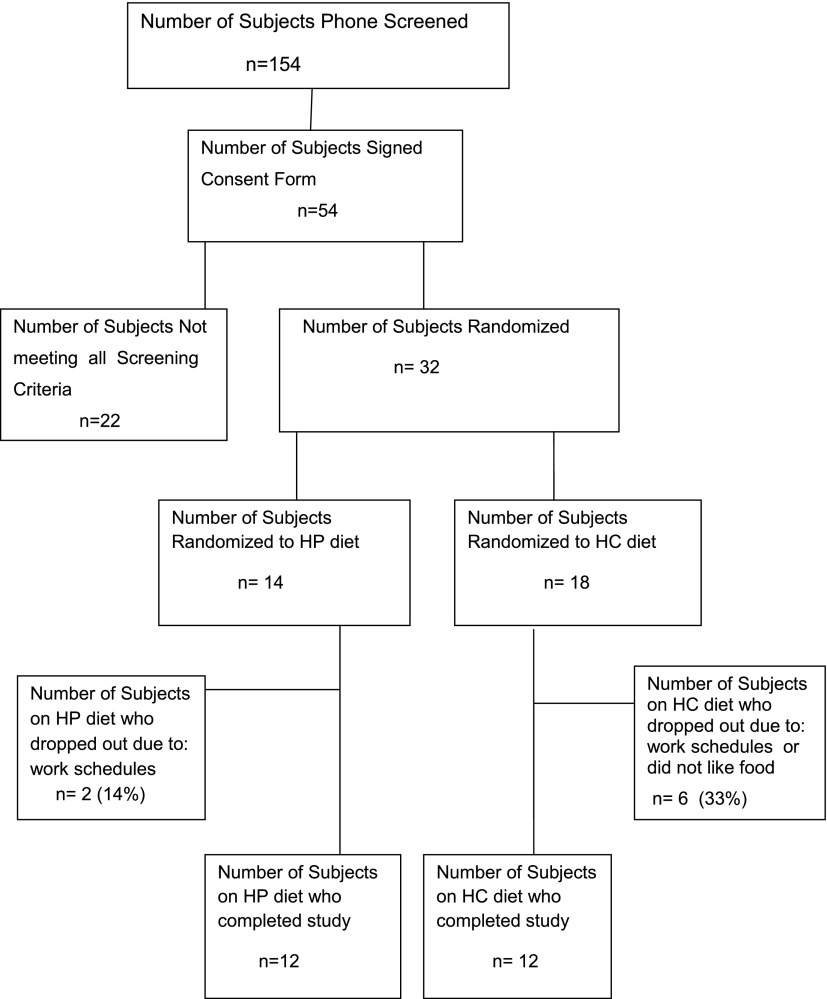
Subjects were screened by phone (n = 154). Fifty-four met the phone-screening criteria, were interested in participating, and signed the consent form. Thirty-two met all of the inclusion criteria and were randomized to a high protein–low carbohydrate (HP) diet (14 subjects) versus high carbohydrate–low protein (HC) diet (18 subjects) for a period of 6 months. Twelve subjects in each group completed the 6-month study (Fig. 1).
Figure 1.
Flowchart of the patient recruitment for the study.
The study was a prospective randomized trial of an HP diet versus HC diet with 30% kcal from fat in both groups for a period of 6 months. The study was approved by the institutional review board of the University of Tennessee Health Science Center. All participants were seen in the Clinical Research Center (CRC) at the University of Tennessee Health Science Center for all their visits. After the patients signed the consent form, a history and physical examination, height and weight, and blood pressure and waist circumference measurements were obtained. At baseline, the study participants underwent an oral glucose tolerance test (OGTT) and mixed-meal tolerance test with glucose and insulin measured at baseline and at 30-min intervals for 2 h. These tests were repeated after 6 months of diet intervention. Dual-energy X-ray absorptiometry (DXA) scan, REE, chemistry profile, complete blood count, vitamin D, and lipid profile were obtained. Urine collections for 24 h were done for creatinine clearance, microalbumin, and urinary urea nitrogen (UUN). After meeting screening criteria, subjects were randomized using a permuted block randomization method generated by the biostatistician to HP and HC diets. All investigators except for the nutritionist were blinded to the diet assignment.
