Abstract
Mitochondrial dysfunction has been reported in a wide array of neurological disorders ranging from neuromuscular to neurodegenerative diseases. Recent studies on neurodegenerative diseases have revealed that mitochondrial pathology is generally found in inherited or sporadic neurodegenerative diseases and is believed to be involved in the pathophysiological process of these diseases. Commonly seen types of mitochondrial dysfunction in neurodegenerative diseases include excessive free radical generation, lowered ATP production, mitochondrial permeability transition, mitochondrial DNA lesions, perturbed mitochondrial dynamics and apoptosis. Mitochondrial medicine as an emerging therapeutic strategy targeted to mitochondrial dysfunction in neurodegenerative diseases has been proven to be of value, though this area of research is still at in its early stage. In this article, we report on recent progress in the development of several mitochondrial therapies including antioxidants, blockade of mitochondrial permeability transition, and mitochondrial gene therapy as evidence that mitochondrial medicine has promise in the treatment of neurodegenerative diseases.
Keywords: Mitochondria, Mitochondrial pathology, Mitochondrial medicine, Neurodegeneration, Neurodegenerative diseases
1. Introduction
Ever since Luft reported the first mitochondrial disease in the 1960s (Luft et al., 1962), increasing numbers of diseases related to the disorders of the cellular “batteries” have been recognized and studied in basic as well as clinical research platforms (Kasiviswanathan et al., 2009; Jacobs et al., 2004; de Magalhaes, 2005; Cortopassi et al., 2006; Haas et al., 2007; Hirano et al., 2004; Kang et al., 2007; Lazarou et al., 2009; McFarland et al., 2002, 2007; McKenzie et al., 2004; Morava et al., 2006; Muravchick, 2008; Noorda et al., 2007; Pons and De Vivo, 2001). As the name suggests, mitochondrial diseases are a series of clinical disorders whose etiology is either directly or indirectly related to mitochondrial pathologies including DNA mutation, mitochondrial redox perturbation, mitochondrial energy production defects and so forth.
In recent years, the recognition of neurodegenerative diseases as mitochondrial diseases has been attracting increasing attention. Free radicals, mitochondrial DNA (mtDNA) mutation, decreased mitochondrial respiration and mitochondrial calcium dysregulation are widely observed, in varying degrees, in many neurodegenerative diseases such as Alzheimer’s disease (AD) (Swerdlow, 2009), Parkinson’s disease (PD) (Banerjee et al., 2009; Esteves et al., 2008), Huntington’s disease (HD) (Almeida et al., 2008; Arenas et al., 1998), amyotrophic lateral sclerosis (ALS) (Damiano et al., 2006; Dupuis et al., 2004; Echaniz-Laguna et al., 2006; Hervias et al., 2006), and progressive supranuclear palsy (PSP) (Albers and Beal, 2002; Albers et al., 2001; Costa et al., 2008). In addition, experiments conducted in animal as well as cellular models of AD, PD, HD, ALS and many other neurodegenerative diseases suggest the benefit of mitochondrial protection for attenuation neuronal degeneration (Faust et al., 2009; Fontaine et al., 2000; Jauslin et al., 2003; Kasparova et al., 2006; Liu and Ames, 2005; Du et al., 2008; Cleren et al., 2008; Martin et al., 2009). Given the appreciation of the prevalent mitochondrial pathology, it is proposed that mitochondrial dysfunction is involved in the pathophysiological process of neurodegenerative diseases. Thus, it has been proponed the emergence of therapeutic approaches targeting mitochondrial dysfunction for the treatment of neurodegenerative diseases. For example, coenzyme Q10 (CoQ10) and vitamin E (Vit E) have been shown to be effective in clinical trials, showing the promise of mitochondrial medicine applications for the treatment of neurodegenerative disease (Kasparova et al., 2006; Shults et al., 2002; Pham and Plakogiannis, 2005).
Here, we review the mitochondrial pathologies in neurodegenerative diseases and discuss several recent applications of mitochondrial medicine in the treatment of neurodegenerative disorders.
2. Mitochondrial pathology in neurodegenerative diseases
As critical organelles in cell survival by their function in modulating energy provision, cellular calcium homeostasis, free radicals and apoptosis, mitochondria play an essential role in eurokyotic cells, including neurons. Neurons, with long processes extending from neuronal soma, have synaptic terminals that are constantly changing to form complex neuronal networks and possess substantive receptors and channels in the neuronal terminals to facilitate neuronal functions. Neuronal mitochondrial function and behavior in normal fashion are the endorsement of the high energy demanding synaptic activity by rapidly providing energy (Onyango et al., 2010; Mattson, 2007; Hollenbeck and Saxton, 2005; Li et al., 2004) and modulating calcium kinetics and metabolism in the strategic neuronal compartments (Mattson et al., 1993; Siklos and Kuhnt, 1994; Mironov et al., 2005; Morris and Hollenbeck, 1995). Notably, mitochondria in neurons are at higher risk for damage. The long lifespan of neurons increases the risk for toxin accumulation during the aging process and other pathological conditions. Further, the stretched length of neuronal protrusions prolongs mitochondrial movement to their destination and can affect mitochondria retrograde to neuron soma in terms of repair and degradation (Reddy and Beal, 2008). Lastly, the high energy demand of neurons increases the risk of mitochondrial oxidative stress. Thus, based on the active function and high risk to detrimental condition of neuronal mitochondria, it is not surprising that mitochondrial pathology is a nexus of many neuronal perturbations.
Mitochondrial pathologies are involved in the pathophysiological process of many neurodegenerative diseases, including AD, PD, HD, ALS and PSP. The predominant mitochondrial dysfunction in many neurodegenerative diseases, in varying degrees, includes massive oxidative stress, mtDNA mutations, low ATP production, dysfunction in calcium homeostasis, mitochondrial permeability transition, mitochondrial movement defect, and mitochondrial fusion and fission imbalance. Although each of the neurodegenerative diseases has its distinct etiological processes and different affected brain regions, neurodegenerative disorders as a whole share similar mitochondrial changes, though in varying degrees, suggesting the connection of disease pathology to the overall character of neurodegeneration. Thus, our knowledge of mitochondrial pathology in the pathogenesis of these diseases will help us to understand these diseases as one category, namely, neurodegeneration related mitochondrial disease.
2.1. Mitochondrial respiratory complexes defects
Mitochondrial respiratory chain complexes present from complex I to IV including NADH dehydrogenase, succinate dehydrogenase, coenzyme Q—cytochrome c reductase and cytochrome c oxidase, respectively (Becker et al., 2009). Electrons transported through the mitochondrial respiratory chain are driven by a proton gradient; ATP is thus produced during the process of oxidative phosphorylation. Furthermore, mitochondrial complexes I and III are known major mitochondrial free radical producing sites, whereas damaged complexes II and IV activities will also lead to burst of reactive oxygen species (ROS) generation in pathological conditions (Liu et al., 2002; Grivennikova and Vinogradov, 2006; Muller et al., 2004). Mitochondrial defects in these respiratory complexes result in increased leakage of electrons from the electron transport chain (ETC) that in turn cause the accumulation of free radicals (especially ROS), exacerbating the existing cellular/mitochondrial perturbations. Therefore, when things go awry, the direct outcomes of damages in the mitochondrial respiratory chain lead to reduced ATP production and increased free radicals accumulation, both of which are commonly seen mitochondrial pathologies in neurodegenerative diseases.
The defects of mitochondrial respiratory complexes are prevalent in many neurodegenerative disorders. Mitochondrial complex I deactivation is predominant in PD substantia nigra mitochondria, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induced PD animal models as well as MPTP treated PD cytoplasma hybrid cells. In addition, decreased mitochondrial complex I activity has also been found in mitochondria from lymphocytes, skeletal muscles and platelets of PD patients (Gu et al., 1998; Alam and Schmidt, 2004; Blin et al., 1994; Banerjee et al., 2009; Hanagasi et al., 2005; Yoshino et al., 1992); and decreased complexes II/III activities have been reported in platelet mitochondria isolated from sporadic PD patients (Haas et al., 1995; Varghese et al., 2009). These studies suggest the mitochondrial respiratory chain dysfunction is a systematic change in PD. Using cybrid technology, several groups reported decreased mitochondrial complex I activity in PSP cybrids (Chirichigno et al., 2002; Swerdlow et al., 2000). Besides, decreased complex I activity has also been detected in HD patients as well as HD animal and cell models (Tabrizi et al., 2000; Turner et al., 2007; Browne, 2008). Symptomatic HD patients demonstrate decreased complex II activity in caudate and putamen (Benchoua et al., 2006; Browne, 2008; Calabresi et al., 2001; Gu et al., 1996) with decreased expression of the complex II iron–sulfur subunit in caudate and putamen tissues from HD patients (Benchoua et al., 2006). Reduced complex III activity has also been detected in mitochondria isolated from affected brain regions as well as platelets of AD and Down’s syndrome patients (Kim et al., 2000; Valla et al., 2006). In AD patients, mitochondria isolated from the temporal pole, hippocampus and platelets showed a reduced mitochondrial complex IV activity (Mutisya et al., 1994; Kish et al., 1992). Damaged complex IV activity also occurred in AD animal models, AD cybrid cells and Aβ-treated cells (Trimmer et al., 2004; Cardoso et al., 2004; Caspersen et al., 2005; Du et al., 2008; Takuma et al., 2005, 2009; Lustbader et al., 2004). The mechanism underlying decreased complex IV activity is largely unknown. A study on Aβ-treated SK-N-SH cells showed a decrease in mtDNA encoded complex IV subunits (subunits I, II and III) at both the mRNA and protein levels, suggesting a possible mechanism for decreased complex IV activity related to mtDNA perturbation (Hong et al., 2007), though this hypothesis has not yet been supported by clinical (AD patients of familial AD subjects) or animal AD model (APP overexpression) studies.
