Abstract
OBJECTIVE
Dietary protein is an important modulator of glucose metabolism. However, studies regarding the association between dietary protein intake and gestational diabetes mellitus (GDM) risk are sparse. This study was to examine the association.
RESEARCH DESIGN AND METHODS
Our study included 21,457 singleton pregnancies reported among 15,294 participants of the Nurses' Health Study II cohort between 1991 and 2001. Included pregnancies were free of chronic diseases before pregnancy or previous GDM. Generalized estimating equations were used to estimate the relative risks (RRs) and 95% CIs.
RESULTS
After adjustment for age, parity, nondietary and dietary factors, and BMI, multivariable RRs (95% CIs) comparing the highest with lowest quintiles were 1.49 (1.03–2.17) for animal protein intake and 0.69 (0.50–0.97) for vegetable protein intake. The substitution of 5% energy from vegetable protein for animal protein was associated with a 51% lower risk of GDM (RR [95% CI], 0.49 [0.29–0.84]). For major dietary protein sources, multivariable RRs (95% CIs) comparing the highest with the lowest quintiles were 2.05 (1.55–2.73) for total red meat and 0.73 (0.56–0.95) for nuts, respectively. The substitution of red meat with poultry, fish, nuts, or legumes showed a significantly lower risk of GDM.
CONCLUSIONS
Higher intake of animal protein, in particular red meat, was significantly associated with a greater risk of GDM. By contrast, higher intake of vegetable protein, specifically nuts, was associated with a significantly lower risk. Substitution of vegetable protein for animal protein, as well as substitution of some healthy protein sources for red meat, was associated with a lower risk of GDM.
Gestational diabetes mellitus (GDM), defined as glucose intolerance with onset or first recognition during pregnancy, is a growing health concern (1). Approximately 7% (ranging from 1 to 14%) of all pregnancies in the U.S. are complicated by GDM, resulting in more than 200,000 cases annually (2). GDM is associated with an increased risk of adverse pregnancy and perinatal outcomes (3) and long-term adverse health consequences for both mothers and their children, including a predisposition to obesity, metabolic syndrome, and type 2 diabetes mellitus (T2DM) (1,2,4); thus, the identification of modifiable risk factors that may contribute to the prevention of GDM is important.
Recently, several dietary and lifestyle factors have been associated with GDM risk, although precise underlying mechanisms have yet to be established (5). Macronutrients including carbohydrates (6) and fats (7) have previously been evaluated for their association with GDM risk. The association with protein, however, remains unclear. Dietary proteins and amino acids are important modulators of glucose metabolism, and a diet high in protein may impact glucose homeostasis by promoting insulin resistance and increasing gluconeogenesis (8). Moreover, emerging data suggest that protein actions may vary by the amino acid types and food sources. For instance, a prospective cohort study in Europeans showed that long-term high intake of animal protein but not vegetable protein was associated with an increased risk of T2DM (9). Additionally, a study of metabolomics recently demonstrated that plasma concentrations of several kinds of amino acids, including branched-chain amino acids (BCAAs) and aromatic amino acids, were strongly and significantly associated with incident T2DM risk (10).
Several major food sources of animal protein, such as red meat, were positively associated with the risk of both T2DM (11) and GDM (12). Conversely, higher intakes of nuts (13) and legumes (14) were associated with a lower risk of T2DM, but their associations with GDM have not yet been evaluated. In addition, the associations between other major sources of animal protein (e.g., poultry, fish, and dairy products) and GDM risk have not been reported.
In this prospective cohort study, we aimed to examine the associations of prepregnancy dietary protein intake (total, animal, and vegetable protein) as well as major dietary protein sources with the risk of GDM. We also estimated the effect of substituting prepregnancy protein for carbohydrates, substituting vegetable protein for animal protein, and substituting other major dietary protein sources for red meat on the risk of GDM.
RESEARCH DESIGN AND METHODS
The Nurses’ Health Study II (NHS II) is an ongoing prospective cohort study of 116,678 female nurses aged 25–44 years at study inception in 1989 (15). The participants are sent a biennial questionnaire regarding disease outcomes and lifestyle characteristics, such as smoking status, medication use, and physical activity. Follow-up for each questionnaire cycle was >90%. This study has been approved by the institutional review board of the Partners Health Care System (Boston, MA), with participants’ consent implied by the return of the questionnaires.
NHS II participants were included in this analysis if they reported at least one singleton pregnancy lasting >6 months between 1991 and 2001. GDM was last captured on the 2001 questionnaire, as the majority of NHS II participants passed reproductive age by then. Pregnancies were excluded if the participant reported GDM in a previous pregnancy, a diagnosis of T2DM, cancer, or a cardiovascular event prior to an otherwise eligible pregnancy. Pregnancies reported after GDM were not included because women with GDM in a previous pregnancy may change their diet and lifestyle during the next pregnancy to prevent recurrent GDM. Pregnancies were also excluded if the participant did not return a prepregnancy food frequency questionnaire (FFQ), left >70 FFQ items blank, or reported unrealistic total energy intake (<600 or >3,500 kcal/day).