Weight loss caloric needs
Weight reduction caloric needs were established for each individual by subtracting 500 kcal/day from their calculated maintenance needs established by REE. Weight loss of 1–2 lbs weekly was targeted. On average, an 1,800 kcal/day diet for a 70-kg individual was used to achieve adequate weight loss. If a subject reached a plateau and did not lose weight for two consecutive weeks, calories were reduced by an additional 200 kcal or to a minimum of 1,200 kcal/day. Caloric adjustments were made by altering the amount of supplement bars and/or shakes. Since these were very similar to the nutrient composition of each diet, they could be easily taken out of the diet to reduce calories but maintain adequate nutrient composition.
Nutrient adequacy
Both the HP and HC diets met the recommended daily intake for vitamins and minerals for women aged 20–50 years. Each diet was assessed for nutrient adequacy using nutrition software (version 1.1; University of Minnesota Nutrition Data System for Research). The study diets provided more than the recommended amount of calcium (1,000 mg/day) for women 20–50 years of age (29) by providing an average of 1,725 mg for HC and 1,684 mg for HP diets. Both HP and HC diets were designed to minimize participant health risks. Dietary fat sources focused on monounsaturated and polyunsaturated fats, i.e., plant oils, semiliquid margarine, and nuts; dietary carbohydrate sources emphasized whole grains, fruits, vegetables, and legumes; and dietary protein sources included lean meats, fish, chicken, eggs, and nonfat dairy foods, i.e., fat-free milk and low-fat cheese, consistent with American Diabetes Association and Institute of Medicine guidelines (17,18). Most of the entrees for the HP diet were Cedarlane The Zone frozen entrees. They were purchased directly from the manufacturer. Additional entrees were purchased from the Whole Foods Market: Boca Chik’n Nuggets and Whole Foods–brand Yellow Fin Tuna Burgers, Alaskan Salmon Burgers, and Mahi Mahi Burgers, which were served with a Rudi’s 100% Whole Wheat Organic bun. Snacks in between meals included Slim Fast High Protein Shakes and Zone Perfect bars. All of the entrees for the HC diet are readily available at most grocery stores. These options included frozen entrees made by Lean Cuisine, Healthy Choice, Smart Ones, and Amy’s frozen burritos. Snacks in between meals include Slim Fast Optima Shakes and Slim Fast Meal Bars. Both diets included 1 cup of frozen vegetables at both lunch and dinner. Participants chose from broccoli, cauliflower, carrots, green beans, “California” blend, and Brussels sprouts. There were five breakfast choices that are adapted by the use of Canadian bacon, margarine, and almonds to adjust for macronutrients: eggs and toast, cold cereal (Cheerios or Special K Protein Plus) and 2% milk, oatmeal, yogurt, and cottage cheese. Each breakfast included canned peaches, canned mixed fruit, or applesauce.
Dispensation of diet assignment food
Participants were given all food needed to meet their dietary assignment for the duration of the study. Without a metabolic kitchen, fresh, prepackaged, and frozen foods were used. Frozen foods were stored in bulk at the CRC to enable easy dispensation to participants. All food was dispensed by the dietitian at the CRC on a weekly basis to each participant.
Diet adherence
Dietary adherence is critical in a feeding study of this nature, so detailed instructions were given to subjects during prestudy orientation and screening visits by the dietitian. After randomization, participants received an in-depth review of their dietary assignment and were given some choice in food selection in order to increase adherence. This meant that each participant had a more personalized meal plan to follow while still maintaining the macronutrient integrity of their dietary assignment. Discussions on issues like traveling and dining out were addressed. Participants had the option of eating one “free meal” each week that was not dispensed by the study. An individualized weekly food diary (which included the participant’s preferences) was printed and given to each subject with instructions for recording all food intake on a daily basis. Subjects were asked to turn in completed food diaries each week in order to pick up the next week’s food. At times, the dietitian adjusted the participant’s meal choices if issues arose regarding compliance. Studies have shown increased dietary compliance with frequent interaction, an individualized diet with food variety, and some form of food-recording system (30,31). The study dietitian assessed compliance by both subjective and objective parameters including weekly individual contact with patients that included a detailed review of their food diaries.
