Abstract
An increasing body of evidence indicates that local axonal translation is required for growing axons to respond appropriately to guidance cues and other stimuli. Recent studies suggest that asymmetrical synthesis of cytoskeletal proteins mediates growth cone turning and that local translation and retrograde transport of transcription factors mediate neuronal survival. Axonal translation is regulated partly by selective axonal localization of mRNAs and by translation initiation factors and RNA-binding proteins. We discuss possible rationales for local axonal translation, including distinct properties of nascent proteins, precise localization, and axonal autonomy.
Introduction
The highly polarized and extended nature of neurons presents a cell biological challenge regarding how distal ends of dendrites and axons communicate with the cell body. On the one hand, these remote cellular outposts need to respond quickly to extracellular stimuli, which makes it impractical to wait for instructions from the cell body. On the other hand, the cell body must be updated on events in the distal reaches of the neuron to appropriately regulate gene expression. Both of these phenomena are crucial during the development of the nervous system, where axons must not only navigate to the correct targets autonomously from the cell body but also instruct their cell bodies about the extracellular signals they are receiving. Although the role of local dendritic translation in the autonomy of distal neuronal processes has been studied for many years [1], the role of local axonal translation in these phenomena has only gained attention more recently. This review will focus on progress in local axonal translation over the past two years.
Function of local translation in axons
Growth cone chemotropic responses
Developing axons are guided to their correct targets by attractive and repulsive guidance cues like the netrins, slits, semaphorins, and ephrins [2]. These cues are received and transduced into turning decisions by a highly motile structure, the growth cone, at the tip of the growing axon. Because these growth cones can be millimeters or centimeters away from the cell body, these turning decisions must be made relatively autonomously from the cell body. Indeed, growth cones still navigate correctly even when the cell body has been removed, both in vivo and in vitro. Local axonal translation, first proposed many years ago [3,4], has received much attention recently as a possible mechanism for this independence [5,6].
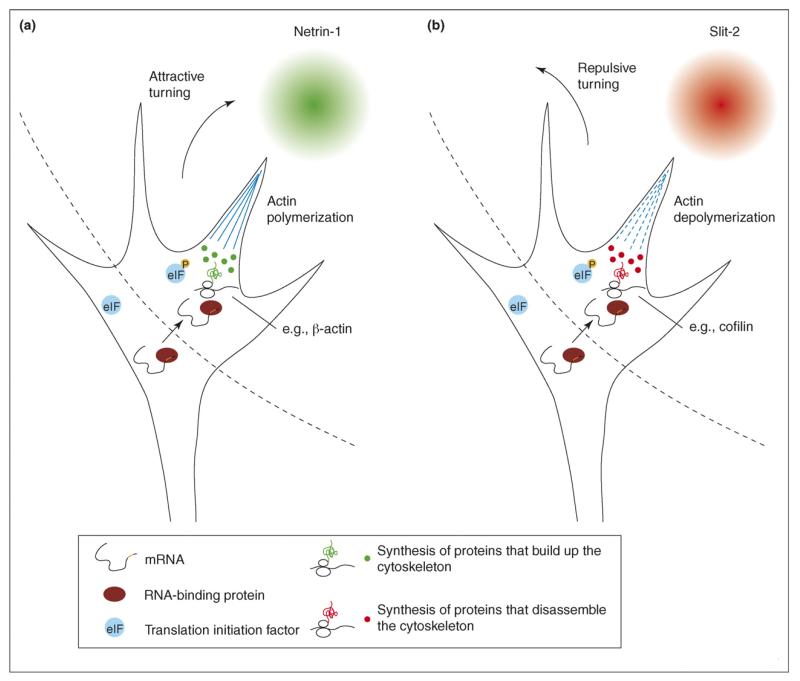
Local protein synthesis is required for the growth cone to respond appropriately to some guidance cues, including netrin-1, Slit-2, and Semaphorin3A [7–10]. An emerging model for local translation in response to guidance cues, termed the ‘differential translation’ model, is that attractive and repulsive cues induce asymmetrical translation of proteins that build up or break down the cytoskeleton, respectively [5,11] (see Figure 1). In support of this model, netrin-1 and brain-derived neurotrophic factor (BDNF) under attractive conditions induce local synthesis of β-actin in axonal growth cones [12••,13••]. This occurs asymmetrically in response to a gradient of netrin-1 or BDNF, and attractive turning toward netrin-1 or BDNF is prevented by morpholinos that block β-actin translation or antisense oligonucleotides that bind to the β-actin 3′ untranslated region (UTR) and thus deregulate β-actin translation. Interestingly, repulsive turning is not affected by morpholino-based blockade of translation, but is prevented by de-regulation of β-actin translation, suggesting that repulsive turning requires repression of β-actin translation. Indeed, netrin-1 and BDNF under repulsive conditions do not induce translation of β-actin.
Figure 1.
The ‘differential translation’ model for local translation in growth cones. (a) A gradient of attractive guidance cue, such as netrin-1, induces asymmetrical activation of translation and transport of mRNAs, causing asymmetrical translation of proteins that build up the cytoskeleton, which leads to attractive turning. (b) A gradient of repulsive guidance cue, such as Slit-2, induces similar asymmetrical activation of translation but induces transport and translation of different mRNAs, causing asymmetrical translation of proteins that disassemble the cytoskeleton, which leads to repulsive turning.
By contrast, repulsive cues stimulate the translation of proteins that disassemble the cytoskeleton. For example, the repellent Sema3A induces local translation of RhoA, a GTPase that mediates neurite retraction, and this is required for growth cone collapse [10]. Local translation of β-thymosin, an actin monomer sequestering protein, in Lymnaea neurons reduces neurite length [14]. Another repellent, Slit-2, induces a translation-dependent increase in ADF/cofilin-1, an actin depolymerizing protein, though whether this is required for collapse remains to be defined [8]. These results suggest that attractants and repellents stimulate the asymmetrical translation of ‘attractive’ and ‘repulsive’ proteins, respectively, close to the site of stimulus. However, it remains to be determined whether a repulsive gradient actually induces asymmetrical translation of proteins like cofilin, β-thymosin, or RhoA. It also remains unknown whether this local translation of ‘attractive’ versus ‘repulsive’ proteins is instructive or permissive. Interestingly, translational regulation of the cytoskeleton in axons may also be complemented by cytoskeletal regulation of translation (see below).
