Abstract
OBJECTIVE
This study evaluated the sex-specific association of plasma fetuin-A levels with prevalent and incident type 2 diabetes in community-dwelling older adults.
RESEARCH DESIGN AND METHODS
Participants were 684 men and 1,058 women (median age, 71 years) whose fetuin-A levels, diabetes prevalence, and diabetes risk factors were evaluated in 1992–1996. The participants were followed for incident diabetes through 2010 (median follow-up, 9 years).
RESULTS
Women with impaired glucose tolerance had elevated fetuin-A levels compared with women with normal glucose tolerance (P = 0.019), but fetuin-A levels were not elevated in women with impaired fasting glucose. Fetuin-A did not vary by glucose tolerance status in men. There were significant interactions of fetuin-A by sex for prevalent (P = 0.007) and incident (P = 0.020) diabetes. For women, each SD (0.10 g/L) higher fetuin-A level was associated with a higher odds of prevalent diabetes (odds ratio [OR] 1.79, 95% CI 1.47–2.17) and greater risk of incident diabetes (hazard ratio [HR] 1.66, 95% CI 1.18–2.34), adjusting for age and estrogen therapy. These associations were not materially altered by adjustment for diabetes risk factors but were attenuated by adjusting for postchallenge glucose levels. Among men, although positive associations with prevalent (OR 1.15 [0.94–1.41]) and incident (HR 1.24 [0.93–1.65]) diabetes were suggested in age-adjusted models, risk estimates attenuated to one after multivariable adjustment.
CONCLUSIONS
Higher fetuin-A concentrations were independently associated with an increased risk of developing type 2 diabetes in older women but were not related to diabetes risk in older men. Fetuin-A may provide novel insights into mechanisms underlying sex differences in glucose homeostasis and diabetes risk in old age.
Type 2 diabetes is now epidemic. In the U.S., there are 1.5 million incident cases annually, and the prevalence in 2011 was almost 26 million (1). Type 2 diabetes is a heterogeneous disorder that results from the complex interplay of genetic susceptibility and lifestyle choices, leading to insulin resistance and impaired β-cell function (2). Available data suggest that glucose regulation and the pathophysiology of diabetes show sex differences that may be particularly relevant in older adults (2–5). For example, a markedly higher prevalence of postchallenge hyperglycemia has been reported in older women compared with that in older men, whereas fasting hyperglycemia is more common in older men than in older women (6–10).
Fetuin-A is a protein secreted primarily by the liver that regulates insulin signaling (11) and circulates in higher concentrations in women than in men (12–15). Only two proteins are known to bind directly to the extracellular domain of the insulin receptor: insulin and fetuin-A. Experimental evidence indicates that fetuin-A binding inhibits the insulin receptor tyrosine kinase (16) and induces insulin resistance in skeletal muscle and fat (11). In epidemiologic studies, we and others have shown that higher fetuin-A levels are associated with the development of type 2 diabetes independent of established markers of insulin resistance and diabetes risk factors (13,14,17,18).
To date, no epidemiologic studies have comprehensively investigated the association of fetuin-A with measures of glucose homeostasis and prevalent and incident diabetes among older men and women from the same population. Examining associations at various stages of the pathway to diabetes may provide novel insights into the biological interaction between this unique liver protein and the development of diabetes in older adults.
In this study, we examined the sex-specific association of plasma fetuin-A levels with prevalent and incident type 2 diabetes among community-dwelling older adults from the Rancho Bernardo Study. We used data from a baseline oral glucose tolerance test to examine the influence of glucose homeostasis as well as a number of other potential mediators and confounders on study findings. Based on the existing literature, we hypothesized that higher fetuin-A levels would be associated with dysglycemia and increased risk of diabetes in both older men and older women.
RESEARCH DESIGN AND METHODS
Study population
Between 1972 and 1974, community-dwelling residents living in Rancho Bernardo, California, 30–79 years of age, were invited to participate in a study of heart disease risk factors; 82% enrolled. Nearly all were middle- to upper-middle class and relatively well-educated. Since, research clinic visits have been conducted at ∼4-year intervals. The present analysis included individuals who participated in the 1992–1996 clinic visits. The study was approved by the Institutional Review Board of the University of California San Diego, and all participants gave written informed consent.
Of the 1,781 participants who attended the 1992–1996 visits, 39 were excluded for insufficient stored plasma for fetuin-A determination and 27 for missing covariates, resulting in sample sizes of 672 men and 1,043 women for the prevalent diabetes analysis. Of these 1,715 participants, 241 were lost to follow-up for incident diabetes. On average, participants lost to follow-up were older (79.8 vs. 70.0 years, P = 0.008) and had lower age-adjusted fetuin-A levels at baseline (0.489 vs. 0.523 g/L, P < 0.001) compared with those followed; 82% of loss to follow-up was caused by death. These individuals, as well as those with prevalent diabetes at baseline (n = 242), were excluded, resulting in sample sizes of 477 men and 782 women for the incident diabetes analysis.
