Abstract
OBJECTIVE
To 1) determine if plasma 25-hydroxyvitamin D (25[OH]D) concentrations differ among obese youth with normal glucose tolerance (NGT) versus prediabetes versus type 2 diabetes and 2) assess the relationships between 25(OH)D and in vivo insulin sensitivity and β-cell function in this cohort.
RESEARCH DESIGN AND METHODS
Plasma 25(OH)D concentrations were examined in banked specimens in 9- to 20-year-old obese youth (n = 175; male 42.3%, black 46.3%) (NGT, n = 105; impaired glucose tolerance [IGT], n = 43; type 2 diabetes, n = 27) who had in vivo insulin sensitivity and secretion measured by hyperinsulinemic-euglycemic and hyperglycemic clamp techniques and had an assessment of total body composition and abdominal adiposity.
RESULTS
The mean age and BMI of the subjects were 14.3 ± 2.1 years and 35.7 ± 5.6 kg/m2, respectively. BMI, plasma 25(OH)D, and the proportion of vitamin D–deficient and –insufficient children did not differ across the three groups. Furthermore, there was no association between 25(OH)D and in vivo insulin sensitivity or β-cell function relative to insulin sensitivity (disposition index) in all groups combined or in each group separately.
CONCLUSIONS
Our data in obese youth show 1) no differences in plasma 25(OH)D concentrations across the glucose tolerance groups and 2) no relationship between 25(OH)D and in vivo insulin sensitivity and β-cell function relative to insulin sensitivity in any of the groups. It remains uncertain if enhancement of the vitamin D status could improve pathophysiological mechanisms of prediabetes and type 2 diabetes in obese youth.
The role of vitamin D in glucose homeostasis remains controversial. The reported relationships between vitamin D and glucose homeostasis have been inconsistent (1–6). Differences in study populations (glucose tolerant vs. intolerant; obese vs. nonobese; children vs. adults), the methodological approaches to the assessment of insulin sensitivity and secretion (surrogate indices vs. clamp studies), and adiposity measures (BMI vs. direct measures of total and/or regional adiposity) may explain some of the inconsistent findings among these studies. In healthy, glucose-tolerant, nonobese and obese youth, we found no independent relationships between plasma 25-hydroxyvitamin D (25[OH]D) and in vivo insulin sensitivity and β-cell function relative to insulin sensitivity (5). However, it remains to be determined if the vitamin D–glucose homeostasis relationships differ under pathophysiological conditions of glucose intolerance. Therefore, we 1) examined if plasma 25(OH)D concentrations differ among obese youth with normal glucose tolerance (NGT) versus prediabetes versus type 2 diabetes and 2) assessed the relationships between 25(OH)D and the pathophysiological components of type 2 diabetes, in vivo insulin sensitivity, and β-cell function.
RESEARCH DESIGN AND METHODS
Subjects
Plasma 25(OH)D concentrations were analyzed in banked specimens in 175 obese 9- to <20-year-old black and white youth (NGT, n = 105; impaired glucose tolerance [IGT], n = 43; type 2 diabetes, n = 27). Subjects had complete data on in vivo insulin sensitivity and secretion measured by the hyperinsulinemic-euglycemic and hyperglycemic clamp. Twenty-two adolescents with NGT and 26 with IGT had untreated polycystic ovary syndrome (PCOS). Subjects with NGT and IGT had fasting glucose <5.6 mmol/L and 2-h glucose levels of <7.8 mmol/L and 7.8–11.0 mmol/L, respectively, during an oral glucose tolerance test (OGTT). They were not on any medications that affected glucose metabolism. The adolescents with type 2 diabetes were clinically diagnosed according to American Diabetes Association and World Health Organization criteria (7), with negative glutamic acid decarboxylase and insulinoma-associated protein 2 (determined by DK harmonization assay) autoantibodies (8). They were on treatments consisting of lifestyle changes alone (n = 7), metformin (n = 14), metformin + insulin (n = 4), or insulin alone (n = 2). Metformin and long-acting insulin were discontinued 48 h before the clamp studies.