Local translation most probably operates in concert with other signaling pathways involved in growth cone turning. For example, protein synthesis is required for growth cone turning induced by local Ca2+ transients [13••]. De novo synthesis of cytoskeletal regulators may cooperate with regulation of pre-existing protein in regulating actin dynamics; for example, in addition to being locally translated, ADF/cofilin is regulated by LIM kinase and Slingshot phosphatase, which helps switch growth cone responses between attraction and repulsion [15]. Similarly, local translation of RhoA in response to Sema3A [10] is consistent with findings that the three small GTPases RhoA, Rac1, and Cdc42 are important for growth cone chemotropic responses [16]. Intriguingly, RhoA function is also required for repulsion by lysophosphatidic acid [17], but translation is not [7], raising the question why guidance cues that seem to have the same effects on growth cones and use the same signaling molecules have different requirements for local translation. Future work on this question may provide further insights into the relationship between local translation and other signaling pathways in the growth cone.
Regulation of axonal responsiveness
Local translation may also mediate changes in axonal responsiveness. Axons en route to their final destination often encounter intermediate targets that change their responsiveness to guidance cues, for example, making them repelled by a previously attractive cue that the growth cone must move beyond. Local synthesis of guidance cue receptors is one suggested mechanism for this: chick commissural axons upregulate both the ephrin receptor EphA2 and a reporter regulated by the EphA2 3′UTR in the postmidline segment. This correlation between EphA2 expression and midline crossing suggests that the midline induces commissural axon growth cones to locally translate EphA2 after crossing, though direct causation remains to be demonstrated [18].
Axons also must adapt to changing levels of guidance cue, for example, in climbing up a gradient, which requires continuous desensitization and resensitization. Resensitization (but not desensitization) requires local protein synthesis [19,20]; it is not yet clear if the increased sensitivity is mediated by local synthesis of new receptors or downstream signaling molecules such as the cytoplasmic regulators described above. Interestingly, local dendritic translation is important for homeostatic regulation of synaptic strength by mini-EPSP frequency [21], suggesting that local translation is a general mechanism for maintaining a dynamic range of responsiveness.
Recent studies also suggest a role for local translation in sensory axons. κ-opioid receptor (kor) is translated locally in dorsal root ganglion (DRG) axons in response to KCl depolarization, and at least in DRG cell bodies in response to netrin-1 [22•,23•]. A recent study suggests that local translation in A-fibers, axons from the DRG that mediate fast nociceptive sensation, is involved in some forms of hyperalgesia, in which pain sensitivity is upregulated in response to previous painful stimuli or nerve injury [24]. Given the role of opioids in regulating pain, these two findings may be linked, but the connection remains unclear for the moment.
Synaptogenesis
Local translation is also important at the end of the axon’s journey, when it must form a synapse. Xenopus spinal neurons can form synapses on muscle cells in vitro, and these synapses are potentiated by local application of a bead coated with BDNF. This synaptic potentiation occurs even with severed axons and requires presynaptic protein synthesis, indicating that local axonal translation is required for synaptic potentiation [25]. In Aplysia, the formation of synapses between sensory and motor neurons induces localization of sensorin mRNA to the presynaptic terminal, and the knockdown of sensorin mRNA abolishes synapse formation [26•], suggesting that local synthesis of sensorin in the presynaptic terminal is required for synapse formation. In Drosophila, axonal arborization requires cytoplasmic protein synthesis, though whether it requires local protein synthesis is unknown [27].
Retrograde signaling
Recent evidence indicates that local axonal translation is also involved in long-range retrograde signaling. According to the neurotrophic hypothesis, overproduction of many types of neurons forces them to compete for survival factors secreted by target cells, such that those not receiving enough die, thus ensuring that the correct number of neurons are generated to innervate the target. This requires a retrograde signal to travel from the site of survival factor reception, at the tip of the axon, back to the cell body, often a distance of many millimeters or centimeters. This signal is thought to be a ‘signaling endosome,’ a signaling platform containing the neurotrophic factor, its activated receptor, and downstream effectors that are retrogradely transported to the cell body [28]. Retrograde signaling is also important for axonal regeneration, as the cell body must be updated on the injured status of the axon to initiate a program of repair [29].
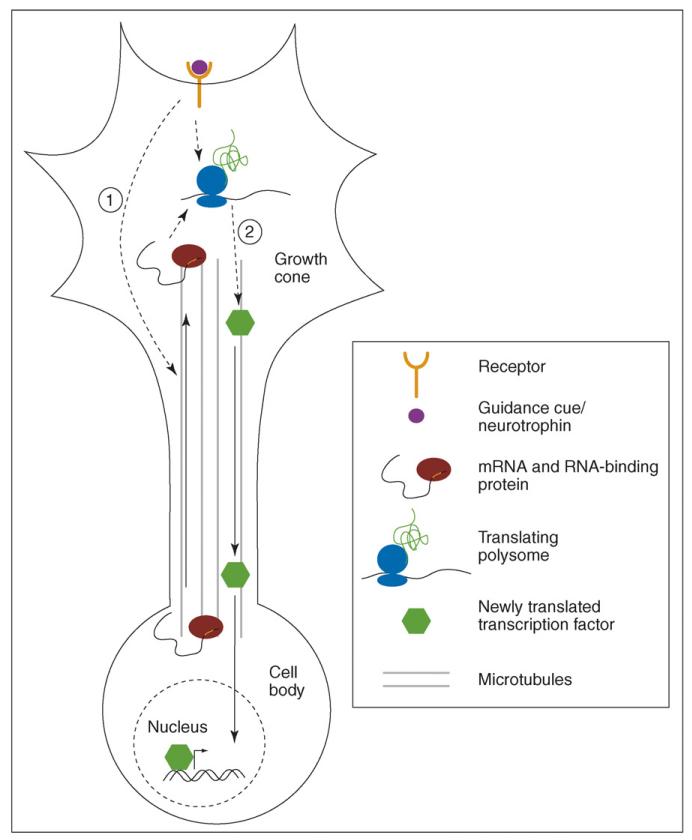
An early indication that local translation is involved in retrograde signaling came from evidence that injured DRG axons locally synthesize importin β, a protein that transports nuclear localization signal (NLS) bearing proteins to the nucleus [30], suggesting that transcription factors might be retrogradely transported from the axon to the nucleus. Indeed, a recent study shows that the transcription factor cAMP response element binding protein (CREB) is locally translated in axons and retrogradely transported to the cell body in response to the neurotrophin nerve growth factor (NGF) [31••] (see Figure 2). Axon-specific application of anti-CREB siRNA revealed that axonally synthesized CREB is required for the phosphorylation of cell body CREB, CREB-mediated transcription, and subsequent cell survival. This suggests the surprising conclusion that axonally synthesized CREB is the source of phosphorylated, and hence active, CREB in the cell body. Together, these studies raise the interesting possibility that injured axons may locally synthesize CREB or other transcription factors to induce expression of genes for axonal repair. Given that CREB-binding protein (CBP) acetylates histones to facilitate CREB-mediated transcription [32], these studies also raise the possibility that local translation and retrograde transport may mediate broader epigenetic changes.