Clinical measurements
During the 1992–1996 clinic visits, information regarding medical history, medication use, physical activity, alcohol consumption, and current smoking was obtained through standard questionnaires. Current medication use was validated by examination of pills and prescriptions brought to the clinic for that purpose. BMI and waist-to-hip ratio (WHR) were used as estimates of overall and central adiposity, respectively. Percent body fat was determined by full body dual energy X-ray absorptiometry scan. Blood pressures were measured twice while participants were seated, according to the Hypertension Detection and Follow-Up Program protocol (19), and the mean used in the analyses.
A 75-g oral glucose tolerance test was administered between 0730 and 1100 h after a minimum 8-h overnight fast. Blood samples were drawn by venipuncture at 0 and 2 h, and serum and plasma were separated and frozen at −70°C. Plasma glucose was measured by the glucose oxidase method, and insulin concentration was determined by radioimmunoassay in a diabetes research laboratory. Fetuin-A concentrations were measured in duplicate in 2010 from archived fasting EDTA plasma samples with a human ELISA kit (Epitope Diagnostics, San Diego, CA). This assay uses a two-site sandwich technique with polyclonal antibodies that bind different epitopes of human fetuin-A. Intra- and interassay coefficients of variation were 2.4–4.7% and 9.5–9.9%, respectively, for the set of assays used for the present sample.
Prevalent conditions and incident diabetes assessment
Impaired fasting glucose (IFG) was defined as a fasting plasma glucose level of 100–125 mg/dL and impaired glucose tolerance (IGT) as a 2-h postchallenge glucose level of 140–199 mg/dL. Prevalent diabetes was defined by self-report of physician diagnosis, fasting plasma glucose ≥126 mg/dL, 2-h postchallenge glucose ≥200 mg/dL, or use of diabetes medications. Incident diabetes was defined as any of the prevalent diabetes criteria with the exception of postchallenge glucose, which was not available at follow-up. Incident diabetes was assessed at five follow-up clinic visits and by four mailed questionnaires over a maximum 15-year follow-up period.
Prevalent cardiovascular disease (CVD) was defined as physician-diagnosed myocardial infarction, coronary artery revascularization, congestive heart failure, stroke or transient ischemic attack, carotid artery surgery, peripheral arterial surgery, or physician-diagnosed intermittent claudication. The metabolic syndrome was defined according to 2002 Adult Treatment Panel III criteria (20). Kidney function was assessed by the estimated glomerular filtration rate (eGFR) based on the Modification of Diet in Renal Disease equation (21) and by the albumin-to-creatinine ratio (ACR). The homeostasis model assessment for insulin resistance (HOMA-IR) was used to estimate insulin resistance (22). Insulin resistance was defined as a HOMA-IR >75th percentile for the study population.
Statistical analysis
Initial combined analyses revealed strong interactions for sex and fetuin-A for both prevalent (P = 0.007) and incident (P = 0.020) diabetes; therefore, all analyses were stratified by sex. Fetuin-A concentration was entered into models as a first-order continuous variable after screening for thresholds and curvilinear associations. The association between fetuin-A and baseline characteristics was evaluated by Pearson correlation coefficient. HDL cholesterol, triglyceride, aspartate transaminase (AST), and alanine transaminase (ALT) levels were log-transformed to reduce the influence of outliers; reported values are geometric means and interquartile ranges. The age-adjusted association of fetuin-A with baseline glycemic status was assessed by ANCOVA.
The association of fetuin-A with prevalent diabetes was determined by logistic regression; the association with incident diabetes was assessed by Cox proportional hazards regressions. Risk estimates for both outcomes are presented per a sex-specific SD (0.10 g/L for both sexes) increment in fetuin-A. Model assumptions were validated using standard statistical methods. Three successive regression models were evaluated. The first adjusted for age and use of oral estrogens (on the basis of our earlier studies that showed higher fetuin-A levels in women taking oral estrogens versus those not taking estrogens [12,23]). The second added adjustment for adiposity and lifestyle, and the third added other diabetes risk factors. Separate secondary models tested the influence of potential confounders and mediators by adding each variable separately to the fully adjusted base model. Biologically plausible effect modifiers were individually tested by interaction terms on a multiplicative scale in both the logistic and the proportional hazards regression models.
All P values presented are two-tailed, with P < 0.05 considered statistically significant for all analyses, including the interaction terms. Data were analyzed using STATA version 12.0 (Stata Corp., College Station, TX) and SPSS version 15 (IBM Corporation, Chicago, IL) statistical software.