Exposure assessment
Beginning in 1991 and every 4 years thereafter, participants were asked to report their food intakes using a semiquantitative FFQ. Answers were provided in nine possible categories ranging from “never” to “6 or more times/day,” with a standard portion size specified for each food. Major protein sources included the following (16,17): unprocessed red meat (beef or lamb as main dish, pork as main dish, hamburger, and beef, pork, or lamb as a sandwich or mixed dish), processed red meat (bacon, beef hot dogs, and sausage, salami, bologna, and other processed meats), poultry (chicken with and without skin, chicken sandwich, and chicken/turkey hot dog), fish (canned tuna, dark- and light-fleshed fish, and breaded fish), dairy products (whole milk, ice cream, hard cheese, full-fat cheese, cream, sour cream, cream cheese, butter, skim/low-fat milk, 1 and 2% milk, yogurt, cottage and ricotta cheeses, low-fat cheese, and sherbet), eggs, nuts (peanuts, peanut butter, walnuts, and other nuts), and legumes (tofu or soybeans, string beans, peas or lima beans, and beans or lentils). Total red meat intake was calculated as the sum of unprocessed and processed red meat intakes.
Intakes of individual nutrients including protein were computed by multiplying the frequency of consumption of each unit of food by the nutrient content of the specified portions based on food composition data from U.S. Department of Agriculture sources (18). The reproducibility and validity of the FFQ have been extensively documented elsewhere (19–21). Pearson correlation coefficient between energy-adjusted protein intakes assessed by the FFQ compared with four 1-week diet records was 0.52 in a similar cohort of U.S. women (20).
Covariate assessment
Participants reported their current weight on each biennial questionnaire. Self-reported weight was highly correlated with measured weight (r = 0.97) in a previous validation study (22). BMI was computed as weight in kilograms divided by the square of height in meters. Total physical activity was ascertained by frequency of engaging in common recreational activities, from which MET hours per week were derived. The questionnaire-based estimates correlated well with detailed activity diaries in a prior validation study (r = 0.56) (23).
Outcome ascertainment
Incident GDM was ascertained by self-report on each biennial questionnaire through 2001. In the case of more than one pregnancy lasting >6 months reported within a 2-year questionnaire period, GDM status was attributed to the first pregnancy. In a prior validation study among a subgroup of the NHS II cohort, 94% of GDM self-reports were confirmed by medical records (15). In a random sample of parous women without GDM, 83% reported a glucose screening test during pregnancy and 100% reported frequent prenatal urine screening, suggesting a high level of GDM surveillance in this cohort (15).
Statistical analysis
Exposure was computed as the percentage of total energy intake from protein using the nutrient-density method (24). Prepregnancy dietary exposure measures were used to calculate the updated cumulative average intake for each individual at each time period to reduce within-subject variation and represent long-term habitual prepregnancy diet (25).
Participants were divided into quintiles according to the cumulative average intakes of dietary protein (% of energy) or major protein sources (servings/day) in their diet. Relative risks (RRs) and 95% CIs were estimated through multivariate logistic regression with generalized estimating equations, specifying an exchangeable correlation structure. Generalized estimating equations allowed us to account for correlations among repeated observations (pregnancies) contributed by a single participant. To compute the test for a significant trend across quintiles, we modeled median values of each quintile as a continuous variable.
Covariates in the multivariable models included age; parity; race/ethnicity; family history of diabetes; cigarette smoking; alcohol intake; physical activity; total energy intake; intakes of saturated fat, monounsaturated fat, polyunsaturated fat, trans fat, dietary cholesterol, glycemic load, and dietary fiber; and updated BMI when total protein intake was modeled as the exposure of interest. Animal protein and vegetable protein were mutually adjusted for one another. For the intake of major dietary protein sources, we adjusted for age; parity; race/ethnicity; family history of diabetes; cigarette smoking; alcohol intake; physical activity; total energy intake; dietary intakes of fruits, sugar-sweetened beverages, whole grains, and other major dietary protein sources (for mutual adjustment); and updated BMI.
To simulate the substitution of dietary protein for carbohydrates, we fit isocaloric models (24) by simultaneously including total energy intake and the percentages of energy (continuous) derived from total fat and protein, as well as the potential confounders listed above. The β-coefficient for total protein from these models estimated the effect of substituting 1% of energy from carbohydrates with 1% of energy from protein (26). For the estimation of substituting animal protein with vegetable protein, we simultaneously included total energy intake and the percentages of energy derived from vegetable protein, as well as the potential confounders listed above in the model. Similarly, we estimated the effect of substituting one major protein source for another by simultaneously modeling all major protein sources (servings/day) with total energy and other potential confounders listed above. RRs and 95% CIs were estimated by computing the difference in the coefficients for two protein sources and their own variances and covariance (16,17,27).
Advanced maternal age is an established risk factor for GDM (28). We evaluated effect modification by age (<35 vs. ≥35 years), parity (nulliparous vs. parous), family history of diabetes (yes vs. no), and physical activity (highest two quintiles vs. lowest three quintiles) by stratified analyses. Since BMI is a possible intermediate between dietary protein and GDM, we estimated the proportion of the association between dietary protein intake and GDM risk that is explained by prepregnancy BMI (modeled continuously) (29) using an SAS macro developed by Dr. D. Spiegelman and colleagues at the Harvard School of Public Health (http://www.hsph.harvard.edu/faculty/donna-spiegelman/software/mediate/).