Given the global defects in mitochondrial respiratory complexes, it is not surprising that decreased ATP production has been reported in disease affected regions in neurodegenerative diseases; for example, in temporal cortex and hippocampus of AD patients, substantia nigra of PD patients, cerebellum of PSP patients and spinal cord of ALS patients. In addition, decreased ATP concentration in animal and/or cellular models of AD, PD, HD and ALS serves as supportive evidence of ATP depletion in neurodegenerative diseases (Lim et al., 2009; Du et al., 2008; Vali et al., 2007; Lin and Beal, 2006). Thus, the impotence of mitochondria to provide sufficient energy is a common causative factor for decreased neuronal function in neurodegenerative diseases.
Increased free radicals production/accumulation and the resulted oxidative stress damages in neurodegenerative diseases are another inevitable outcome of mitochondrial respiratory complexes defects. Oxidative stress disrupts neuronal homeostasis as it causes lipid oxidation, protein modification, DNA mutation and triggers the formation of mitochondrial permeability transition pore (mPTP), thus, leading to low energy provision, dysregulated mitochondrial dynamics, disturbed mitochondrial calcium handling capacity, decreased neuronal plasticity and eventually neuron death. Oxidative damage occurs in very early stages of AD ahead of massive Aβ deposition and causes oxidative modification of proteins, mtDNA mutations and mitochondrial permeability transition perturbations (Butterfield et al., 2006; Du et al., 2008). Massive oxidative stress is also seen in dopaminergic neurons in PD, substantia nigra and cerebellum of PSP, striatum of HD and disease insulted brain regions of many other neurodegenerative diseases (Tsang and Chung, 2009; Tasset et al., 2009; Albers et al., 2000; Aoyama et al., 2006).
The defect in mitochondrial respiratory chain function is, therefore, a crucial detrimental mitochondrial pathology during neurodegeneration, ultimately, resulting to decreased mitochondrial ATP provision and increased free radical damages in neurodegenerative disorders. However, we should be reminded that the massive oxidative stress seen in these diseases is not only due to the increased free radicals accumulation, but also due to the reduced free radicals eliminating ability of mitochondria. Thus, it is of critical importance to look into the change of mitochondrial antioxidants defense system in neurodegenerative disorders.
2.2. Decreased mitochondrial free radical clearing ability
The mitochondrial antioxidant defense system, normally effective for clearing endogenous free radicals generated in mitochondria, is composed of small reducing molecules such as reduced glutathione, coenzyme Q, vitamin C and vitamin E and enzymes catalyzing oxidation, such as copper/zinc SOD, manganese SOD, catalase and glutathione peroxidase. Increasing evidence demonstrates decreased mitochondrial free radical clearing ability in neurodegenerative diseases. For example, both familial and sporadic ALS patients demonstrate SOD1 aggregation in mitochondria and similarly a featured mitochondrial change seen in familial ALS is a mutation in the gene encoding Cu/Zn SOD1, exhibiting a decreased SOD activity (Higgins et al., 2003; Dal Canto and Gurney, 1995). Mutant SOD1 transgenic mice mimic ALS syndrome, adding further evidence for the role of decreased SOD1 activity in the pathogenesis of ALS (Mattiazzi et al., 2002). Further, decreased mitochondrial SOD expression level is found in AD patients (Zubenko and Sauer, 1989). Additionally, one of the first studies to identify Aβ binding alcohol dehydrogenase (ABAD) as an Aβ binding mitochondrial protein showed that the interaction of Aβ with its binding partner promotes ROS generation and leakages from mitochondria, leading to severe neuronal perturbation in AD (Ren et al., 2008; Yao et al., 2007; Lustbader et al., 2004; Takuma et al., 2005). Coenzyme Q (CoQ) deficiency has been observed in PD patients in substantia nigra, cerebellum, cortex and striatum (Beal, 2002) and AD patients showed decreased CoQ in peripheral tissues as well as in brains (Moreira et al., 2005).
Therefore, impairment in scavenging mitochondrial free radicals would also contribute to the massive oxidative damage in the affected brain regions of neurodegenerative diseases sufferers.
2.3. Mitochondrial DNA (mtDNA) lesions
mtDNA is an apparent feature of mitochondria to retain their identity from other cellular organelles. Each mitochondrion contains 2-10 copies of circular DNA encoding 13 key mitochondria associated proteins, 2 ribosomal RNAs and 22 tRNAs. mtDNA is maternally inherited, and is at high risk for lesion development induced by multiple factors, of which oxidative stress is the most common one (Shoffner et al., 1993; Beal, 2005) due to the extreme physical proximity of mtDNA to free radical generation sites. There are multiple presentations of mtDNA defects in neurodegenerative diseases, including point mutation, nucleic acid modification, large-scale deletion, and decreased mtDNA copies (Yang et al., 2008a; Swerdlow, 2002; Gu et al., 2002; Murata et al., 2008). Damaged mtDNA results in deficiency in mitochondrial key enzyme activities, leading to mitochondrial respiration defects, excessive ROS generation, increased mitophagy, and eventually apoptosis and cell death. Although there seems to be a repair and clearance strategy for damaged DNA (Stuart et al., 2004; Bohr, 2002), accumulation of abnormal mtDNA will eventually reach a threshold that induces mitochondrial respiratory chain defects as seen in aging and a wide range neurological diseases. Abnormally high levels of mtDNA lesions are a hallmark in many neurodegenerative diseases, including AD, PD, HD, ALS and Friedrich’s ataxia (Coskun et al., 2004; Hutchin et al., 1997; Wiedemann et al., 2002; Polidori et al., 1999). Despite the consensus on mtDNA lesions as a prevalent pathology in neurodegenerative disorders, the exact role that mtDNA plays in the pathological process of sporadic neurodegenerative diseases is still under debate. The question is whether mtDNA lesions are a primary and disease-specific change in sporadic neurodegenerative diseases. PD cybrids studies suggest that mtDNA deletion in PD affected neurons leads to mitochondrial complex I deficiency (Richter et al., 2002; Swerdlow et al., 1998). Results from cybrids studies imply that PD and AD have specific mtDNA mutations that correlate with defects in certain mitochondrial respiratory complexes (Cardoso et al., 2004). Thus, it is proposed that disease-specific mtDNA mutation is a primary change in neurodegenerative diseases. However, this hypothesis has been challenged by many other studies showing the similarity in mtDNA damage in neurodegenerative diseases patients as compared to aged controls (Howell et al., 2005; Schapira, 1998), implying that the distribution and severity of mtDNA lesions, presumably, in many neurodegenerative diseases are likely not to be disease specific but may instead be dependent upon the stresses that affected neurons undergo. However, due to the difficulties that exist for the study of mtDNA replication and degradation in neurons in live individuals suffering sporadic neurodegenerative diseases, it is still unclear if: (1) mtDNA mutation is a primary change; and (2) if patients have inherited mtDNA disorders. Further studies on this issue will help to address these key questions.
2.4. Mitochondrial calcium dyshomeostasis and mitochondrial permeability transition pore (mPTP)
Mitochondria are essential organelles to modulate intracellular calcium homeostates. Perturbation in mitochondrial calcium handling capacity enhances free radical production; and the resultant increase in ROS modulates ion channels in mitochondrial as well as cytoplasmic membrane and then exacerbates intracellular calcium perturbation (Gordeeva et al., 2003; Brookes et al., 2004); thus, eventually leads to severe cellular disturbances. Mitochondrial calcium disturbance is one of the hallmarks of mitochondrial pathology in neurodegenerative disorders. Increased mitochondrial calcium loading and decreased mitochondrial calcium buffering capacity have been observed in affected regions of AD, PD, HD and ALS patients as well as the animal and/or cellular models of these disorders (Sheehan et al., 1997a,b; Yu et al., 2009; Panov et al., 2005; Quintanilla and Johnson, 2009; Jaiswal et al., 2009). So far, there is insufficient evidence to show calcium perturbation is an initiative factor of any neurodegenerative disease, whereas increasing lines of studies accentuated that disturbed calcium metabolism plays a role as a nexus of mitochondrial dysfunctions including free radicals, mitochondrial respiratory chain defect, apoptosis and so forth during neurodegeneration. For example, Aβ causes increased cytoplasmic calcium concentration and leads to mitochondrial calcium overloading (Mattson et al., 1998; Begley et al., 1999); calcium overloading then causes increased free radicals accumulation and triggers the formation of mitochondrial permeability transition pore (mPTP) to initiate calcium efflux and exacerbate cytoplasmic calcium disturbance, thus eventually induces neuronal death. mPTP is, therefore, a crucial pathological change in mitochondria induced by severe mitochondrial calcium disturbance.