Participants were enrolled in the National Institutes of Health (NIH)–funded K24 Childhood Insulin Resistance and R01 Childhood Metabolic Markers of Adult Morbidity in Blacks studies in Pittsburgh, PA (latitude, 40.4° north). Data from some of the participants were reported previously (5,9,10). In the current dataset, 88 of the obese NGT participants’ data for 25(OH)D and insulin sensitivity and secretion were reported in our previous publication, which, in addition, included healthy prepubertal normal-weight youth (5). Vitamin D data of subjects with dysglycemia (IGT = 43 and type 2 DM = 27) have not been published before. The studies were approved by the institutional review board of the University of Pittsburgh. Signed parental informed consent and participant assent were obtained prior to participation.
Study design
Research procedures were completed at the Children’s Hospital of Pittsburgh NIH-funded Pediatric Clinical and Translational Research Center (PCTRC). Subjects underwent medical assessment by history and physical examination including pubertal staging according to Tanner criteria and routine hematological and biochemical testing. Weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively, using a weighing balance and wall-mounted stadiometer. Age- and sex-based BMI cutoffs were used for categorizing children as obese (≥95th percentile).
Body composition and visceral fat distribution
Body composition (fat mass and percent of body fat) was assessed in 166 subjects by dual-energy X-ray absorptiometry (DEXA) as described previously (11). Abdominal subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) were measured in 140 subjects by a 10-mm single axial computed tomography (CT) scan at the L4–5 intervertebral space as previously reported (11,12), and in 26 subjects with magnetic resonance imaging (MRI) (13). Data are not available for subjects who exceeded the weight limit for DEXA scan, CT scan, or MRI (n = 9).
Clamp studies
A 3-h hyperinsulinemic-euglycemic clamp (14,15) and a 2-h hyperglycemic clamp (15,16) were performed in a random sequence within a 1–4-week period at the PCTRC in all participants (n = 175) after 10–12 h of overnight fasting as described previously. Each participant underwent a 2-h OGTT (1.75 g/kg of glucola [max 75 g]) the day before the first clamp to assess glucose tolerance status.
In vivo insulin sensitivity
A 3-h hyperinsulinemic-euglycemic clamp with crystalline insulin (Humulin; Eli Lilly, Indianapolis, IN) was infused at a constant rate of 80 mU/m2/min to suppress hepatic glucose production as described previously (14–17). Plasma glucose was clamped at 5.6 mmol/L with a variable rate infusion of 20% dextrose based on arterialized plasma glucose determinations every 5 min.
In vivo insulin secretion
First- and second-phase insulin secretion were measured during a 2-h hyperglycemic clamp (15). Plasma glucose was rapidly raised to 12.5 mmol/L by a bolus infusion of dextrose and maintained at that level by a variable rate infusion of 20% dextrose for 2 h (15).
Biochemical measurements
Plasma glucose was measured using a glucose analyzer (YSI, Yellow Springs, OH), and insulin concentrations were measured by radioimmunoassay (17). Hemoglobin A1c (HbA1c) was measured by high-performance liquid chromatography (Tosoh Medics, Inc.). Plasma 25(OH)D was measured in M.F.H.’s laboratory at Boston University using a noncommercial 25(OH)D competitive protein binding assay as described previously (18). The 25(OH)D competitive protein binding assay recognizes both 25(OH)D2 and 25(OH)D3 equally well and has been validated by liquid chromatography tandem mass spectrophotometry, which measures the contributions of serum 25(OH)D2 and 25(OH)D3 (19). The intra-assay and interassay coefficients of variation of the 25(OH)D assay were 8 and 10%, respectively. The lower limit of detection was 10 nmol/L.
Calculations
Insulin-stimulated glucose disposal was calculated using the average exogenous glucose infusion rate during the final 30 min of the 3-h hyperinsulinemic-euglycemic clamp as previously described (17). Insulin sensitivity was calculated by dividing the insulin-stimulated glucose disposal rate by steady-state plasma insulin concentrations, during the last 30 min of the clamp multiplied by 100 (16,17). The first-phase insulin concentration was calculated as the mean of five determinations from 2.5 to 12.5 min, and second-phase insulin concentration was calculated as the mean of eight determinations from 15 to 120 min of the 2-h hyperglycemic clamp (16). Disposition index (DI) was calculated as the product of insulin sensitivity from the euglycemic clamp and first-phase insulin concentration (15).
