Abstract
OBJECTIVE
Carbohydrate nutrition during periods of physiological insulin resistance such as puberty may affect future risk of type 2 diabetes. This study examined whether the amount or the quality (dietary glycemic index [GI], glycemic load [GL], and added sugar, fiber, and whole-grain intake) of carbohydrates during puberty is associated with risk markers of type 2 diabetes in younger adulthood.
RESEARCH DESIGN AND METHODS
The analysis was based on 226 participants (121 girls and 105 boys) from the Dortmund Nutritional and Anthropometric Longitudinally Designed Study (DONALD) with an average of five 3-day weighed dietary records (range 2–6) during puberty (girls, age 9–14 years; boys, age 10–15 years) and fasting blood samples in younger adulthood (age 18–36 years) (average duration of follow-up 12.6 years). Multivariable linear regression was used to analyze the associations between carbohydrate nutrition and homeostasis model assessment–insulin resistance (HOMA-IR) as well as the liver enzymes alanine aminotransferase (ALT) and γ-glutamyltransferase (GGT) (n = 214).
RESULTS
A higher dietary GI was prospectively related to greater values of HOMA-IR (Ptrend = 0.03), ALT (Ptrend = 0.02), and GGT (Ptrend = 0.04). After adjustment for sex, adult age, baseline BMI, and early life and socioeconomic factors as well as protein and fiber intake, predicted mean HOMA-IR values in energy-adjusted tertiles of GI were 2.37 (95% CI 2.16–2.60), 2.47 (2.26–2.71), and 2.59 (2.35–2.85). The amount of carbohydrates, GL, and added sugar, fiber, and whole-grain intake were not related to the analyzed markers.
CONCLUSIONS
Our data indicate that a habitually higher dietary GI during puberty may adversely affect risk markers of type 2 diabetes in younger adulthood.
Concern has been raised that the commonly advocated low-fat, high-carbohydrate diet may be detrimental for the growing number of persons with impaired glucose tolerance even among youths, since it induces postprandial rises in glucose and insulin and may thereby increase the risk the risk of developing type 2 diabetes (1,2). Observational evidence suggests that dietary glycemic index (GI) and glycemic load (GL) are related to risk of type 2 diabetes (3,4), yet it remains to be determined whether the relevance of postprandial rises in glucose and insulin extends to puberty—a period characterized by a physiological insulin resistance (5).
Chronic postprandial hyperglycemia and hyperinsulinemia can also exacerbate hepatic insulin resistance: enhanced glucose uptake by the liver subsequently leads to increased hepatic fat accumulation through upregulated de novo lipogenesis. In fact, hepatic fat accumulation is frequently observed in patients with insulin resistance or type 2 diabetes (6). The liver enzymes alanine aminotransferase (ALT) and γ-glutamyltransferase (GGT) are commonly used as surrogate parameters for hepatic fat content and are now recognized as risk markers for type 2 diabetes (7,8). Furthermore, preliminary evidence supports a role of carbohydrate nutrition for hepatic steatosis and these indirect markers of liver fat (9).
This study addressed the hypothesis that recurring postprandial glycemic excursions during puberty are of specific relevance for later risk of type 2 diabetes. Since calculated dietary GI is a valid predictor of glycemic responses (10,11), we postulate that dietary GI estimated from 3-day dietary records repeatedly collected during puberty is a better predictor of type 2 diabetes risk in younger adulthood than intakes of dietary fiber, whole grain, or added sugar. This hypothesis was addressed using data from a cohort of healthy young Germans. The homeostasis model assessment–insulin resistance (HOMA-IR) index and the liver enzymes ALT and GGT was used as risk markers of type 2 diabetes.
RESEARCH DESIGN AND METHODS
The present analysis is based on data from the Dortmund Nutritional and Anthropometric Longitudinally Designed Study (DONALD), an ongoing open cohort study conducted at the Research Institute of Child Nutrition in Dortmund, Germany (12). This study has previously been described in detail (12). Briefly, since 1985, detailed data on diet, growth, development, and metabolism have been collected from >1,300 healthy children. Participants are recruited in the city of Dortmund and surrounding communities via personal contacts, maternity wards, or pediatric practices. On average, 40 infants are newly recruited every year and first examined at the age of 3 months. Each child returns for three more visits during the first year, two in the second, and then annually until adulthood. Since 2005, participants over the age of 18 years are invited for subsequent examinations with fasting blood withdrawal. The study was approved by the ethics committee of the University of Bonn, and all examinations are performed with written parental and adult participants’ consent (12).
Because of the open cohort design, many children had not yet reached younger adulthood, and among those who did age varied from 18 to 36 years. At the time of this analysis, one measurement of insulin and glucose was available for 319 participants (mean age 22.7 years), who were term (36–43 weeks’ gestation) singletons with a birth weight ≥2,500 g. ALT and GGT values were available for 309 participants. Of these, 229 participants (for HOMA analysis) and 221 (for ALT and GGT analysis), respectively, had provided at least two plausible 3-day weighed dietary records during the adolescent baseline period (chronological age: girls 9–14 years, boys 10–15 years), allowing the estimation of habitual dietary intake. Participants who consistently underreported their energy intake (i.e., they had provided more implausible than plausible food records) were excluded from the study (n = 20) (13). A 3-day weighed dietary record was considered plausible when the total recorded energy intake was adequate in relation to the basal metabolic rate (13). For inclusion in the study sample, participants also had to have anthropometric measures taken in adolescence and adulthood as well as information on relevant covariates. This resulted in a final sample of 226 participants for analysis of insulin or related outcomes and of 214 for the liver enzymes.