Although the detailed structure of mPTP is still under debate, it is generally accepted that the basic composition of mPTP contains voltage-dependent anion channel (VDAC) in outer mitochondrial membrane (OMM), and adenine nucleotide translocase (ANT) in inner mitochondrial membrane (IMM) and cyclophilin D (CypD) in matrix (Halestrap, 2006). Recent studies showed that phosphate carrier (PiC) might also be involved in the composition of mPTP (Leung and Halestrap, 2008). The physiological function in mPTP is unclear. It is generally believed that mPTP is a portal to other pathological conditions (Petersen et al., 2000; Halestrap, 2006). Oxidative stress, mitochondrial calcium and phosphate overloading induce the translocation of CypD to IMM (Halestrap et al., 1993; Kantrow et al., 2000; Leung and Halestrap, 2008). CypD translocation to bind ANT is the initiating step for the formation of mPTP. Once bound with CypD, ANT undergoes conformation change to form a channel. The ANT formed channel along with the channel formed by VDAC constitutes a nonselective pore across the two mitochondrial membranes. The main consequences of mPTP include rapid efflux of calcium from mitochondria, decreased proton gradient, damaged mitochondrial respiration, increased reactive oxygen species (ROS) generation, the release of proapoptotic molecules to cytoplasma, mitochondrial swelling and mitochondrial membrane rupture (Du et al., 2008; Baines et al., 2005; Petersen et al., 2000). The outcome of massive mPTP formation in neurons is eventually apoptosis and cell death (Du and Yan, 2010).
In line with the notion that abundant endogenous ROS production and calcium overloading are common pathological changes in neurodegenerative diseases, massive mPTP perturbation in neurons is widely observed in affected regions in many neurodegenerative diseases. For example, the levels of CypD were significantly elevated in AD-affected regions (temporal pole and hippocampi) but not in AD-spared region (cerebellum) of AD patients. Similarly, brains from the transgenic AD mice, including hippocampus and cortex, also demonstrated up-regulation of CypD in addition to the aged mice (Du et al., 2008, 2009). Aβ interacts with CypD to form complexes in mitochondria. In the presence of mPTP inducers, Aβ decreases the mPTP threshold in a dose-dependent manner in isolated mitochondria. As a result, increased CypD translocation to IMM occurs in cortical mitochondria isolated from brains of AD patients and transgenic AD mouse mice (Du et al., 2008). The genetic depletion of CypD or the addition of the CypD inhibitor, cyclosporine A efficiently attenuates mitochondrial and neuronal dysfunctions in the aged AD mouse model and rescues Aβ-induced reduction of long term potential (LTP) and learning/memory (Du et al., 2008). Recent experiments in the G93A-mSOD1 ALS mouse model revealed increased CypD immunostaining in motor neurons with mitochondrial IMM remodeling and matrix vesiculation in the spinal cord of even presymptomatic animals. In contrast, genetic depletion of CypD delayed the onset of disease and substantially prolonged the survival of the ALS mice (Martin et al., 2009). Abramov’s group, using the PTEN-induced putative kinase 1 (PINK1) deficient mouse model to study age-related dopaminergic neuronal loss and mitochondrial dysfunctions in PD, found that PINK1 deficiency enhances calcium and ROS perturbation and leads to a deceased mPTP threshold and massive mPTP formation in the mice, thereby suggesting the involvement of mPTP formation in the pathogenesis of PD (Wang et al., 2007; Gandhi et al., 2009). A study on CypD expression comparison in different brain regions has reported increased CypD expression levels and decreased mPTP thresholds in striatum of rats. The increased CypD level in the disease affected brain region is proposed as the reason for high susceptibility of mPTP formation and consequent mitochondrial dysfunction. This regional difference in CypD expression might be a possible risk for the etiology of HD (Brustovetsky et al., 2003). Increased mPTP formation and decreased mitochondrial calcium handling capacity have been reported in various HD animal models, the STHdhQ111/Q111 striatal cell line and lymphocytes of HD patients (Gellerich et al., 2008; Lim et al., 2008; Fernandes et al., 2007; Milakovic et al., 2006; Panov et al., 2005). The mitochondrial complex II inhibitor, 3-nitropropionic acid induced HD animal model demonstrated obvious increased mPTP formation in brain mitochondria (Rosenstock et al., 2004), while the application of mPTP inhibitor efficiently attenuated calcium disturbance and oxidative stress in this model (Quintanilla and Johnson, 2009).
2.5. Dysregulated mitochondrial dynamics
Mitochondrial dynamics is an essential function in maintaining cell viability. The regulation of mitochondrial dynamics requires a delicate balance of fusion and fission proteins (Chan, 2006). Disturbed balance of mitochondrial dynamics causes severe mitochondrial behavioral change and thus leads to cellular perturbation, for example, apoptosis. The imbalance of mitochondrial fusion and fission is currently a subject of intense study in neurodegenerative diseases. A series of recent studies found decreased expression levels of mitochondrial fusion proteins, including MFN-1, MFN-2 and OPA1 in AD hippocampal tissues, but increased levels of mitochondrial fission protein FIS1. The decrease in mitochondrial fusion proteins and increase in fission protein correlate well with observations of mitochondrial morphology change in autopsied AD brain tissues. Of interest, mitochondrial fission protein, DLP1 level was also decreased in AD hippocampal neurons (Wang et al., 2008a,b). DLP1 is a cytoplasmic protein, which triggers mitochondrial fission upon its translocation to mitochondria to interact with FIS1(Chan, 2006). Detailed studies showed that DLP1 translocation increased in AD, despite the decrease in the expression level of total DLP1 (Wang et al., 2008b). Furthermore, there is significantly increased S-nitrosylated DLP1 and phosphorylated (S616) DLP1 in AD mitochondria (Cho et al., 2009; Wang et al., 2009b).
PINK1 mutation is associated with hereditary PD (Abou-Sleiman et al., 2006; Sim et al., 2006) and is known to genetically interact with mitochondrial fusion and fission machinery. Deficiency in PINK1 causes decreased mitochondrial cristae and elongated large mitochondria (Yang et al., 2008c). A recent study conducted on Hela cells expressing htt proteins containing 74 polyglutamine repeats found that mitochondria displayed significantly reduced movement and increased fragmentation (Wang et al., 2009a). Based on the evidence above, we propose that perturbations in mitochondrial fusion/fission balance are significant featured mitochondrial pathology in neurodegenerative disorders, and ultimately accompany with other mitochondrial stresses, leading to neuronal injury.
3. Mitochondria-based interventional medicine
Mitochondrial medicine is developing rapidly with the increasingly deepened appreciation of mitochondrial diseases. Mitochondria-based interventional medicine, though a relatively nascent area, takes great benefit from the expanding knowledge on the mitochondria-related molecular basis of mitochondrial diseases. It has demonstrated the priority through its specific manipulation targeting on the originals of mitochondrial diseases, the mitochondrial dysfunction; which is comparative to many current medicinal therapeutic strategies only focusing on extinguishing the consequent syndromes. The current mitochondrial medicine strategies can be divided into two categories: (1) preventing on-going mitochondrial dysfunction in diseases with determined mitochondria-related etiology; this is of essential significance for the treatment of inherited mitochondrial diseases such as Leber’s hereditary optic neuropathy (LHON) (Martin-Kleiner et al., 2006); and (2) manipulating on diseases with prevalent mitochondrial defects, for example sporadic neurodegenerative diseases (Cleren et al., 2008). Strategies of mitochondria-based interventional medicine is springing up, aiming at prevalent mitochondrial pathologies including perturbed cellular bioenergetics, oxidative stress, mtDNA mutations, impaired mitochondrial calcium handling capacity, mitochondria-originated apoptosis, and defected mitochondrial behavior, although the studies on most of those strategies are still at early stages. Nevertheless, with more discoveries of secrete underlying mitochondrial diseases the interventional medicine specifically targeting on mitochondrial changes will be the prospective trend in the treatment of mitochondrial diseases.