Statistical analysis
Differences in clinical characteristics, body composition, and metabolic parameters among the glucose tolerance groups (NGT, IGT, and type 2 diabetes) were examined by ANOVA with Bonferroni post hoc correction or Kruskal-Wallis test for quantitative variables depending on data distribution and χ2 test or Fisher exact test for categorical variables. Pearson or Spearman correlation, depending on data distribution, was used to assess bivariate relationships between 25(OH)D and clamp-derived measures (insulin sensitivity, first-phase insulin, and DI). The independent effect of plasma 25(OH)D on insulin sensitivity, first-phase insulin, and DI was examined through multiple regression analysis adjusting for age, race, Tanner stage, season of assessment, sex, and any one of the adiposity measures (BMI, fat mass, VAT, or SAT). In the model examining the independent effect of plasma 25(OH)D on insulin secretion, further adjustments were also made for insulin sensitivity. All statistical assumptions were met. Continuous variables are expressed as mean ± SD or median and interquartile range (IQR) depending on data distribution. Statistical significance was set at P < 0.05. The statistical analysis was performed using PASW Statistics (version 18; SPSS Inc., Chicago, IL).
RESULTS
A total of 175 black (n = 81 [46.3%]) and white (n = 94 [53.7%]) 9- to <20-year-old obese youth were studied during winter/spring (n = 84 [48%]) or summer/fall (n = 91 [52%]). The study cohort included 42% males and had a mean age (±SD) of 14.3 ± 2.1 years. The glucose tolerance status was as follows: NGT, n = 105 (60%); IGT, n = 43 (25%); and type 2 diabetes, n = 27 (15%). The mean (±SD) plasma 25(OH)D concentration was 43.5 ± 19.0 nmol/L; vitamin D status was classified as follows: deficient (<50 nmol/L), n = 121 (69.1%); insufficient (50 to <75 nmol/L), n = 40 (22.9%); and sufficient (≥75 nmol/L), n = 14 (8.0%) (20).