Blood analysis
Venous blood samples were drawn after an overnight fast, centrifuged within 15 min, and frozen at –80°C in the Research Institute. For the present analysis, blood samples were transported to the technical laboratory of the German Diabetes Center to determine serum activities of ALT and GGT using the COBAS C311 analyzer (Roche, Mannheim, Germany). Serum insulin concentrations were measured with an immunoradiometric assay in the Laboratory for Translational Hormone Analytics in Pediatric Endocrinology at the University of Giessen. Based on these values, HOMA-IR and secretion (HOMA of β-cell function [HOMA-β]) were calculated (14).
Anthropometric measurements
From the age of 2 years onward, standing height is measured to the nearest 0.1 cm using a digital stadiometer (Harpenden, Crymych, U.K.). Body weight is measured to the nearest 100 g with an electronic scale (Seca 753E; Seca Weighing and Measuring Systems, Hamburg, Germany). Measurements are taken at each visit according to standard procedures. Skinfold thicknesses are measured from the age of 6 months onward at four different sites (suprailiacal, subscapular, biceps, and triceps) on the right side of the body to the nearest 0.1 mm using a Holtain caliper (Holtain, Crosswell, U.K.). Waist circumference in younger adulthood was measured at the midpoint between the lower rip and the iliac crest to the nearest 0.1 cm. Sex- and age-specific SD scores (SDs) were calculated for the adolescent BMI values using the German BMI standards (15). For definition of overweight during puberty, values proposed by the International Obesity Task Force were used (16). Percentage body fat (%BF) for pubescent children was derived using the equations of Slaughter et al. (17), and excess body fatness was defined according to the %BF standard (18). For estimation of %BF in adulthood, equations of Durnin and Womersley were used (19).
Dietary assessment
During 3 days, the participants or their parents weighed and recorded all foods and beverages consumed as well as leftovers to the nearest 1 g using electronic food scales (initially, Soehnle Digita 8000; Leifheit, Nassau, Germany; now, WEDO digi 2000; Werner Dorsch, Münster/Dieburg, Germany). For this analysis, dietary variables were calculated as individual means of the 3-day weighed dietary records using LEBTAB (20), the in-house database. As we aimed to describe the habitual dietary intake, an individual average intake during puberty was calculated from at least two records (average of 5 records per participant).
Each carbohydrate-containing food recorded in the dietary records was assigned a published GI value (21) (based on glucose as a reference food) according to a standardized procedure (22). The carbohydrate content (in grams) of each consumed food was then multiplied by the food’s GI to obtain the respective GL. The overall dietary GI is obtained by dividing total daily GL by total daily carbohydrate intake.
The following foods were defined as added sugars: white sugar, brown sugar, raw sugar, corn syrup, corn syrup solids, high-fructose corn syrup, malt syrup, maple syrup, pancake syrup, fructose sweetener, liquid fructose, honey, molasses, anhydrous dextrose, and crystal dextrose (23). Fruit syrups commonly used as sweeteners in Germany also were considered added sugars. Dietary fiber content was calculated using the LEBTAB database. Whole-grain intake was estimated by assigning whole-grain content in grams to each carbohydrate-containing food using the respective recipe and ingredient information available at the time of recording. The definition of whole grain followed the whole-grain label statements of the U.S. Food and Drug Administration (24).
Statistical analysis
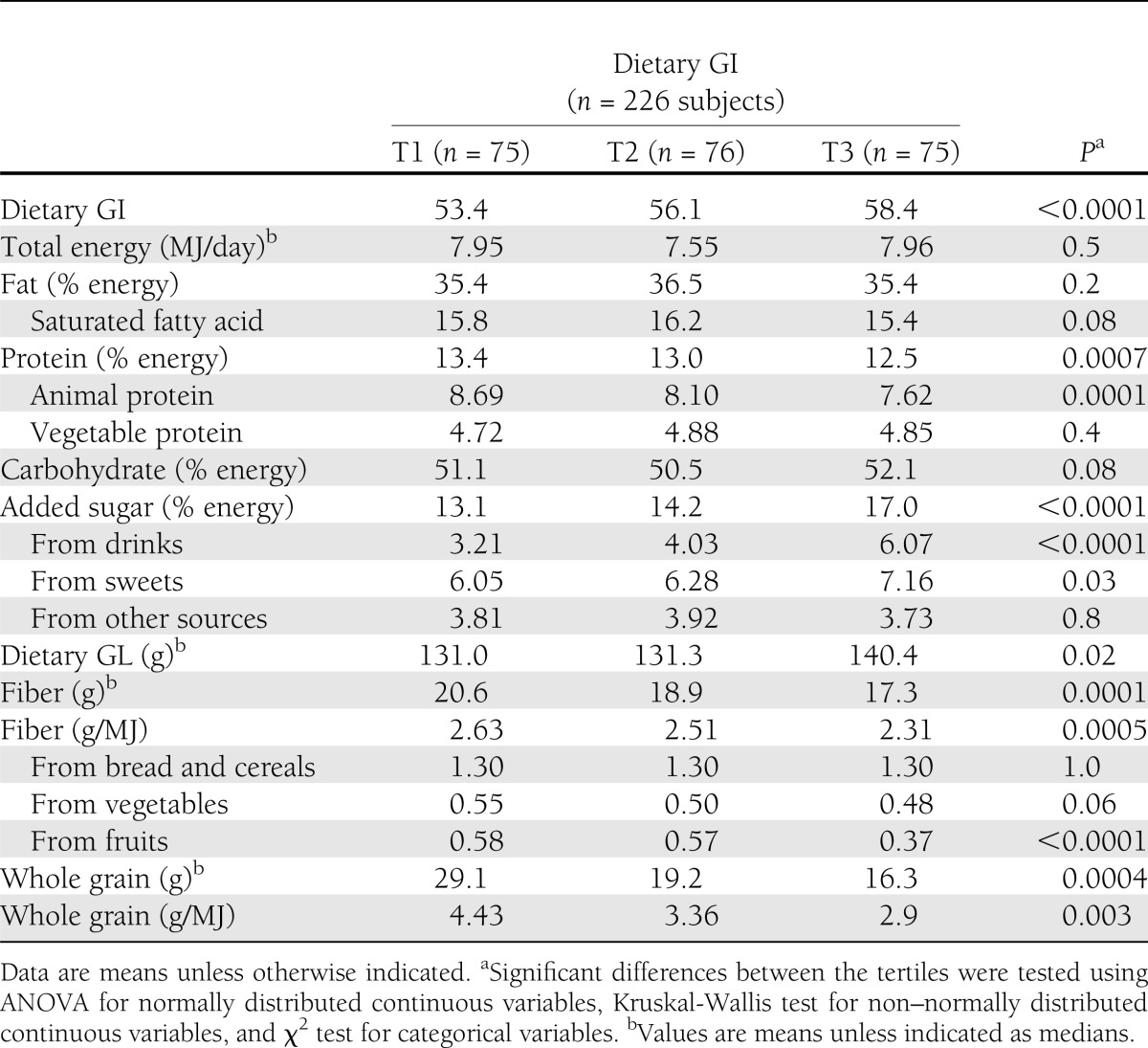