RESULTS
Baseline characteristics
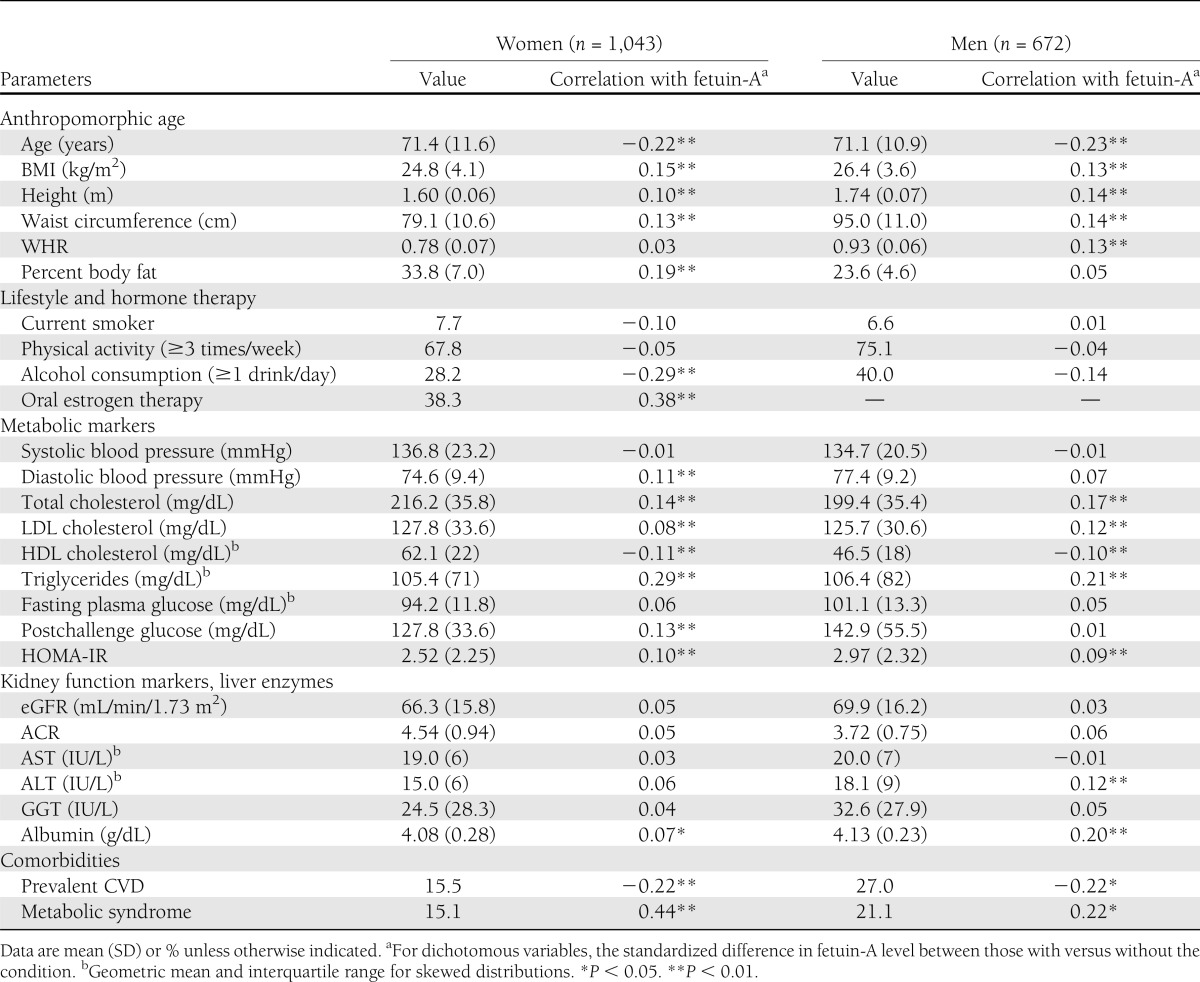
The mean age of the 1,715 participants was 71.3 years; 61% (n = 1,043) were women of whom 401 (38%) reported current oral estrogen therapy. Fetuin-A levels were highest in women taking oral estrogens (median 0.54 g/L, interquartile range 0.47–0.63 g/L), intermediate for women not taking oral estrogens (0.51, 0.44–0.58), and lowest for men (0.50, 0.44–0.56) (P < 0.001 for all comparisons). Baseline characteristics and the correlation of individual variables with fetuin-A levels are shown in Table 1. In general, fetuin-A associations were similar for men and women. Triglycerides, total cholesterol, BMI, and waist circumference showed the strongest positive associations with fetuin-A, whereas age and HDL cholesterol showed the strongest negative associations. Men and women with the metabolic syndrome had higher fetuin-A levels, whereas those with prevalent CVD had lower fetuin-A levels.
Table 1.