Clinical characteristics, body composition, and metabolic profile across the spectrum of glucose tolerance
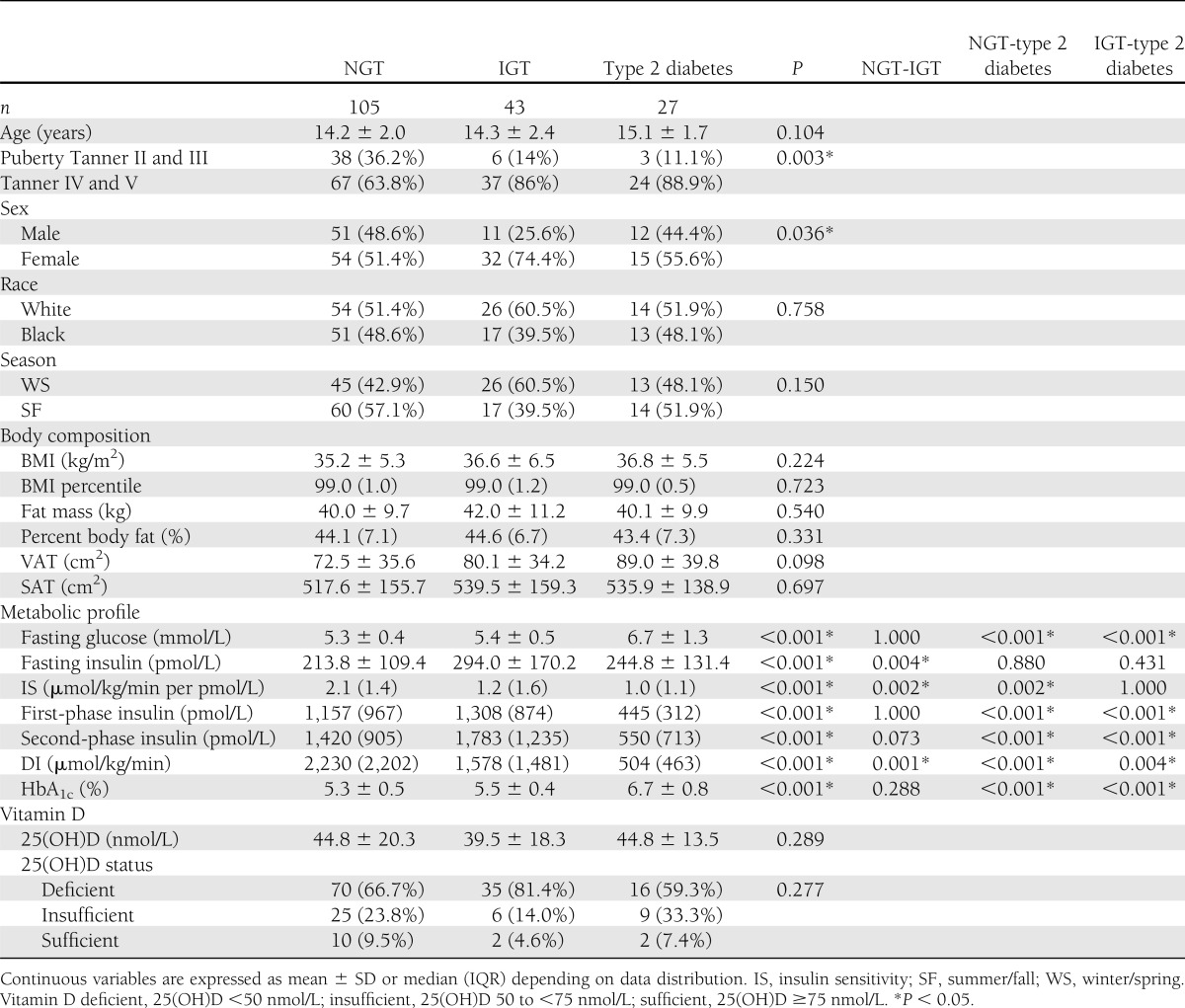
There were no differences among the groups in age, race, or season of assessment. Participants with dysglycemia (IGT or type 2 diabetes) had a higher proportion of subjects with advanced pubertal status. All participants were obese, with no significant differences in adiposity measures (BMI, BMI percentile, fat mass, percent body fat, VAT, and SAT) among the three groups. There were no significant differences in circulating 25(OH)D concentrations among NGT versus IGT versus type 2 diabetes or vitamin D status among the three groups (Table 1).
Table 1.
Clinical characteristics, body composition, metabolic profile, and plasma 25(OH)D in obese adolescents with NGT, IGT, and type 2 diabetes
Glucose homeostasis parameters in vitamin D–deficient and –nondeficient subjects
When comparing vitamin D–deficient (<50 nmol/L) versus –nondeficient (≥50 nmol/L) participants, there were no differences in in vivo insulin sensitivity (µmol/kg/min per pmol/L) (median 1.8 [IQR 1.6] vs. 1.6 [1.1], P = 0.759], first-phase insulin (pmol/L) (926.4 [984] vs. 765.3 [904.6], P = 0.213), or DI (µmol/kg/min) (1,641.3 [1,629.1] vs. 1,387.5 [1,789.1], P = 0.423). Further, there were no differences in OGTT glucose area under the curve (mmol/L) (median 937.1 [IQR 388.8] vs. 918.1 [368.1], P = 0.540) and insulin area under the curve (pmol/L) (116,401.5 [125,956.5] vs. 123,102.0 [130,179.0], P = 0.417) between vitamin D–deficient and –nondeficient subjects. The results for clamp-derived and OGTT-derived analyses were similar when the comparisons were performed separately in each glucose tolerance group (data not shown).
