Abstract
OBJECTIVE
The role of inflammation in the increased risk of cardiovascular disease in type 1 diabetes is unclear. We examined the association of inflammation and progression of coronary artery calcification (CAC)—a marker of subclinical atherosclerosis—in adults with and without type 1 diabetes.
RESEARCH DESIGN AND METHODS
A nested case-control study was performed within the prospective cohort of the Coronary Artery Calcification in Type 1 Diabetes (CACTI) study. Participants underwent two CAC measurements ∼2.5 years apart. Case subjects (n = 204) were those with significant progression of CAC. Control subjects (n = 258) were frequency-matched to case subjects on diabetes status, sex, age, and baseline CAC status. Inflammatory marker assessments were performed on stored blood samples from baseline. A principal components analysis (PCA) was performed and a composite score derived from that analysis. The composite score was constructed by assigning a value of 1 for each PCA component where at least one of the markers exceeded the 75th percentile (range 0–4). Conditional logistic regression was used for the matching strategy.
RESULTS
The first two components of the PCA were modestly (odds ratio 1.38 [95% CI 1.08–1.77] and 1.27 [1.02–1.59], respectively) associated with CAC progression after adjustment for other risk factors. The composite score was more strongly associated with CAC progression for those with elevated markers in three or four of the principal components compared with those with none.
CONCLUSIONS
Measures of inflammation were associated with progression of CAC in a population of adults with and without type 1 diabetes.
Individuals with type 1 diabetes are at a dramatically increased risk of cardiovascular disease (CVD) compared with those without diabetes (1). Inflammation is a factor in the pathogenesis of atherosclerosis (2) and is elevated in type 1 diabetes (3,4), but the role it may play in the increased risk of CVD in type 1 diabetes is unclear.
Increased levels of high-sensitivity C-reactive protein (hsCRP), interleukin (IL)-6, and tumor necrosis factor (TNF)-α have been shown to be associated with microvascular complications in type 1 diabetes (5–8); however, these cross-sectional studies do not prove causality. Large prospective studies have so far suggested that measures of inflammation are associated with microvascular and macrovascular complications, but the inflammatory markers measured have been limited. An analysis of data collected from the Pittsburgh Epidemiology of Diabetes Complications (EDC) study found that elevated hsCRP levels were associated with an increased risk of coronary artery disease (CAD) during 18 years of follow-up, particularly in certain haptoglobin genotypes (9). The Diabetes Control and Complications Trial/Epidemiology of Diabetes Intervention and Complications study found that plasma fibrinogen and soluble cell adhesion molecules were associated with the progression in carotid intimal-medial thickness and diabetic nephropathy, respectively, but no association was found for either of these outcomes with hsCRP (10).
Coronary artery calcification (CAC), a subclinical measure of CVD, predicts cardiac events and has been a valuable tool for quantifying the burden of atherosclerosis (11–13). Short-term progression of CAC predicts all-cause mortality (14) independently of the baseline CAC levels. Associations between inflammatory markers and the amount of CAC have been reported for the general population (15,16), but few studies have looked at the association between inflammation and the progression of CAC (17). One study found that plasma homocysteine predicted progression of CAC, but a significant association was not found with CRP (18). In the Multi-Ethnic Study of Atherosclerosis, fibrinogen and CRP were not associated with incident CAC after adjusting for BMI (19). Analyses of data from the Coronary Artery Calcification in Type 1 Diabetes (CACTI) study found significant relationships between soluble IL-2 receptor (sIL-2R), fibrinogen, and progression of CAC (20,21).
The goal of this study was to examine the prospective association of markers of inflammation with the progression of CAC over time in adults with and without type 1 diabetes.
RESEARCH DESIGN AND METHODS
Study design
Study subjects for this nested case-control study were selected from participants of the CACTI Study, a prospective cohort study examining the prevalence of CAC—a marker of subclinical atherosclerosis—in adults with type 1 diabetes and a comparable group of control subjects. Detailed descriptions of the study design have been published (22). Briefly, the full cohort consisted of 1,416 participants (652 with type 1 diabetes, 764 control subjects) who reported no history of CVD and were asymptomatic for CVD at enrollment. All study participants provided informed consent, and the protocol was reviewed and approved by the Colorado Multiple Institutional Review Board.
Examination measurements
Physical examination measurements included height, weight, waist and hip circumference, and systolic and diastolic blood pressure. BMI was calculated as weight in kilograms divided by the square of height in meters. Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or treatment with antihypertensive medication. A fasting blood sample was collected and stored at −80°C until assayed for measurement of cholesterol (total and HDL) and triglyceride levels. LDL cholesterol was calculated using the Friedewald equation. Retinopathy was assessed in subjects with diabetes only. Nephropathy was defined as a glomerular filtration rate <60 mL/min/1.73 m2.
All subjects were given standardized questionnaires to obtain demographics (age, sex, race/ethnicity, and years in school), medical history, medication use, current and past smoking status, insulin dose, and family medical history. Subjects were also asked whether they were ill within 24 h at the time of the blood draw (e.g., with a cold, flu, fever, or vomiting). Subjects were not excluded from participating if they reported being ill. A single 6-mm-thick image at the L4-L5 levels was obtained using abdominal computed tomography (CT) during suspended respiration to measure intra-abdominal and subcutaneous fat as previously described (22).