Figure 2.
Local translation and communication between the axon and cell body. (1) Stimulation of axons leads to transcription-independent differential localization of mRNAs to the axon through transport on microtubules, changing the population of mRNAs available for local axonal translation. (2) Newly synthesized transcription factors can be retrogradely transported on microtubules to the cell body where they influence transcription.
Why local translation?
Why do proteins need to be locally synthesized in axons rather than transported from the cell body or held inactive until needed? We and others have previously discussed possible rationales for local translation [5,33,34]. RNA provides a more flexible format for the regulation of localization and activation than protein, as regulatory elements in the 5′UTR or 3′UTR do not affect the function of the protein, while regulatory elements in the protein itself do [35]. Indeed, RNA localization may be the only way to correctly localize some proteins, like tau [36] or myelin basic protein [37]. As discussed above, local translation may give the growth cone autonomy to respond to signals quickly without waiting for input from the cell body. In addition, axons may be limited by macromolecular crowding [38] — there is simply not enough room to store all the proteins that may be needed, so it is more efficient to store mRNA and to synthesize and degrade proteins as needed. In this context, it is intriguing to note that in synaptic plasticity, a phenomenon conceptually similar to axon guidance (see below), a balance of protein synthesis and degradation is required for long-term potentiation (LTP) [39], and during memory retrieval, protein degradation mediates memory destabilization and protein synthesis mediates memory reconsolidation [40]. These results suggest that the rapid regulation of the local proteome is a general rationale for local translation. We focus here on possible rationales for local axonal translation highlighted by recent research: distinct properties of newly synthesized proteins, precise localization, and homeostatic control.
Unique properties of nascent proteins
One puzzle of local axonal translation is that in some cases, the newly synthesized protein apparently has a crucial function in a part of the cell where that protein is already abundant. Local β-actin synthesis is required for growth cone turning even though pre-existing β-actin is abundant in the growth cone [12••,13••]. Acute knockdown of sensorin mRNA in Aplysia sensory neurons blocks synapse formation, even though synaptic sensorin protein levels remain normal [26•]. Axon-specific knockdown of CREB mRNA leaves cell body CREB levels intact, but abolishes cell survival induced by axonal application of NGF, indicating that only axonally synthesized CREB is competent to induce cell survival [31••]. These examples suggest that locally synthesized proteins may have unique properties that allow them to function differently from pre-existing proteins in the axon or cell body. For example, newly synthesized β-actin presumably lacks post-translational modifications such as arginylation [41] or glutathionylation [42] that can affect polymerization. In the absence of biochemical characterization of locally translated proteins, however, this possibility remains speculative.
Protein localization
Another possible rationale for local translation is precise spatial localization: proteins may need to be synthesized in close proximity to their binding or signaling partners. In the case of β-actin, local synthesis could lead to increased local concentration of actin monomers, which would aid in actin filament nucleation, if synthesis takes place in a restricted volume like a lamellipodium, or if an mRNP contains multiple β-actin mRNAs that might be released and translated simultaneously in close proximity. Indeed, a recent work on peripherin, an intermediate filament protein, suggests that peripherin mRNPs contain multiple peripherin mRNAs and that peripherin protein is assembled into particles cotranslationally in PC12 cells [43]. Localized synthesis of β-actin, and hence nucleation of actin filaments, could create a spatial ‘tag’ instructing actin monomers where to polymerize, biasing the formation of filopodia and ultimately the direction of growth. In DRG axons, CREB colocalizes with components of the signaling endosome, suggesting that after the signaling endosome induces axonal synthesis of new CREB protein, the two remain associated as they are retrogradely trafficked to the cell body [31••]. This physical proximity would make the axonally synthesized CREB uniquely available for phosphorylation, in contrast to pre-existing cell body CREB.
Homeostatic control
Finally, some of the autonomy ceded to the axon may be required for homeostatic control or metabolic health of the axon. Many of the mRNAs localized to DRG axons encode metabolic proteins, such as enolase, cytochrome oxidase, and ferritin [44••]. Given that the tip of an axon may be a long distance from the cell body — up to a meter for some human sensory and motor axons — metabolic conditions in the axon may be different from those in the cell body. For example, ferritin sequesters free iron, which is essential for many enzymes yet encourages formation of toxic free radicals, and its translation is regulated by iron concentration through iron-regulatory proteins acting on the iron responsive element in its 5′UTR [45]. Local regulation of ferritin levels may thus be necessary to maintain axonal free iron concentration in an acceptable range. Axonal localization of nuclear-encoded mRNAs for mitochondrial proteins [44••] may be a function of targeting of mitochondrial mRNAs to the vicinity of mitochondria [46] and the presence of mitochondria in the axon [47,48]. Local regulation of mitochondrial protein synthesis would allow fine-tuned control of relative levels of each complex in the electron transport chain to prevent the buildup of free electrons and production of free radicals, a rationale suggested for why mitochondria have retained their own genomes [49]. These local translational mechanisms for preventing free radical production and oxidative damage could be important for axonal health and perhaps even axonal degeneration. In this context, it is interesting to note that DRG axons also contain the mRNA for superoxide dismutase [44••], an antioxidant enzyme implicated in familial amyotrophic lateral sclerosis [50].