Association between 25(OH)D and in vivo insulin sensitivity, first-phase insulin, and DI
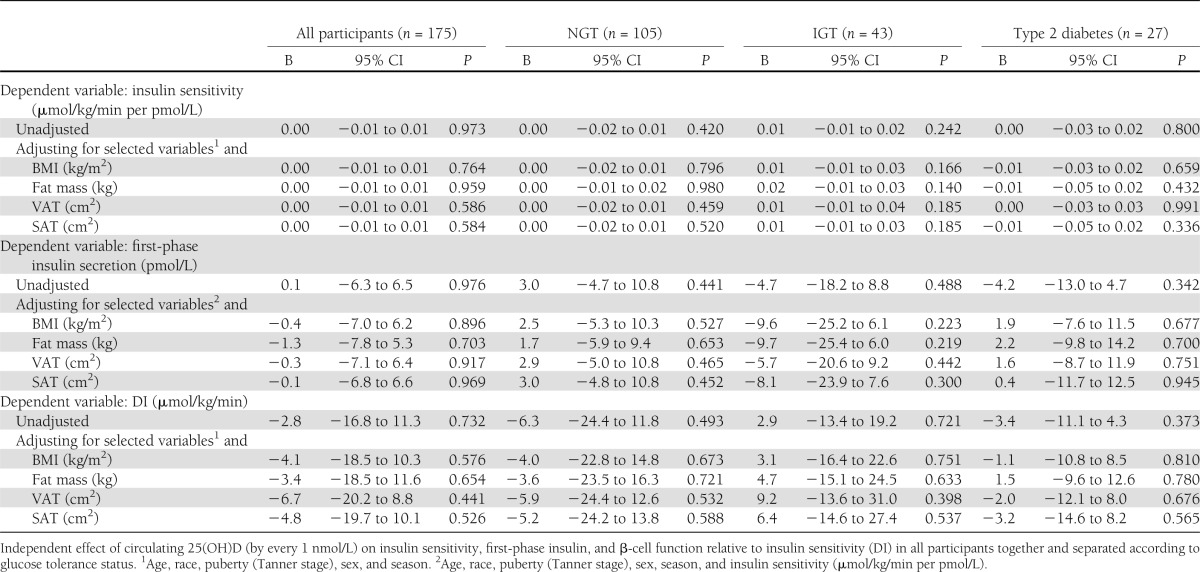
Circulating 25(OH)D did not correlate with in vivo insulin sensitivity, first-phase insulin, or DI in all participants combined or within each glucose tolerance group separately (Supplementary Fig. 1). The results did not differ when analyzing males and females separately (data not shown). Plasma 25(OH)D was not associated with in vivo insulin sensitivity, first-phase insulin, or DI after adjusting for age, race, Tanner stage, sex, season of assessment, and adiposity (and insulin sensitivity in the models for insulin secretion) in all participants together or in each group separately (NGT, IGT, and type 2 diabetes) (Table 2). The results did not change after excluding the PCOS subjects (Supplementary Table 1).
Table 2.
Multiple linear regression analysis
CONCLUSIONS
The present investigation of obese youth showed 1) no differences in circulating 25(OH)D among NGT versus IGT versus type 2 diabetes, and 2) no relationship between 25(OH)D concentrations and pathophysiological components of type 2 diabetes, in vivo insulin sensitivity, and β-cell function relative to insulin sensitivity.
Contrary to our findings, Chiu et al. (21) reported an independent positive association between serum 25(OH)D and the insulin sensitivity index (ISI), derived from the hyperglycemic clamp, in glucose-tolerant, multiethnic young adults, after adjusting for confounders. However, plasma 25(OH)D was not independently associated with the first- or second-phase insulin response. In a follow-up study of this cohort with inclusion of additional subjects and reexamining the plasma 25(OH)D concentration with the liquid chromatography tandem mass spectrophotometry assay, Karnchanasorn et al. (22) reported a favorable association between plasma 25(OH)D and β-cell function contrary to their prior findings. Plasma 25(OH)D concentrations were positively associated with β-cell function adjusted for the ISI, and these associations remained significant after adjustment for confounders. The discordance in the findings between our study and the latter two studies could be due to the differences in mean age (14 vs. 26 years), BMI (35 vs. 25 kg/m2), and the measure of adiposity. Whereas we used total body adiposity measured by DEXA and/or abdominal visceral or subcutaneous adiposity, they only had BMI as a measure of adiposity. In our previous study (5) of healthy glucose-tolerant youth, the unadjusted positive association between 25(OH)D and in vivo insulin sensitivity was nullified when adjusted for fat mass, VAT, or SAT, in agreement with other studies (23,24). Furthermore, BMI does not necessarily robustly reflect total or abdominal adiposity, especially in different ethnic groups (25), thus potentially explaining the contrast in the findings.
On the other hand, and consistent with our findings, Lamendola et al. (26), using the insulin suppression test, found no difference in plasma 25(OH)D concentrations between insulin-sensitive and insulin-resistant obese adults matched for BMI. Furthermore, similar to our data, in a cohort of nondiabetic obese adults, the positive association between 25(OH)D and insulin sensitivity, measured by the hyperinsulinemic-euglycemic clamp, was confounded by BMI and negated when controlled for BMI (24). In a nested case-control study of glucose-tolerant adults, comparing the 25(OH)D and hyperglycemic clamp–derived ISI revealed that vitamin D–sufficient subjects had a higher ISI than vitamin D–deficient subjects, but adjustment for physical activity negated this difference (18). Most importantly, however, the authors reported, in a randomized placebo-controlled trial, that 20,000 IU of vitamin D3 twice weekly for 6 months in vitamin D–deficient adults failed to improve insulin sensitivity or secretion (23).