Body composition by DXA assessment
For measurement of lean mass (LM) and fat mass (FM) of the whole body, we performed DXA, using the Hologic Discovery QDR Bone Densitomiter (version 8.3). A DXA-certified nurse performed the DXA measurements using a standardized protocol. All scans at the end of the intervention were compared with baseline for positioning. For those individuals whose left arm did not fit within the region of scan acquisition, the right arm LM and FM were imputed for the left arm, and total body values were recalculated. The coefficient of variation (CV) in our research group is 0.012% for LM, 0.02% for FM, and 0.03% for total mass. All DXA scans were reviewed for quality assurance by one of the study coinvestigators (F.A.T.).
Laboratory procedures
Determination of plasma metabolic hormones, cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation.
Glucose and insulin areas under the curve for OGTT and MTT were measured and calculated for 0, 30, 60, 90, and 120 min. Proinflammatory cytokines (tumor necrosis factor-α and interleukin-6), markers of oxidative stress (dichlorofluorescein), lipid peroxidation (malondialdehyde), cardiovascular risk factors (blood pressure, BMI, C-reactive protein, triglycerides, and FFAs) were measured by previously established methods (25) at baseline and 6 months of diet intervention. E-selectin and adipokines (high–molecular weight adiponectin and leptin) were measured at baseline by ELISA methods (Millipore, ALPCO Diagnostics, and R&D Systems). The CVs of the assays were all <5%.
Complete metabolic profile, lipid profile, UUN, thyrotropin, and cortisol, microalbumin, and pregnancy tests (to exclude chemical and metabolic abnormalities) were determined by standard laboratory procedures. Protein and muscle mass catabolism were assessed with the use of 24-h UUN and creatinine clearance at baseline and at 6 months.
Calcium balance was assessed by 24-h urine calcium excretion in addition to serum Ca, 25 OH-vitamin D, and parathyroid hormone at baseline and after 6 months of dietary interventions. BMI and waist circumference were measured by standard methods.
Insulin sensitivity and β-cell function.
Insulin resistance was determined by homeostasis model assessment of insulin resistance (HOMA-IR) (32). Insulin sensitivity (ISI) was derived from plasma glucose and insulin measurements obtained during the OGTT by the Matsuda insulin index (33,34). β-Cell function was calculated as the index of insulin secretion factored by insulin resistance (ΔI0–120/ΔG0–120 × Matsuda index) during the OGTT (34).
REE
Each participant underwent indirect calorimetry to assess REE at the beginning and end of the study to determine the caloric intake for the diets. A Cardio Coach (Korr Medical Technologies) was used for the determination of the REE. After 10 min of absolute rest, respiratory exchanges were measured continuously for up to 15 min (35).
Statistical analysis
The primary outcomes were cardiovascular risk factors, markers of insulin sensitivity, β-cell function, oxidative stress, lipid peroxidation, and proinflammatory cytokines from baseline to 6 months. Initially, changes were compared between the two arms using Wilcoxon rank sum test to compare the effects of the two diets. In addition, Wilcoxon signed rank test was used to compare baseline and 6-month data to assess effects of each diet.
To assess the effectiveness of randomization, Wilcoxon rank sum test was used to compare baseline variables between the two arms. A P value <0.05 was considered statistically significant. If important baseline differences were identified, they were included in adjusted analysis using generalized linear models.
All analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC). Results are presented as means ± SE. Statistical significance was declared if the two-sided P value was <0.01 to account for multiple comparisons. The study was designed to recruit 12 subjects in each arm. Power analysis was done according to two scenarios at 5% significance level. In the first objective, the changes were compared between the two arms. Assuming the baseline follow-up correlation is 0.25 and the effect size (the ratio of variance of interaction effects to within-cell variance) is 0.40, interaction effects could be tested with at least 80% power. Just as important, in the absence of interaction effects, focus was shifted to assessing marginal treatment arm differences. Assuming again a baseline-follow up correlation of 0.25 and an effect size of 0.80 (ratio of mean weight change to within SD), the changes could be tested with at least 80% power. Note that extended models including baseline covariates should lead to greater statistical power under both scenarios.
RESULTS
The two groups are not different at baseline (Tables 1 and 2). Table 1 shows the mean ± SE of various parameters monitored at baseline and 6 months of HP or HC diets and their significance. The weight loss, BMI, blood pressure, HOMA-IR, and FM and LM (36) were all significantly improved at 6 months from baseline in both the HP and HC diets, but the differences between the two diets were not significant. However, the HP diet exhibited significantly greater improvement in insulin sensitivity (Matsuda index) and β-cell function. The HP diet also exhibited greater increase in REE than the HC diet after 6 months of diet intervention, confirming similar findings in other HP studies (6–10).