All statistical analyses were performed with SAS software (version 9.1; SAS Institute). P < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics
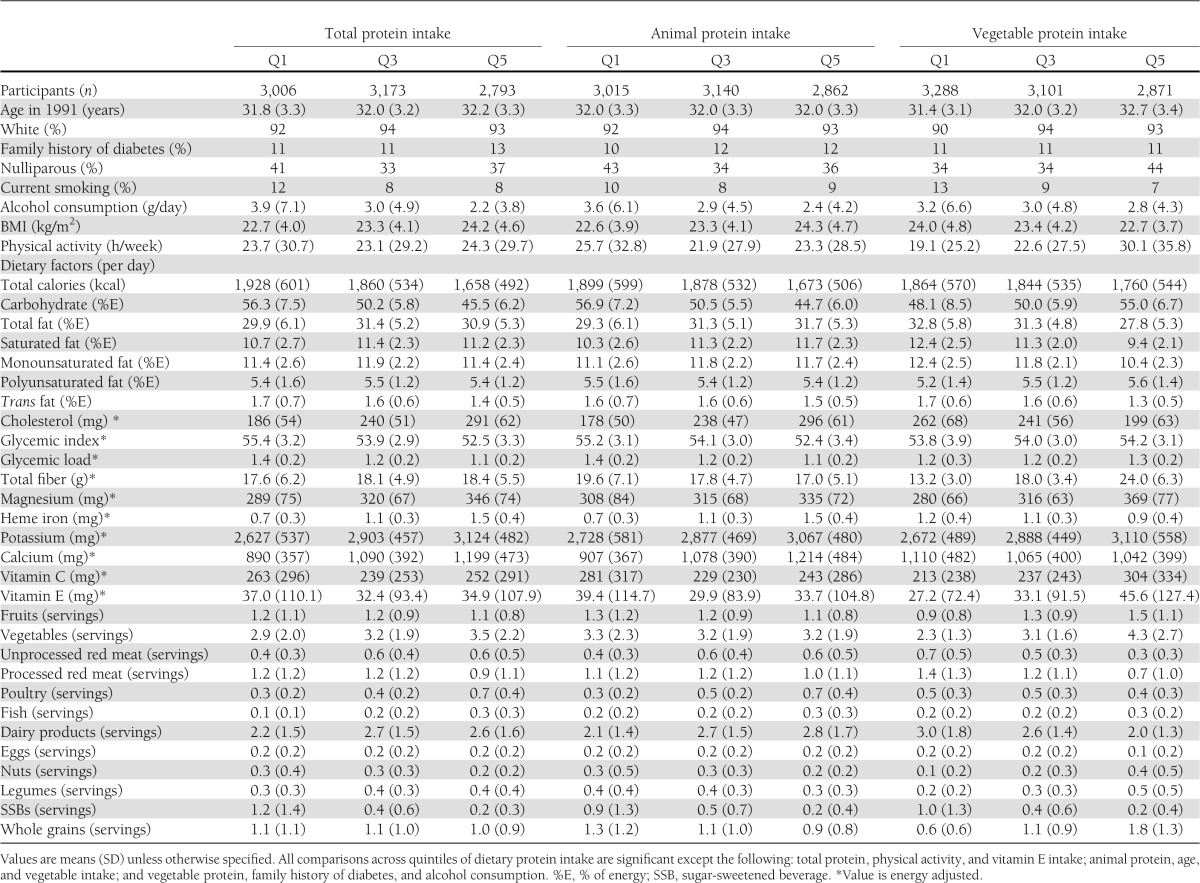
Among the 21,457 eligible singleton pregnancies from the 15,294 women, during the 10 years of follow-up we documented 870 incident GDM pregnancies. Compared with the participants with lower total protein intake, those with higher protein intake were more likely to be nonsmokers and consumed more cholesterol, dietary fiber, magnesium, heme iron, potassium, calcium, meat, vegetables, and dairy products but less alcohol, carbohydrate, trans fat, and sugar-sweetened beverages during the prepregnancy time period (Table 1). Women who consumed more animal protein were likely to consume more total fat, saturated fat, cholesterol, heme iron, calcium, red meat, poultry, and dairy products. Women who consumed more vegetable proteins were likely to consume less of these food and nutrients.
Table 1.