CAC measurement
Two sets of images for scoring of CAC were obtained using an ultrafast Imatron C-150XLP electron beam CT scanner (Imatron, San Francisco, CA). The CAC score using the Agatston method and the total volume score using the volumetric method were calculated from the images, as described previously (23). The volume score was used to identify progression of CAC, described below. Scans were repeated in the follow-up examination, which occurred an average of 2.5 years (range 1.1–4.3) after the baseline scans.
Subject selection
Selection of subjects for this nested case-control study occurred after the second visit, which was an average of 2.5 years (range 1.1–4.3) after the baseline. Eligible case subjects for this study were those with significant progression of CAC (n = 204), defined as an increase in volume of CAC between the baseline and follow-up visits of ≥2.5 square root transformed units. Significant regression would be a reduction in CAC volume of ≥2.5 square root transformed units, but this has not been observed in this population (CAC volume in 53 subjects decreased by less than that). Previous work has shown that this definition of change in CAC volume represents meaningful differences in CAC that are unlikely to be due to interscan variability (23,24). Prevalence of CAC at baseline was not a factor in whether a subject was eligible to be a case; thus, case subjects could have had no CAC at baseline.
Participants with no significant progression in the volume of CAC were eligible to be selected as a control subject. A binary indicator was used to identify the presence of CAC at baseline. Control subjects were frequency matched to case subjects on diabetes status, sex, and age-group at the follow-up visit (<30, 30–39, 40–59, and >60 years) and the presence of CAC at baseline (n = 258).
Laboratory measurements
Laboratory assessments were performed in the laboratory of Dr. Russell P. Tracy at the University of Vermont on the stored plasma or serum specimens obtained at baseline. Plasma or serum was deemed optimal for different analytes. IL-6 was measured using a multiplex panel (BioRad). TNF-α and sIL-1RA were measured on a cytokine panel (Millipore/Linco). Commercially available ELISA kits (R&D Systems, Alpco Diagnostics) were used to measure hsCRP, sIL-6R, soluble TNF-α receptor type II (sTNFR2), sIL-2R, IL-18, soluble intercellular adhesion molecule-1 (ICAM-1), P-selectin, soluble matrix metalloproteinase-3 (MMP-3), and osteoprotegerin (OPG). These biomarkers were chosen to represent different inflammatory pathways. Baseline measures for the inflammatory markers were used to examine the association of inflammation on the progression of CAC from baseline. Detailed information regarding the assays, range of detection, and standard values for each marker are presented in the Supplementary Data.
Statistical analyses
Distributions of all variables were examined to determine departure from normality. All of the inflammatory markers, with the exception of P-selectin, were skewed and were log-transformed for all analyses.
Differences were compared by diabetes and case status. Parametric continuous data are presented as means ± SD. Nonparametric data are presented as the median and interquartile range, with the exception of the log-transformed inflammatory markers, which are presented as the geometric mean and the interquartile range. Categorical data are presented as the number of subjects and the percent. Statistical testing to detect differences between groups included the t test for parametric continuous data, the Wilcoxon rank sum test for nonparametric data, and the χ2 test for categorical data.
Principal components analysis
Many cytokines exhibit pleiotropic and synergistic effects. Evaluating single independent markers would likely underestimate these effects; thus, a method that considers the combined influence of multiple markers, and therefore the overall inflammatory burden, would be preferable. However, combining markers into a composite measure needs to avoid overestimating the association due to the potentially high correlation between markers. We therefore used principal components analysis (PCA) with orthogonal rotation to derive uncorrelated linear transformations of the biomarkers. We considered the eigen values (≥1), scree plots, and interpretability (variables with factor loads ≥0.40) of the final solution in determining the minimum number of components to use for further analysis. Markers with factor loads ≥0.40 on multiple components (ICAM-1, OPG, and MMP-3) were dropped, and the PCA was run again. In the final PCA, the first four components explained 65% of the total variance and were used in the multivariate regression analysis.
Inflammatory marker composite score
To test a measure of the combined effects (inflammatory burden), a composite score was constructed using the component interpretation from the PCA. Subjects were assigned a score of 1 for each component in which one or more of the markers with a factor load ≥0.40 in that component had a value exceeding the 75th percentile. The scores were added across the four components to arrive at the summed composite score. Composite scores could therefore range from 0 (in which none of the markers exceeded the 75th percentile) to 4 (in which at least one marker from each component exceeded the 75th percentile). In this way, the composite score captured subjects with high levels of inflammatory markers in multiple components, without inflating the score due to the correlation between markers within components. We used the distribution of the composite score to categorize the score into the following: 0, 1–2, and 3–4.
Multivariate modeling
Conditional logistic regression to adjust for the matching of case subjects and control subjects was used to determine the independent effect of the principal components and the composite score on the progression of CAC. All continuous variables were examined for the best functional form before model testing using the −2 log-likelihood ratio test and visual inspection of plots. Potential confounding variables were considered for inclusion in the models based on a priori criteria: significance in previous work, significant contribution to the model fit (P value of the Wald χ2 <0.05), or confounding the association between the main variable of interest and the outcome by more than 10%. The age-group, diabetes status, presence of CAC at baseline, and sex were included as the conditional variables to account for the matching. Systolic blood pressure, duration of diabetes, the number of years in school, age at baseline (continuous), HbA1c, LDL cholesterol, and BMI were included in the final models. Additional variables that were considered for inclusion but did not meet the a priori criteria were race, Hispanic ethnicity, years of follow-up, smoking status, waist circumference, waist-to-hip ratio, intra-abdominal fat, subcutaneous fat, hypertension, retinopathy, nephropathy, total cholesterol, HDL, and triglycerides. Interaction terms were tested in the final models between diabetes status, sex, and race with the principal components and composite score to determine if diabetes status, sex, or race was a significant modifier of the relationship between the inflammatory markers and progression of CAC. All analyses were performed using SAS/STAT 9.2 software (SAS Institute Inc., Cary, NC).