Baseline characteristics of the study population are presented by sex-specific tertiles of dietary GI. Tests for differences between these tertiles were performed using ANOVA for normally distributed continuous variables, Kruskal-Wallis test for non–normally distributed continuous variables, and χ2 test for categorical variables.
For analysis of the prospective association between carbohydrate nutrition during puberty and risk markers for type 2 diabetes in younger adulthood, multivariable linear regression models were used. As the outcome variables were not normally distributed, HOMA-IR was log transformed prior to analysis, and liver enzymes ALT and GGT were log transformed twice to obtain normal distribution. All dietary variables except dietary GI were energy adjusted using the residual method. To account for age-dependent nutritional differences, we standardized all variables by age-group and sex (mean ± SD 0 ± 1).
Covariates considered as potentially affecting the association between carbohydrate nutrition and risk markers of type 2 diabetes were birth weight, gestational age, breast-feeding for >2 weeks, firstborn child (yes/no), BMI SDs or %BF at baseline, maternal overweight (BMI ≥25 kg/m2), high maternal educational status (≥12 years of schooling), maternal occupation (yes/no), smoking in the household, parental history of diabetes (yes/no [questionnaire based]), physical activity level (light, moderate, or high [questionnaire based]), and intakes of protein (total, animal, or vegetable) and fat (total and saturated fat). Vice versa adjustment for added sugar, fiber, and GI was also considered. Each potential confounder was initially examined separately and included only if it 1) substantially altered the association of the principal dietary variables with the outcome in the unadjusted models (>10%), 2) significantly predicted the outcome, or 3) improved the coefficient of determination (>5%). In the basic model (model A), sex and age were included, since age at blood withdrawal in younger adulthood varied considerably (18–35 years). In a second model (model B), we further adjusted for early life and socioeconomic as well as other nutritional factors. Finally, we ran a conditional model (additionally including waist circumference in younger adulthood) to assess whether the observed associations are partly attributable to effects of carbohydrate nutrition on body composition. Verification of the linear regression modeling assumptions showed that these were appropriate for the analyzed longitudinal data.
As associations between carbohydrate nutrition and risk markers of type 2 diabetes did not differ by sex (P for interaction >0.2), data were pooled for analysis. The adjusted means are presented by tertiles with the corresponding 95% CIs. P values <0.05 were considered statistically significant. All statistical analyses were carried out using SAS procedures (version 9.1.3; SAS Institute, Cary, NC).
RESULTS
Subjects who were excluded from the study sample because of missing information (dietary intake data or covariates) (n = 93) did not differ from those included (n = 226) with respect to early life factors or anthropometric or metabolic characteristics in younger adulthood (data not shown).
Participants with a higher dietary GI during adolescence were more likely to be exposed to smoking in the household (Table 1). There were no other differences in anthropometric, early life, or socioeconomic factors during puberty between the dietary GI tertiles. Regarding data from younger adulthood, participants with a higher dietary GI during puberty had higher ALT and GGT values (Table 1). In terms of nutritional intake data during puberty, those in the higher dietary GI tertiles consumed less (animal) protein, (fruit) fiber, and whole grain, as well as more added sugar, especially from drinks (Table 2).
Table 1.
