Abstract
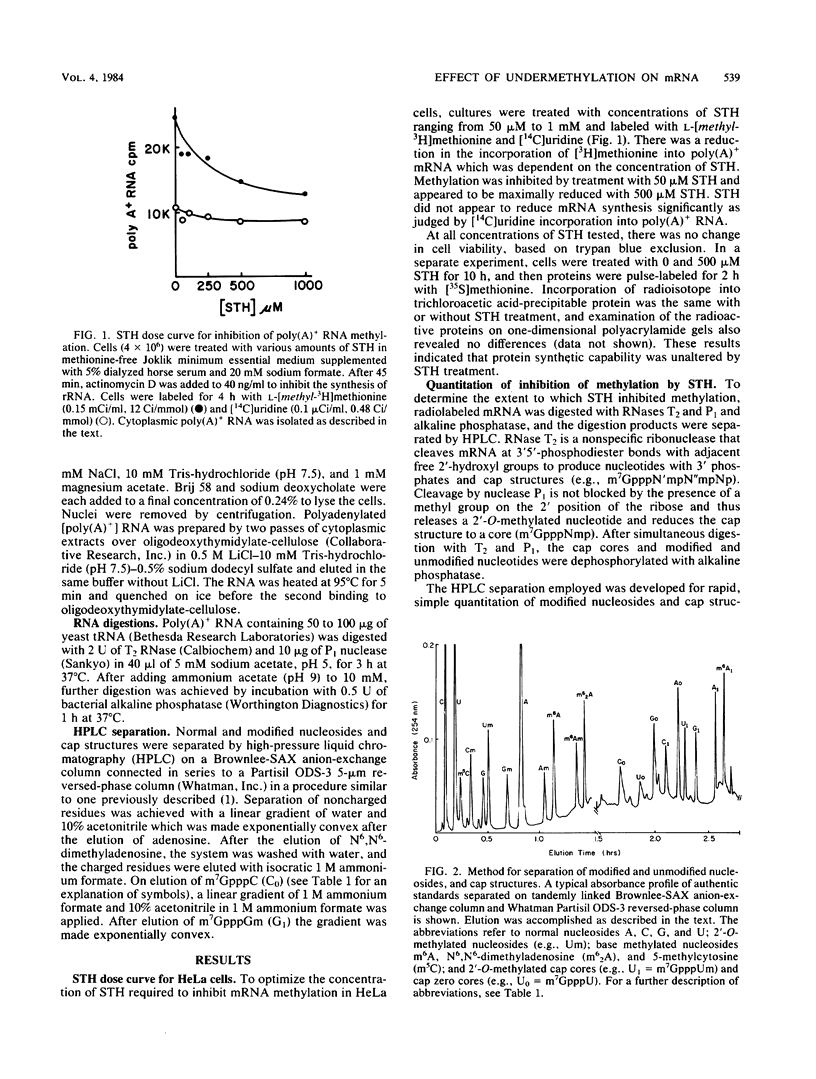
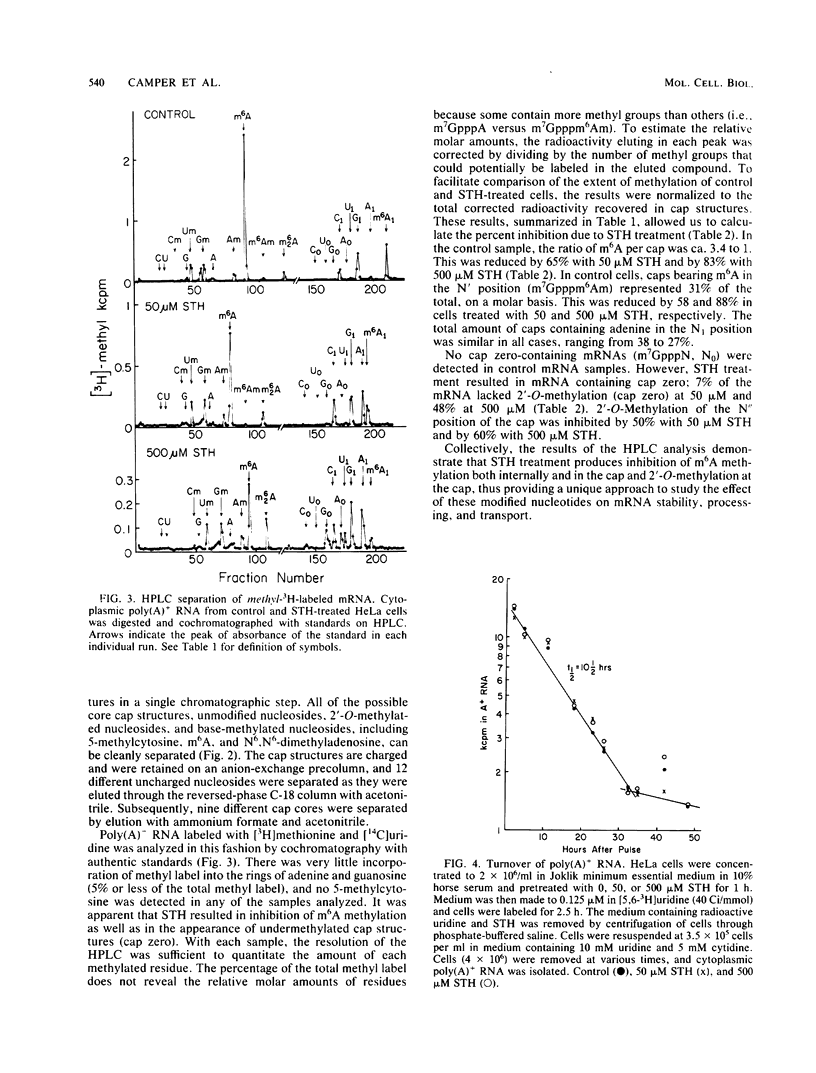
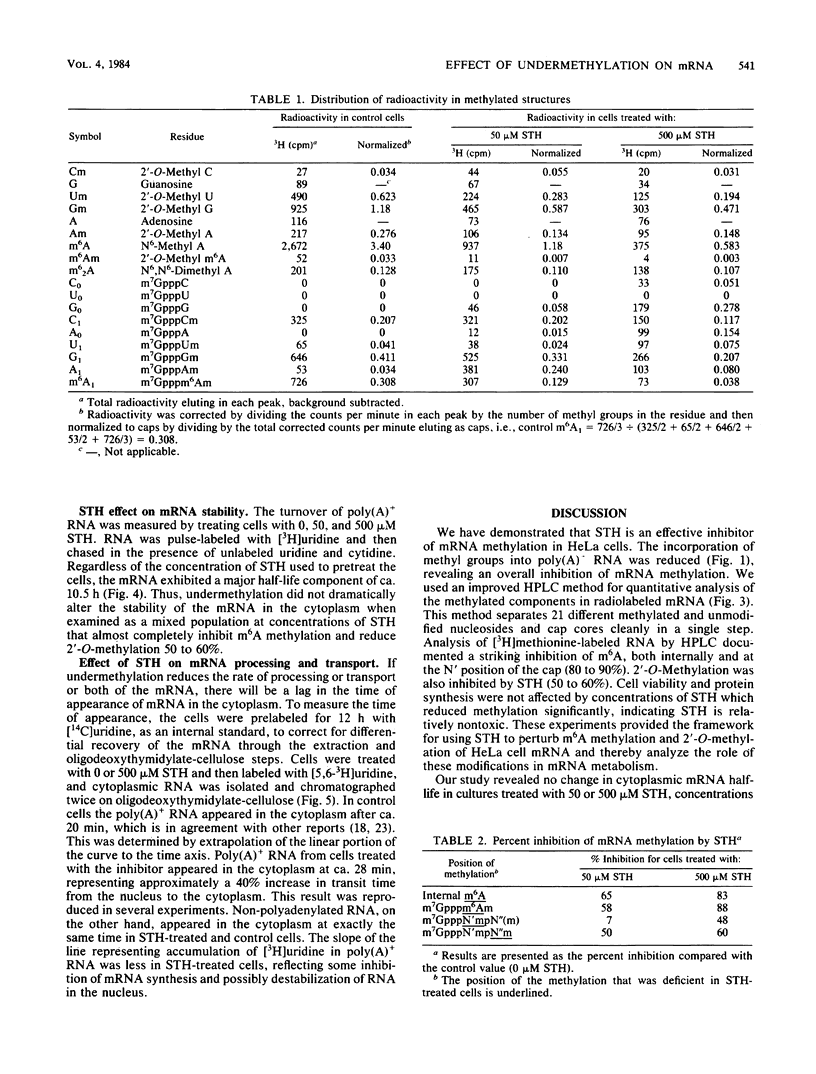
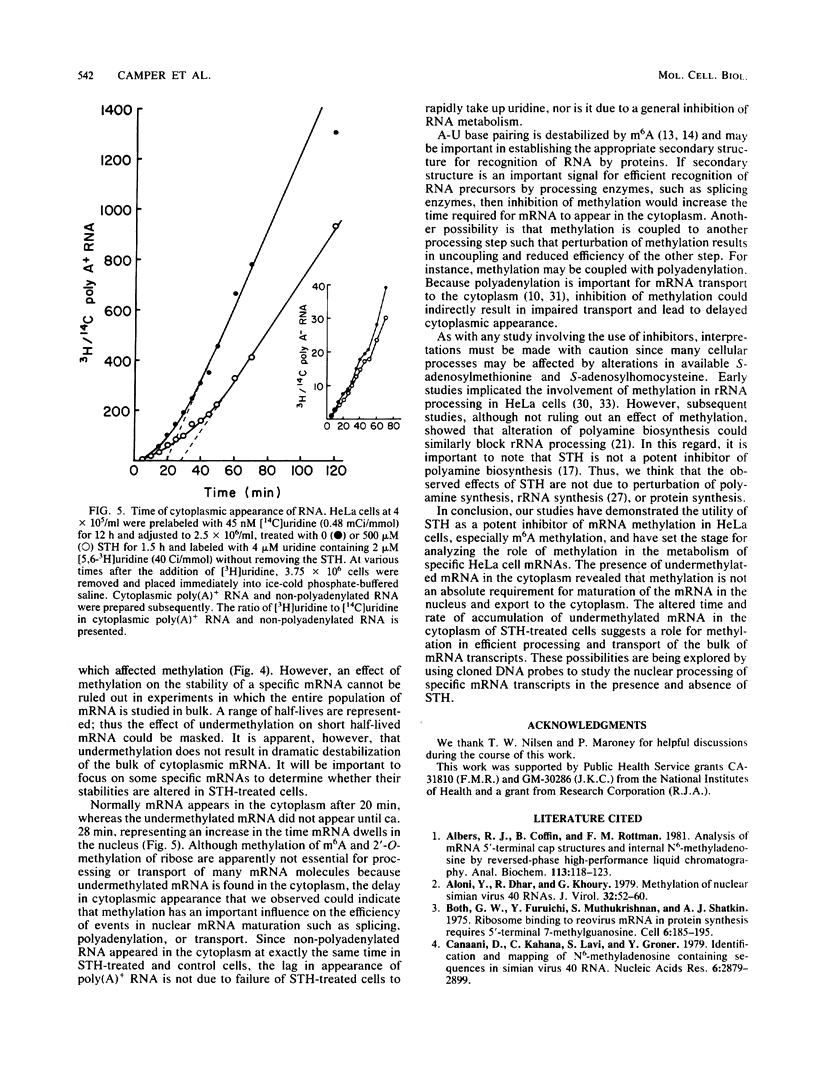
S-Tubercidinylhomocysteine (STH) is a structural analog of S-adenosylhomocysteine and a potent inhibitor of S-adenosylmethionine-dependent methyltransferase reactions. We investigated the effects of STH on HeLa cell mRNA metabolism. Dual labeling studies reveal that STH dramatically inhibits the methylation of HeLa mRNA in a dose-dependent manner. Analysis of the modified nucleosides and 5'-terminal cap structures in radiolabeled mRNA by high-pressure liquid chromatography indicated that internal N6-methylation of adenosine was reduced by 65% at 50 microM STH and by 83% at 500 microM STH. The N6-methylation of adenosine contained in cap structures was similarly reduced at both concentrations of STH. Substantial amounts of cap structures lacking 2'-O-methylated nucleosides (m7GpppN, cap zero) were detected at the higher level of STH. To test the possibility that methylation affects mRNA stability, cytoplasmic mRNA half-life was measured in a pulse-chase experiment. The half-life of undermethylated mRNA, produced as a consequence of STH treatment, was unchanged compared with the control. To determine whether mRNA methylation is coupled to nuclear processing or transport, the time of cytoplasmic appearance of polyadenylated RNA in STH-treated HeLa cells was compared with untreated cells. STH caused a significant lag in the time of appearance of the polyadenylated RNA, suggesting that mRNA methylation may be required for efficient processing or transport.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers R. J., Coffin B., Rottman F. M. Analysis of mRNA 5'-terminal cap structures and internal N6-methyladenosine by reversed-phase high-performance liquid chromatography. Anal Biochem. 1981 May 1;113(1):118–123. doi: 10.1016/0003-2697(81)90053-1. [DOI] [PubMed] [Google Scholar]