Table 1.
Effect of HP or HC diet on weight loss, insulin sensitivity, β-cell function, and compliance
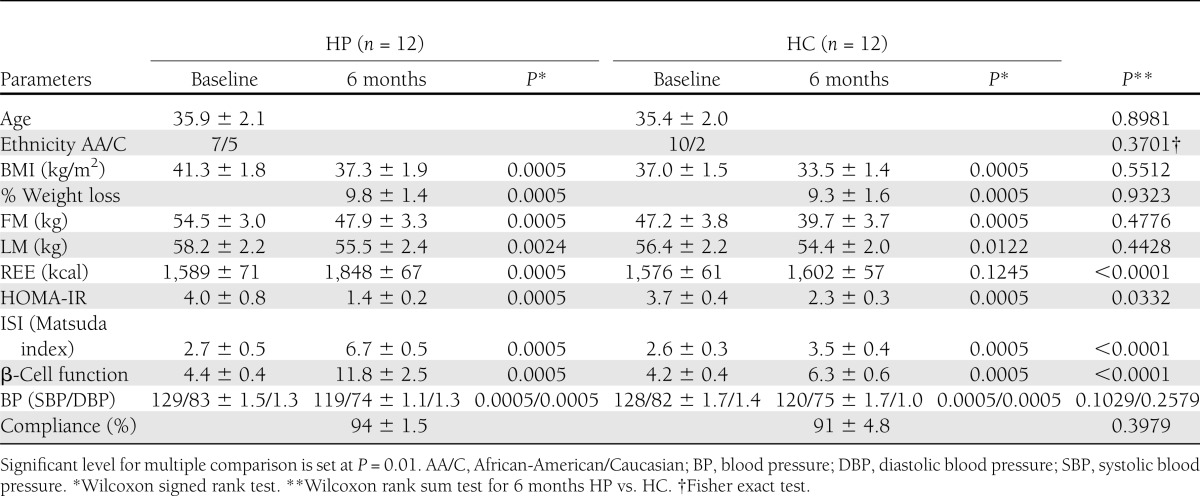
Table 2.
Effect of HP and HC diets on markers of oxidative stress, inflammation, cardiovascular risk factors, and adipokines
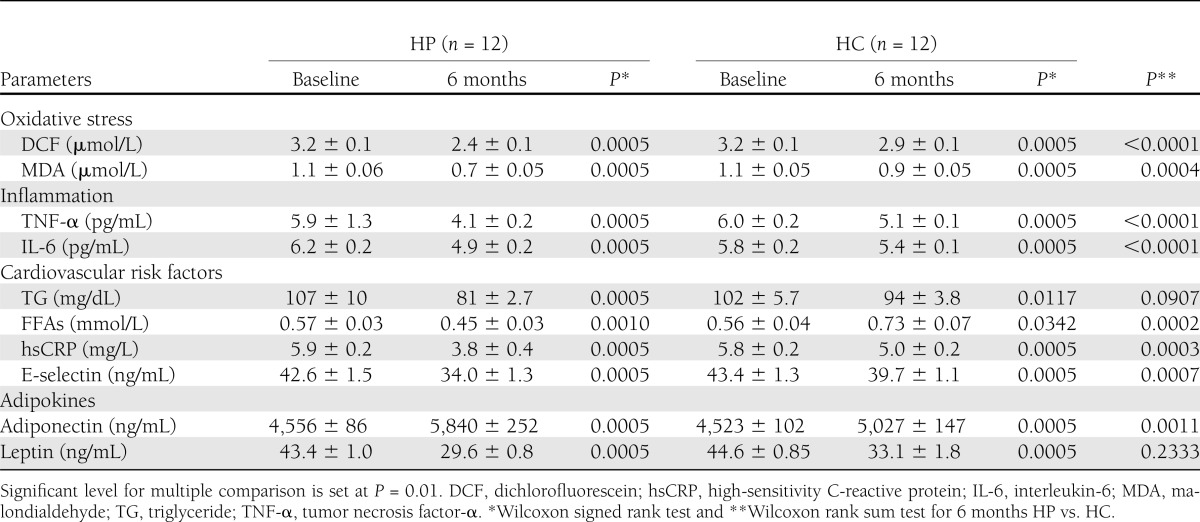
Table 2 shows the effect of the HP and HC diets after 6 months compared with baseline. The HP diet produced a significantly greater decrease than the HC diet in the levels of oxidative stress, inflammatory markers, and cardiovascular risk factors (except for triglycerides). The difference in triglyceride changes was not significantly greater with HP than HC diet. Comparison of FFA results were of interest, indicating a significant increase after 6 months on the HC diet suggesting greater lipolysis with HC diet after 6 months (P < 0.001) as contrasted to levels of FFA, which were significantly lower in the HP group after 6 months. Table 2 also demonstrates the effect of macronutrients on adiponectin, which was significantly increased in the HP diet at 6 months compared with the baseline. Of interest was the level of total adiponectin, which, as expected, was opposite to leptin: the former being significantly increased at 6 months in both the HP and HC diet groups with greater increase in the HP diet (1,284 vs. 504, respectively; P = 0.0011). Interestingly, leptin in the HP diet group was not significantly lower than in the HC diet group at 6 months. 25-OH vitamin D, parathyroid hormone, and serum and urinary Ca levels did not change significantly in either the HP or HC groups from baseline to 6 months (36) (data not shown).