4. Mitochondria-targeted therapeutics for neurodegenerative diseases
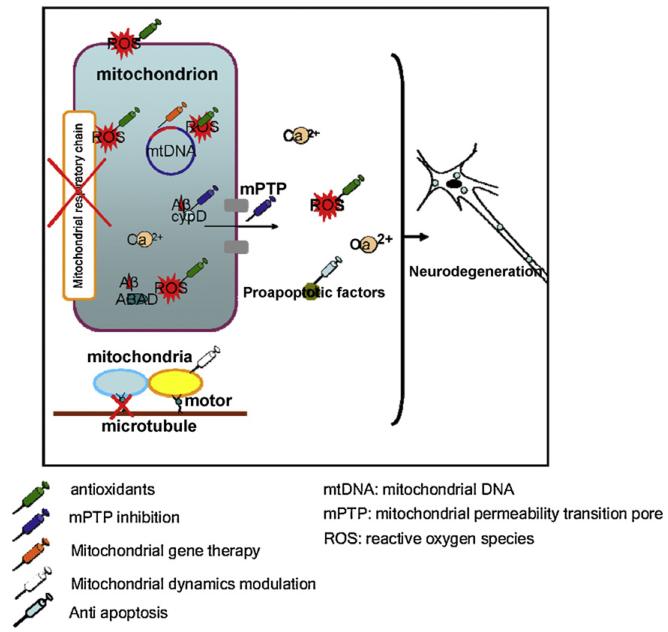
As aforementioned discussion, mitochondrial dysfunction, including oxidative stress, low ATP generation, mtDNA mutation, calcium perturbation, mPTP, and mitochondrial dynamic malfunction are commonly seen in neurodegenerative diseases, such as AD, PD, HD, ALS and PSP. Since damaged mitochondria exacerbate and participate in the pathogenesis of these diseases, it would be logical to choose mitochondria-targeted intervention as a therapeutic approach to treat or delay the onset of pathophysiological process of these diseases; indeed, several mitochondria-targeted treatments have been applied in clinical practice or to disease related animal models. The efficiency of these treatments have been evaluated and proven to be effective. The prevailing strategies for mitochondrial medicine include oxidant removal, mitochondrial gene therapy, and mPTP inhibition (Fig. 1).
4.1. Redox therapy
Based on the premise that oxidative stress is one of the most predominant mitochondrial pathologies involved in neurodegenerative diseases, and on the convenience for implementation of an antioxidant strategy, oxygen free radical scavengers have been intensively studied as a therapeutic approach for neurodegenerative diseases.
4.1.1. Supplementation of small reducing molecules
Small reducing molecules, such vitamins C and E, glutathione, and coenzyme Q10 (CoQ10), play an important role in the endogenous defensive strategy against oxygen free radicals in mitochondria. Decreased expression of these molecules observed in neurodegenerative diseases such as AD, PD, and ALS (Young et al., 2007; Beal, 2002; Paraskevas et al., 1997; Yapa, 1992). Thus, the idea of supplementing these small reducing molecules to scavenge oxidative stressors in neurodegenerative diseases has attracted the interest of several research groups.
4.1.1.1. Vitamins C and E (Vit C and Vit E) and MitoVit E
Vit C and Vit E are essential nutrients for human physiological process; Vit C, also known as l-ascorbate, is a strong intracellular reducing molecule, while Vit E is a major scavenger of lipid oxidation in brain. Vit C and Vit E have been reported to be neuroprotective in vivo and in vitro against the insult of oxidative stress (Calderon Guzman et al., 2007; Gurel et al., 2005; Zaidi and Banu, 2004).
Vit E protects neuoblastoma SK-N-SH cells from Aβ-induced oxidative stress, when administered prior to or simultaneously with the exposure of Aβ (Green et al., 1996; Butterfield, 2002). Four weeks of Vit E supplementation in an AD mouse model (Tg2576 mice at 11 months old) improved mice cognitive function and reduced the Aβ deposition (Conte et al., 2004). Administration of Vit E to young Tg2576 mice significantly decreased amyloidosis (Sung et al., 2004). Daily i.p. injection of vitamin C in APP/PSN1 mice at middle (12 months) to old (24 months) age significantly attenuated deficits in spatial learning/memory ability (Harrison et al., 2009). Daily doses of 2000 IU Vit E has been observed to be beneficial to mild-to-moderate stage AD patients (Petersen et al., 2005; Sano et al., 1997). Furthermore, administration of Vit E alone or concomitant administration of Vit C and Vit E can decrease the risk of AD (Boothby and Doering, 2005), while the combination of Vit E and Vit C has been reported to reduce the prevalence of AD in aged people (Logroscino et al., 1996; Kontush et al., 2001; Zandi et al., 2004).
Treatment with Vit E (20 mg/kg/day) efficiently protected mitochondrial complex II activity and prevented oxidative damage in a 3-nitropropionic acid induced HD mouse model (Rosenstock et al., 2004). Further, vitamin E intake delayed neuropathology onset and progression in a SOD1 transgenic ALS mouse model (Kruman et al., 1999). A large study on ALS showed that regulatory use of Vit E decreases the risk of ALS in humans (Ascherio et al., 2005). Similarly, diet rich in Vit E has been reported to reduce the risk of PD (Etminan et al., 2005; Zhang et al., 2002). Clinical trials on ALS patients showed that regular intake of Vit E significantly decreased the risk of death in ALS patients (Ascherio et al., 2005). The combination of Vit E and Vit C has been reported to delay the progression of PD (Fahn, 1992; Fahn and Cohen, 1992).
There exist, however, contradictory reports on the efficacy of Vit E and Vit C as a treatment for neurodegenerative diseases. A recent study reported by Zhang focusing on the effect of Vit E and Vit C intake on the risk of PD found no decrease in risk of the disorder (Zhang et al., 2002). He proposed that the protective effect of high levels of dietary Vit E may be due to other active molecules in the food, instead of Vit E. Several other independent studies also showed that the administration of Vit E or Vit C alone or in combination failed to demonstrate any benefits for the progression or risk of AD and PD (Luchsinger et al., 2003; McIntosh et al., 1997; Etminan et al., 2005; Weber and Ernst, 2006). Further, a few case-controlled studies showed no protection from ALS patients as well as ALS animal models with vitamin administration (Longnecker et al., 2000; Nelson et al., 2000; Desnuelle et al., 2001). In addition, high dosage of Vit E supplementation (>150 IU/day) may increase the mortality of receivers (Miller et al., 2005).
In considering the discrepancies in the results for vitamins treatment, it is proposed that the limitations of vitamin treatment might affect the efficiency of vitamins treatment and thus causes the discrepancy. The limitations include: (1) poor transport of Vit E and Vit C through the blood–brain barrier resulting in difficulties in accumulating therapeutic concentration of vitamins in neuronal mitochondria; (2) rapid oxidation of Vit C that reduces the efficacy of Vit C in treatment; and (3) potential side effects of high dosage of Vit E. Thus, mitochondrial targeted vitamin E has been developed to overcome the limitations.
MitoVit E is a newly developed vitamin E (engineered chemically from natural vitamin E) derivative that is highly efficient in accumulating in mitochondria due to its triphenylphosphonium cation moiety (Jauslin et al., 2003). Incubation of MitoVit E with human osteosarcoma cells showed that most of the MitoVit E localized inside mitochondria, suggesting its high cell permeability and preference for mitochondrial penetration that enables its utility in scavenging for mitochondrial oxidants. There is no obvious toxic effect of MitoVit E on mitochondrial membrane potential and respiration function in vitro at a concentration of 10 μM (Smith et al., 1999). Oxidatively stressed (iron/ascorbate) mitochondria demonstrated 75% protection in mitochondrial membrane potential rescued by 5 μM MitoVit E, significantly better protection in comparison to vitamin E at the same concentration. Kalyanaraman’s group found that 1 μM MitoVit E efficiently reduced lipid and protein oxidation, apoptosis, and mitochondrial iron uptake in glucose oxidase insulted bovine aortic endothelial cells by scavenging mitochondrial ROS (Dhanasekaran et al., 2004). A study related to fetal alcohol syndrome, conducted on primary cultured cerebella granule cells (Siler-Marsiglio et al., 2005), demonstrating suppressed cellular and mitochondrial antioxidation ability and increased lipid oxidation by alcohol, found that the oxidative damages caused by a concentration of alcohol at as high as 16 g/L was significantly attenuated by 1 nM MitoVit E. MitoVit E-treated cells displayed an increase in cell viability and GSH–GPx activity. However, due to scant in vivo evidence of animal models and clinical trials, the efficacy of the application of MitoVit E in the treatment of neurodegenerative diseases is still largely unclear. Nevertheless, the high performance of oxidant scavenging of MitoVit E makes it a potentially significant therapeutic target for neurodegenerative disease treatment.