Baseline characteristics according to quintiles of prepregnancy dietary protein intake among 15,294 participants in the Nurses’ Health Study II
Prepregnancy dietary protein intake and the risk of GDM
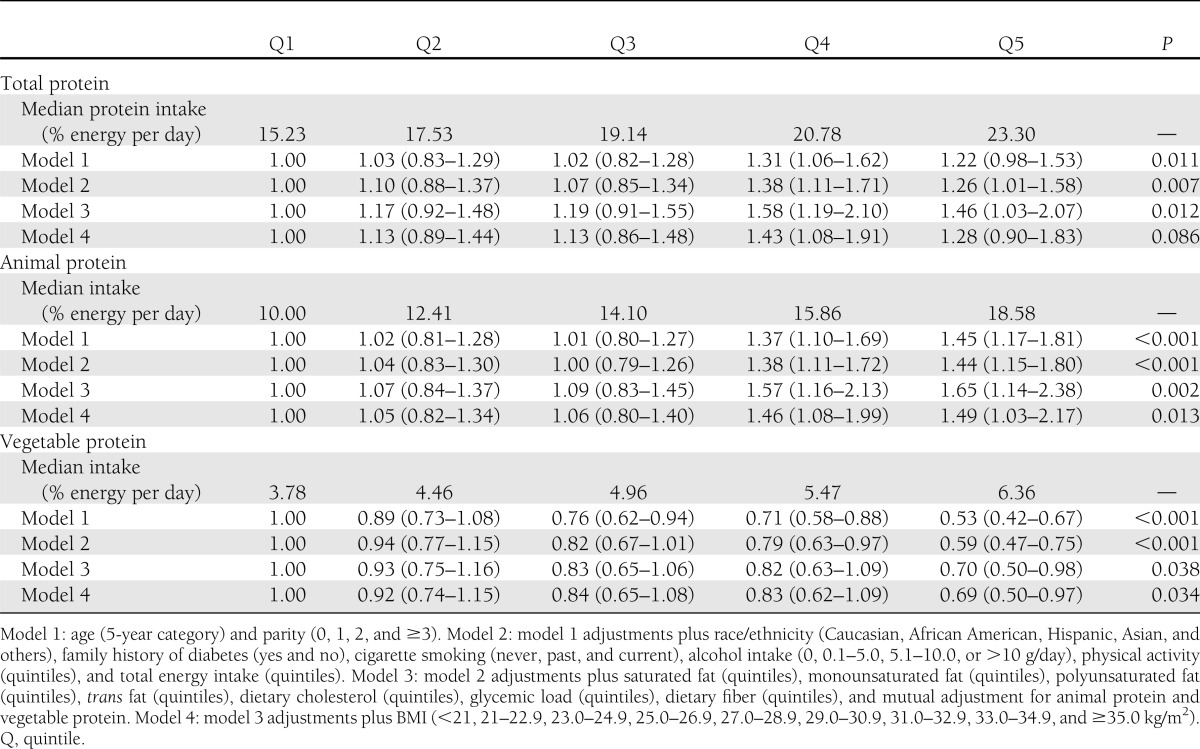
The median intakes of prepregnancy total calories from protein in this population were 15.2 and 23.3% of energy in the lowest and the highest quintile, respectively. Animal protein accounted for the majority of total protein intake. After adjustment for age, parity, nondietary and dietary factors, and BMI, animal protein intake was significantly and positively associated with GDM risk while vegetable protein intake was significantly and inversely associated with the risk; multivariable RRs (95% CIs), comparing the highest with lowest quintiles were 1.28 (0.90–1.83) for total protein intake, 1.49 (1.03–2.17) for animal protein intake, and 0.69 (0.50–0.97) for vegetable protein intake (Table 2).
Table 2.
RRs (95% CIs) for GDM according to quintiles of prepregnancy dietary protein intake
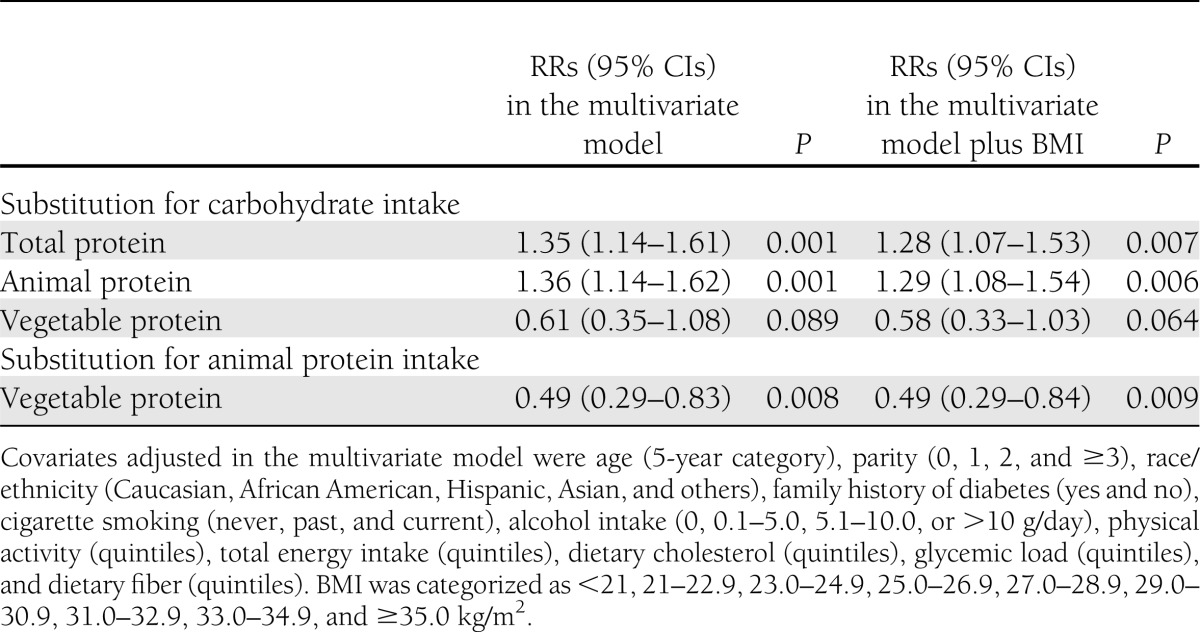
Substituting 5% of energy from carbohydrates with animal protein was associated with a significant 29% greater risk of GDM (multivariable RR [95% CI], 1.29 [1.08–1.54]; P = 0.006). Substituting 5% of energy from vegetable protein for animal protein was associated with a 51% lower risk (0.49 [0.29–0.84]; P = 0.009) (Table 3).