Baseline characteristics
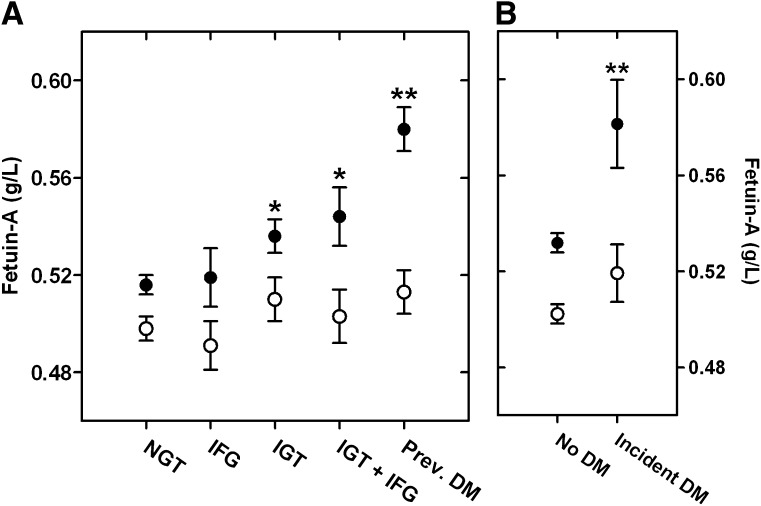
Fetuin-A levels by glucose tolerance status
Figure 1 shows age-adjusted fetuin-A levels by glucose tolerance status at baseline (Fig. 1A) and in nondiabetic participants in whom incident diabetes did and did not develop during follow-up (Fig. 1B). Compared with women with normal glucose tolerance (NGT), age-adjusted levels of fetuin-A were elevated in women with isolated IGT (P = 0.019) or IGT plus IFG (P = 0.030) and were highest in women with prevalent diabetes (P < 0.001). Fetuin-A levels did not differ by IFG in women (P = 0.81) and did not vary by any measure of glucose tolerance status in men. Baseline fetuin-A levels were also significantly higher in women (P = 0.008), but not men, in whom incident diabetes developed during follow-up compared with those who remained diabetes free (Fig. 1B).
Figure 1.
Mean (± SEM) age-adjusted fetuin-A levels by glycemic status (A) and by incident diabetes status (B) in women (●) and men (○). Number of women with each status: NGT = 578, IFG = 75, IGT = 204, IGT + IFG = 72, prevalent diabetes = 129, no diabetes = 750, and incident diabetes = 32. Number of men with each status: NGT = 294, IFG = 96, IGT = 109, IGT + IFG = 72, prevalent diabetes = 113, no diabetes = 424, and incident diabetes = 53. *P < 0.05 versus NGT, **P < 0.01 versus NGT (A) or no diabetes (B).
Fetuin-A associations with prevalent and incident diabetes
At baseline, 113 (17%) men and 129 (12%) women had prevalent diabetes. Overall, 42% (n = 102) of prevalent cases were based on postchallenge glucose alone, 4% (n = 9) on fasting glucose alone, and 38% (n = 92) only on self-report of physician diagnosis; the remaining 16% (n = 39) were based on more than one criterion. During the median 9.1-year follow-up, incident diabetes developed in 32 women and 53 men. Overall, 33% (n = 26) of incident cases were based on fasting plasma glucose, 13% (n = 11) on the use of diabetes medications, and 52% (n = 44) on self-report of physician diagnosis; four cases were based on combined criteria. The distribution of diagnostic categories that identified cases of prevalent and incident diabetes did not differ significantly by sex (χ2 P > 0.17), although the proportion of prevalent cases identified by postchallenge glucose alone was higher in women than in men (48 vs. 35%, P = 0.047).
Table 2 presents odds ratios (ORs) for prevalent diabetes and hazard ratios (HRs) for incident diabetes per SD (0.10 g/L) higher fetuin-A level. Among women, each SD higher fetuin-A level was associated with a 79% greater odds of prevalent diabetes and a 66% greater risk of incident diabetes, adjusting for age and oral estrogen therapy. Risk estimates for women were only modestly attenuated in sequential models that added adjustment for alcohol consumption, current smoking, exercise, BMI, and WHR (model 2) and other diabetes risk factors (triglycerides, HDL cholesterol, and systolic and diastolic blood pressures) (model 3). In contrast, among men, a positive association was suggested in age-adjusted models, but risk estimates were attenuated to one in multivariable models.
Table 2.
Odds of prevalent diabetes and risk of incident diabetes per SD (0.10 g/L) greater fetuin-A level
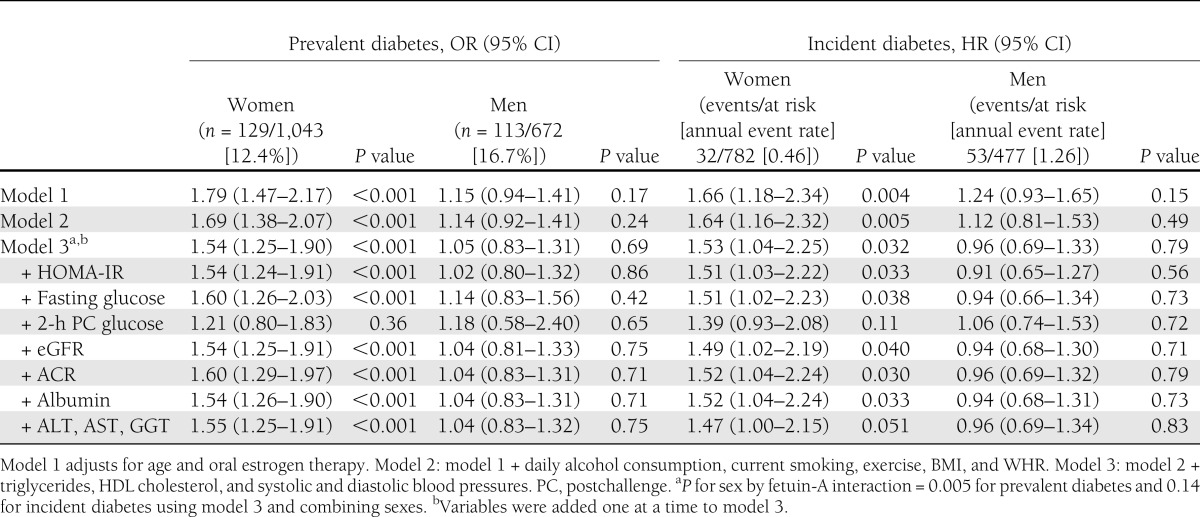
In secondary models, individually adding adjustment for HOMA-IR, fasting plasma glucose, 2-h postchallenge glucose, eGFR, ACR, albumin, or liver enzymes (ALT, AST, and γ-glutamyl transpeptidase [GGT]) to the multivariable base model (model 3) had minimal influence on results in either sex, with one exception. In women, adjusting for postchallenge glucose attenuated associations to nonsignificance for both prevalent and incident diabetes. Separate added adjustment for height and percent fat mass did not alter results for either diabetes outcome in either sex (data not shown).