4.1.1.2. Coenzyme Q10 and MitoQ
Coenzyme Q10 (CoQ10) also known as ubiquinone, coenzyme Q and ubidecarenone, locates mainly in mitochondrial inner membrane. The biochemical significance of CoQ10 lies in the fact that this small molecule participates in transferring electrons from mitochondrial complexes I and II to mitochondrial complex III (Littarru and Tiano, 2007). In addition to its important role in aerobic respiration, CoQ10 is a strong lipid oxidants scavenger similar to Vit E. When fibroblast and HEK293 cells are exposed to oxidative stress, CoQ10 helps to: stabilize mitochondrial membrane potential; prevent cytochrome c release; inhibit mitochondrial permeability transition pore; and block Bax translocation to mitochondria, thereby suggesting multiple biochemical roles for this enzyme (Naderi et al., 2006). CoQ10 has been shown to protect neurons from oxidative stress and severe mitochondrial dysfunction in affected regions of experimental models of PD, epilepsy and stroke (Beal, 2003; Horvath et al., 2003; Chaturvedi and Beal, 2008).
Due to the multi-faceted mitochondrial protective effects of CoQ10, it has been widely evaluated as a therapy for neurodegenerative diseases in both experimental animal models and clinical research studies. Administration of CoQ10 to aged mice rescued striatal dopamine loss and dopaminergic axonal damage (Beal et al., 1998). A recent study showed the neuroprotective effects of CoQ10 and its reduced form on MPTP induced PD animal models (Cleren et al., 2008). Dietary CoQ10 (1600 mg/kg/day for 2 months) protected striatal dopamine (DA) from MPTP toxicity two folds as compared to MPTP-untreated mice. CoQ10 also significantly preserved tyrosine hydroxylase (TH)-positive neurons in substantia nigra of these mice and attenuated α-synuclein aggregation depletion in dopaminergic neurons. The neuroprotective role of CoQ was also observed in a MPTP induced primate PD model. Similarly, an AD mouse model with a presenilin 1 mutation revealed a decrease in the Aβ deposition and improvement in the brain mitochondrial SOD activity after the CoQ10 dosing of 1200 mg/kg/day for 2 months (Yang et al., 2008b).
Importantly, clinical trials for CoQ10 administration to treat PD, HD and ALS have been resulted in positive data regarding neuroprotection (Matthews et al., 1998; Beal et al., 1998; Ferrante et al., 2002, 2005; Shults et al., 2004). CoQ10 treatment significantly ameliorates cognitive impairment (Senin et al., 1992; Gutzmann and Hadler, 1998).
MitoQ, an analogue of Co1Q10, that has the addition of a lipophilic triphenylphosphonium cation, has high lipid membrane permeability and easily accumulates in mitochondria (James et al., 2007; Reddy, 2008). MitoQ accumulates rapidly and preferentially in mitochondria when added to cultured cells in media. Cell toxicity lessened at concentrations up to 10 μM of MitoQ, though there was a report that MitoQ damaged N2a cells at concentrations higher than 0.3 μM (Reddy, 2008). MitoQ is a strong antioxidant molecule and can be recycled through the respiratory chain after detoxifying reactive oxygen species (James et al., 2007). MitoQ has been shown to be effective for preventing apoptosis, even in the presence of multiple apoptosis inducers.
Reddy recently reported that MitoQ enhances neurite out-growth of N2a cells in serum-free medium at a concentration of 0.3 μM, which supports the beneficial effect of MitoQ on neuronal function (Reddy, 2008). However, it should be noted that the uptake of MitoQ relies on mitochondrial membrane potential; thus, the efficiency of MitoQ to clear free radicals in severely damaged mitochondria may be compromised.
The safety of MitoQ application has been evaluated in an in vivo C57BL/6 mice. 50 μM MitoQ was added in the drinking water of the mice for up to 28 weeks. The administration of MitoQ on the mice did not show any measurable deleterious effect on mice tissues (brain, heart and liver). Besides, there were a few beneficial effects such as decrease in liver fat and decreased blood triacylglyceride, which suggest the safety of long term oral administration of MitoQ (Rodriguez-Cuenca et al., 2010). The safety and efficacy of human administration needs further clinical studies. Given the promising data derived from in vitro and in vivo animal experiments as well as the safety of long term MitoQ administration, MitoQ is proposed to be a potentially promising therapy for neuronal protection from oxidative stress and related mitochondrial dysfunction. More studies will be required to further evaluate the efficacy of MitoQ on the treatment of neurodegenerative diseases, such as AD or PD.
4.1.2. Mitochondrial targeted small peptide antioxidants
SS peptides are a series of newly designed free radical scavenging aromatic-cationic peptides (Petri et al., 2006; Szeto, 2006). The sequence motif of these peptides enables them to directly target to mitochondria and bind to IMM irrespective of mitochondrial membrane potential. Animal studies showed that SS02 quickly penetrates the mouse blood–brain barrier after subcutaneous administration as well as after intravenous injection. SS02 and SS31 demonstrate the ability to remove free radical removals in tert butyhydroperoxide (tBHP) induced mitochondrial ROS production in cell cultures (Szeto, 2006). SS peptides showed significant neuronal protection on animal models of brain ischemia and ALS (Cho et al., 2007; Petri et al., 2006). Its ability to efficiently scavenge free radicals, its water solubility and high blood–brain barrier permeability make these peptides pharmaceutically applicable for neurological diseases (Szeto, 2006). Although the evaluation of SS peptides effect on neurological diseases is still at the early stages, the application of SS peptides as promising antioxidants has been attracting increasing attention.
4.1.3. Natural antioxidants
Polyphenols, isoflavones, ginsenosides and flavonoids, extracted from medicinal plants, have antioxidant function and are proved to have protective effects on mitochondrial function. Green tea polyphenols are believed to be a strong antioxidant against hydroxyl radicals, nitric oxide and lipid oxidation (Nanjo et al., 1996; Panickar et al., 2009). The administration of green tea polyphenols to 6-hydroxydopamine (6-OHDA) or MPTP induced PD cell/animal models has been proven to effectively attenuate oxidative stress and apoptosis in cells (Guo et al., 2005) or in neurons of affected brain regions of animal models (Levites et al., 2001). Administration of green tea extracts reduced Aβ production in mice overexpressing APP/Aβ and in APP/Aβ overexpressed primary cultured neurons in vitro through enhancing α-secretase activity. These results suggest the potential of green extracts to protect against Aβ related mitochondrial dysfunction and other AD pathologies (Rezai-Zadeh et al., 2005).
Ginsenosides are steroid like compounds, comprising two sub-groups, Rb1 and Rg1. Studies from in vitro and in vivo experiments demonstrate that ginsenosides scavenge oxidants and convey protection mitochondrial and neuronal functions (Surh et al., 2002). Isoflavones and flavonoids are also thought to offer neuroprotection by removing superoxide and hydroxyl radicals (Ishige et al., 2001; Kurzer and Xu, 1997; Chan and Yu, 2000). Published reports demonstrated that isoflavones protect neurons from the Aβ-induced toxicity in vitro (Zheng et al., 2003). However, due to the limited studies to date, the efficacy and side effects of natural antioxidants for the treatment of neurodegenerative diseases are yet to be determined.
4.2. Mitochondrial permeability transition inhibition
The formation of mitochondrial permeability transition pore (mPTP) collapses mitochondrial membrane potential, enhances ROS generation, induces mitochondrial calcium perturbation and facilitates mitochondrial proapoptotic molecule release. mPTP-mediated severe mitochondrial dysfunction is believed to exacerbate pathological injuries in neurons, undergoing aforementioned neurodegeneration. The protective effects of mPTP blockade have been studied in animal models of a variety of neurodegenerative diseases and current studies are focusing on inhibition on cyclophilin D (CypD) or voltage-dependent anion channel (VDAC).
CypD is the intra-mitochondria component of mPTP, which triggers its translocation to IMM to ANT upon the presence of mPTP inducers, such as ROS and mitochondrial calcium overloading. Genetic CypD deficiency significantly reduces mPTP threshold, consequently, enhances mitochondrial calcium buffering capacity, and reduces cytochrome c release and ROS generation, especially in the presence of mPTP inducers (Baines et al., 2005; Du et al., 2008). Increasing evidence has shown the therapeutic effects of CypD depletion in neurodegenerative diseases (Du et al., 2008; Forte et al., 2007; Du et al., 2009). Transgenic AD mice lacking CypD displayed the protective effects on mitochondrial and behavioral function, as evidenced by increased mitochondrial membrane potential, reduced mitochondrial ROS production, preserved mitochondrial cytochrome c oxidase activity and respiration control ratio, increased long term potentiation and improved spatial learning/memory (Du et al., 2008). Notably, CypD depletion confers lifelong mitochondrial and neuronal protection against Aβ toxicity in an AD mouse model in mice up to 24 months of age (Du et al., 2009). Another example is an experiment conducted on EAE mice. In an experimental multiple sclerosis (MS) mouse model, it has been shown that CypD depletion ameliorates the severity of symptoms and preserves axonal functions, suggesting that mPTP blockade via reducing CypD translocation is a practical treatment strategy to restore mitochondrial function and to prevent neuronal degeneration. Several CypD inhibitors such as cyclosporine A (CsA), Sanglifehrin A (SfA), and FK506 have been developed and are reported to be capable of inhibiting mPTP formation and its consequent damages (Halestrap et al., 1997; Cassarino et al., 1998; Setkowicz et al., 2009). The application of these agents significantly protects neurons from oxidative stress-induced injury, ER stress and apoptosis induced by H2O2, Aβ, MPP+ and many other toxins (Luchowska et al., 2003; Fall and Bennett, 1998; Baines et al., 2005; Du et al., 2008). Administration of CsA to brain via intracerebroventricular CsA injections (dosage, of 20 mg/week) in a familial ALS (FALS) mouse model (G93A) alleviated symptoms and reversed pathological changes (Keep et al., 2001). CsA treatment delayed the onset of hindlimb weakness and weight loss, extended the time from the onset of weakness to paralysis and prolonged the lifespan. Further, CsA treatment preserved both cervical and lumbar spine motor neurons and tyrosine hydroxylase-positive dopaminergic neurons in substantia nigra. There were no adverse effects such as systemic immunosuppression or nephrotoxicity noted in this study, suggesting the safety of CsA treatment at these concentrations; the protective effect of CsA administration is mostly due to its inhibition of mPTP but not immunological effects.