The majority of the data reporting a favorable 25(OH)D-glucose homeostasis relationship in children used surrogate indices of insulin sensitivity and controlled for the influence of adiposity by using BMI instead of direct measures of total and/or regional adiposity (1,6). The association between adiposity and vitamin D is well established (10,27), and increasing evidence suggests that the observed relationship between 25(OH)D and glucose homeostasis is mediated through adiposity (5,24). In a cross-sectional study of 1,882 nondiabetic subjects, the significant inverse association between 25(OH)D and insulin resistance indices (fasting glucose, serum insulin, and homeostasis model assessment of insulin resistance) present with the adjustment for BMI attenuated after adjustment for SAT and disappeared after adjustment for VAT (27). These findings highlight the important mediating effect of abdominal adiposity in the relationship between vitamin D status and glucose homeostasis. Our participant characteristics explain the absence of an association between adiposity and 25(OH)D. All subjects were markedly obese (mean BMI 35.7 ± 5.6 kg/m2) and mostly vitamin D deficient (69%) or insufficient (23%). However, in our prior work, which included normal-weight and obese children, we demonstrated an important inverse relationship between adiposity and 25(OH)D concentrations, particularly between abdominal adiposity and 25(OH)D (10).
In the current study, 48 girls with untreated PCOS (22 with NGT and 26 with IGT) were included. Some authors have postulated a role for vitamin D in the pathogenesis of insulin resistance associated with PCOS (28). The only study addressing this with clamp-derived glucose homeostasis parameters and direct measures of adiposity concluded that 25(OH)D deficiency in PCOS does not directly affect the development of insulin resistance but rather results from the presence of obesity, independent of PCOS (29). Nevertheless, excluding the PCOS subjects in our study did not change the results.
The strengths of our study are the accurate assessment of total and regional adiposity and state-of-the-art measures of insulin sensitivity and β-cell function. Furthermore, the homogeneous nature of our three groups in terms of adiposity and BMI across the spectrum of glucose tolerance enhances the possibility of finding an independent association between 25(OH)D and glucose homeostasis. However, it is possible that an overall overrepresentation of vitamin D–deficient and –insufficient subjects in our cohort may have resulted in a lack of a relationship between 25(OH)D and glucose homeostasis parameters. The overrepresentation of recognized risk factors for vitamin D deficiency in our sample (obesity, 100%; puberty, 100%; black race, 46%) may explain the low percentage of vitamin D–sufficient youth in our study. However, our study cohort reflects the clinical picture of youth with dysglycemia, as adolescents with IGT or type 2 diabetes are typically pubertal, obese, and overrepresented by blacks. The relatively small numbers of youth with IGT and type 2 diabetes and the resultant unequal glucose tolerance group sizes could also be a limiting factor. However, this is unlikely because there were no statistical trends for differences in 25(OH)D concentrations among the three groups or for any relationships between 25(OH)D and glucose homeostasis parameters. Moreover, power analysis shows that in the most unfavorable case (n = 27 participants in each group), the power would be 0.9999. Furthermore, irrespective of group size, the proportion of children classified as deficient, insufficient, or sufficient were similar across the three glucose tolerance groups.
Our study has several limitations, including the cross-sectional study design, lack of information regarding participant dietary intake of vitamin D and calcium, sunlight exposure, skin pigmentation, physical activity and dietary caloric intake, and concentrations of parathyroid hormone, 1,25-dihydroxyvitamin D, and vitamin D–binding protein. Prospective long-term studies in adults have shown an inverse association between hypovitaminosis D and subsequent risk of fasting hyperglycemia and insulin resistance (homeostasis model assessment of insulin resistance) after 5 (30) and 10 years (3) of follow-up. Our cross-sectional study design precludes us from inferring the potential time-dependent effects of vitamin D deficiency on glucose homeostasis, as it is possible that vitamin D deficiency, when long standing, can be detrimental to glucose homeostasis. Also, the vitamin D deficiency in our study population may not have been severe or long standing enough to impact insulin secretion or sensitivity.
In conclusion, our data show no differences in plasma 25(OH)D concentrations among obese youth across the glucose tolerance categories, and no relationship to in vivo insulin sensitivity and β-cell function relative to insulin sensitivity. Future randomized, controlled trials in obese vitamin D–deficient youth, with or without dysglycemia, should examine if vitamin D replenishment could improve insulin sensitivity and β-cell function and lessen the metabolic risk for type 2 diabetes.