Demographic, anthropometric, birth, and socioeconomic characteristics by sex-specific tertiles of dietary glycemic index: DONALD, Germany
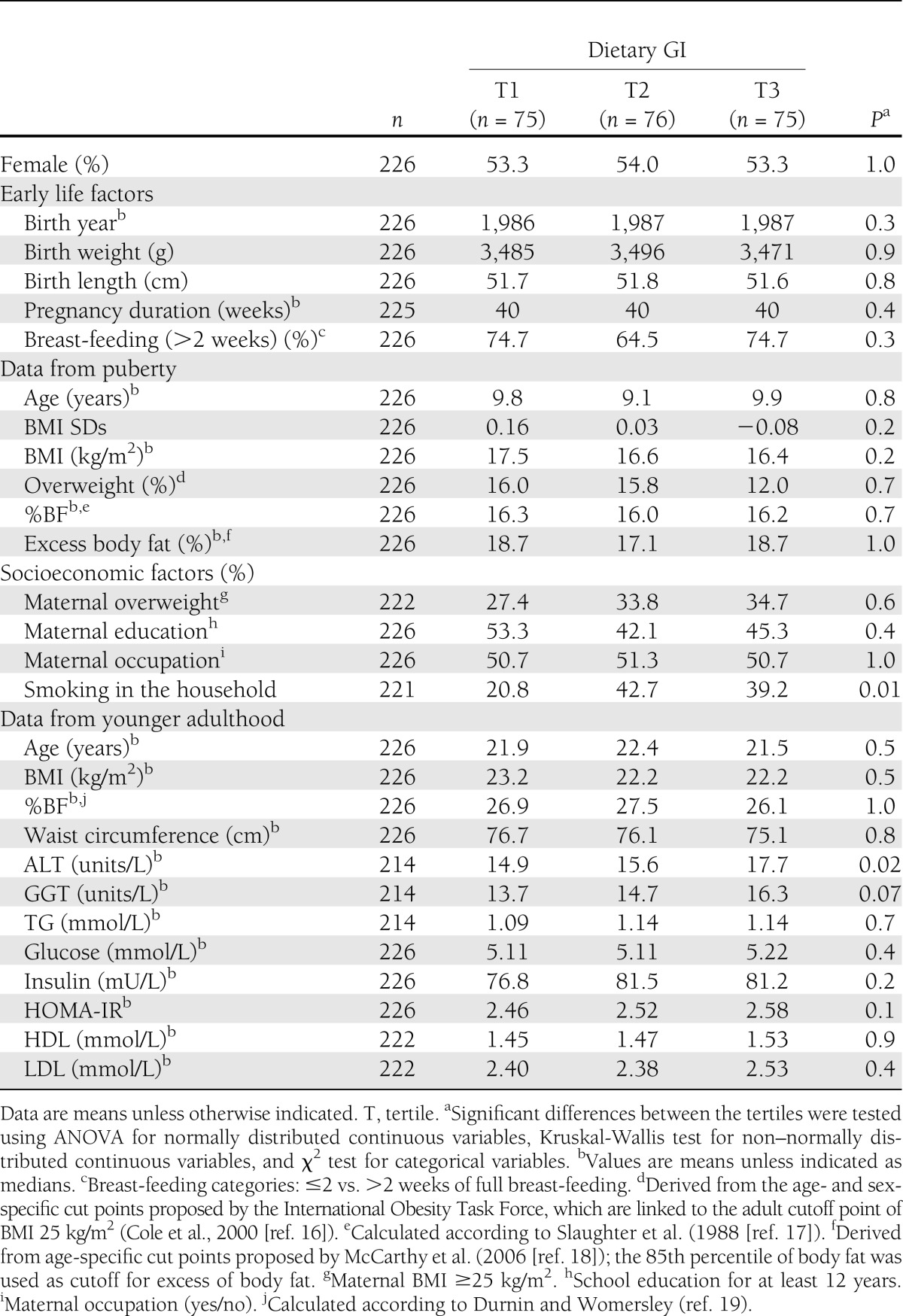
Table 2.
Baseline nutritional data by sex-specific tertiles of dietary glycemic index: DONALD, Germany
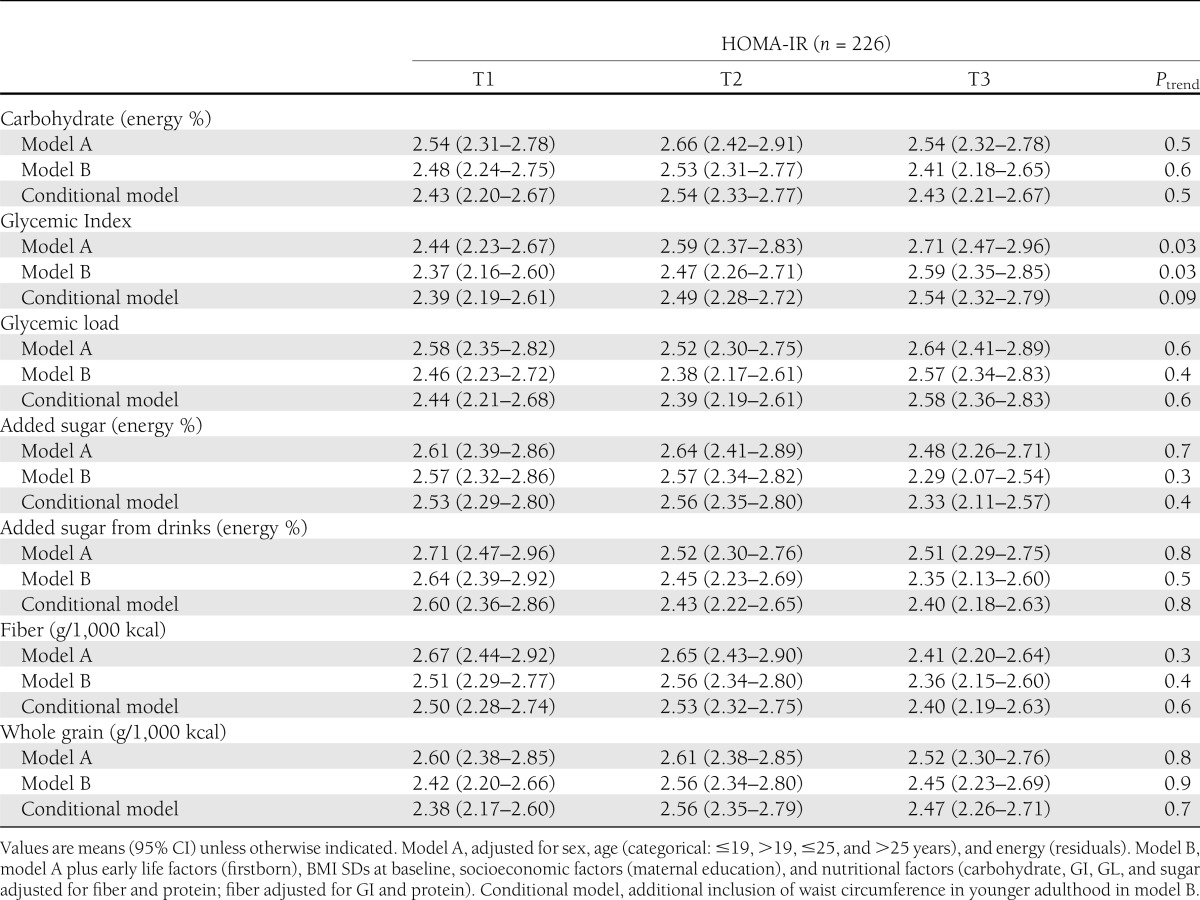
The amount of carbohydrates, dietary GL, added sugar, fiber, and whole-grain intake during puberty was not associated with HOMA-IR in younger adulthood (Table 3). A higher dietary GI during puberty was prospectively related to higher values of HOMA-IR in multivariable analysis (P for trend = 0.03 [model A]). This association was not explained by baseline BMI, early life or socioeconomic factors, or protein or fiber intake (P for trend = 0.03 [model B]). No prospective associations were observed between carbohydrate nutrition and HOMA-β (P for trend ≥0.2) (data not shown).
Table 3.
HOMA-IR in younger adulthood by tertiles of carbohydrate nutrition parameters during puberty
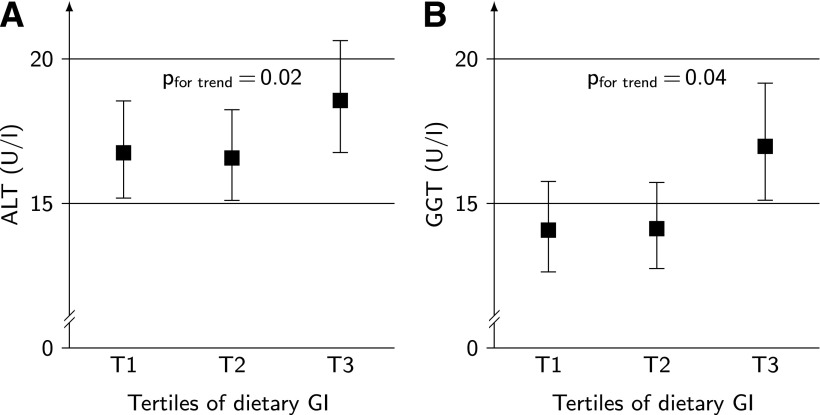
A higher dietary GI was also independently associated (adjustment for baseline BMI and socioeconomic and nutritional factors) with higher values of both ALT (P for trend = 0.02 [model B]) and GGT (P for trend = 0.04 [model B]) (Fig. 1). Amount of carbohydrates, dietary GL, total added sugar, dietary fiber, and whole-grain intake were not related to liver enzymes. Higher intakes of added sugar from drinks during puberty were independently related to higher levels of GGT in adulthood (P for trend = 0.04 [model B]) (data not shown).
Figure 1.