FK506, a non-immunosuppressant derivative FK1706, at a non-immunosuppressant dose significantly ameliorated damage in spinal cord and protected axonal loss in a MS mice model (Gold et al., 2004). These treatments using mPTP inhibitors such as FK506 and CsA have also been proved to be effective in HD animal models, due to, at least in part, their effects on mPTP blockade (Kumar and Kumar, 2009).
VDAC, the mPTP component in OMM, is another potential target for therapy. A recent study reported that cholest-4-en-3-one, oxim (TRO19622) significantly extended the lifespan and attenuated the symptoms of G93A SOD1 ALS mice. The authors proposed that protection of TRO19622 on ALS mice is due to its interaction with VDAC (Bordet et al., 2007). This study supports mPTP involvement in the pathogenesis of ALS and the potential pharmaceutical effect of the MPTP interference by VDAC in ALS.
Blockade of mPTP formation has a promising potential as an effective chemotherapeutic approach to protect against neurodegeneration and restore mitochondrial function in neurodegenerative diseases. However, issues in the development of mPTP inhibitors exist. First, CypD inhibitors have multiple biological side effects, including immunosuppression and inhibition of calcineurin, both of which should be considered in clinical practice (Mohebbi et al., 2009; Borlongan et al., 2000). Secondly, mitochondrial delivery of the drugs requires further chemical studies to increase the permeability of these drugs through the blood–brain barrier and to enhance their accumulation in mitochondria. So far, investigations on mPTP inhibitor have shown CsA has the potential to be a candidate for mitochondrial medicine. Recent study exhibited that administration of CsA intraperitoneally to the mice extended the life of the ALS mouse model. CsA accumulated in the brain and spinal cord of this model (Kirkinezos et al., 2004), suggesting that CsA is able to cross BBB in the impaired blood–brain barrier. Keep’s study showed that CsA at the experimental dosage did not demonstrate immunosuppression (Keep et al., 2001). However, the long-term side effect and the administration dosage of CsA still need further investigation. Nevertheless, given the increasing evidence of protective role of mPTP inhibition in treating neurodegenerative diseases, it is likely that mPTP blockers will be investigated for their potential as pharmacological therapeutics in clinical treatment of neurodegenerative diseases.
4.3. Mitochondrial gene therapy
Given the high incidence of pathogenic mtDNA in neurodegenerative disease, studies targeting to ameliorate mtDNA lesion, specifically genetic manipulation of mtDNA and its downstream gene are called for. Several promising developments in this field have been demonstrated and can be divided into three categories: selective inhibition of mutant mtDNA, recombinant mtDNA substitution and allotropic expression of mitochondrial proteins (Bacman et al., 2007; Bayona-Bafaluy et al., 2005; Chen et al., 2003; Krieg, 2000; Zullo et al., 2005).
Elimination of mtDNA by restriction endonuclease selection and inhibition of mutant mtDNA using antisense are the two well-investigated strategies of selective inhibition of mutant mtDNA. COX-PstI, COX8-ApaLI and ScaI have been studied and show restorative effect on mitochondrial function (Chinnery et al., 1999; Flierl et al., 2003; Bacman et al., 2007). The principle of antisense inhibition is to genetically block the replication of mutant mtDNA and thus reduce the expression of defective proteins. DNA, RNA or chemical analogues have been studied as antisense molecules to bind their complementary DNA or RNA target. The most studied type of antisense molecules so far are DNA-like peptide nucleic acids (PNA), which are effective for treatment of heteroplasmic disorders. The discoveries of short hairpin RNA (shRNA) and small interfering RNA (siRNA) also may be promising approaches to modulate mitochondrial genome defects at the mRNA level. Substitution of defective mtDNA using gene-carrying vectors to send recombinant mtDNA into cells is another approach for mitochondrial gene therapy.
The replacement of defective mtDNA with “healthy” recombinant mtDNA genome will benefit not only heteroplasmy but also homoplasmy. Although the therapeutic efficacy of recombinant mtDNA has been evaluated in a few studies, the lack of availability of human mtDNA constructs, poor mitochondrial import of large constructs, and the competition for recombinant mtDNA with functional resident mtDNA greatly limits the application of this method as a therapeutic strategy.
Recent findings on the application of allotropic expression of mitochondrial proteins suggest the promising future of this method. An example is the allotropic expression of NDI1, a rotenone-insensitive NADH-Q-oxidoreductase. By incorporating NDI1 into mitochondria, a group revealed that the expression of NDI1 restores the NADH dehydrogenase activity and as a result, protects neurons in the substantia nigra of a Parkinson’s disease rat model from severe degeneration (Marella et al., 2008). Another example is that the allotropic expression of ND1 attenuates mitochondrial dysfunction in cell model of LHON (Leber’s hereditary optic neuropathy) or the allotropic expression of ND4 prevents visual impairment of LHON animal model (Ellouze et al., 2008; Park et al., 2007). Most recent studies using allotropic expressing of interfering on nucleic genome encoded mitochondrial proteins provide a novel approach to improve mitochondrial function. Hayashi’s finding showed that overexpressing mitochondrial transcriptional factor A restores memory impairments including working memory and hippocampal long term potentiation (LTP), attenuates mitochondrial ROS production and mtDNA damages in aged mice (Hayashi et al., 2008). However, investigations in this field are still at very early stage and many applications are theoretical concepts to date.
5. Mitochondrial medicine delivery
Blood–brain barrier permeability still remains a significant obstacle in the development of mitochondrial pharmacotherapeutics for neurodegenerative diseases. Similarly, targeting drugs directly to mitochondria to achieve efficacious accumulation remains a stumbling block to successful therapies. Development of big constructs and transporting these constructs to neurons remains problematic. Therefore, novel methodologies for manipulation of mitochondrial drugs are needed for mitochondria-targeted treatment (Murphy and Smith, 2000; Murphy, 1996; Muratovska et al., 2001).
Modification of drugs to increase their cellular as well as mitochondrial permeability is a feasible undertaking. Lipophilic cations have been proved to be effective to facilitate molecular transport across the hydrophobic barrier of the lipid bilayers (Murphy, 2008; Adlam et al., 2005). Applications such as MitoQ (Graham et al., 2009; Supinski et al., 2009) and MitoVit E (Leo et al., 2008) have been extensively studied and shown to be effective.
Another approach is to enable big molecules to enter mitochondria through mitochondrial import machinery. The addition of a mitochondrial signaling peptide to molecules is a practical methodology (Hurt et al., 1985), and is complementary with lipophilic cations, especially for molecules that are too large or excessively polar so as to be attached by lipophilic cations. However, the introduction of recombinant mtDNA directly into mitochondria has not yet been successfully performed. It is extremely difficult to deliver a big construct containing human mtDNA into neuronal mitochondria, but one possibility course of action is to use oligonucleotides to attach to a mitochondrial signaling peptide. To date, there is little progress in the study of mitochondrial gene therapy carriers, preventing the clinical application of mitochondrial gene therapy and appealing further investigation.
6. Conclusion
Mitochondria are major neuronal organelles that play a critical role in ATP production to sustain neuronal survival. Functional mitochondrial distribution and energy provision in neuronal strategic sites are the prerequisite for the formation of massive neuron information exchange networks in brain. Mitochondrial dysfunction, including increased ROS generation, mitochondrial permeability transition, collapsed mitochondrial membrane potential, decreased mitochondrial ATP production and the release of proapoptotic factors, leads to severe synaptic dysfunction and eventually neuronal death. Recent studies have highlighted mitochondrial malfunction as a hallmark pathological change in neurodegenerative diseases, such as AD, PD, ALS, and HD; researchers have therefore postulated that mitochondrial stress is significantly involved in the pathophysiological process of neurodegeneration. Mitochondria-targeted interventional medicine is, therefore, strongly implied to be a prospective therapeutic approach in neurodegenerative diseases.