Acknowledgments
This project was supported by National Institutes of Health grants 1UL1-RR-025771 CTSI, K24-HD-01357 (S.A.A.), K23-HD-052550 (K.R.), MO1-RR-00084 (General Clinical Research Center), UL1-RR-024153, and UL1-TR-000005; the United States Public Health Service Grant RO1-HD-27503 (S.A.A.); the Thrasher Research Fund (F.B.); the Richard L. Day Endowed Chair (S.A.A.); and the Río Hortega contract from the Instituto de Salud Carlos III of the Spanish Ministry of Health (CM07/00211 to J.d.l.H.).
No potential conflicts of interest relevant to this article were reported.
J.d.l.H. analyzed and interpreted data and wrote the manuscript. K.R. interpreted data and wrote the manuscript. S.J.L. conducted analyses of abdominal CT and MRI. F.B. contributed participants and reviewed the manuscript. M.F.H. conducted the 25(OH)D assays and provided review of the manuscript. S.A.A. conceived and designed the study; acquired, analyzed, and interpreted data; obtained funding; provided administrative, technical, and material support and study supervision; drafted and critically revised the manuscript for important intellectual content; and was primarily responsible for the final manuscript content. All authors read and approved the final draft of the manuscript. S.A.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 93rd Annual Meeting of The Endocrine Society, Boston, Massachusetts, 4–7 June 2011, and the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011.
These studies would not have been possible without the nursing staff of the PCTRC, the devotion of Nancy Guerra (Children's Hospital of Pittsburgh of UPMC, Pittsburgh, PA) and the project coordinators, the laboratory expertise of Resa Stauffer (Children's Hospital of Pittsburgh of UPMC, Pittsburgh, PA), the assistance of past endocrine fellows with some of the clamp experiments, and most importantly, the commitment of the study participants and their parents.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1288/-/DC1.
References
- 1.Delvin EE, Lambert M, Levy E, et al. Vitamin D status is modestly associated with glycemia and indicators of lipid metabolism in French-Canadian children and adolescents. J Nutr 2010;140:987–991 [DOI] [PubMed] [Google Scholar]
- 2.Ford ES, Ajani UA, McGuire LC, Liu S. Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care 2005;28:1228–1230 [DOI] [PubMed] [Google Scholar]
- 3.Forouhi NG, Luan J, Cooper A, Boucher BJ, Wareham NJ. Baseline serum 25-hydroxy vitamin d is predictive of future glycemic status and insulin resistance: the Medical Research Council Ely Prospective Study 1990-2000. Diabetes 2008;57:2619–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gulseth HL, Gjelstad IMF, Tierney AC, et al. Serum vitamin D concentration does not predict insulin action or secretion in European subjects with the metabolic syndrome. Diabetes Care 2010;33:923–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajakumar K, de las Heras J, Lee S, Holick MF, Arslanian SA. 25-hydroxyvitamin D concentrations and in vivo insulin sensitivity and β-cell function relative to insulin sensitivity in black and white youth. Diabetes Care 2012;35:627–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reis JP, von Mühlen D, Miller ER, 3rd, Michos ED, Appel LJ. Vitamin D status and cardiometabolic risk factors in the United States adolescent population. Pediatrics 2009;124:e371–e379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genuth S, Alberti KG, Bennett P, et al. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26:3160–3167 [DOI] [PubMed] [Google Scholar]
- 8.Tfayli H, Bacha F, Gungor N, Arslanian S. Phenotypic type 2 diabetes in obese youth: insulin sensitivity and secretion in islet cell antibody-negative versus -positive patients. Diabetes 2009;58:738–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bacha F, Gungor N, Lee S, Arslanian SA. In vivo insulin sensitivity and secretion in obese youth: what are the differences between normal glucose tolerance, impaired glucose tolerance, and type 2 diabetes? Diabetes Care 2009;32:100–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajakumar K, de las Heras J, Chen TC, Lee S, Holick MF, Arslanian SA. Vitamin D status, adiposity, and lipids in black American and Caucasian children. J Clin Endocrinol Metab 2011;96:1560–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee S, Kuk JL, Hannon TS, Arslanian SA. Race and gender differences in the relationships between anthropometrics and abdominal fat in youth. Obesity (Silver Spring) 2008;16:1066–1071 [DOI] [PubMed] [Google Scholar]