ALT (units/L) (A) and GGT (units/L) (B) levels in younger adulthood by energy-adjusted tertiles of dietary glycemic (GI) (mean dietary GI across tertiles [T]: tertile 1, 53.5; 2, 56.2; and 3, 58.5) during puberty (baseline) for 214 subjects. Data are geometric means (95% CI) adjusted for sex, age (categorical ≤19, >19, ≤25, and >25 years), BMI SDs at baseline, socioeconomic factors (maternal overweight), energy (residuals), and protein and fiber intake. See the text for results from the conditional model additionally considering waist circumference in younger adulthood. Note that the slight U-shape in A results from illustration of least square means by GI tertiles, the association is linear, and all assumptions of linear regression modeling are met. (See the Statistical analysis.)
We also examined the association between carbohydrate nutrition and fasting insulin levels; similarly, this analysis revealed a prospective positive relation for dietary GI only (P for trend = 0.045). Further adjustment for breast-feeding status, birth weight, physical activity level, or parental history of type 2 diabetes did not change any of the results.
The additional inclusion of waist circumference in adulthood attenuated the associations between dietary GI and risk markers of type 2 diabetes toward a trend (conditional model [Table 3]). The corresponding mean predicted ALT and GGT values in sex-specific tertiles of GI were 16.7 units/L (95% CI 15.3–18.4), 16.3 units/L (15.0–17.8), and 18.0 units/L (16.4–19.9) (P for trend = 0.07) and 14.1 units/L (12.7–15.7), 14.0 units/L (12.6–15.5), and 16.6 units/L (14.8–18.7) (P for trend = 0.09), respectively.
CONCLUSIONS
This study provides new epidemiological evidence of a detrimental role of postprandial glycemic excursions during puberty for risk markers of type 2 diabetes in younger adulthood. Dietary GI was the only feature of carbohydrate nutrition that was consistently related to different diabetes risk markers. As a low-GI diet is characterized by an average of ≤45 (25), the dietary GI in the present sample (56.0 ± 2.4) can be considered moderate.