Although most of the treatment approaches described in this article is far from fruition, they have been shown to have a great potential for therapeutic applications. Antioxidant treatment has shed light on mitochondria-targeted medicine, with several drugs currently undergoing clinical trials on patients with neurodegenerative diseases and some have demonstrated significant efficacy in clinical practice. Based on current experimental observations, despite the mitochondria-targeted interventions discussed afore-mentioned, modulations on mitochondrial dynamics and apoptotic factors hold promise as potential efficacious treatment strategies, though most of the relevant studies are still concepts. Nevertheless, given the frequent occurrence and similarity of mitochondrial pathology in early onset inherited and late onset sporadic neurodegenerative diseases, mitochondrial medicine, targeting on the essential pathology in neurodegeneration, is expected to be a promising future therapeutic strategy in the treatment of neurodegenerative diseases.
Fig. 1.
Schematic figure of therapeutic strategies to protect mitochondria against neurodegeneration. In neurodegenerative diseases such as AD, PD, HD and ALS, mitochondria undergo increased ROS production, suppressed respiratory function, mtDNA lesions, mPTP formation, preapoptotic factors release and damaged dynamics/motility. These deleterious factors will eventually lead to neurodegeneration. Mitochondrial medicine, including oxidant scavenge, mPTP inhibition, mitochondrial gene therapy, anti-apoptosis and mitochondrial dynamics modulation, are promising therapeutic strategies to attenuate neurodegeneration and to halt the progression of neuronal injury in the neurodegenerative diseases.
Acknowledgment
This work was supported by the USPHS (PO1 AG17490 and P50 AG008702) and Alzheimer Association.
References
- Abou-Sleiman PM, Muqit MM, McDonald NQ, Yang YX, Gandhi S, Healy DG, et al. A heterozygous effect for PINK1 mutations in Parkinson’s disease? Ann Neurol. 2006;60:414–9. doi: 10.1002/ana.20960. [DOI] [PubMed] [Google Scholar]
- Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP, et al. Targeting an antioxidant to mitochondria decreases cardiac ischemia–reperfusion injury. FASEB J. 2005;19:1088–95. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- Alam M, Schmidt WJ. Mitochondrial complex I inhibition depletes plasma testosterone in the rotenone model of Parkinson’s disease. Physiol Behav. 2004;83:395–400. doi: 10.1016/j.physbeh.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Albers DS, Augood SJ, Park LC, Browne SE, Martin DM, Adamson J, et al. Frontal lobe dysfunction in progressive supranuclear palsy: evidence for oxidative stress and mitochondrial impairment. J Neurochem. 2000;74:878–81. doi: 10.1046/j.1471-4159.2000.740878.x. [DOI] [PubMed] [Google Scholar]
- Albers DS, Beal MF. Mitochondrial dysfunction in progressive supranuclear palsy. Neurochem Int. 2002;40:559–64. doi: 10.1016/s0197-0186(01)00126-7. [DOI] [PubMed] [Google Scholar]
- Albers DS, Swerdlow RH, Manfredi G, Gajewski C, Yang L, Parker WD, Jr, et al. Further evidence for mitochondrial dysfunction in progressive supranuclear palsy. Exp Neurol. 2001;168:196–8. doi: 10.1006/exnr.2000.7607. [DOI] [PubMed] [Google Scholar]
- Almeida S, Sarmento-Ribeiro AB, Januario C, Rego AC, Oliveira CR. Evidence of apoptosis and mitochondrial abnormalities in peripheral blood cells of Huntington’s disease patients. Biochem Biophys Res Commun. 2008;374:599–603. doi: 10.1016/j.bbrc.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Aoyama K, Matsubara K, Kobayashi S. Aging and oxidative stress in progressive supranuclear palsy. Eur J Neurol. 2006;13:89–92. doi: 10.1111/j.1468-1331.2006.01139.x. [DOI] [PubMed] [Google Scholar]
- Arenas J, Campos Y, Ribacoba R, Martin MA, Rubio JC, Ablanedo P, et al. Complex I defect in muscle from patients with Huntington’s disease. Ann Neurol. 1998;43:397–400. doi: 10.1002/ana.410430321. [DOI] [PubMed] [Google Scholar]
- Ascherio A, Weisskopf MG, O’Reilly EJ, Jacobs EJ, McCullough ML, Calle EE, et al. Vitamin E intake and risk of amyotrophic lateral sclerosis. Ann Neurol. 2005;57:104–10. doi: 10.1002/ana.20316. [DOI] [PubMed] [Google Scholar]
- Bacman SR, Williams SL, Hernandez D, Moraes CT. Modulating mtDNA heteroplasmy by mitochondria-targeted restriction endonucleases in a ‘differential multiple cleavage-site’ model. Gene Ther. 2007;14:1309–18. doi: 10.1038/sj.gt.3302981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–62. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- Banerjee R, Starkov AA, Beal MF, Thomas B. Mitochondrial dysfunction in the limelight of Parkinson’s disease pathogenesis. Biochim Biophys Acta. 2009;1792:651–63. doi: 10.1016/j.bbadis.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayona-Bafaluy MP, Blits B, Battersby BJ, Shoubridge EA, Moraes CT. Rapid directional shift of mitochondrial DNA heteroplasmy in animal tissues by a mitochondrially targeted restriction endonuclease. Proc Natl Acad Sci USA. 2005;102:14392–7. doi: 10.1073/pnas.0502896102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal MF. Coenzyme Q10 as a possible treatment for neurodegenerative diseases. Free Radic Res. 2002;36:455–60. doi: 10.1080/10715760290021315. [DOI] [PubMed] [Google Scholar]
- Beal MF. Bioenergetic approaches for neuroprotection in Parkinson’s disease. Ann Neurol. 2003;53(Suppl. 3):S39–47. doi: 10.1002/ana.10479. [DOI] [PubMed] [Google Scholar]
- Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann Neurol. 2005;58:495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- Beal MF, Matthews RT, Tieleman A, Shults CW. Coenzyme Q10 attenuates the 1-methyl-4-phenyl-1,2,3-tetrahydropyridine (MPTP) induced loss of striatal dopamine and dopaminergic axons in aged mice. Brain Res. 1998;783:109–14. doi: 10.1016/s0006-8993(97)01192-x. [DOI] [PubMed] [Google Scholar]
- Becker T, Gebert M, Pfanner N, van der Laan M. Biogenesis of mitochondrial membrane proteins. Curr Opin Cell Biol. 2009;21:484–93. doi: 10.1016/j.ceb.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Begley JG, Duan W, Chan S, Duff K, Mattson MP. Altered calcium homeostasis and mitochondrial dysfunction in cortical synaptic compartments of presenilin-1 mutant mice. J Neurochem. 1999;72:1030–9. doi: 10.1046/j.1471-4159.1999.0721030.x. [DOI] [PubMed] [Google Scholar]
- Benchoua A, Trioulier Y, Zala D, Gaillard MC, Lefort N, Dufour N, et al. Involvement of mitochondrial complex II defects in neuronal death produced by N-terminus fragment of mutated huntingtin. Mol Biol Cell. 2006;17:1652–63. doi: 10.1091/mbc.E05-07-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin O, Desnuelle C, Rascol O, Borg M, Peyro Saint, Paul H, Azulay JP, et al. Mitochondrial respiratory failure in skeletal muscle from patients with Parkinson’s disease and multiple system atrophy. J Neurol Sci. 1994;125:95–101. doi: 10.1016/0022-510x(94)90248-8. [DOI] [PubMed] [Google Scholar]
- Bohr VA. Repair of oxidative DNA damage in nuclear and mitochondrial DNA, and some changes with aging in mammalian cells. Free Radic Biol Med. 2002;32:804–12. doi: 10.1016/s0891-5849(02)00787-6. [DOI] [PubMed] [Google Scholar]
- Boothby LA, Doering PL. Vitamin C and vitamin E for Alzheimer’s disease. Ann Pharmacother. 2005;39:2073–80. doi: 10.1345/aph.1E495. [DOI] [PubMed] [Google Scholar]
- Bordet T, Buisson B, Michaud M, Drouot C, Galea P, Delaage P, et al. Identification and characterization of cholest-4-en-3-one, oxime (TRO19622), a novel drug candidate for amyotrophic lateral sclerosis. J Pharmacol Exp Ther. 2007;322:709–20. doi: 10.1124/jpet.107.123000. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Stahl CE, Keep MF, Elmer E, Watanabe S. Cyclosporine-A enhances choline acetyltransferase immunoreactivity in the septal region of adult rats. Neurosci Lett. 2000;279:73–6. doi: 10.1016/s0304-3940(99)00962-3. [DOI] [PubMed] [Google Scholar]
- Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–33. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- Browne SE. Mitochondria and Huntington’s disease pathogenesis: insight from genetic and chemical models. Ann NY Acad Sci. 2008;1147:358–82. doi: 10.1196/annals.1427.018. [DOI] [PubMed] [Google Scholar]