Regulation of local axonal translation
We have previously reviewed models of how local axonal translation is regulated [5]. Axon guidance cues induce global activation of translation through initiation factors like eukaryotic initiation factor 4E (eIF-4E) and eIF-4E-binding protein [7,8,31••,51], and guidance cue gradients induce asymmetrical activation of such global translation regulators [12••]. However, estimates for the number of mRNAs in axons range from ~100 [6] to ~200 [44••], and guidance cues do not stimulate the translation of all of them [12••,31••]; indeed, stimulus-induced axonal translation is probably highly selective. Our working model is that this global activation ‘opens the gates’ to translation, and the specific response of the growth cone (attraction, repulsion, etc.) is determined by mRNA-specific regulation by RNA-binding proteins and miRNAs [5,6] (see Figure 1). RNA-binding proteins and miRNAs are thought to repress translation when bound to their target mRNAs, often in large ribonucleoprotein (RNP) complexes called RNA granules, and activate translation by releasing their target mRNAs to ribosomes [52,53]. For example, Grb7 represses kor mRNA translation, but releases kor mRNA upon netrin-1 stimulation to allow translation [54]. Vg1RBP/ZBP binds to β-actin mRNA in Xenopus axons and moves asymmetrically within growth cones in response to a netrin-1 or BDNF gradient [12••,13••]. Other candidate axonal RNA-binding proteins include FMRP [55] and CPEB [18]. We focus here on mechanisms highlighted by recent research.
Axonal mRNA localization
Local translation can be regulated by mRNA localization, that is, the population of mRNAs present in the axon (see Figure 2). Axonal localization of kor mRNA in DRG neurons is mediated by Copb1, a subunit of COPI vesicle coatomer complexes, in association with kinesin and the RNA-binding protein HuR [56]. Neurotrophin stimulation has been known for some time to induce transport of β-actin mRNA to growth cones [57]. More generally, stimulus-driven changes in mRNA localization could result in changes in axon responsiveness, in a mechanism perhaps complementary to local synthesis of new receptors. For example, a guidance cue expressed in an early part of an axon’s pathway may induce axonal localization of an mRNA that will be needed to respond to a guidance cue in the next part of the pathway.
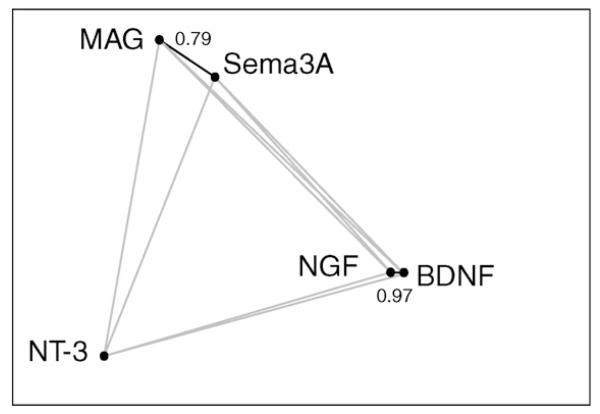
Recently, Twiss and colleagues conducted a large-scale study of transcription-independent axonal mRNA localization, revealing differential regulation of many mRNAs by the neurotrophins NGF, BDNF, and neurotrophin-3 (NT-3) and the repulsive guidance cues Sema3A and myelin-associated glycoprotein (MAG) [44••]. To examine quantitatively whether different cues induce the same or distinct populations of mRNAs to be trafficked into axons, we ran a correlation analysis on the data from this study (see Table 1 in [44••]), and visualized the results by plotting correlations between the mRNA responses to pairs of stimuli as distances between points (Figure 3). This analysis indicates that mRNA responses to the broadly attractive stimuli BDNF and NGF are correlated, as are responses to the broadly repulsive stimuli Sema3A and MAG, but these two groups are not correlated to each other. Interestingly, NT-3 diverges from the other two neurotrophins, even though all three are broadly ‘attractive’ stimuli, and does not correlate with any of the other stimuli tested. These findings might reflect differences across cell types of the heterogeneous DRG, which are sensitive to different neurotrophins and carry different sensory modalities [58], and thus may have different mRNA trafficking ‘regimes’. The overall clustering of responses to cues of similar effects suggests a possible coregulation of functionally related mRNAs, though elucidating the molecular logic underlying these responses will require understanding the function of local axonal translation of these mRNAs, most of which are not obviously ‘attractive’ or ‘repulsive’.
Figure 3.
Correlation analysis of mRNA localization data from [44••] indicates clustering of axonal mRNA trafficking responses to related stimuli. In this network graph, correlations between responses to each stimulus are represented as distances (high correlation = short distance). The fold changes (x) in the axonal localization of 51 mRNAs in response to extracellular stimuli were taken from [44••] and converted to a linear scale y according to y = x – |x|/x. For each pair of stimuli, the Pearson correlation coefficient r was calculated for y across the 51 genes. The five stimulation conditions were drawn as points where each pair of stimuli is connected by a line of length of approximately 1 – r, using Graphviz software, with values of significant correlations shown. Line lengths are not exact because of geometrical constraints. Lines representing correlations that are not statistically significant (p > 0.05) are shaded gray.
Interaction with the cytoskeleton
Axonal translation, in addition to regulating the cytoskeleton as described above, can also be regulated by the cytoskeleton [59]. Many mRNAs, polysomes, and translation factors are associated with the actin cytoskeleton [60], suggesting that the cytoskeleton acts as an anchor or platform for translation. Indeed, disrupting the actin cytoskeleton impairs protein synthesis in general [61] and in goldfish Mauthner axons in particular [62]. Recent genetic evidence in Drosophila suggests that a translational regulator, Krasavietz, must associate with the F-actin to function correctly in midline repulsion [63•], in which longitudinal axons in the ventral nerve cord avoid crossing the midline because of repulsion from the axon guidance cue Slit. Krasavietz (kra) is a novel translational repressor that seems to act by binding to eIF2β, a tRNA-recruiting initiation factor, and preventing eIF2B-epsilon and eIF5 from activating eIF2β. Kra interacts with an F-actin-microtubule crosslinking protein, Short stop (shot), both physically and genetically, and both proteins are required for axons to avoid crossing the midline. Notably, both the protein domains that mediate the interaction between Kra and Shot, and those that mediate the interaction between Shot and F-actin, are required for midline repulsion, suggesting that translational repression takes place on a ‘platform’ linked to the cytoskeleton. However, whether this mechanism operates locally in axons awaits future development of tools that can restrict mutations to acting in axons.
Translational regulation of translation?