- 12.Danadian K, Balasekaran G, Lewy V, Meza MP, Robertson R, Arslanian SA. Insulin sensitivity in African-American children with and without family history of type 2 diabetes. Diabetes Care 1999;22:1325–1329 [DOI] [PubMed] [Google Scholar]
- 13.Lee S, Guerra N, Arslanian S. Skeletal muscle lipid content and insulin sensitivity in black versus white obese adolescents: is there a race differential? J Clin Endocrinol Metab 2010;95:2426–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burns SF, Lee S, Arslanian SA. In vivo insulin sensitivity and lipoprotein particle size and concentration in black and white children. Diabetes Care 2009;32:2087–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tfayli H, Lee S, Arslanian S. Declining beta-cell function relative to insulin sensitivity with increasing fasting glucose levels in the nondiabetic range in children. Diabetes Care 2010;33:2024–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bacha F, Saad R, Gungor N, Arslanian SA. Are obesity-related metabolic risk factors modulated by the degree of insulin resistance in adolescents? Diabetes Care 2006;29:1599–1604 [DOI] [PubMed] [Google Scholar]
- 17.Arslanian SA, Saad R, Lewy V, Danadian K, Janosky J. Hyperinsulinemia in african-american children: decreased insulin clearance and increased insulin secretion and its relationship to insulin sensitivity. Diabetes 2002;51:3014–3019 [DOI] [PubMed] [Google Scholar]
- 18.Chen TC, Turner AK, Holick MF. Methods for the determination of the circulating concentration of 25-hydroxyvitamin D. J Nutr Biochem 1990;1:315–319 [DOI] [PubMed] [Google Scholar]
- 19.Holick MF, Siris ES, Binkley N, et al. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab 2005;90:3215–3224 [DOI] [PubMed] [Google Scholar]
- 20.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Endocrine Society Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–1930 [DOI] [PubMed] [Google Scholar]
- 21.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr 2004;79:820–825 [DOI] [PubMed] [Google Scholar]
- 22.Karnchanasorn R, Ou H-Y, Chiu KC. Plasma 25-hydroxyvitamin D levels are favorably associated with β-cell function. Pancreas 2012;41:863–868 [DOI] [PubMed] [Google Scholar]
- 23.Grimnes G, Figenschau Y, Almås B, Jorde R. Vitamin D, insulin secretion, sensitivity, and lipids: results from a case-control study and a randomized controlled trial using hyperglycemic clamp technique. Diabetes 2011;60:2748–2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muscogiuri G, Sorice GP, Prioletta A, et al. 25-Hydroxyvitamin D concentration correlates with insulin-sensitivity and BMI in obesity. Obesity (Silver Spring) 2010;18:1906–1910 [DOI] [PubMed] [Google Scholar]
- 25.Lee S, Kim Y, Kuk JL, Boada FE, Arslanian S. Whole-Body MRI and Ethnic Differences in Adipose Tissue and Skeletal Muscle Distribution in Overweight Black and White Adolescent Boys. Journal of Obesity 2011;2011. [DOI] [PMC free article] [PubMed]
- 26.Lamendola CA, Ariel D, Feldman D, Reaven GM. Relations between obesity, insulin resistance, and 25-hydroxyvitamin D. Am J Clin Nutr 2012;95:1055–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng S, Massaro JM, Fox CS, et al. Adiposity, cardiometabolic risk, and vitamin D status: the Framingham Heart Study. Diabetes 2010;59:242–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li HWR, Brereton RE, Anderson RA, Wallace AM, Ho CKM. Vitamin D deficiency is common and associated with metabolic risk factors in patients with polycystic ovary syndrome. Metabolism 2011;60:1475–1481 [DOI] [PubMed] [Google Scholar]
- 29.Muscogiuri G, Policola C, Prioletta A, et al. Low levels of 25(OH)D and insulin-resistance: 2 unrelated features or a cause-effect in PCOS? Clin Nutr 2012;31:476–480 [DOI] [PubMed] [Google Scholar]
- 30.Husemoen LLN, Thuesen BH, Fenger M, et al. Serum 25(OH)D and type 2 diabetes association in a general population: a prospective study. Diabetes Care 2012;35:1695–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]