Table 3.
Multivariate RRs (95% CIs) of GDM associated with increases in 5% of energy from types of protein
The associations between prepregnancy dietary protein intake and GDM risk were not significantly modified by age, parity, family history of diabetes, or physical activity. Mediation analyses estimated that prepregnancy BMI explained 35.7% (95% CI 10.6–60.8; P = 0.005) and 31.1% (10.7–51.6; P = 0.003) of the total effects of total protein and animal protein on GDM risk, respectively. The effect of vegetable protein intake on GDM risk was not significantly mediated by BMI (12.2% [−21.1 to 45.4]; P = 0.47).
Major prepregnancy dietary protein sources and the risk of GDM
Prepregnancy red meat consumption was significantly and positively associated with the risk of GDM. Multivariable RRs (95% CIs) for GDM among participants with the highest compared with the lowest quintiles of intakes were 2.46 (1.86–3.25), 1.89 (1.43–2.48), and 1.48 (1.13–1.95) for total red meat, unprocessed red meat, and processed red meat, respectively. These associations were attenuated but remained significant after additional adjustment for BMI, with RRs of 2.05 (1.55–2.73), 1.60 (1.21–2.12), and 1.36 (1.03–1.80), respectively. By contrast, greater prepregnancy nut consumption was significantly associated with a lower risk of GDM; the fully adjusted RR comparing the highest with lowest quintiles of intake was 0.73 (0.56–0.95) (Table 4).
Table 4.
RRs (95% CIs) for GDM according to quintiles of prepregnancy intake (servings per day) of major sources of dietary protein
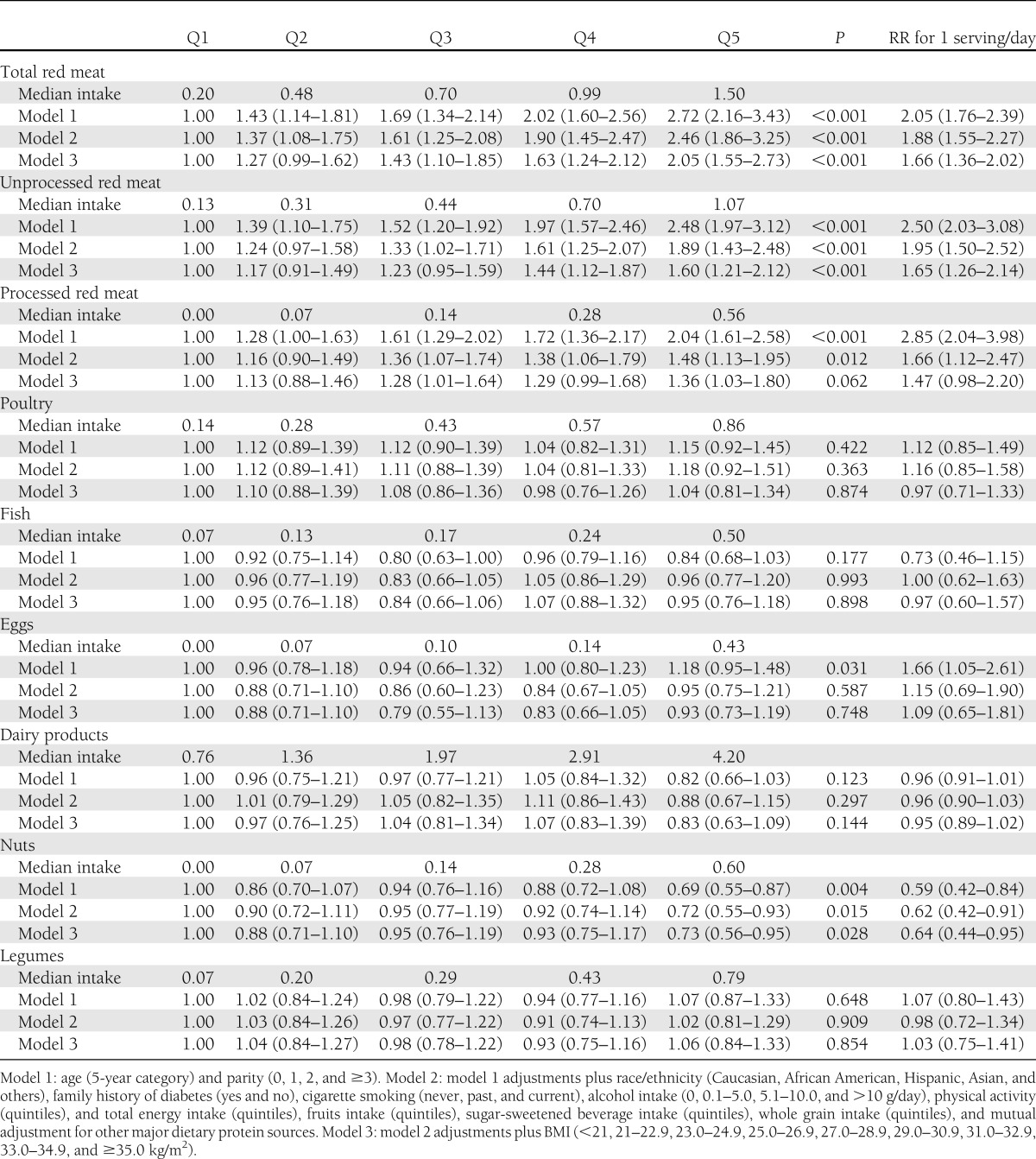
Substituting one serving per day of total red meat with some healthy protein sources was significantly associated with a lower risk of GDM: 29% lower risk for poultry (RR [95% CI], 0.71 [0.54–0.94]), 33% for fish (0.67 [0.46–0.98]), 51% for nuts (0.49 [0.36–0.66]), and 33% for legumes (0.67 [0.51–0.88]). Similar substitution estimates were observed for the replacement of unprocessed red meat and processed red meat (Supplementary Fig. 1).
CONCLUSIONS
In this prospective cohort study, we observed that a prepregnancy intake of animal protein, in particular red meat, was significantly and positively associated with GDM risk, while vegetable protein intake, specifically nuts, was significantly and inversely associated with GDM risk. Substituting 5% of energy from vegetable protein for animal protein and substitution of poultry, fish, nuts, or legumes for red meat were associated with a lower GDM risk.
Although protein may have beneficial effects on energy homeostasis by promoting thermogenesis, inducing satiety, and possibly increasing energy expenditure, it may also have detrimental effects on glucose homeostasis (8). Consumption of a high-protein diet for 6 months in healthy individuals induced a higher glucose-stimulated insulin secretion due to reduced glucose threshold of the endocrine β-cells, increased endogenous glucose output and plasma glucagon level, and enhanced gluconeogenesis (30). Recent studies examining different types and sources of dietary protein suggest that animal protein and vegetable protein may have divergent effects on diabetes. A prospective cohort study with 10 years of follow-up showed that the risk of T2DM increased with higher intakes of total protein and animal protein, but vegetable protein intake was not related to T2DM risk (9). It has been proposed that elevated incidence of diabetes in relation to high protein intake, in particular animal protein, might result from an accelerated “fatigue” or “failure” of pancreas islets (8).