Local axonal translation may also be autoregulatory, in the sense that some stimuli may regulate the translation of factors involved in translation, such as initiation factors, elongation factors, ribosomal proteins, and chaperones. Intuitively, incoming signals that increase axonal translation may need to increase the ‘translational capacity’ of the axon. For example, DRG axons locally translate chaperones and heat shock proteins [64] and upregulate or downregulate axonal localization of mRNAs for calreticulin, heat shock proteins, and ribosomal proteins in response to neurotrophins or repulsive guidance cues [44••]. Chaperones and heat shock proteins might increase the axon’s effective translational capacity by folding newly translated proteins; indeed, the secretion of heterologously expressed proteins in yeast is greatly enhanced by increasing the expression of chaperones to overcome an ER ‘bottleneck’ [65]. Growth-promoting stimuli, which correspond to a need for ribosomes, promote the translation of ribosomal protein mRNAs through 5′UTR regulatory elements [66,67], but the mechanism of regulation is unclear, as ribosomal protein mRNA translation is apparently not sensitive to the concentration of free ribosomal proteins [68].
Insights from dendrites
Local translation in dendrites also provides insights into possible mechanisms for regulating translation in axons. The two processes are conceptually similar: both are cellular outposts that must respond quasi-autonomously to impinging signals — synaptic transmission for dendrites, and guidance cues and neurotrophins for axonal growth cones. Recent work in dendrites may foreshadow discoveries just around the corner in axons. For example, in hippocampal dendritic spines, the microRNA miR-134 regulates spine size by repressing the translation of Lim kinase 1, a cofilin inhibitor; this repression is relieved by BDNF stimulation and thus might mediate synaptic plasticity [69]. In Drosophila, the synaptic activity induces degradation of elements of the RNA interference complex, relieving miRNA repression of CaMKII translation, and this process is required for long-term memory [70]. These results implicate miRNAs in dendritic translation and raise the possibility that endogenous miRNAs may regulate axonal translation. Indeed, axons contain functional RNA interference machinery [71]. The degradation of RNA interference machinery in Drosophila is especially interesting in light of the requirement for local protein degradation in growth cone turning [7]. Reports that Drosophila neurons contain RNP granules similar to P bodies [72], which are involved in mRNA degradation and miRNA-mediated silencing, raise the question of whether axons contain P bodies or other types of RNA granules, such as stress granules [73]. Outposts of the Golgi complex secretory pathway have been observed in dendrites but not in axons [74,75], raising the question of how locally synthesized membrane and secreted proteins are processed in axons. Axons might contain noncanonical Golgi outposts, perhaps only in certain cell types or at certain developmental stages, which may differ from those in dendrites because of the more dynamic nature of axonal growth cones. Finally, a recent study suggests that dendritic arc mRNA is bound by an exon junction complex factor, leading to nonsense-mediated degradation after its first round of translation in dendrites or elsewhere, a phenomenon the authors term translation-dependent degradation [76]. The authors suggest that this process could assist in preventing inappropriate mRNA translation, or enable large, but temporally limited, bursts of translation. A limited burst of translation might be useful when axons reach intermediate or final targets, or if axonal translation plays a role in synaptic plasticity [25,77].
Conclusions
In summary, recent years have seen a remarkable amount of progress in local axonal translation. The ‘differential translation’ model posits that guidance cues induce both global and mRNA-specific activation of translation to elicit asymmetrical synthesis of ‘attractive’ proteins like β-actin for attractive turning, or ‘repulsive’ proteins like RhoA for repulsive turning. In addition, local translation of transcription factors functions in retrograde axonal signaling. Mechanisms of local regulation of translation include translation initiation factors, RNA-binding proteins, mRNA localization, interactions with the cytoskeleton, and possibly miRNAs. Why some proteins must be synthesized locally remains mostly speculative, but some possible rationales are macromolecular crowding, RNA flexibility, novel properties of nascent proteins, precise spatial localization, and axonal autonomy.
Several important challenges remain. The mechanisms by which different guidance cues selectively induce translation of specific mRNAs remain poorly understood. In addition, most of the work described here has been in vitro, and the in vivo work has not distinguished axonal from somatic protein synthesis. Understanding the role of local axonal translation in vivo will require new in vivo tools to interfere with translation specifically in axons, ideally in an acute and mRNA-specific manner. It will also be important to identify the populations of proteins locally synthesized in response to specific axon guidance cues to test the hypothesis that attractive and repulsive cues elicit synthesis of ‘attractive’ and ‘repulsive’ proteins, respectively.
Acknowledgement
The authors thank members of the Holt and Harris labs for helpful discussions.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Sutton MA, Schuman EM. Local translational control in dendrites and its role in long-term synaptic plasticity. J Neurobiol. 2005;64:116–131. doi: 10.1002/neu.20152. [DOI] [PubMed] [Google Scholar]
- 2.Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- 3.Koenig E, Giuditta A. Protein-synthesizing machinery in the axon compartment. Neuroscience. 1999;89:5–15. doi: 10.1016/s0306-4522(98)00282-6. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez J, Giuditta A, Koenig E. Protein synthesis in axons and terminals: significance for maintenance, plasticity and regulation of phenotype, with a critique of slow transport theory. Prog Neurobiol. 2000;62:1–62. doi: 10.1016/s0301-0082(99)00062-3. [DOI] [PubMed] [Google Scholar]
- 5.Lin AC, Holt CE. Local translation and directional steering in axons. EMBO J. 2007;26:3729–3736. doi: 10.1038/sj.emboj.7601808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hengst U, Jaffrey SR. Function and translational regulation of mRNA in developing axons. Semin Cell Dev Biol. 2007;18:209–215. doi: 10.1016/j.semcdb.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell DS, Holt CE. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32:1013–1026. doi: 10.1016/s0896-6273(01)00551-7. [DOI] [PubMed] [Google Scholar]
- 8.Piper M, Anderson R, Dwivedy A, Weinl C, van Horck F, Leung KM, Cogill E, Holt C. Signaling mechanisms underlying Slit2-induced collapse of Xenopus retinal growth cones. Neuron. 2006;49:215–228. doi: 10.1016/j.neuron.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunet I, Weinl C, Piper M, Trembleau A, Volovitch M, Harris W, Prochiantz A, Holt C. The transcription factor Engrailed-2 guides retinal axons. Nature. 2005;438:94–98. doi: 10.1038/nature04110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu KY, Hengst U, Cox LJ, Macosko EZ, Jeromin A, Urquhart ER, Jaffrey SR. Local translation of RhoA regulates growth cone collapse. Nature. 2005;436:1020–1024. doi: 10.1038/nature03885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Horck FP, Weinl C, Holt CE. Retinal axon guidance: novel mechanisms for steering. Curr Opin Neurobiol. 2004;14:61–66. doi: 10.1016/j.conb.2004.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12 ••.Leung KM, van Horck FP, Lin AC, Allison R, Standart N, Holt CE. Asymmetrical beta-actin mRNA translation in growth cones mediates attractive turning to netrin-1. Nat Neurosci. 2006;9:1247–1256. doi: 10.1038/nn1775. This study along with [13••] shows that local asymmetrical synthesis of β-actin in the growth cone is required for attractive turning, and not for repulsive turning. Two mechanisms are proposed for asymmetrical regulation of β-actin translation: asymmetrical activation of translation initiation via eIF-4EBP, and asymmetric movement and activation of Vg1RBP/ZBP, which binds β-actin mRNA.