The association between dietary GI and diabetes risk seen in our study is in accordance with observational evidence in adulthood linking dietary GI to risk of developing type 2 diabetes (3,4). Our study is, however, the first to suggest that this association emerges already during puberty. In view of the relatively large 95% CIs, the observed associations have to be interpreted cautiously. In our study, a 5-unit increase of dietary GI was accompanied by a 9% increase in HOMA-IR and an 11% increase in ALT values. This is in line with evidence from large observational studies, where moderate GI differences between extreme quantiles were also associated with relatively large differences in type 2 diabetes risk (3). Importantly, there was no strong correlation between HOMA-IR, ALT, and GGT in our study (r <0.4), which argues against the possibility of chance findings.
Of note, the relation between dietary GI and diabetes risk markers appeared to be partly attributable to body composition, since associations were attenuated toward a trend in the conditional model. Nonetheless, a trend was maintained, suggesting an additional mechanism independent of body composition. In fact, a previous analysis of ours did not reveal an independent association between GI during puberty and body composition in younger adulthood (26). Another mechanism by which dietary GI may affect diabetes risk independently of body composition is oxidative stress: Increased postprandial glycemia can exert prooxidative and proinflammatory effects (27). Hyperglycemia-induced oxidative stress could impair mitochondrial function (28). In turn, impaired mitochondrial function may cause both hepatocyte injury and subsequently increased release of ALT and GGT (28) and contribute to insulin resistance independently of hepatic lipid content (29). Moreover, excessive postprandial glycemia increases the strain on β-cell mass, which can be particularly detrimental in a phase of decreased insulin sensitivity such as puberty (30). Our data indicate a long-term relevance of dietary GI for both systemic and hepatic insulin resistance, as reflected by associations with HOMA-IR and insulin as well as GGT and ALT. Moreover, in our healthy sample, habitual dietary GI seems to be of long-term relevance for insulin sensitivity only, since GI was not prospectively related to β-cell function (e.g., HOMA-β).
The results of our study dismiss the relevance of total carbohydrate intake for later insulin sensitivity and corroborate the rising awareness that carbohydrate quality is more important for risk of type 2 diabetes than carbohydrate quantity—at least for healthy persons. We cannot, however, exclude the possibility that lower carbohydrate intake may offer some benefits for obese adolescents, since they cannot adapt appropriately to high-carbohydrate diets by increasing their insulin sensitivity and may, hence, need to increase insulin secretion further (31).
We observed no prospective association between consumption of added sugar from drinks or fiber intake and adult type 2 diabetes risk markers except for an association between added sugar from drinks and GGT. Observational studies in adults support a relation of both consumption of sugar-sweetened beverages (32) and cereal fiber (33) to type 2 diabetes risk, while mechanistic studies point to specific benefits of viscous fiber on insulin sensitivity (34). This discrepancy may to some degree result from residual confounding. In the present analysis, confounding is less likely because the DONALD population is comparably homogeneous with a higher socioeconomic status. In addition, benefits of higher fiber intakes are partly attributed to lower postprandial glycemia. This response is, however, better described by dietary GI: In a recent study using 121 foods and 13 meals, postprandial glycemia was related to GI and GL but not fiber content (35). It is therefore possible that exposure to postprandial glycemia during puberty (as estimated by dietary GI) is of particular relevance for diabetes risk in younger adulthood, whereas other mechanisms linking fiber intake to diabetes risk become more important in later adulthood.
The main strengths of our study are its prospective design and the detailed repeated measurements of dietary intake during puberty. Assessment of dietary intake during puberty is notoriously difficult, but the present analysis was based on an average of five dietary records during puberty (range 2–6 per participant), which allowed estimation of habitual dietary intake. Comparisons of our carbohydrate-intake data with other studies in adolescents showed similar intake levels with respect to total carbohydrate, added sugar (36,37), and dietary GI (38). The availability of data on several potential confounders, such as parental characteristics, including self-reported parental history of type 2 diabetes, further strengthens our analysis. However, we cannot preclude residual confounding, resulting from imprecisely measured or unmeasured confounding factors. Importantly, only crude questionnaire-based data were available for physical activity levels.
Our study also has several limitations. First, risk markers of type 2 diabetes were only measured once in younger adulthood. Second, the relatively elaborate DONALD study design results in a socioeconomic status above average, and extremes of diet or behavior might not be represented, which is likely to introduce selection bias. Thirdly, estimation of the dietary GI from the GI values of individual foods is discussed controversially (10,39). However, in contrast to most epidemiological studies using food-frequency questionnaires, the GI estimates in this study stem from direct assignment of GI values to all carbohydrate-containing foods recorded during 3 days (22).
Relating our results to those from other studies, the lack of data on the longer-term influence of adolescent nutrition on later health becomes very evident. Our study provides new evidence for a long-term impact of postprandial glycemic excursions during puberty on later diabetes risk. The absence of such associations for other measures of carbohydrate quality suggests that advice focusing solely on dietary fiber and added sugar intake is insufficient. Further large-scale studies, preferably in at-risk populations (e.g., overweight or insulin-resistant adolescents) are needed to support the present findings and confirm their public health relevance.
In conclusion, our data indicate that a habitually higher dietary GI during puberty may adversely affect risk markers of type 2 diabetes in younger adulthood. Advice for preferred selection of low-GI carbohydrates during puberty may need to be incorporated into preventive dietary recommendations given to adolescents.