- Aloni Y., Dhar R., Khoury G. Methylation of nuclear simian virus 40 RNAs. J Virol. 1979 Oct;32(1):52–60. doi: 10.1128/jvi.32.1.52-60.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Furuichi Y., Muthukrishnan S., Shatkin A. J. Ribosome binding to reovirus mRNA in protein synthesis requires 5' terminal 7-methylguanosine. Cell. 1975 Oct;6(2):185–195. doi: 10.1016/0092-8674(75)90009-4. [DOI] [PubMed] [Google Scholar]
- Canaani D., Kahana C., Lavi S., Groner Y. Identification and mapping of N6-methyladenosine containing sequences in simian virus 40 RNA. Nucleic Acids Res. 1979 Jun 25;6(8):2879–2899. doi: 10.1093/nar/6.8.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Kiang S., Nevins J. R., Darnell J. E., Jr N-6-methyl-adenosine in adenovirus type 2 nuclear RNA is conserved in the formation of messenger RNA. J Mol Biol. 1979 Dec 15;135(3):733–752. doi: 10.1016/0022-2836(79)90174-8. [DOI] [PubMed] [Google Scholar]
- Chiang P. K., Richards H. H., Cantoni G. L. S-Adenosyl-L-homocysteine hydrolase: analogues of S-adenosyl-L-homocysteine as potential inhibitors. Mol Pharmacol. 1977 Sep;13(5):939–947. [PubMed] [Google Scholar]
- Coward J. K., Bussolotti D. L., Chang C. D. Analogs of S-adenosylhomocysteine as potential inhibitors of biological transmethylation. Inhibition of several methylases by S-tubercidinylhomocysteine. J Med Chem. 1974 Dec;17(12):1286–1289. doi: 10.1021/jm00258a011. [DOI] [PubMed] [Google Scholar]