- 13 ••.Yao J, Sasaki Y, Wen Z, Bassell GJ, Zheng JQ. An essential role for beta-actin mRNA localization and translation in Ca2+-dependent growth cone guidance. Nat Neurosci. 2006;9:1265–1273. doi: 10.1038/nn1773. See annotation to [12••].
- 14.van Kesteren RE, Carter C, Dissel HM, van Minnen J, Gouwenberg Y, Syed NI, Spencer GE, Smit AB. Local synthesis of actin-binding protein beta-thymosin regulates neurite outgrowth. J Neurosci. 2006;26:152–157. doi: 10.1523/JNEUROSCI.4164-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen Z, Han L, Bamburg JR, Shim S, Ming GL, Zheng JQ. BMP gradients steer nerve growth cones by a balancing act of LIM kinase and Slingshot phosphatase on ADF/cofilin. J Cell Biol. 2007;178:107–119. doi: 10.1083/jcb.200703055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallo G, Letourneau PC. Regulation of growth cone actin filaments by guidance cues. J Neurobiol. 2004;58:92–102. doi: 10.1002/neu.10282. [DOI] [PubMed] [Google Scholar]
- 17.Yuan XB, Jin M, Xu X, Song YQ, Wu CP, Poo MM, Duan S. Signalling and crosstalk of Rho GTPases in mediating axon guidance. Nat Cell Biol. 2003;5:38–45. doi: 10.1038/ncb895. [DOI] [PubMed] [Google Scholar]
- 18.Brittis PA, Lu Q, Flanagan JG. Axonal protein synthesis provides a mechanism for localized regulation at an intermediate target. Cell. 2002;110:223–235. doi: 10.1016/s0092-8674(02)00813-9. [DOI] [PubMed] [Google Scholar]
- 19.Piper M, Salih S, Weinl C, Holt CE, Harris WA. Endocytosis-dependent desensitization and protein synthesis-dependent resensitization in retinal growth cone adaptation. Nat Neurosci. 2005;8:179–186. doi: 10.1038/nn1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ming GL, Wong ST, Henley J, Yuan XB, Song HJ, Spitzer NC, Poo MM. Adaptation in the chemotactic guidance of nerve growth cones. Nature. 2002;417:411–418. doi: 10.1038/nature745. [DOI] [PubMed] [Google Scholar]
- 21.Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125:785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 22 •.Bi J, Tsai NP, Lin YP, Loh HH, Wei LN. Axonal mRNA transport and localized translational regulation of kappa-opioid receptor in primary neurons of dorsal root ganglia. Proc Natl Acad Sci U S A. 2006;103:19919–19924. doi: 10.1073/pnas.0607394104. This study along with [23•] shows that κ-opioid receptor (kor) mRNA is localized to axons, where its translation is locally regulated by KCl depolarization, and that kor mRNA is translated in cell bodies in response to netrin-1. Whether netrin-1 locally regulates axonal kor translation remains unknown.
- 23 •.Tsai NP, Bi J, Loh HH, Wei LN. Netrin-1 signaling regulates de novo protein synthesis of kappa opioid receptor by facilitating polysomal partition of its mRNA. J Neurosci. 2006;26:9743–9749. doi: 10.1523/JNEUROSCI.3014-06.2006. See annotation to [22•].
- 24.Jimenez-Diaz L, Geranton SM, Passmore GM, Leith JL, Fisher AS, Berliocchi L, Sivasubramaniam AK, Sheasby A, Lumb BM, Hunt SP. Local translation in primary afferent fibers regulates nociception. PLoS ONE. 2008;3:e1961. doi: 10.1371/journal.pone.0001961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Poo MM. Localized synaptic potentiation by BDNF requires local protein synthesis in the developing axon. Neuron. 2002;36:675–688. doi: 10.1016/s0896-6273(02)01023-1. [DOI] [PubMed] [Google Scholar]
- 26 •.Lyles V, Zhao Y, Martin KC. Synapse formation and mRNA localization in cultured Aplysia neurons. Neuron. 2006;49:349–356. doi: 10.1016/j.neuron.2005.12.029. This study shows that synapse formation between sensory and motor neurons induces the localization of sensorin mRNA to the presynaptic terminal, and the knockdown of sensorin mRNA abolishes synapse formation, suggesting that local synthesis of sensorin in the presynaptic terminal is required for synapse formation.
- 27.Chihara T, Luginbuhl D, Luo L. Cytoplasmic and mitochondrial protein translation in axonal and dendritic terminal arborization. Nat Neurosci. 2007;10:828–837. doi: 10.1038/nn1910. [DOI] [PubMed] [Google Scholar]
- 28.Howe CL, Mobley WC. Long-distance retrograde neurotrophic signaling. Curr Opin Neurobiol. 2005;15:40–48. doi: 10.1016/j.conb.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Hanz S, Fainzilber M. Retrograde signaling in injured nerve — the axon reaction revisited. J Neurochem. 2006;99:13–19. doi: 10.1111/j.1471-4159.2006.04089.x. [DOI] [PubMed] [Google Scholar]
- 30.Hanz S, Perlson E, Willis D, Zheng JQ, Massarwa R, Huerta JJ, Koltzenburg M, Kohler M, van-Minnen J, Twiss JL, et al. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron. 2003;40:1095–1104. doi: 10.1016/s0896-6273(03)00770-0. [DOI] [PubMed] [Google Scholar]
- 31 ••.Cox LJ, Hengst U, Gurskaya NG, Lukyanov KA, Jaffrey SR. Intra-axonal translation and retrograde trafficking of CREB promotes neuronal survival. Nat Cell Biol. 2008;10:149–159. doi: 10.1038/ncb1677. This study adds to the existing model that the retrograde neurotrophic signal is carried by a ‘signaling endosome’ that activates transcription factors in the cell body, by showing that the transcription factor CREB is itself synthesized in axons and retrogradely trafficked to the cell body. Surprisingly, axonally synthesized CREB is required for cell survival, suggesting that pre-existing CREB in the cell body does not function in this pathway.