Modifiers of the fetuin-A–diabetes associations
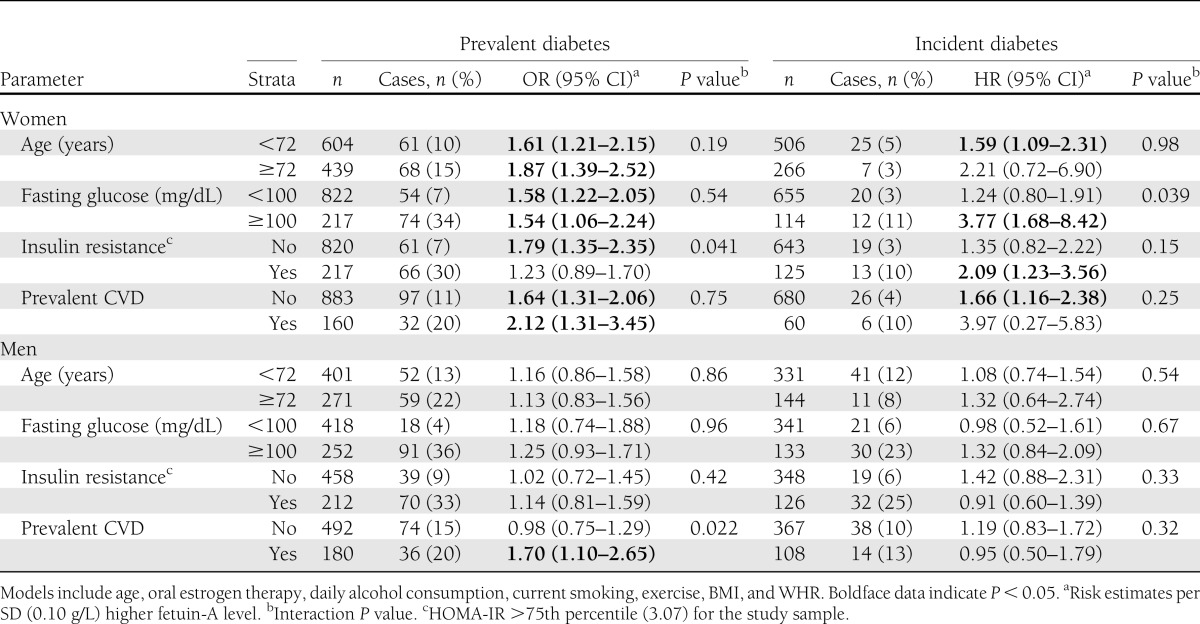
Next we examined whether the associations of fetuin-A with prevalent and incident diabetes differed across strata of selected risk factors (Table 3). For women, the association of fetuin-A with prevalent diabetes was stronger for those with insulin resistance compared with those without (P for interaction = 0.041). An interaction was also observed for fasting glucose such that each SD (0.10 g/L) higher fetuin-A level was associated with a nearly fourfold higher risk of incident diabetes in women with fasting glucose >100 mg/dL, but this was not significantly related to diabetes risk in women with lower fasting glucose levels (P for interaction = 0.039). There was no statistical evidence of effect modification by age, prevalent CVD, or oral estrogen therapy. Among men, the association of fetuin-A with prevalent and incident diabetes was similar across all subgroups except for prevalent CVD status. An SD (0.10 g/L) higher fetuin-A level was associated with a 70% greater odds of prevalent diabetes for men with prevalent CVD but was not related to prevalent diabetes among men without known CVD (P for interaction = 0.022).
Table 3.