Meat consumption has continued to rise over the past century in the U.S., with the largest proportion from red meat (58%) (31). Red meat consumption has been found to be positively associated with long-term weight gain (32) and risk of T2DM (11), coronary heart disease (16), stroke (17), and all-cause mortality (27). In this study, red meat consumption was significantly associated with an increased risk of GDM, which is consistent with our previous findings with shorter follow-up (12). Although no significant association was observed for other animal foods, the substitution of fish and poultry for red meat was associated with a lower risk of GDM. By contrast, a significant and inverse association was found between nut consumption and risk of GDM. An inverse association between nut consumption and risk of T2DM was previously observed in the NHS participants (13). Nuts have a healthful nutritional profile; in addition to being a good source of vegetable protein, nuts are rich in monounsaturated fatty acids, polyunsaturated fatty acids, fiber, and magnesium and have a relatively low glycemic index (13,33). These factors, either individually or in combination, have been associated with improved insulin sensitivity and lower diabetes risk (33).
The distinct effects of animal protein and vegetable protein on the incidence of GDM could be attributable to other nutrients coexistent in foods rich in protein, e.g., the co-occurrence of cholesterol and saturated fat in foods rich in animal protein. However, in the current study, the association of animal protein and GDM risk remained significant even after the adjustment of dietary cholesterol and saturated fat intakes. The distinct effects could be also due to variations of amino acid composition in these foods. Several in vitro and in vivo studies support an important role of amino acids in glucose homeostasis through regulation of glucose uptake and glycogen synthesis in skeletal muscle, hepatic glucose production, and insulin secretion (8). It has long been recognized that the plasma concentrations of BCAAs are dramatically raised to ~200% of fasting values after the ingestion of an animal protein-rich meal (34). A recent study showed that dietary proteins isolated from beef and pork meat resulted in significantly higher plasma concentrations of BCAAs than soy protein (35). Although the BCAAs constitute only around 20% of the total amino acid content in red meat, they represent the majority of the amino acids entering the systemic circulation after a red meat meal (34). Recently, a BCAAs-related metabolic signature has been implicated in the development of insulin resistance among both obese (36) and nonobese (37) individuals, and elevated plasma levels of BCAAs, tyrosine, and phenylalanine have been linked to incident diabetes in a metabolomics study (10). The associations of BCAAs and other amino acid intakes with GDM risk need to be elucidated in future studies.