Acknowledgments
This work was financially supported by the German Federal Ministry of Food, Agriculture, and Consumer Protection through the Federal Office for Agriculture and Food (Grant 2810HS035). Furthermore, insulin measurements were funded by the Wereld Kanker Onderzoek Fonds (WCRF NL) (Grant 2010/248). The DONALD study is supported by the Ministry of Science and Research of North Rhine Westphalia, Germany. The German Diabetes Center is funded by the German Federal Ministry of Health, the Ministry of School, Science, and Research of the State of North-Rhine-Westphalia, and the German Center for Diabetes Research.
No potential conflicts of interest relevant to this article were reported.
J.G. assigned the GI values, conducted the statistical analysis, wrote the manuscript, contributed to the interpretation of the results, critically revised the manuscript, and approved the final version of the manuscript. C.H. gave detailed assistance in the drafting process, contributed to the interpretation of the results, critically revised the manuscript, and approved the final version of the manuscript. G.J. assigned the GI values, contributed to the interpretation of the results, critically revised the manuscript, and approved the final version of the manuscript. K.B. conducted the statistical analysis, contributed to the interpretation of the results, critically revised the manuscript, and approved the final version of the manuscript. T.R. contributed to the interpretation of the results, critically revised the manuscript, and approved the final version of the manuscript. S.A.W. ensured correct determination of plasma glucose, contributed to the interpretation of the results, critically revised the manuscript, and approved the final version of the manuscript. Triglycerides, ALT, and GGT values were measured in the laboratory of M.R., and M.R. contributed to the interpretation of the results, critically revised the manuscript, and approved the final version of the manuscript. W.R. contributed to the interpretation of the results, critically revised the manuscript, and approved the final version of the manuscript. A.E.B. conceived the research project, supervised the project, gave detailed assistance in the drafting process, contributed to the interpretation of the results, critically revised the manuscript, and approved the final version of the manuscript. A.E.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of the study were presented as a poster session at the 48th Annual Meeting of the European Association for the Study of Diabetes, Berlin, Germany, 1–5 October 2012.
The authors thank the staff of the Research Institute of Child Nutrition for carrying out the anthropometric measurements and for collecting and coding the dietary records. The authors also thank all the participants of the DONALD study. The authors thank the staff of the technical laboratory of the German Diabetes Center, Düsseldorf, and the Laboratory for Translational Hormone Analytics in Paediatric Endocrinology, Giessen, for carrying out blood analysis.
References
- 1.Buyken AE, Mitchell P, Ceriello A, Brand-Miller J. Optimal dietary approaches for prevention of type 2 diabetes: a life-course perspective. Diabetologia 2010;53:406–418 [DOI] [PubMed] [Google Scholar]
- 2.Hite AH, Feinman RD, Guzman GE, Satin M, Schoenfeld PA, Wood RJ. In the face of contradictory evidence: report of the Dietary Guidelines for Americans Committee. Nutrition 2010;26:915–924 [DOI] [PubMed] [Google Scholar]
- 3.Barclay AW, Petocz P, McMillan-Price J, et al. Glycemic index, glycemic load, and chronic disease risk—a meta-analysis of observational studies. Am J Clin Nutr 2008;87:627–637 [DOI] [PubMed] [Google Scholar]
- 4.Dong JY, Zhang L, Zhang YH, Qin LQ. Dietary glycaemic index and glycaemic load in relation to the risk of type 2 diabetes: a meta-analysis of prospective cohort studies. Br J Nutr 2011;106:1649–1654 [DOI] [PubMed] [Google Scholar]
- 5.Goran MI, Gower BA. Longitudinal study on pubertal insulin resistance. Diabetes 2001;50:2444–2450 [DOI] [PubMed] [Google Scholar]
- 6.Roden M. Mechanisms of disease: hepatic steatosis in type 2 diabetes—pathogenesis and clinical relevance. Nat Clin Pract Endocrinol Metab 2006;2:335–348 [DOI] [PubMed] [Google Scholar]
- 7.Ford ES, Schulze MB, Bergmann MM, Thamer C, Joost HG, Boeing H. Liver enzymes and incident diabetes: findings from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes Care 2008;31:1138–1143 [DOI] [PubMed] [Google Scholar]
- 8.Bonnet F, Ducluzeau PH, Gastaldelli A, et al. RISC Study Group Liver enzymes are associated with hepatic insulin resistance, insulin secretion, and glucagon concentration in healthy men and women. Diabetes 2011;60:1660–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valtuena S, Pellegrini N, Ardigo D, et al. Dietary glycemic index and liver steatosis. Am J Clin Nutr 2006;84:136–142 [DOI] [PubMed]
- 10.Fabricatore AN, Ebbeling CB, Wadden TA, Ludwig DS. Continuous glucose monitoring to assess the ecologic validity of dietary glycemic index and glycemic load. Am J Clin Nutr 2011;94:1519–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kochan AM, Wolever TM, Chetty VT, Anand SS, Gerstein HC, Sharma AM. Glycemic index predicts individual glucose responses after self-selected breakfasts in free-living, abdominally obese adults. J Nutr 2012;142:27–32 [DOI] [PubMed] [Google Scholar]