Effect modifiers of the fetuin-A association with prevalent and incident diabetes for women and men
CONCLUSIONS
In this cohort of white community-dwelling older adults, we observed a strong and persistent sex difference in the association of fetuin-A with stages of glucose tolerance, prevalent diabetes, and the development of new diabetes over a median 9-year follow-up. An SD greater fetuin-A level was associated with 66% higher odds of prevalent diabetes and a 79% greater risk of incident diabetes in women but was not related to diabetes in men. This sex difference extended to prediabetic conditions; women, but not men, with IGT had elevated fetuin-A concentrations compared with their counterparts with NGT. These findings were independent of established diabetes risk factors and markers of liver function and chronic kidney disease, with one notable exception: Adjustment for postchallenge glucose concentrations attenuated associations with prevalent and incident diabetes in women.
Evidence of sex differences in the regulation of glucose homeostasis and the primary risk factors for diabetes is accumulating, particularly for older adults. Early studies in the Rancho Bernardo cohort showed that more than one-half of type 2 diabetes cases would be undiagnosed without a glucose tolerance test, that a diagnosis based solely on postchallenge hyperglycemia was more frequent in women than in men (comprising more than one-half of diabetes diagnoses in women when a glucose tolerance test was included), and that older women had more pronounced glucose intolerance but lower fasting glucose concentrations than men (9,10,24). A markedly higher prevalence of IGT in older women than in older men, contrasted with more IFG in men than in women, has been observed in epidemiologic studies from various countries around the world, including Germany (25), Mauritius (6), Denmark (7), and England (26). Although the precise mechanisms underlying these sex differences remain uncertain, Basu et al. (4) observed higher rates of both glucose appearance and glucose disappearance in response to a fixed meal in elderly women than in elderly men.
Consistent with prior epidemiologic studies (27–29), fetuin-A levels were positively associated with insulin resistance in both sexes in the current study. Several lines of evidence suggest that IGT may be a specific link between fetuin-A and type 2 diabetes, especially for older women. First, in this and prior studies (30,31), fetuin-A levels are elevated in middle-aged and elderly individuals with isolated IGT but are not related to IFG. Second, the association of fetuin-A with IGT in the current study was only observed in women, and IGT is more common in older women than in older men (6,7,10,25,26). Third, postchallenge glucose was the only risk factor among many tested that explained a significant proportion of the association of fetuin-A with both prevalent and incident diabetes in the women in this study. The apparently dominant role of IGT in defining diabetes in older women, whereas IFG seems to be more important for older men, may explain the absence of an association of fetuin-A with diabetes in the men in this study.
The sex differences identified in the current study conflict with earlier epidemiologic studies. A link between elevated fetuin-A levels and the development of type 2 diabetes was first reported in 2008 in our pilot study of older white and black adults from the Health, Aging, and Body Composition (Health ABC) population (17) and in a larger study of middle-aged adults from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study (14). We recently confirmed these early findings with data from the Cardiovascular Health Study among community-dwelling adults aged ≥65 years. Higher fetuin-A levels predicted incident diabetes regardless of sex, race, or prevalent CVD status (13). Effect modification by sex was also examined in the Health ABC and EPIC-Potsdam studies, and none was found. The Health ABC was a pilot study, and the sample size may have been limiting. The EPIC-Potsdam study and Cardiovascular Health Study were not able to exclude prevalent diabetes cases on the basis of postchallenge glucose alone, and incident diabetes cases in EPIC-Potsdam were identified by self-report and examination of medical records without fasting glucose measurements; thus, undiagnosed cases may have confounded identification of sex differences in these studies. It is also notable that the Rancho Bernardo Study population is distinct in its high degree of racial and socioeconomic homogeneity, which may have reduced sources of confounding and optimized our ability to detect sex differences.
Consonant with findings from the EPIC-Potsdam study (14), the association between circulating fetuin-A and incident diabetes in the current study was modified by fasting glucose levels, but only in women. Higher fetuin-A levels were associated with an increased risk of diabetes among women whose fasting glucose levels fell to within the prediabetic range but were not related to diabetes among women with normal glucose levels, suggesting that fetuin-A elevation might be more important later in the progression to diabetes.
The clinical implications of the current findings are uncertain. Although evidence from this study along with that from several others strongly suggests that fetuin-A is an independent marker of type 2 diabetes risk in humans, whether the relation is causal is still unknown. The fact that the gene encoding fetuin-A is located on chromosome arm 3q27, a region mapped as a diabetes susceptibility locus (32), supports a role for fetuin-A in the regulation of glucose homeostasis. That plasma fetuin-A levels and protein expression are increased in fatty liver disease (29), a strong predictor of diabetes (33), also points to a role for fetuin-A in the pathogenesis of diabetes. Nonetheless, experimental evidence that selective lowering of fetuin-A levels improves insulin sensitivity and reduces the risk for type 2 diabetes in humans is still lacking. More investigations are needed to determine whether fetuin-A is a viable target for diabetes prevention or treatment.