RESULTS
Characteristics of the population stratified by diabetes status are reported in Table 1. Among subjects with type 1 diabetes, case subjects with significant CAC progression were older, had a longer duration of diabetes, higher HbA1c, higher systolic and diastolic blood pressure, and higher triglycerides. Among subjects without diabetes, case subjects had a higher BMI, higher waist circumference, and higher measures of intra-abdominal and subcutaneous fat. Among both groups, the presence of CAC at baseline was significantly associated with CAC progression. No significant differences were found in either group for sex, race, smoking status, total cholesterol, HDL, or LDL.
Table 1.
Characteristics of study subjects
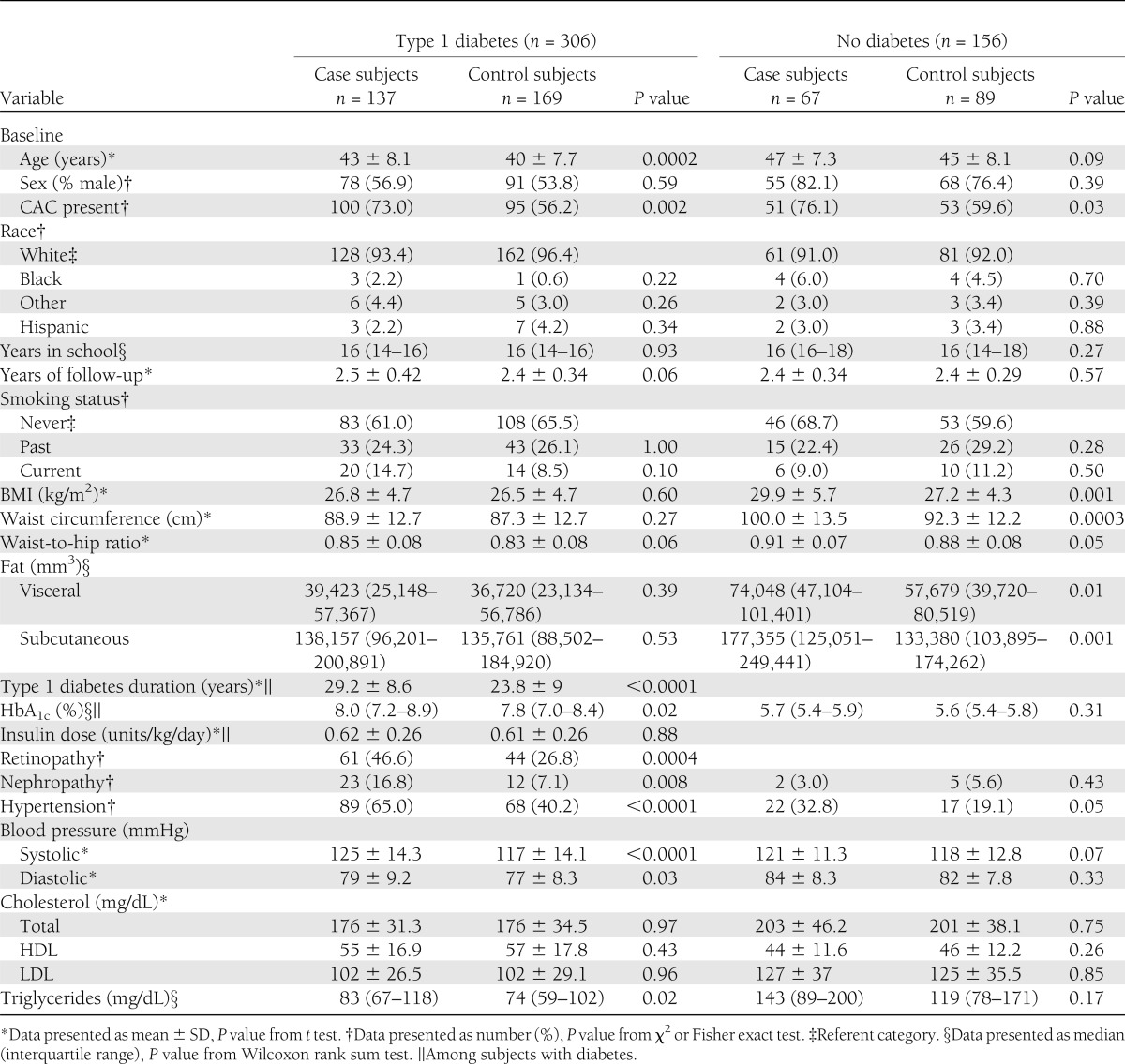
Table 2 summarizes the distributions for the inflammatory markers stratified by diabetic status. Among subjects with diabetes, geometric means of sIL-2R, TNF-α, sTNFR2, sP-selectin, and MMP-3 were higher in case subjects than in control subjects. Geometric means of IL-6 and sIL-1RA were higher in case subjects than in control subjects among those without diabetes. The means of the other markers (hsCRP, sIL-6R, IL-18, ICAM-1, and OPG) were not significantly different or of borderline significance in both groups. Relative to subjects with a composite score of 0, case subjects were more likely to have elevated biomarkers in three or four components than control subjects in those with and without diabetes. The percentage of subjects who reported being ill within 24 h of the blood draw did not significantly differ by the composite score (P = 0.14; data not shown).