- Crooks P. A., Dreyer R. N., Coward J. K. Metabolism of S-adenosylhomocysteine and S-tubercidinylhomocysteine in neuroblastoma cells. Biochemistry. 1979 Jun 12;18(12):2601–2609. doi: 10.1021/bi00579a026. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Dimock K., Stoltzfus C. M. Processing and function of undermethylated chicken embryo fibroblast mRNA. J Biol Chem. 1979 Jul 10;254(13):5591–5594. [PubMed] [Google Scholar]
- Dimock K., Stoltzfus C. M. Sequence specificity of internal methylation in B77 avian sarcoma virus RNA subunits. Biochemistry. 1977 Feb 8;16(3):471–478. doi: 10.1021/bi00622a021. [DOI] [PubMed] [Google Scholar]
- Engel J. D., von Hippel P. H. Effects of methylation on the stability of nucleic acid conformations. Studies at the polymer level. J Biol Chem. 1978 Feb 10;253(3):927–934. [PubMed] [Google Scholar]
- Engel J. D., von Hippel P. H. Effects of methylation on the stability of nucleic acid conformations: studies at the monomer level. Biochemistry. 1974 Sep 24;13(20):4143–4158. doi: 10.1021/bi00717a013. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., LaFiandra A., Shatkin A. J. 5'-Terminal structure and mRNA stability. Nature. 1977 Mar 17;266(5599):235–239. doi: 10.1038/266235a0. [DOI] [PubMed] [Google Scholar]
- Green M. R., Maniatis T., Melton D. A. Human beta-globin pre-mRNA synthesized in vitro is accurately spliced in Xenopus oocyte nuclei. Cell. 1983 Mar;32(3):681–694. doi: 10.1016/0092-8674(83)90054-5. [DOI] [PubMed] [Google Scholar]
- Hibasami H., Borchardt R. T., Chen S. Y., Coward J. K., Pegg A. E. Studies of inhibition of rat spermidine synthase and spermine synthase. Biochem J. 1980 May 1;187(2):419–428. doi: 10.1042/bj1870419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. F., Williams J. G., Abelson