The strengths of this study are its prospective design, the relatively large sample size, inclusion of both sexes, and the availability of measurements of a wide spectrum of potential mediators and confounders. Our ability to accurately identify prevalent diabetes cases was enhanced by having postchallenge glucose concentrations at baseline. The study also has important limitations. Associations were based on fetuin-A concentrations measured at a single time point; nonetheless, we identified a strong signal for both prevalent and incident diabetes in women that was robust to statistical adjustment for multiple covariates. Because the number of diabetes cases in this study was relatively small, it is possible that the findings, including the sex differences, were due to chance. Like most epidemiologic studies, postchallenge glucose levels were not available at follow-up, which may have exerted a stronger limitation on incident diabetes diagnosis in women than in men but is unlikely to account for the sex-specific associations. We also had no information on family history of diabetes, which may have confounded results, but whether this is the case to a greater degree in one sex than the other is not clear (34). Finally, the majority of participants were white elderly men and women, and the results may not generalize to younger adults or those of different races/ethnicities.
In conclusion, higher fetuin-A levels were associated with IGT and prevalent and incident type 2 diabetes in community-dwelling older women independent of most established and several emerging diabetes risk factors. Fetuin-A levels were not associated with IGT or diabetes prevalence and incidence in men. If the findings are substantiated in larger epidemiologic studies with postchallenge glucose levels available, these sex-specific fetuin-A associations may provide new insights into mechanisms responsible for sex differences in glucose homeostasis and diabetes risk with advancing age.
Acknowledgments
This study was supported by grant R01-HL-096851 from the National Heart, Lung, and Blood Institute. The Rancho Bernardo Study was funded by grants AG-028507 and AG-018339 from the National Institute on Aging and by grant DK-31801 from the National Institute of Diabetes and Digestive and Kidney Diseases. G.A.L. was supported by a grant from the American Heart Association.
No potential conflicts of interest relevant to this article were reported.
G.A.L. directed the data analysis and wrote the manuscript. E.B.-C., as principal investigator of the Rancho Bernardo Study, had overall responsibility for collection of the diabetes data and reviewed and edited the manuscript. K.M.C. analyzed the data, wrote the statistical analysis section, and reviewed and edited the manuscript. L.B.D. and C.L.W. reviewed and edited the manuscript. J.H.I., as principal investigator of the ancillary study of fetuin-A in the Rancho Bernardo Study, designed the study, reviewed the analyses, contributed to the discussion, and reviewed and edited the manuscript. G.A.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Centers for Disease Control and Prevention. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States Atlanta, GA, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011 [Google Scholar]
- 2.DECODE Study Group Age- and sex-specific prevalences of diabetes and impaired glucose regulation in 13 European cohorts. Diabetes Care 2003;26:61–69 [DOI] [PubMed] [Google Scholar]
- 3.Shaw JE, de Courten M, Boyko EJ, Zimmet PZ. Impact of new diagnostic criteria for diabetes on different populations. Diabetes Care 1999;22:762–766 [DOI] [PubMed] [Google Scholar]
- 4.Basu R, Dalla Man C, Campioni M, et al. Effects of age and sex on postprandial glucose metabolism: differences in glucose turnover, insulin secretion, insulin action, and hepatic insulin extraction. Diabetes 2006;55:2001–2014 [DOI] [PubMed] [Google Scholar]
- 5.Regitz-Zagrosek V, Lehmkuhl E, Weickert MO. Gender differences in the metabolic syndrome and their role for cardiovascular disease. Clin Res Cardiol 2006;95:136–147 [DOI] [PubMed] [Google Scholar]
- 6.Williams JW, Zimmet PZ, Shaw JE, et al. Gender differences in the prevalence of impaired fasting glycaemia and impaired glucose tolerance in Mauritius. Does sex matter? Diabet Med 2003;20:915–920 [DOI] [PubMed] [Google Scholar]
- 7.Glümer C, Jørgensen T, Borch-Johnsen K, Inter99 Study Prevalences of diabetes and impaired glucose regulation in a Danish population: the Inter99 study. Diabetes Care 2003;26:2335–2340 [DOI] [PubMed] [Google Scholar]
- 8.Sicree RA, Zimmet PZ, Dunstan DW, Cameron AJ, Welborn TA, Shaw JE. Differences in height explain gender differences in the response to the oral glucose tolerance test- the AusDiab study. Diabet Med 2008;25:296–302 [DOI] [PubMed] [Google Scholar]
- 9.Barrett-Connor E. Factors associated with the distribution of fasting plasma glucose in an adult community. Am J Epidemiol 1980;112:518–523 [DOI] [PubMed] [Google Scholar]
- 10.Wingard DL. E. B-C, McPhillips JB: Community-based study of prevalence of NIDDM in older adults. Diabetes Care 1990;13:3–8 [Google Scholar]