Table 2.
Distribution of inflammatory markers
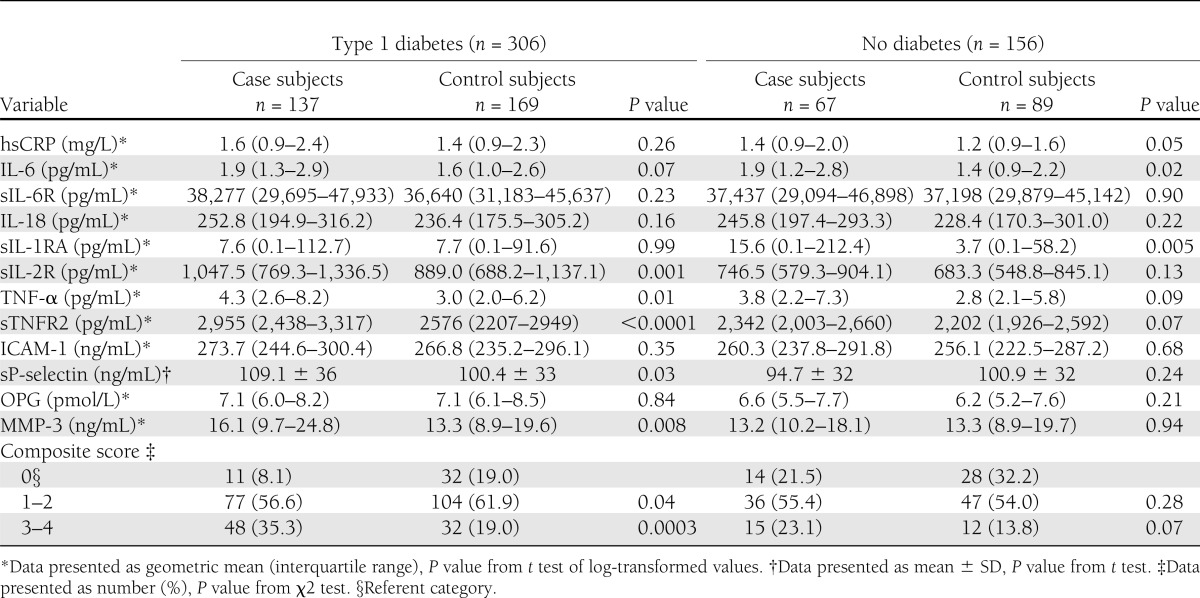
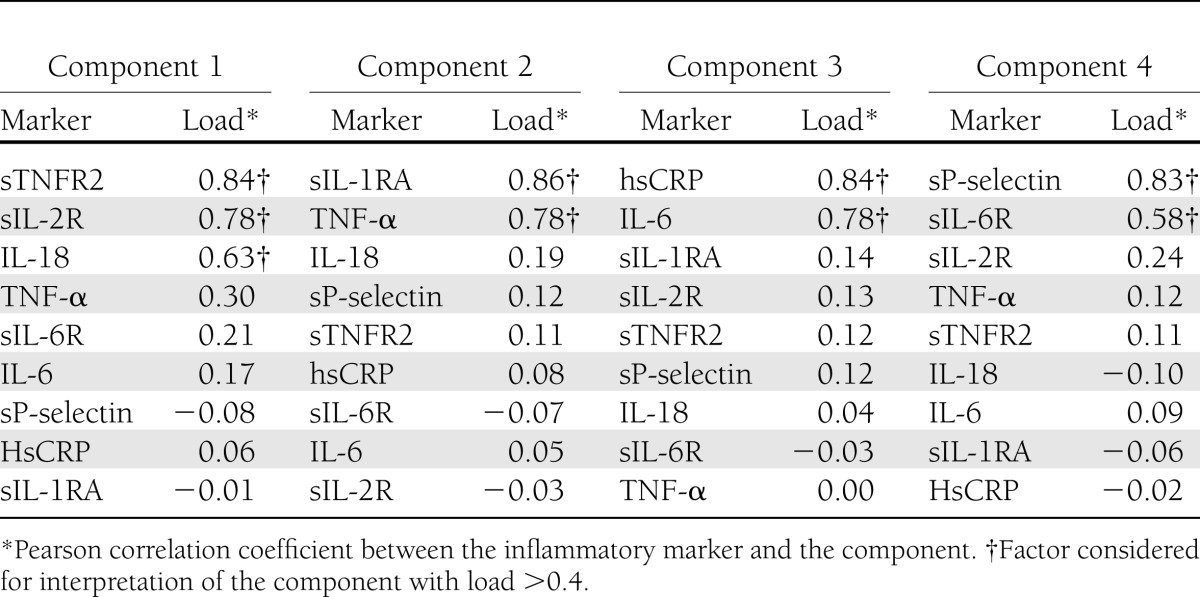
Table 3 reports the inflammatory marker patterns derived from the PCA. Values for sTNFR2, sIL-2R, and IL-18 were positively correlated with component 1; that is, subjects with higher scores on this component would have higher values of these inflammatory markers (factor loads of 0.84, 0.78, and 0.63, respectively). Similarly, scores on component 2 were correlated with the values of sIL-1RA and TNF-α (factor loads of 0.86, and 0.78, respectively). Finally, hsCRP and IL-6 were highly correlated with component 3 (factor loads of 0.84 and 0.78, respectively), and sP-selectin and sIL-6R were highly correlated with component 4 (factors loads of 0.83 and 0.58, respectively).