Strengths of this study include the large sample size, the high response rates of long-term follow-up, and the detailed prospective dietary assessments with FFQs that have been extensively validated against multiple weeks of food records in previous studies (19–21). We acknowledge that there are several limitations. First, misclassification of dietary protein intake is possible. However, the random within-person error would be nondifferential, given that the prepregnancy dietary information was captured prospectively; therefore, our observed associations may underestimate the true RRs. Furthermore, the use of cumulative averages of dietary intakes for participants with more than one prepregnancy FFQ reduces random error. Second, the NHS II cohort did not assess diet during pregnancy. Therefore, we are unable to assess the association of prepregnancy protein intake with GDM risk, independent of diet during pregnancy. However, evidence suggests that the overall dietary patterns and dietary intakes of major protein sources change little from prepregnancy to during pregnancy (38,39). In addition, although GDM is a pregnancy complication (usually diagnosed in 24–28 weeks of gestation), increasing evidence suggests that most women with GDM seem to have a chronic β-cell defect before pregnancy (40). Women who develop GDM are thought to have a compromised capacity to adapt to the metabolic challenges of pregnancy, which serves to unmask a predisposition to glucose metabolic disorders in these women (40,41). Therefore, prepregnancy dietary factors implicated in glucose homeostasis are also physiologically relevant to the development of GDM. Third, our study population consisted mostly of Caucasian American women; thus, the generalization of our findings to other races and ethnic groups may be limited. However, the relative homogeneity of our study population reduces potential confounding. Fourth, plasma amino acid data were not available thus far in this population. The inclusion of plasma amino acids data may help better understand the underlying mechanisms for the divergent effects of animal and vegetable protein intakes on GDM risk. Finally, although major potential confounders have been adjusted in the current study, we cannot completely rule out the possibility of residual confounding from unmeasured factors. In addition, because of the high correlations among nutrients coexisting with protein in common food sources, we could not exclude the possibility of overadjustment, which may lead to an underestimate of the real associations of animal and vegetable protein intake with GDM risk.
In summary, our findings indicate that prepregnancy intake of animal protein, in particular red meat, is significantly and positively associated with GDM risk, whereas consumption of vegetable protein, specifically nuts, is inversely associated with the risk. Moreover, our findings suggest that among women of reproductive age, substitution of vegetable protein for animal protein, as well as substitution of some healthy protein sources (e.g., nuts, legumes, poultry, and fish) for red meat may potentially lower GDM risk. Along with our previous findings on associations of GDM risk with carbohydrates (6) and fats (7), the joint effects of different amounts and types of these macronutrients on the risk of GDM warrant further investigation in future studies.
Acknowledgments
This study was funded by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (contract no. HHSN275201000020C). The Nurses’ Health Study II was funded by research grants DK58845, CA50385, and P30 DK46200 from the National Institutes of Health.
No potential conflicts of interest relevant to this article were reported.
W.B. contributed to the design and analysis of the study and wrote the manuscript. K.B. contributed to the data analysis, and reviewed and edited the manuscript. D.K.T. conducted the technique review and reviewed and edited the manuscript. F.B.H. interpreted the results and reviewed and edited the manuscript. C.Z. contributed to the design and analysis of the study, and reviewed and edited the manuscript. W.B. and C.Z. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 45th Society for Epidemiologic Research Annual Meeting, Minneapolis, Minnesota, 27–30 June 2012.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-2018/-/DC1.