- 32.Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- 33.Du TG, Schmid M, Jansen RP. Why cells move messages: the biological functions of mRNA localization. Semin Cell Dev Biol. 2007;18:171–177. doi: 10.1016/j.semcdb.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 34.Condeelis J, Singer RH. How and why does beta-actin mRNA target? Biol Cell. 2005;97:97–110. doi: 10.1042/BC20040063. [DOI] [PubMed] [Google Scholar]
- 35.St Johnston D. Moving messages: the intracellular localization of mRNAs. Nat Rev Mol Cell Biol. 2005;6:363–375. doi: 10.1038/nrm1643. [DOI] [PubMed] [Google Scholar]
- 36.Aronov S, Aranda G, Behar L, Ginzburg I. Axonal tau mRNA localization coincides with tau protein in living neuronal cells and depends on axonal targeting signal. J Neurosci. 2001;21:6577–6587. doi: 10.1523/JNEUROSCI.21-17-06577.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boggs JM. Myelin basic protein: a multifunctional protein. Cell Mol Life Sci. 2006;63:1945–1961. doi: 10.1007/s00018-006-6094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ellis RJ. Macromolecular crowding: obvious but underappreciated. Trends Biochem Sci. 2001;26:597–604. doi: 10.1016/s0968-0004(01)01938-7. [DOI] [PubMed] [Google Scholar]
- 39.Fonseca R, Vabulas RM, Hartl FU, Bonhoeffer T, Nagerl UV. A balance of protein synthesis and proteasome-dependent degradation determines the maintenance of LTP. Neuron. 2006;52:239–245. doi: 10.1016/j.neuron.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 40.Lee SH, Choi JH, Lee N, Lee HR, Kim JI, Yu NK, Choi SL, Lee SH, Kim H, Kaang BK. Synaptic protein degradation underlies destabilization of retrieved fear memory. Science. 2008;319:1253–1256. doi: 10.1126/science.1150541. [DOI] [PubMed] [Google Scholar]
- 41.Karakozova M, Kozak M, Wong CC, Bailey AO, Yates JR, III, Mogilner A, Zebroski H, Kashina A. Arginylation of beta-actin regulates actin cytoskeleton and cell motility. Science. 2006;313:192–196. doi: 10.1126/science.1129344. [DOI] [PubMed] [Google Scholar]
- 42.Wang J, Boja ES, Tan W, Tekle E, Fales HM, English S, Mieyal JJ, Chock PB. Reversible glutathionylation regulates actin polymerization in A431 cells. J Biol Chem. 2001;276:47763–47766. doi: 10.1074/jbc.C100415200. [DOI] [PubMed] [Google Scholar]
- 43.Chang L, Shav-Tal Y, Trcek T, Singer RH, Goldman RD. Assembling an intermediate filament network by dynamic cotranslation. J Cell Biol. 2006;172:747–758. doi: 10.1083/jcb.200511033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44 ••.Willis DE, van Niekerk EA, Sasaki Y, Mesngon M, Merianda TT, Williams GG, Kendall M, Smith DS, Bassell GJ, Twiss JL. Extracellular stimuli specifically regulate localized levels of individual neuronal mRNAs. J Cell Biol. 2007;178:965–980. doi: 10.1083/jcb.200703209. The authors identify >200 mRNA localized to DRG axons and show that different extracellular stimuli upregulate or downregulate axonal localization of different mRNAs. Local stimulation with stimulus-coated beads also locally regulates mRNA localization within axons.
- 45.Hentze MW, Kuhn LC. Molecular control of vertebrate iron metabolism: mRNA-based regulatory circuits operated by iron, nitric oxide, and oxidative stress. Proc Natl Acad Sci U S A. 1996;93:8175–8182. doi: 10.1073/pnas.93.16.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Margeot A, Blugeon C, Sylvestre J, Vialette S, Jacq C, Corral-Debrinski M. In Saccharomyces cerevisiae, ATP2 mRNA sorting to the vicinity of mitochondria is essential for respiratory function. EMBO J. 2002;21:6893–6904. doi: 10.1093/emboj/cdf690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bunge MB. Fine structure of nerve fibers and growth cones of isolated sympathetic neurons in culture. J Cell Biol. 1973;56:713–735. doi: 10.1083/jcb.56.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Allen JF. The function of genomes in bioenergetic organelles. Philos Trans R Soc Lond B Biol Sci. 2003;358:19–37. doi: 10.1098/rstb.2002.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boillee S, Vande Velde C, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 51.Campbell DS, Holt CE. Apoptotic pathway and MAPKs differentially regulate chemotropic responses of retinal growth cones. Neuron. 2003;37:939–952. doi: 10.1016/s0896-6273(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 52.Krichevsky AM, Kosik KS. Neuronal RNA granules: a link between RNA localization and stimulation-dependent translation. Neuron. 2001;32:683–696. doi: 10.1016/s0896-6273(01)00508-6. [DOI] [PubMed] [Google Scholar]
- 53.Huttelmaier S, Zenklusen D, Lederer M, Dictenberg J, Lorenz M, Meng X, Bassell GJ, Condeelis J, Singer RH. Spatial regulation of beta-actin translation by Src-dependent phosphorylation of ZBP1. Nature. 2005;438:512–515. doi: 10.1038/nature04115. [DOI] [PubMed] [Google Scholar]