Table 3.
Inflammatory marker patterns derived by PCA
To determine the association of the inflammatory markers on progression of CAC, conditional logistic regression models were fit (as described above) for the principal components and the composite score as a measure of inflammatory burden. The results are presented in Table 4. In unadjusted analyses, components 1, 2, and 3 were modestly but significantly associated with progression of CAC. Component 4 was not significantly associated. After adjustment for age, systolic blood pressure, LDL, BMI, years in school, duration of diabetes, and HbA1c, the association was similar for components 1 and 2, but the association was no longer statistically significant for component 3.
Table 4.
Multiple logistic regression of principal components with progression of CAC
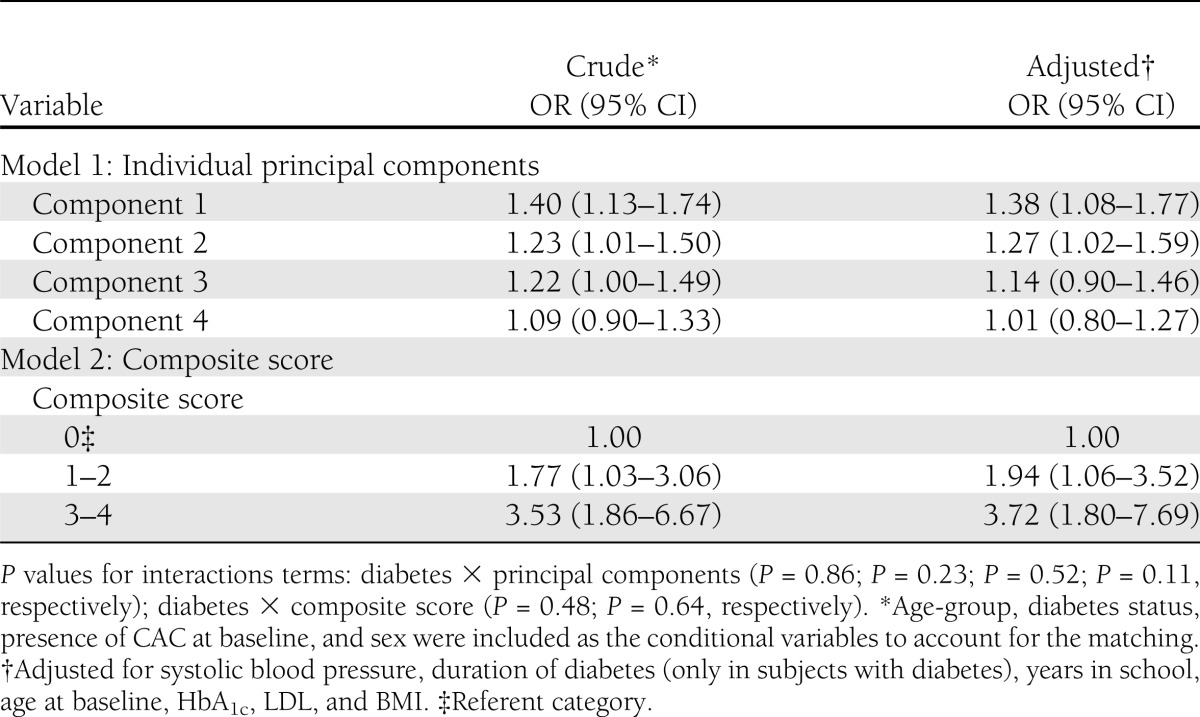
In separate models, the odds ratios (ORs) for the composite score were strongly associated with progression of CAC, even after adjustment, and showed a dose-response effect with the strongest effect of composite score 3–4. Interaction terms for diabetes, sex, and race with the principal components and for the composite score were tested in the final models. Diabetes, sex, and race were not significant modifiers (P > 0.05). Exclusion of subjects who reported being ill within 24 h of the blood draw did not substantially alter these results (data not shown).
CONCLUSIONS
These results demonstrate that markers of inflammation are prospectively associated with progression of CAC in a population of adults with and without type 1 diabetes. Two principal components were associated with the progression of CAC. The inflammatory markers that loaded strongly on these components were sTNFR2, sIL-2R, IL-18, sIL-1RA, and TNF-α. A composite score measuring inflammatory burden was also strongly associated with progression of CAC.
Prevalent coronary calcium has been associated with incident coronary heart disease events (12,13). Progression of CAC is significantly related to all-cause mortality independent of prevalent coronary calcium (14). No prospective studies have been published on the relationship between CAC and cardiac events in patients with type 1 diabetes; however, the EDC study found that CAC was strongly correlated with clinical CAD, myocardial infarction, and obstructive CAD (25). In subjects with type 1 diabetes, certain inflammatory markers (hsCRP, IL-6, and TNF-α) have been cross-sectionally associated with microvascular and macrovascular complications (5–8). In the EDC study, hsCRP was associated with an increased risk of CAD (9).
Only two prior studies from independent samples have examined the relationship between inflammation and progression of CAC (18,19) and did not find an association between hsCRP and progression (18) or incident (19) CAC after adjusting for other risk factors. Hokanson et al. (24) previously showed that interscan variability increases with increasing calcium volume scores, which may lead to biased estimates of the change in calcium scores over time. The definition of progression used for this report (≥2.5 square root transformed units) has been shown to be representative of actual progression and is <1% likely to be due to interscan variability (23,24). By using this approach, our study was better able to identify differences in markers of inflammation that were masked when using other approaches to assessing CAC progression.