H. T., Green H., Penman S. Changes in RNA in relation to growth of the fibroblast. III. Posttranscriptional regulation of mRNA formation in resting and growing cells. Cell. 1975 Jan;4(1):69–75. doi: 10.1016/0092-8674(75)90135-x. [DOI] [PubMed] [Google Scholar]
- Kaehler M., Coward J., Rottman F. Cytoplasmic location of undermethylated messenger RNA in Novikoff cells. Nucleic Acids Res. 1979 Mar;6(3):1161–1175. doi: 10.1093/nar/6.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaehler M., Coward J., Rottman F. In vivo inhibition of Novikoff cytoplasmic messenger RNA methylation by S-tubercidinylhomocysteine. Biochemistry. 1977 Dec 27;16(26):5770–5775. doi: 10.1021/bi00645a019. [DOI] [PubMed] [Google Scholar]
- Levin E. G., Clark J. L. Defect in polyamine metabolism in a BHK cell mutant temperature-sensitive for rRNA maturation. J Cell Physiol. 1979 Dec;101(3):361–368. doi: 10.1002/jcp.1041010303. [DOI] [PubMed] [Google Scholar]
- Muthukrishnan S., Moss B., Cooper J. A., Maxwell E. S. Influence of 5'-terminal cap structure on the initiation of translation of vaccinia virus mRNA. J Biol Chem. 1978 Mar 10;253(5):1710–1715. [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Messenger RNA turnover in mouse L cells. J Mol Biol. 1973 Oct 5;79(4):681–696. doi: 10.1016/0022-2836(73)90071-5. [DOI] [PubMed] [Google Scholar]
- Pugh C. S., Borchardt R. T. Effects of S-adenosylhomocysteine analogues on vaccinia viral messenger ribonucleic acid synthesis and methylation. Biochemistry. 1982 Mar 30;21(7):1535–1541. doi: 10.1021/bi00536a011. [DOI] [PubMed] [Google Scholar]
- Pugh C. S., Borchardt R. T., Stone H. O. Inhibition of Newcastle disease virion messenger RNA (guanine-7-)-methyltransferase by analogues of S-adenosylhomocysteine. Biochemistry. 1977 Aug 23;16(17):3928–3932. doi: 10.1021/bi00636a032. [DOI] [PubMed] [Google Scholar]