- 54.Tsai NP, Bi J, Wei LN. The adaptor Grb7 links netrin-1 signaling to regulation of mRNA translation. EMBO J. 2007;26:1522–1531. doi: 10.1038/sj.emboj.7601598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Antar LN, Li C, Zhang H, Carroll RC, Bassell GJ. Local functions for FMRP in axon growth cone motility and activity-dependent regulation of filopodia and spine synapses. Mol Cell Neurosci. 2006;32:37–48. doi: 10.1016/j.mcn.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 56.Bi J, Tsai NP, Lu HY, Loh HH, Wei LN. Copb1-facilitated axonal transport and translation of kappa opioid-receptor mRNA. Proc Natl Acad Sci U S A. 2007;104:13810–13815. doi: 10.1073/pnas.0703805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang HL, Singer RH, Bassell GJ. Neurotrophin regulation of beta-actin mRNA and protein localization within growth cones. J Cell Biol. 1999;147:59–70. doi: 10.1083/jcb.147.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ladle DR, Pecho-Vrieseling E, Arber S. Assembly of motor circuits in the spinal cord: driven to function by genetic and experience-dependent mechanisms. Neuron. 2007;56:270–283. doi: 10.1016/j.neuron.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 59.van Horck FPG, Holt CE. A cytoskeletal platform for local translation in axons. Sci Signal. 2008;1:pe11. doi: 10.1126/stke.18pe11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hovland R, Hesketh JE, Pryme IF. The compartmentalization of protein synthesis: importance of cytoskeleton and role in mRNA targeting. Int J Biochem Cell Biol. 1996;28:1089–1105. doi: 10.1016/1357-2725(96)00059-3. [DOI] [PubMed] [Google Scholar]
- 61.Stapulionis R, Kolli S, Deutscher MP. Efficient mammalian protein synthesis requires an intact F-actin system. J Biol Chem. 1997;272:24980–24986. doi: 10.1074/jbc.272.40.24980. [DOI] [PubMed] [Google Scholar]
- 62.Sotelo-Silveira J, Crispino M, Puppo A, Sotelo JR, Koenig E. Myelinated axons contain beta-actin mRNA and ZBP-1 in periaxoplasmic ribosomal plaques and depend on cyclic AMP and F-actin integrity for in vitro translation. J Neurochem. 2008;104:545–557. doi: 10.1111/j.1471-4159.2007.04999.x. [DOI] [PubMed] [Google Scholar]
- 63 •.Lee S, Nahm M, Lee M, Kwon M, Kim E, Zadeh AD, Cao H, Kim HJ, Lee ZH, Oh SB, et al. The F-actin-microtubule crosslinker Shot is a platform for Krasavietz-mediated translational regulation of midline axon repulsion. Development. 2007;134:1767–1777. doi: 10.1242/dev.02842. This study identifies a novel translational regulator, Krasavietz, as a binding partner for the F-actin-microtubule crosslinker, Short stop, and shows that both are required for Slit/Robo-mediated midline repulsion in Drosophila, suggesting a functional connection between the cytoskeleton and translational regulation in axon guidance.
- 64.Willis D, Li KW, Zheng JQ, Chang JH, Smit A, Kelly T, Merianda TT, Sylvester J, van Minnen J, Twiss JL. Differential transport and local translation of cytoskeletal, injury-response, and neurodegeneration protein mRNAs in axons. J Neurosci. 2005;25:778–791. doi: 10.1523/JNEUROSCI.4235-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang W, Zhao HL, Xue C, Xiong XH, Yao XQ, Li XY, Chen HP, Liu ZM. Enhanced secretion of heterologous proteins in Pichia pastoris following overexpression of Saccharomyces cerevisiae chaperone proteins. Biotechnol Prog. 2006;22:1090–1095. doi: 10.1021/bp060019r. [DOI] [PubMed] [Google Scholar]
- 66.Mariottini P, Amaldi F. The 5′ untranslated region of mRNA for ribosomal protein S19 is involved in its translational regulation during Xenopus development. Mol Cell Biol. 1990;10:816–822. doi: 10.1128/mcb.10.2.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Loreni F, Amaldi F. Translational regulation of ribosomal protein synthesis in Xenopus cultured cells: mRNA relocation between polysomes and RNP during nutritional shifts. Eur J Biochem. 1992;205:1027–1032. doi: 10.1111/j.1432-1033.1992.tb16870.x. [DOI] [PubMed] [Google Scholar]
- 68.Pierandrei-Amaldi P, Beccari E, Bozzoni I, Amaldi F. Ribosomal protein production in normal and anucleolate Xenopus embryos: regulation at the posttranscriptional and translational levels. Cell. 1985;42:317–323. doi: 10.1016/s0092-8674(85)80127-6. [DOI] [PubMed] [Google Scholar]
- 69.Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, Greenberg ME. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439:283–289. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- 70.Ashraf SI, McLoon AL, Sclarsic SM, Kunes S. Synaptic protein synthesis associated with memory is regulated by the RISC pathway in Drosophila. Cell. 2006;124:191–205. doi: 10.1016/j.cell.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 71.Hengst U, Cox LJ, Macosko EZ, Jaffrey SR. Functional and selective RNA interference in developing axons and growth cones. J Neurosci. 2006;26:5727–5732. doi: 10.1523/JNEUROSCI.5229-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barbee SA, Estes PS, Cziko AM, Hillebrand J, Luedeman RA, Coller JM, Johnson N, Howlett IC, Geng C, Ueda R, et al. Staufen-and FMRP-containing neuronal RNPs are structurally and functionally related to somatic P bodies. Neuron. 2006;52:997–1009. doi: 10.1016/j.neuron.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kiebler MA, Bassell GJ. Neuronal RNA granules: movers and makers. Neuron. 2006;51:685–690. doi: 10.1016/j.neuron.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 74.Horton AC, Ehlers MD. Dual modes of endoplasmic reticulum-to-Golgi transport in dendrites revealed by live-cell imaging. J Neurosci. 2003;23:6188–6199. doi: 10.1523/JNEUROSCI.23-15-06188.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ye B, Zhang Y, Song W, Younger SH, Jan LY, Jan YN. Growing dendrites and axons differ in their reliance on the secretory pathway. Cell. 2007;130:717–729. doi: 10.1016/j.cell.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Giorgi C, Yeo GW, Stone ME, Katz DB, Burge C, Turrigiano G, Moore MJ. The EJC factor eIF4AIII modulates synaptic strength and neuronal protein expression. Cell. 2007;130:179–191. doi: 10.1016/j.cell.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 77.Yin HH, Davis MI, Ronesi JA, Lovinger DM. The role of protein synthesis in striatal long-term depression. J Neurosci. 2006;26:11811–11820. doi: 10.1523/JNEUROSCI.3196-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]