- 11.Rauth G, Pöschke O, Fink E, et al. The nucleotide and partial amino acid sequences of rat fetuin. Identity with the natural tyrosine kinase inhibitor of the rat insulin receptor. Eur J Biochem 1992;204:523–529 [DOI] [PubMed] [Google Scholar]
- 12.Laughlin GA, Cummins KM, Wassel CL, Daniels LB, Ix JH. The association of fetuin-A with cardiovascular disease mortality in older community-dwelling adults: the Rancho Bernardo Study. J Am Coll Cardiol 2012;59:1688–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ix JH, Biggs ML, Mukamal KJ, et al. Association of fetuin-A with incident diabetes mellitus in community-living older adults: the Cardiovascular Health Study. Circulation 2012;125:2316–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stefan N, Fritsche A, Weikert C, et al. Plasma fetuin-A levels and the risk of type 2 diabetes. Diabetes 2008;57:2762–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ix JH, Katz R, de Boer IH, et al. Fetuin-A is inversely associated with coronary artery calcification in community-living persons: the Multi-Ethnic Study of Atherosclerosis. Clin Chem 2012;58:887–895 [DOI] [PubMed] [Google Scholar]
- 16.Auberger P, Falquerho L, Contreres JO, et al. Characterization of a natural inhibitor of the insulin receptor tyrosine kinase: cDNA cloning, purification, and anti-mitogenic activity. Cell 1989;58:631–640 [DOI] [PubMed] [Google Scholar]
- 17.Ix JH, Wassel CL, Kanaya AM, et al. Health ABC Study Fetuin-A and incident diabetes mellitus in older persons. JAMA 2008;300:182–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun Q, Cornelis MC, Manson JE, Hu FB. Plasma levels of fetuin-A and hepatic enzymes and risk of type 2 diabetes in women in the U.S. Diabetes 2013;62:49–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hypertension Detection and Follow-up Program Cooperative Group. The Hypertension Detection and Follow-up Program.. Prev Med 1976;5:207–215 [DOI] [PubMed] [Google Scholar]
- 20.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143–3421 [PubMed] [Google Scholar]
- 21.Levey AS, Greene T, Kusek JW, Beck GJ. A simplified equation to predict glomerular filtration rate from serum creatinine (Abstract). J Am Soc Nephrol 2001;11:A0828 [Google Scholar]
- 22.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 23.Ix JH, Barrett-Connor E, Wassel CL, et al. The associations of fetuin-A with subclinical cardiovascular disease in community-dwelling persons: the Rancho Bernardo Study. J Am Coll Cardiol 2011;58:2372–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barrett-Connor E. The prevalence of diabetes mellitus in an adult community as determined by history or fasting hyperglycemia. Am J Epidemiol 1980;111:705–712 [DOI] [PubMed] [Google Scholar]
- 25.Hanefeld M, Koehler C, Fuecker K, Henkel E, Schaper F, Temelkova-Kurktschiev T, Impaired Glucose Tolerance for Atherosclerosis and Diabetes Study Insulin secretion and insulin sensitivity pattern is different in isolated impaired glucose tolerance and impaired fasting glucose: the risk factor in Impaired Glucose Tolerance for Atherosclerosis and Diabetes study. Diabetes Care 2003;26:868–874 [DOI] [PubMed] [Google Scholar]
- 26.Pomerleau J, McKeigue PM, Chaturvedi N. Relationships of fasting and postload glucose levels to sex and alcohol consumption. Are American Diabetes Association criteria biased against detection of diabetes in women? Diabetes Care 1999;22:430–433 [DOI] [PubMed] [Google Scholar]
- 27.Ix JH, Shlipak MG, Brandenburg VM, Ali S, Ketteler M, Whooley MA. Association between human fetuin-A and the metabolic syndrome: data from the Heart and Soul Study. Circulation 2006;113:1760–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mori K, Emoto M, Yokoyama H, et al. Association of serum fetuin-A with insulin resistance in type 2 diabetic and nondiabetic subjects. Diabetes Care 2006;29:468. [DOI] [PubMed] [Google Scholar]
- 29.Stefan N, Hennige AM, Staiger H, et al. Alpha2-Heremans-Schmid glycoprotein/fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care 2006;29:853–857 [DOI] [PubMed] [Google Scholar]
- 30.Tönjes A, Fasshauer M, Kratzsch J, Stumvoll M, Blüher M. Adipokine pattern in subjects with impaired fasting glucose and impaired glucose tolerance in comparison to normal glucose tolerance and diabetes. PLoS One 2010;5:e13911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ou HY, Yang YC, Wu HT, Wu JS, Lu FH, Chang CJ. Serum fetuin-A concentrations are elevated in subjects with impaired glucose tolerance and newly diagnosed type 2 diabetes. Clin Endocrinol (Oxf) 2011;75:450–455 [DOI] [PubMed] [Google Scholar]
- 32.Vionnet N, Hani EH, Dupont S, et al. Genomewide search for type 2 diabetes-susceptibility genes in French whites: evidence for a novel susceptibility locus for early-onset diabetes on chromosome 3q27-qter and independent replication of a type 2-diabetes locus on chromosome 1q21-q24. Am J Hum Genet 2000;67:1470-1480 [DOI] [PMC free article] [PubMed]
- 33.Shibata M, Kihara Y, Taguchi M, Tashiro M, Otsuki M. Nonalcoholic fatty liver disease is a risk factor for type 2 diabetes in middle-aged Japanese men. Diabetes Care 2007;30:2940-2944 [DOI] [PubMed]
- 34.Harrison TA, Hindorff LA, Kim H, et al. Family history of diabetes as a potential public health tool. Am J Prev Med 2003;24:152–159 [DOI] [PubMed] [Google Scholar]