References
- 1.Reece EA, Leguizamón G, Wiznitzer A. Gestational diabetes: the need for a common ground. Lancet 2009;373:1789–1797 [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Gestational diabetes mellitus. Diabetes Care 2004;27(Suppl 1):S88–S90 [DOI] [PubMed] [Google Scholar]
- 3.Metzger BE, Lowe LP, Dyer AR, et al. HAPO Study Cooperative Research Group Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002 [DOI] [PubMed] [Google Scholar]
- 4.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 2009;373:1773–1779 [DOI] [PubMed] [Google Scholar]
- 5.Zhang C, Ning Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: review of epidemiologic evidence. Am J Clin Nutr 2011;94(Suppl.):1975S–1979S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang C, Liu S, Solomon CG, Hu FB. Dietary fiber intake, dietary glycemic load, and the risk for gestational diabetes mellitus. Diabetes Care 2006;29:2223–2230 [DOI] [PubMed] [Google Scholar]
- 7.Bowers K, Tobias DK, Yeung E, Hu FB, Zhang C. A prospective study of prepregnancy dietary fat intake and risk of gestational diabetes. Am J Clin Nutr 2012;95:446–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tremblay F, Lavigne C, Jacques H, Marette A. Role of dietary proteins and amino acids in the pathogenesis of insulin resistance. Annu Rev Nutr 2007;27:293–310 [DOI] [PubMed] [Google Scholar]
- 9.Sluijs I, Beulens JW, van der A DL, Spijkerman AM, Grobbee DE, van der Schouw YT. Dietary intake of total, animal, and vegetable protein and risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-NL study. Diabetes Care 2010;33:43–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011;17:448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan A, Sun Q, Bernstein AM, et al. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr 2011;94:1088–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang C, Schulze MB, Solomon CG, Hu FB. A prospective study of dietary patterns, meat intake and the risk of gestational diabetes mellitus. Diabetologia 2006;49:2604–2613 [DOI] [PubMed] [Google Scholar]
- 13.Jiang R, Manson JE, Stampfer MJ, Liu S, Willett WC, Hu FB. Nut and peanut butter consumption and risk of type 2 diabetes in women. JAMA 2002;288:2554–2560 [DOI] [PubMed] [Google Scholar]
- 14.Villegas R, Gao YT, Yang G, et al. Legume and soy food intake and the incidence of type 2 diabetes in the Shanghai Women’s Health Study. Am J Clin Nutr 2008;87:162–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solomon CG, Willett WC, Carey VJ, et al. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA 1997;278:1078–1083 [PubMed] [Google Scholar]
- 16.Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC. Major dietary protein sources and risk of coronary heart disease in women. Circulation 2010;122:876–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernstein AM, Pan A, Rexrode KM, et al. Dietary protein sources and the risk of stroke in men and women. Stroke 2012;43:637–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu FB, Stampfer MJ, Manson JE, et al. Dietary protein and risk of ischemic heart disease in women. Am J Clin Nutr 1999;70:221–227 [DOI] [PubMed] [Google Scholar]
- 19.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65 [DOI] [PubMed] [Google Scholar]
- 20.Willett WC, Sampson L, Browne ML, et al. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol 1988;127:188–199 [DOI] [PubMed] [Google Scholar]
- 21.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858–867 [DOI] [PubMed] [Google Scholar]
- 22.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology 1990;1:466–473 [DOI] [PubMed] [Google Scholar]
- 23.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol 1994;23:991–999 [DOI] [PubMed] [Google Scholar]
- 24.Willett WC. Nutritional Epidemiology. 2nd ed. New York, Oxford University Press, 1998 [Google Scholar]
- 25.Hu FB, Stampfer MJ, Rimm E, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol 1999;149:531–540 [DOI] [PubMed] [Google Scholar]
- 26.Hu FB, Stampfer MJ, Manson JE, et al. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med 1997;337:1491–1499 [DOI] [PubMed] [Google Scholar]
- 27.Pan A, Sun Q, Bernstein AM, et al. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med 2012;172:555–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lao TT, Ho LF, Chan BC, Leung WC. Maternal age and prevalence of gestational diabetes mellitus. Diabetes Care 2006;29:948–949 [DOI] [PubMed] [Google Scholar]
- 29.Lin DY, Fleming TR, De Gruttola V. Estimating the proportion of treatment effect explained by a surrogate marker. Stat Med 1997;16:1515–1527 [DOI] [PubMed] [Google Scholar]
- 30.Linn T, Santosa B, Grönemeyer D, et al. Effect of long-term dietary protein intake on glucose metabolism in humans. Diabetologia 2000;43:1257–1265 [DOI] [PubMed] [Google Scholar]
- 31.Daniel CR, Cross AJ, Koebnick C, Sinha R. Trends in meat consumption in the USA. Public Health Nutr 2011;14:575–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 2011;364:2392–2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kendall CW, Josse AR, Esfahani A, Jenkins DJ. Nuts, metabolic syndrome and diabetes. Br J Nutr 2010;104:465–473 [DOI] [PubMed] [Google Scholar]
- 34.Adeva MM, Calviño J, Souto G, Donapetry C. Insulin resistance and the metabolism of branched-chain amino acids in humans. Amino Acids 2012;43:171–181 [DOI] [PubMed] [Google Scholar]
- 35.Brandsch C, Shukla A, Hirche F, Stangl GI, Eder K. Effect of proteins from beef, pork, and turkey meat on plasma and liver lipids of rats compared with casein and soy protein. Nutrition 2006;22:1162–1170 [DOI] [PubMed] [Google Scholar]
- 36.Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009;9:311–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tai ES, Tan ML, Stevens RD, et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia 2010;53:757–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cucó G, Fernández-Ballart J, Sala J, et al. Dietary patterns and associated lifestyles in preconception, pregnancy and postpartum. Eur J Clin Nutr 2006;60:364–371 [DOI] [PubMed] [Google Scholar]
- 39.Crozier SR, Robinson SM, Godfrey KM, Cooper C, Inskip HM. Women’s dietary patterns change little from before to during pregnancy. J Nutr 2009;139:1956–1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buchanan TA, Xiang AH, Page KA. Gestational diabetes mellitus: risks and management during and after pregnancy. Nat Rev Endocrinol 2012;8:639–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang C, Solomon CG, Manson JE, Hu FB. A prospective study of pregravid physical activity and sedentary behaviors in relation to the risk for gestational diabetes mellitus. Arch Intern Med 2006;166:543–548 [DOI] [PubMed] [Google Scholar]