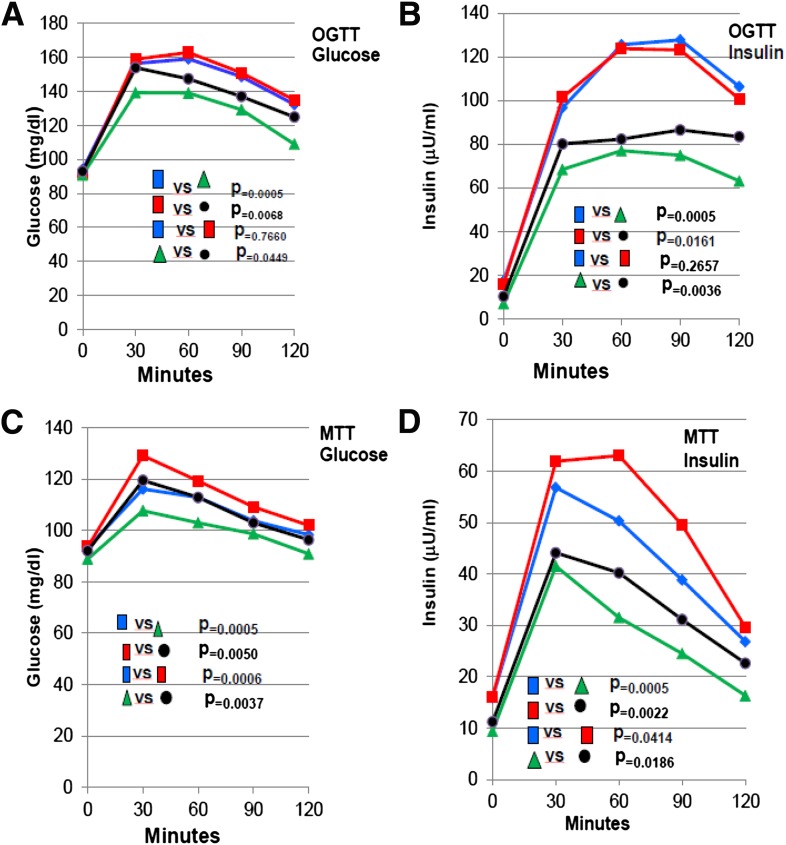
Figure 2A–D shows the mean for glucose and insulin excursions for the OGTT and MTT from 0 to 120 min for the baseline-HP, baseline-HC, 6 months–HP, and 6 months–HC diets for the 12 HP subjects and the 12 HC subjects. P values give the significance of area under the curve for glucose and insulin for OGTT and MTT comparing baseline-HP with 6 months–HP, baseline-HC with 6 months–HC, baseline-HP with baseline-HC, and 6 months–HP with 6 months–HC diets. These studies clearly indicate that subjects on HC diet during OGTT exhibited greater excursions for glucose and insulin after 6 months than HP diet. These excursions were less with respective meal tolerance test (Fig. 2C and D) for insulin and glucose, as the glycemic indices for HP diet is greatly decreased.
Figure 2.