A previous report from the CACTI cohort found that fibrinogen was significantly associated with the progression of CAC (OR 2.92 [95% CI 1.36–6.27]) in subjects with type 1 diabetes. In the nested case-control sample used for this study, log-transformed fibrinogen was not significantly associated with progression of CAC in the whole sample (P = 0.34) or in those with type 1 diabetes (P = 0.29; data not shown) after adjusting for other risk factors. The discrepancies are likely explained by the differences in the study designs between the two reports. The previous study involved samples from the entire cohort, and the definition of progression included subjects who developed clinical CAD during the follow-up period. For these reasons, we did not include fibrinogen in the PCA or models for this report. However, both reports lend support to the notion that an association exists between inflammation and progression of CAC.
TNFR2 is typically expressed in immune system cells and is important for the activation and proliferation of certain types of T cells (26,27). The generation of the soluble receptor, sIL-2R, depends on cell activation (28), and serum levels have been used in studies as a measure of T-cell activation (29). IL-18 was initially described as a potent inducer of interferon-γ production and was subsequently realized to be important in T-cell differentiation (30). A previous analysis of data from a smaller nested case-control sample of the CACTI cohort (98 case subjects and 173 control subjects) found a significant association between sIL-2R and progression of CAC independently of other risk factors among subjects with and without type 1 diabetes (20). In the current study, these three cytokines loaded highly on component 1 and may reflect a T-cell activation construct associated with progression of CAC.
TNF-α and sIL-1RA loaded highly on component 2. sIL-1RA is produced primarily by the liver as an acute-phase protein and acts as a natural inhibitor of the effects of IL-1β (31). The diverse effects of TNF-α include cell death and proinflammatory changes to vascular endothelial cells, which promote leukocyte adhesion and thrombosis (27). IL-1 and TNF-α act synergistically to promote the inflammatory response (30); therefore, component 2, which is significantly associated with progression of CAC, may represent IL-1/TNF-α–mediated inflammatory processes.
hsCRP is an acute-phase protein produced in response to infection or physical trauma. Production of hsCRP is predominantly controlled by IL-6 (32). IL-6 is the primary regulator of the hepatic acute-phase response, is elevated in systemic inflammation, and increases with obesity (2). Some studies have not found any association between hsCRP and prevalence or severity of CAC (17,33,34) or CAC progression (18). The study by Kronmal et al. (19) found that the association between hsCRP and incident coronary calcium was attenuated by BMI. Other studies have also found an obesity effect, albeit specific to sex, where hsCRP was significantly associated with prevalent coronary calcium in men, but not in women, after controlling for BMI (35,36). A recently published cross-sectional study by Raaz-Schrauder et al. (16) demonstrated that IL-6 was associated with prevalent coronary calcium but did not find a consistent association with hsCRP. Jenny et al. (15) found modest significant associations between prevalent coronary calcium and IL-6 and a composite score that included IL-6 and hsCRP. In our study population, hsCRP and IL-6 were most highly correlated with component 3, which may represent a construct for the acute-phase response and which was not significantly associated with progression of CAC in the adjusted model (OR 1.14 [95% CI 0.90–1.46]). Although hsCRP and IL-6 were not independently associated with progression of CAC in the current study, they did contribute to the composite score, which had a stronger association with progression of CAC than the individual principal components.
IL-6/sIL-6R trans-signaling has been shown in mice to promote endothelial adhesion molecules and macrophage infiltration into vascular lesions (37). P-selectin is an endothelial adhesion molecule that promotes leukocyte adhesion and migration (38). P-selectin and sIL-6R loaded highly on component 4 and may represent an endothelial adhesion and leukocyte migration component of CAC progression.
Similar to P-selectin, ICAM-1 promotes leukocyte adhesion and migration and has been suggested to be a biomarker of CAC burden (39). OPG is typically thought to inhibit atherosclerosis by acting as a decoy substrate for receptor activator of nuclear factor-κB ligand; however, in inflamed tissues, OPG enhanced MMP activity in vascular smooth muscle cells, which is important in atherosclerosis because MMPs degrade the matrix in atherosclerotic plaques (40). ICAM-1, MMP-3, and OPG loaded highly (factor loads >0.4) on multiple components, were dropped from the PCA analysis, and did not contribute to the composite score.
This study has several limitations. Not all potentially influential inflammatory markers were measured. However, owing to the common biologic pathways of many cytokines, we chose to combine markers into a composite score, which could strengthen a single measure with multiple possible pathways. These data were collected as part of a nested case-control study. Some bias might have existed in the selection of subjects for this sample. There were too few controls to get a true one-to-one match on age, sex, diabetes status, and baseline CAC. Frequency matching resulted in a sample of control subjects that did not exactly match the case subjects on these important variables. However, we adjusted for the sampling using conditional logistic regression and additionally controlled for age as a continuous variable in the final models.
In summary, this study demonstrated a significant prospective association between inflammatory markers and progression of CAC in a population of adults with and without type 1 diabetes. The PCA identified two components, potentially representing T-cell activation and IL-1/TNF-α–mediated inflammatory processes, that were independently but modestly associated with progression of CAC. Subjects with at least one elevated marker in three to four of the components were 3.7-times more likely to experience progression of CAC during the follow-up period. These results lend support to the idea that measurement of inflammatory markers may improve risk estimation of CVD progression. They also demonstrate that consideration should be made for inflammatory burden when considering the association of inflammatory markers and CAC. Considering the increased burden of CVD in the type 1 diabetic population, anti-inflammatory therapies could prove to be a valuable tool in this population.