- 12.Kroke A, Manz F, Kersting M, et al. The DONALD Study. History, current status and future perspectives. Eur J Nutr 2004;43:45–54 [DOI] [PubMed] [Google Scholar]
- 13.Sichert-Hellert W, Kersting M, Schöch G. Underreporting of energy intake in 1 to 18 year old German children and adolescents. Z Ernahrungswiss 1998;37:242–251 [DOI] [PubMed] [Google Scholar]
- 14.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 15.Kromeyer-Hauschild K, Wabitsch M, Kunze D, et al. Perzentile für den Body-mass-Index für das Kindes- und Jugendalter unter Heranziehung verschiedener deutscher Stichproben. Monatsschr Kinderheilkd 2001;149:807–818 [Google Scholar]
- 16.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000;320:1240–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slaughter MH, Lohman TG, Boileau RA, et al. Skinfold equations for estimation of body fatness in children and youth. Hum Biol 1988;60:709–723 [PubMed] [Google Scholar]
- 18.McCarthy HD, Cole TJ, Fry T, Jebb SA, Prentice AM. Body fat reference curves for children. Int J Obes (Lond) 2006;30:598–602 [DOI] [PubMed] [Google Scholar]
- 19.Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr 1974;32:77–97 [DOI] [PubMed] [Google Scholar]
- 20.Sichert-Hellert W, Kersting M, Chahda C, Schäfer R, Kroke A. German food composition database for dietary evaluations in children and adolescents. J Food Compost Anal 2007;20:63–70 [Google Scholar]
- 21.Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008;31:2281–2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buyken AE, Dettmann W, Kersting M, Kroke A. Glycaemic index and glycaemic load in the diet of healthy schoolchildren: trends from 1990 to 2002, contribution of different carbohydrate sources and relationships to dietary quality. Br J Nutr 2005;94:796–803 [DOI] [PubMed] [Google Scholar]
- 23.Institute of Medicine of the National Academies, Food and Nutrition Board, Panel of Macronutrients, Panel on the Definition of Dietary Fiber, Subcommittee on Upper Reference Levels of Nutrients, Subcommittee on Interpretation and Uses of Dietary Reference Intakes, and Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary References Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC, National Academies Press, 2005 [Google Scholar]
- 24.Food Labeling and Standards Staff. Whole-grain label statements-guidance for industry and FDA staff [article online], 2006. Available from http://www.fda.gov/Food/GuidanceComplianceRegulatoryInformation/GuidanceDocuments/FoodLabelingNutrition/ucm059088.htm Accessed 28 September 2012
- 25.Brand-Miller J. Glycaemic index and glycaemic load: Crunch time? Nutrition & Dietetics 2009;66:136–137 [Google Scholar]
- 26.Joslowski G, Goletzke J, Cheng G, et al. Prospective associations of dietary insulin demand, glycemic index, and glycemic load during puberty with body composition in young adulthood. Int J Obes (Lond) 2012;36:1463–1491 [DOI] [PubMed] [Google Scholar]
- 27.Hu Y, Block G, Norkus EP, Morrow JD, Dietrich M, Hudes M. Relations of glycemic index and glycemic load with plasma oxidative stress markers. Am J Clin Nutr 2006;84:70–76; quiz 266–267 [DOI] [PubMed] [Google Scholar]
- 28.Dey A, Swaminathan K. Hyperglycemia-induced mitochondrial alterations in liver. Life Sci 2010;87:197–214 [DOI] [PubMed] [Google Scholar]
- 29.Szendroedi J, Chmelik M, Schmid AI, et al. Abnormal hepatic energy homeostasis in type 2 diabetes. Hepatology 2009;50:1079–1086 [DOI] [PubMed] [Google Scholar]
- 30.Goran MI, Ball GD, Cruz ML. Obesity and risk of type 2 diabetes and cardiovascular disease in children and adolescents. J Clin Endocrinol Metab 2003;88:1417–1427 [DOI] [PubMed] [Google Scholar]
- 31.Sunehag AL, Toffolo G, Campioni M, Bier DM, Haymond MW. Effects of dietary macronutrient intake on insulin sensitivity and secretion and glucose and lipid metabolism in healthy, obese adolescents. J Clin Endocrinol Metab 2005;90:4496–4502 [DOI] [PubMed] [Google Scholar]
- 32.Malik VS, Popkin BM, Bray GA, Després JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 2010;33:2477–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schulze MB, Schulz M, Heidemann C, Schienkiewitz A, Hoffmann K, Boeing H. Fiber and magnesium intake and incidence of type 2 diabetes: a prospective study and meta-analysis. Arch Intern Med 2007;167:956–965 [DOI] [PubMed] [Google Scholar]
- 34.Weickert MO, Pfeiffer AF. Metabolic effects of dietary fiber consumption and prevention of diabetes. J Nutr 2008;138:439–442 [DOI] [PubMed] [Google Scholar]
- 35.Bao J, Atkinson F, Petocz P, Willett WC, Brand-Miller JC. Prediction of postprandial glycemia and insulinemia in lean, young, healthy adults: glycemic load compared with carbohydrate content alone. Am J Clin Nutr 2011;93:984–996 [DOI] [PubMed] [Google Scholar]
- 36.Nicklas TA, Elkasabany A, Srinivasan SR, Berenson G. Trends in nutrient intake of 10-year-old children over two decades (1973-1994): the Bogalusa Heart Study. Am J Epidemiol 2001;153:969–977 [DOI] [PubMed] [Google Scholar]
- 37.Linseisen J, Gedrich K, Karg G, Wolfram G. Sucrose intake in Germany. Z Ernahrungswiss 1998;37:303–314 [DOI] [PubMed] [Google Scholar]
- 38.Louie JC, Buyken AE, Heyer K, Flood VM. Dietary glycaemic index and glycaemic load among Australian children and adolescents. Br J Nutr 2011;106:1273–1282 [DOI] [PubMed] [Google Scholar]
- 39.Flint A, Møller BK, Raben A, et al. The use of glycaemic index tables to predict glycaemic index of composite breakfast meals. Br J Nutr 2004;91:979–989 [DOI] [PubMed] [Google Scholar]