A–D: Mean for glucose and insulin values for the OGTT and MTT from 0 to 120 min for the baseline-HP, baseline-HC, 6 months–HP, and 6 months–HC diets for the 12 HP subjects and the 12 HC subjects. P values give the significance of area under the curve for glucose and insulin for OGTT and MTT comparing baseline-HP with 6 months–HP, baseline-HC with 6 months–HC, baseline-HP with baseline-HC, and 6 months–HP with 6 months–HC diets. The colored symbols represent: blue, baseline-HP; red, baseline-HC; green, 6 months–HP; and black, 6 months–HC.
CONCLUSIONS
The subjects on HP diet exhibited greater improvement in markers of insulin sensitivity and β-cell function (A), oxidative stresss (B), lipid peroxidation (C), and inflammatory cytokines (D) than the HC diet. In addition, FFA, an indication of lipolysis, was increased with HC diet compared with HP diet. C-reactive protein and E-selectin were decreased with greater improvement in HP than HC (8.6 vs. 3.7 [P = 0.007]).
Dietary composition, particularly the ratio of protein to carbohydrate, can affect the plasma lipid profile and its metabolism (7,14,37–41). Our study shows changes in triglyceride for both HP and HC diets in normal obese women, but these changes were not significant between the two diets. Of interest was greater improvement in REE in HP versus HC diets (Table 1), confirming other works in the literature (7,10).
The HP and HC diet subjects all had minimal exercise activity, and both lost FM and LM to the same extent. Possibly, if the subjects had been on an exercise program the LM would not have been affected as much, as the protein-preserving effect of HP diet has been shown by many investigators, as cited above. Consumption of HP diet has been reported to cause negative calcium balance, increased calcium loss in the urine, and an adverse effect on the bone (8). However, our studies of both HP and HC diets, which contained more than the U.S. Food and Drug Administration–recommended amount of Ca/day (29), showed no such loss (data not shown). We were able to demonstrate that reduction of daily kilocalories in both diets, as expected, leads to equal amount of weight loss.
The important features of our studies not reported previously are as follows. 1) HP diet compared with HC diet provided greater advantages for β-cell function, insulin sensitivity, selected cardiovascular risk factors, protection against oxidative stress, and improvement in adiponectin. 2) The diets were delivered to each patient from our CRC on a weekly basis, with appropriate survey of food consumption. This method resulted in high level of compliance of >90% (Table 1). 3) No physical activity modifier was involved in the 6 months of follow-up, as study subjects were told to maintain a level of physical activity similar to that prior to dietary intervention; therefore, we were able to study the direct effects of the two diets per se.
In summary, our study demonstrates major findings in that the higher ratio of protein to carbohydrate has significant positive effect on proinflammatory cytokines and oxidative stress with improvement of insulin sensitivity and β-cell function and adipokines. These findings, to our knowledge, have not been reported previously in premenopausal, nondiabetic, obese women, and thus, our results provide new insight into the effects of various macronutrients on metabolic parameters in nondiabetic women. As we did not study obese, nondiabetic males or prediabetic subjects, our findings cannot be generalized until additional data are available in such subjects.
Acknowledgments
This study was funded by the American Diabetes Association (1-09-CR-32 [A.E.K., prinicipal investigator]). The following 2nd-year medical students participated in various aspects of this study and received a stipend during summer fellowship funded by a Medical Student Research Fellowship from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (C5T35DK007405-28 [S. Solomon, principal investigator]): M. Haddad, T. Littleton, L. Napatalung, K. Shah, and D. Wheeler. All students are affiliated with the University of Tennessee Health Science Center (UTHSC).
No potential conflicts of interest relevant to this article were reported.
A.E.K. wrote the original manuscript, which was subsequently reviewed by all authors with some modifications. K.A.M. provided information on the diets to participants and delivered the randomized food daily. J.Y.W. was the biostatistician in charge of statistical analysis. F.A.T. participated in discussion and evaluation of DXA data. C.A.J. provided evaluation of laboratory data and examination of subjects. C.W.S. conducted history and physical examination of subjects. E.A.N. was responsible for evaluation of subjects’ β-cell function. F.B.S. is the laboratory director who supervised recruitment of participants and assay of metabolic end points. A.E.K. and F.B.S. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank J. Fisher, MD, and A. Brewer, LD, MS, both of UTHSC, for reviewing the manuscript; T. Bea, also of UTHSC, for her administrative work; J. Crisler, of UTHSC, for assay of hormones and cytokines; and all of the nursing and recruiting staff of the Clinical Research Center, all of whom are affiliated with UTHSC, for their efforts. The authors are grateful to the study volunteers for participating in the study.