- Rottman F., Shatkin A. J., Perry R. P. Sequences containing methylated nucleotides at the 5' termini of messenger RNAs: possible implications for processing. Cell. 1974 Nov;3(3):197–199. doi: 10.1016/0092-8674(74)90131-7. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J. Capping of eucaryotic mRNAs. Cell. 1976 Dec;9(4 Pt 2):645–653. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- Stoltzfus C. M., Dane R. W. Accumulation of spliced avian retrovirus mRNA is inhibited in S-adenosylmethionine-depleted chicken embryo fibroblasts. J Virol. 1982 Jun;42(3):918–931. doi: 10.1128/jvi.42.3.918-931.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan M. H., Jr, Soeiro R., Warner J. R., Darnell J. E., Jr The effects of methionine deprivation on ribosome synthesis in HeLa cells. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1527–1534. doi: 10.1073/pnas.58.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal L. P., White R. T. A splice junction deletion deficient in the transport of RNA does not polyadenylate nuclear RNA. Mol Cell Biol. 1983 Aug;3(8):1381–1388. doi: 10.1128/mcb.3.8.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C. M., Gershowitz A., Moss B. 5'-Terminal and internal methylated nucleotide sequences in HeLa cell mRNA. Biochemistry. 1976 Jan 27;15(2):397–401. doi: 10.1021/bi00647a024. [DOI] [PubMed] [Google Scholar]
- Wolf S. F., Schlessinger D. Nuclear metabolism of ribosomal RNA in growing, methionine-limited, and ethionine-treated HeLa cells. Biochemistry. 1977 Jun 14;16(12):2783–2791. doi: 10.1021/bi00631a031. [DOI] [PubMed] [Google Scholar]