Acknowledgments
The study was performed at the Barbara Davis Center for Childhood Diabetes in Denver, Colorado, and at the Clinical Translational Research Center (CTRC) at the University of Colorado Hospital. Support was provided by the National Institutes of Health (NIH) National Heart, Lung, and Blood Institute grants R01 HL-61753 and R01 HL-079611, American Diabetes Association postdoctoral fellowship 7-09-CVD-06 to A.C.A., American Diabetes Association Junior Faculty Award 1-10-JF-50 to J.K.S.-B., and Diabetes Endocrinology Research Center Clinical Investigation Core P30 DK-57516. The study performed at the CTRC at the University of Colorado Denver was supported by NIH Grant M01 RR-000051.
No potential conflicts of interest relevant to this article were reported.
A.C.A. analyzed the data and wrote the manuscript. G.L.K. and J.K.S.-B. designed the case/control study, collected data, assisted in the analysis, and edited the manuscript. R.P.T. designed the case/control study, performed the marker assays, and edited the manuscript. D.M.M., J.E.H., and M.J.R. designed the case/control study and edited the manuscript. J.K.S.-B. is the guarantor for this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1874/-/DC1.
References
- 1.Laing SP, Swerdlow AJ, Slater SD, et al. Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia 2003;46:760–765 [DOI] [PubMed] [Google Scholar]
- 2.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis 2000;148:209–214 [DOI] [PubMed] [Google Scholar]
- 3.Gomes MB, Piccirillo LJ, Nogueira VG, Matos HJ. Acute-phase proteins among patients with type 1 diabetes. Diabetes Metab 2003;29:405–411 [DOI] [PubMed] [Google Scholar]
- 4.Devaraj S, Glaser N, Griffen S, Wang-Polagruto J, Miguelino E, Jialal I. Increased monocytic activity and biomarkers of inflammation in patients with type 1 diabetes. Diabetes 2006;55:774–779 [DOI] [PubMed] [Google Scholar]
- 5.Targher G, Bertolini L, Zoppini G, Zenari L, Falezza G. Increased plasma markers of inflammation and endothelial dysfunction and their association with microvascular complications in type 1 diabetic patients without clinically manifest macroangiopathy. Diabet Med 2005;22:999–1004 [DOI] [PubMed] [Google Scholar]
- 6.Devaraj S, Cheung AT, Jialal I, et al. Evidence of increased inflammation and microcirculatory abnormalities in patients with type 1 diabetes and their role in microvascular complications. Diabetes 2007;56:2790–2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schram MT, Chaturvedi N, Schalkwijk CG, Fuller JH, Stehouwer CD, EURODIAB Prospective Complications Study Group Markers of inflammation are cross-sectionally associated with microvascular complications and cardiovascular disease in type 1 diabetes—the EURODIAB Prospective Complications Study. Diabetologia 2005;48:370–378 [DOI] [PubMed] [Google Scholar]
- 8.Hayaishi-Okano R, Yamasaki Y, Katakami N, et al. Elevated C-reactive protein associates with early-stage carotid atherosclerosis in young subjects with type 1 diabetes. Diabetes Care 2002;25:1432–1438 [DOI] [PubMed] [Google Scholar]
- 9.Miller RG, Costacou T, Orchard TJ. Lipoprotein-associated phospholipase A2, C-reactive protein, and coronary artery disease in individuals with type 1 diabetes and macroalbuminuria. Diab Vasc Dis Res 2010;7:47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopes-Virella MF, Carter RE, Gilbert GE, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Intervention and Complications Cohort Study Group Risk factors related to inflammation and endothelial dysfunction in the DCCT/EDIC cohort and their relationship with nephropathy and macrovascular complications. Diabetes Care 2008;31:2006–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeboah J, McClelland RL, Polonsky TS, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA 2012;308:788–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Church TS, Levine BD, McGuire DK, et al. Coronary artery calcium score, risk factors, and incident coronary heart disease events. Atherosclerosis 2007;190:224–231 [DOI] [PubMed] [Google Scholar]
- 13.Taylor AJ, Fiorilli PN, Wu H, et al. Relation between the Framingham Risk Score, coronary calcium, and incident coronary heart disease among low-risk men. Am J Cardiol 2010;106:47–50 [DOI] [PubMed] [Google Scholar]
- 14.Budoff MJ, Hokanson JE, Nasir K, et al. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging 2010;3:1229–1236 [DOI] [PubMed] [Google Scholar]
- 15.Jenny NS, Brown ER, Detrano R, et al. Associations of inflammatory markers with coronary artery calcification: results from the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 2010;209:226–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raaz-Schrauder D, Klinghammer L, Baum C, et al. Association of systemic inflammation markers with the presence and extent of coronary artery calcification. Cytokine 2012;57:251–257 [DOI] [PubMed] [Google Scholar]