Footnotes
Clinical trial reg. no. NCT0164284, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1912/-/DC1.
References
- 1.Centers for Disease Control and Prevention. Adult obesity [article online], 2011. Available from www.cdc.gov/obesity Accessed 10 January 2012
- 2.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. JAMA 2002;288:1723–1727 [DOI] [PubMed] [Google Scholar]
- 3.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001;345:790–797 [DOI] [PubMed] [Google Scholar]
- 4.Alford BB, Blankenship AC, Hagen RD. The effects of variations in carbohydrate, protein, and fat content of the diet upon weight loss, blood values, and nutrient intake of adult obese women. J Am Diet Assoc 1990;90:534–540 [PubMed] [Google Scholar]
- 5.Larsen TM, Dalskov SM, van Baak M, et al. Diet, Obesity, and Genes (Diogenes) Project Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med 2010;363:2102–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bray GA, Smith SR, de Jonge L, et al. Effect of dietary protein content on weight gain, energy expenditure, and body composition during overeating: a randomized controlled trial. JAMA 2012;307:47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krieger JW, Sitren HS, Daniels MJ, Langkamp-Henken B. Effects of variation in protein and carbohydrate intake on body mass and composition during energy restriction: a meta-regression 1. Am J Clin Nutr 2006;83:260–274 [DOI] [PubMed] [Google Scholar]
- 8.Eisenstein J, Roberts SB, Dallal G, Saltzman E. High-protein weight-loss diets: are they safe and do they work? A review of the experimental and epidemiologic data. Nutr Rev 2002;60:189–200 [DOI] [PubMed] [Google Scholar]
- 9.Li Z, Heber D. Overeating and overweight: extra calories increase fat mass while protein increases lean mass. JAMA 2012;307:86–87 [DOI] [PubMed] [Google Scholar]
- 10.Pereira MA, Swain J, Goldfine AB, Rifai N, Ludwig DS. Effects of a low-glycemic load diet on resting energy expenditure and heart disease risk factors during weight loss. JAMA 2004;292:2482–2490 [DOI] [PubMed] [Google Scholar]
- 11.Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 2002;287:2414–2423 [DOI] [PubMed] [Google Scholar]
- 12.Robinson SM, Jaccard C, Persaud C, Jackson AA, Jequier E, Schutz Y. Protein turnover and thermogenesis in response to high-protein and high-carbohydrate feeding in men. Am J Clin Nutr 1990;52:72–80 [DOI] [PubMed] [Google Scholar]
- 13.Samaha FF, Iqbal N, Seshadri P, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med 2003;348:2074–2081 [DOI] [PubMed] [Google Scholar]
- 14.Jenkins DJ, Kendall CW, Vidgen E, et al. High-protein diets in hyperlipidemia: effect of wheat gluten on serum lipids, uric acid, and renal function. Am J Clin Nutr 2001;74:57–63 [DOI] [PubMed] [Google Scholar]
- 15.Nuttall FQ, Mooradian AD, Gannon MC, Billington C, Krezowski P. Effect of protein ingestion on the glucose and insulin response to a standardized oral glucose load. Diabetes Care 1984;7:465–470 [DOI] [PubMed] [Google Scholar]
- 16.Piatti PM, Montia LD, Magni F, et al. Hypocaloric high-protein diet improves glucose oxidation and spares lean body mass: comparison to hypocaloric high-carbohydrate diet. Metabolism 2011;43:1481–1487 [DOI] [PubMed]
- 17.Executive summary: standards of medical care in diabetes. Diabetes Care 2011;34(Suppl. 1):S4–S10 [DOI] [PMC free article] [PubMed]
- 18.Institute of Medicine. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acid. National Academy Press, Washington D.C., 2005 [DOI] [PubMed] [Google Scholar]
- 19.Boden G, Sargrad K, Homko C, Mozzoli M, Stein TP. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann Intern Med 2005;142:403–411 [DOI] [PubMed] [Google Scholar]
- 20.Bray GA. Diet and exercise for weight loss. JAMA 2012;307:2641–2642 [DOI] [PubMed] [Google Scholar]
- 21.Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab 2000;85:2970–2973 [DOI] [PubMed] [Google Scholar]
- 22.Tripathy D, Mohanty P, Dhindsa S, et al. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes 2003;52:2882–2887 [DOI] [PubMed] [Google Scholar]
- 23.Dandona P, Mohanty P, Ghanim H, et al. The suppressive effect of dietary restriction and weight loss in the obese on the generation of reactive oxygen species by leukocytes, lipid peroxidation, and protein carbonylation. J Clin Endocrinol Metab 2001;86:355–362 [DOI] [PubMed] [Google Scholar]
- 24.Stentz FB, Kitabchi AE. Hyperglycemia-induced activation of human T-lymphocytes with de novo emergence of insulin receptors and generation of reactive oxygen species. Biochem Biophys Res Commun 2005;335:491–495 [DOI] [PubMed] [Google Scholar]
- 25.Kitabchi AE, Stentz FB, Umpierrez GE. Diabetic ketoacidosis induces in vivo activation of human T-lymphocytes. Biochem Biophys Res Commun 2004;315:404–407 [DOI] [PubMed] [Google Scholar]
- 26.Stentz FB, Kitabchi AE. Palmitic acid-induced activation of human T-lymphocytes and aortic endothelial cells with production of insulin receptors, reactive oxygen species, cytokines, and lipid peroxidation. Biochem Biophys Res Commun 2006;346:721–726 [DOI] [PubMed] [Google Scholar]
- 27.Nyenwe EA, Jerkins TW, Umpierrez GE, Kitabchi AE. Management of type 2 diabetes: evolving strategies for the treatment of patients with type 2 diabetes. Metabolism 2011;60:1–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stentz FB, Umpierrez GE, Cuervo R, Kitabchi AE. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes 2004;53:2079–2086 [DOI] [PubMed] [Google Scholar]
- 29.NIH Consensus Conference. Optimal calcium intake. NIH Consensus Development Panel on Optimal Calcium Intake. JAMA 1994;272:1942–1948 [PubMed]
- 30.Larkin FA, Metzner HL, Guire KE. Comparison of three consecutive-day and three random-day records of dietary intake. J Am Diet Assoc 1991;91:1538–1542 [PubMed] [Google Scholar]
- 31.Gleason JA, Bourdet KL, Koehn K, Holay SY, Schaefer EJ. Cardiovascular risk reduction and dietary compliance with a home-delivered diet and lifestyle modification program. J Am Diet Assoc 2002;102:1445–1451 [DOI] [PubMed] [Google Scholar]
- 32.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 33.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 34.DeFronzo RA, Tripathy D, Schwenke DC, et al. ACT NOW Study Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 2011;364:1104–1115 [DOI] [PubMed] [Google Scholar]
- 35.Ferrannini E. The theoretical bases of indirect calorimetry: a review. Metabolism 1988;37:287–301 [DOI] [PubMed] [Google Scholar]
- 36.Tylavsky F, Kitabchi AE, Stentz FB, et al. Effect of high-protein and high carbohydrate weight reduction diets on bone density and body composition in obese, non-diabetic, pre-menopausal women (Abstract). Diabetes 2011;60(Suppl. 1):A511.
- 37.Layman Dk, Shiue H, Sather C, Erickson DJ, Baum JI. Increased dietary protein modifies glucose and insulin homeostasis in adult women during weight loss. J Nutr 2003 ;133:405–410 [DOI] [PubMed]
- 38.Blouet C, Mariotti F, Azzout-Marniche D, et al. The reduced energy intake of rats fed a high-protein low-carbohydrate diet explains the lower fat deposition, but macronutrient substitution accounts for the improved glycemic control. J Nutr 2006;136:1849–1854 [DOI] [PubMed] [Google Scholar]
- 39.Devkota S, Layman DK. Increased ratio of dietary carbohydrate to protein shifts the focus of metabolic signaling from skeletal muscle to adipose. Nutr Metab (Lond) 2011;8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Layman DK, Baum JI. Dietary protein impact on glycemic control during weight loss. J Nutr 2004;134:968S–973S [DOI] [PubMed] [Google Scholar]
- 41.Walker-Lasker DA, Evans EM, Layman DK. Moderate carbohydrate, moderate protein weight loss diet reduces cardiovascular disease risk compared to high carbohydrate, low protein diet in obese adults: A randomized clinical trial. Nutr Metab (Lond) 2008;5:30. [DOI] [PMC free article] [PubMed] [Google Scholar]