- 17.Hamirani YS, Pandey S, Rivera JJ, et al. Markers of inflammation and coronary artery calcification: a systematic review. Atherosclerosis 2008;201:1–7 [DOI] [PubMed] [Google Scholar]
- 18.Rasouli ML, Nasir K, Blumenthal RS, Park R, Aziz DC, Budoff MJ. Plasma homocysteine predicts progression of atherosclerosis. Atherosclerosis 2005;181:159–165 [DOI] [PubMed] [Google Scholar]
- 19.Kronmal RA, McClelland RL, Detrano R, et al. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2007;115:2722–2730 [DOI] [PubMed] [Google Scholar]
- 20.Wadwa RP, Kinney GL, Ogden L, et al. Soluble interleukin-2 receptor as a marker for progression of coronary artery calcification in type 1 diabetes. Int J Biochem Cell Biol 2006;38:996–1003 [DOI] [PubMed] [Google Scholar]
- 21.Rodrigues TC, Snell-Bergeon JK, Maahs DM, Kinney GL, Rewers M. Higher fibrinogen levels predict progression of coronary artery calcification in adults with type 1 diabetes. Atherosclerosis 2010;210:671–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dabelea D, Kinney G, Snell-Bergeon JK, et al. Coronary Artery Calcification in Type 1 Diabetes Study Effect of type 1 diabetes on the gender difference in coronary artery calcification: a role for insulin resistance? The Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study. Diabetes 2003;52:2833–2839 [DOI] [PubMed] [Google Scholar]
- 23.Snell-Bergeon JK, Hokanson JE, Jensen L, et al. Progression of coronary artery calcification in type 1 diabetes: the importance of glycemic control. Diabetes Care 2003;26:2923–2928 [DOI] [PubMed] [Google Scholar]
- 24.Hokanson JE, MacKenzie T, Kinney G, et al. Evaluating changes in coronary artery calcium: an analytic method that accounts for interscan variability. AJR Am J Roentgenol 2004;182:1327–1332 [DOI] [PubMed] [Google Scholar]
- 25.Olson JC, Edmundowicz D, Becker DJ, Kuller LH, Orchard TJ. Coronary calcium in adults with type 1 diabetes: a stronger correlate of clinical coronary artery disease in men than in women. Diabetes 2000;49:1571–1578 [DOI] [PubMed] [Google Scholar]
- 26.Kim EY, Priatel JJ, Teh SJ, Teh HS. TNF receptor type 2 (p75) functions as a costimulator for antigen-driven T cell responses in vivo. J Immunol 2006;176:1026–1035 [DOI] [PubMed] [Google Scholar]
- 27.Bradley JR. TNF-mediated inflammatory disease. J Pathol 2008;214:149–160 [DOI] [PubMed] [Google Scholar]
- 28.Rubin LA, Nelson DL. The soluble interleukin-2 receptor: biology, function, and clinical application. Ann Intern Med 1990;113:619–627 [DOI] [PubMed] [Google Scholar]
- 29.Takeshita S, Isshiki T, Ochiai M, et al. Systemic inflammatory responses in acute coronary syndrome: increased activity observed in polymorphonuclear leukocytes but not T lymphocytes. Atherosclerosis 1997;135:187–192 [DOI] [PubMed] [Google Scholar]
- 30.Dinarello CA. Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. Am J Clin Nutr 2006;83:447S–455S [DOI] [PubMed] [Google Scholar]
- 31.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev 2002;13:323–340 [DOI] [PubMed] [Google Scholar]
- 32.Jialal I, Devaraj S, Venugopal SK. C-reactive protein: risk marker or mediator in atherothrombosis? Hypertension 2004;44:6–11 [DOI] [PubMed] [Google Scholar]
- 33.Khera A, de Lemos JA, Peshock RM, et al. Relationship between C-reactive protein and subclinical atherosclerosis: the Dallas Heart Study. Circulation 2006;113:38–43 [DOI] [PubMed] [Google Scholar]
- 34.Reilly MP, Wolfe ML, Localio AR, Rader DJ, Study of Inherited Risk of Coronary Atherosclerosis C-reactive protein and coronary artery calcification: the Study of Inherited Risk of Coronary Atherosclerosis (SIRCA). Arterioscler Thromb Vasc Biol 2003;23:1851–1856 [DOI] [PubMed] [Google Scholar]
- 35.Colhoun HM, Schalkwijk C, Rubens MB, Stehouwer CD. C-reactive protein in type 1 diabetes and its relationship to coronary artery calcification. Diabetes Care 2002;25:1813–1817 [DOI] [PubMed] [Google Scholar]
- 36.Wang TJ, Larson MG, Levy D, et al. C-reactive protein is associated with subclinical epicardial coronary calcification in men and women: the Framingham Heart Study. Circulation 2002;106:1189–1191 [DOI] [PubMed] [Google Scholar]
- 37.Schuett H, Oestreich R, Waetzig GH, et al. Transsignaling of interleukin-6 crucially contributes to atherosclerosis in mice. Arterioscler Thromb Vasc Biol 2012;32:281–290 [DOI] [PubMed] [Google Scholar]
- 38.Blann AD, Nadar SK, Lip GY. The adhesion molecule P-selectin and cardiovascular disease. Eur Heart J 2003;24:2166–2179 [DOI] [PubMed] [Google Scholar]
- 39.Tang W, Pankow JS, Carr JJ, et al. Association of sICAM-1 and MCP-1 with coronary artery calcification in families enriched for coronary heart disease or hypertension: the NHLBI Family Heart Study. BMC Cardiovasc Disord 2007;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandberg WJ, Yndestad A, Øie E, et al. Enhanced T-cell expression of RANK ligand in acute coronary syndrome: possible role in plaque destabilization. Arterioscler Thromb Vasc Biol 2006;26:857–863 [DOI] [PubMed] [Google Scholar]