- Brustovetsky N, Brustovetsky T, Purl KJ, Capano M, Crompton M, Dubinsky JM. Increased susceptibility of striatal mitochondria to calcium-induced permeability transition. J Neurosci. 2003;23:4858–67. doi: 10.1523/JNEUROSCI.23-12-04858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA. Amyloid beta-peptide (1–42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer’s disease brain. A review. Free Radic Res. 2002;36:1307–13. doi: 10.1080/1071576021000049890. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Perluigi M, Sultana R. Oxidative stress in Alzheimer’s disease brain: new insights from redox proteomics. Eur J Pharmacol. 2006;545:39–50. doi: 10.1016/j.ejphar.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Gubellini P, Picconi B, Centonze D, Pisani A, Bonsi P, et al. Inhibition of mitochondrial complex II induces a long-term potentiation of NMDA-mediated synaptic excitation in the striatum requiring endogenous dopamine. J Neurosci. 2001;21:5110–20. doi: 10.1523/JNEUROSCI.21-14-05110.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon Guzman D, Trujillo Jimenez F, Hernandez Garcia E, Juarez Olguin H. Assessment of antioxidant effect of 2,5-dihydroxybenzoic acid and vitamin A in brains of rats with induced hyperoxia. Neurochem Res. 2007;32:1036–40. doi: 10.1007/s11064-006-9269-6. [DOI] [PubMed] [Google Scholar]
- Cardoso SM, Santana I, Swerdlow RH, Oliveira CR. Mitochondria dysfunction of Alzheimer’s disease cybrids enhances Abeta toxicity. J Neurochem. 2004;89:1417–26. doi: 10.1111/j.1471-4159.2004.02438.x. [DOI] [PubMed] [Google Scholar]
- Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, et al. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer’s disease. FASEB J. 2005;19:2040–1. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- Cassarino DS, Swerdlow RH, Parks JK, Parker WD, Jr, Bennett JP., Jr Cyclosporin A increases resting mitochondrial membrane potential in SY5Y cells and reverses the depressed mitochondrial membrane potential of Alzheimer’s disease cybrids. Biochem Biophys Res Commun. 1998;248:168–73. doi: 10.1006/bbrc.1998.8866. [DOI] [PubMed] [Google Scholar]
- Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–52. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Chan WH, Yu JS. Inhibition of UV irradiation-induced oxidative stress and apoptotic biochemical changes in human epidermal carcinoma A431 cells by genistein. J Cell Biochem. 2000;78:73–84. doi: 10.1002/(sici)1097-4644(20000701)78:1<73::aid-jcb7>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Chaturvedi RK, Beal MF. Mitochondrial approaches for neuroprotection. Ann NY Acad Sci. 2008;1147:395–412. doi: 10.1196/annals.1427.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, He CY, Ehrhardt A, Kay MA. Minicircle DNA vectors devoid of bacterial DNA result in persistent and high-level transgene expression in vivo. Mol Ther. 2003;8:495–500. doi: 10.1016/s1525-0016(03)00168-0. [DOI] [PubMed] [Google Scholar]
- Chinnery PF, Taylor RW, Diekert K, Lill R, Turnbull DM, Lightowlers RN. Peptide nucleic acid delivery to human mitochondria. Gene Ther. 1999;6:1919–28. doi: 10.1038/sj.gt.3301061. [DOI] [PubMed] [Google Scholar]
- Chirichigno JW, Manfredi G, Beal MF, Albers DS. Stress-induced mitochondrial depolarization and oxidative damage in PSP cybrids. Brain Res. 2002;951:31–5. doi: 10.1016/s0006-8993(02)03101-3. [DOI] [PubMed] [Google Scholar]
- Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, et al. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324:102–5. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J, Won K, Wu D, Soong Y, Liu S, Szeto HH, et al. Potent mitochondria-targeted peptides reduce myocardial infarction in rats. Coron Artery Dis. 2007;18:215–20. doi: 10.1097/01.mca.0000236285.71683.b6. [DOI] [PubMed] [Google Scholar]
- Cleren C, Yang L, Lorenzo B, Calingasan NY, Schomer A, Sireci A, et al. Therapeutic effects of coenzyme Q10 (CoQ10) and reduced CoQ10 in the MPTP model of Parkinsonism. J Neurochem. 2008;104:1613–21. doi: 10.1111/j.1471-4159.2007.05097.x. [DOI] [PubMed] [Google Scholar]
- Conte V, Uryu K, Fujimoto S, Yao Y, Rokach J, Longhi L, et al. Vitamin E reduces amyloidosis and improves cognitive function in Tg2576 mice following repetitive concussive brain injury. J Neurochem. 2004;90:758–64. doi: 10.1111/j.1471-4159.2004.02560.x. [DOI] [PubMed] [Google Scholar]
- Cortopassi G, Danielson S, Alemi M, Zhan SS, Tong W, Carelli V, et al. Mitochondrial disease activates transcripts of the unfolded protein response and cell cycle and inhibits vesicular secretion and oligodendrocyte-specific transcripts. Mitochondrion. 2006;6:161–75. doi: 10.1016/j.mito.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Coskun PE, Beal MF, Wallace DC. Alzheimer’s brains harbor somatic mtDNA control-region mutations that suppress mitochondrial transcription and replication. Proc Natl Acad Sci USA. 2004;101:10726–31. doi: 10.1073/pnas.0403649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa C, Belcastro V, Tozzi A, Di Filippo M, Tantucci M, Siliquini S, et al. Electrophysiology and pharmacology of striatal neuronal dysfunction induced by mitochondrial complex I inhibition. J Neurosci. 2008;28:8040–52. doi: 10.1523/JNEUROSCI.1947-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Canto MC, Gurney ME. Neuropathological changes in two lines of mice carrying a transgene for mutant human Cu,Zn SOD, and in mice overexpressing wild type human SOD: a model of familial amyotrophic lateral sclerosis (FALS) Brain Res. 1995;676:25–40. doi: 10.1016/0006-8993(95)00063-v. [DOI] [PubMed] [Google Scholar]
- Damiano M, Starkov AA, Petri S, Kipiani K, Kiaei M, Mattiazzi M, et al. Neural mitochondrial Ca2+ capacity impairment precedes the onset of motor symptoms in G93A Cu/Zn-superoxide dismutase mutant mice. J Neurochem. 2006;96:1349–61. doi: 10.1111/j.1471-4159.2006.03619.x. [DOI] [PubMed] [Google Scholar]
- de Magalhaes JP. Human disease-associated mitochondrial mutations fixed in non-human primates. J Mol Evol. 2005;61:491–7. doi: 10.1007/s00239-004-0258-6. [DOI] [PubMed] [Google Scholar]
- Desnuelle C, Dib M, Garrel C, Favier A, ALS riluzole-tocopherol Study Group A double-blind, placebo-controlled randomized clinical trial of alpha-tocopherol (vitamin E) in the treatment of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2001;2:9–18. doi: 10.1080/146608201300079364. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran A, Kotamraju S, Kalivendi SV, Matsunaga T, Shang T, Keszler A, et al. Supplementation of endothelial cells with mitochondria-targeted antioxidants inhibit peroxide-induced mitochondrial iron uptake, oxidative damage, and apoptosis. J Biol Chem. 2004;279:37575–87. doi: 10.1074/jbc.M404003200. [DOI] [PubMed] [Google Scholar]
- Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, et al. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat Med. 2008;14:1097–105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Guo L, Zhang W, Rydzewska M, Yan S. Cyclophilin D deficiency improves mitochondrial function and learning/memory in aging Alzheimer disease mouse model. Neurobiol Aging. 2009 Apr; doi: 10.1016/j.neurobiolaging.2009.03.003. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Yan SS. Mitochondrial permeability transition pore in Alzheimer’s disease: cyclophilin D and amyloid beta. Biochim Biophys Acta. 2010;1802(January (1)):198–204. doi: 10.1016/j.bbadis.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis L, Gonzalez de Aguilar JL, Oudart H, de Tapia M, Barbeito L, Loeffler JP. Mitochondria in amyotrophic lateral sclerosis: a trigger and a target. Neurodegener Dis. 2004;1:245–54. doi: 10.1159/000085063. [DOI] [PubMed] [Google Scholar]
- Echaniz-Laguna A, Zoll J, Ponsot E, N’Guessan B, Tranchant C, Loeffler JP, et al. Muscular mitochondrial function in amyotrophic lateral sclerosis is progressively altered as the disease develops: a temporal study in man. Exp Neurol. 2006;198:25–30. doi: 10.1016/j.expneurol.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Ellouze S, Augustin S, Bouaita A, Bonnet C, Simonutti M, Forster V, et al. Optimized allotopic expression of the human mitochondrial ND4 prevents blindness in a rat model of mitochondrial dysfunction. Am J Hum Genet. 2008;83:373–87. doi: 10.1016/j.ajhg.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteves AR, Domingues AF, Ferreira IL, Januario C, Swerdlow RH, Oliveira CR, et al. Mitochondrial function in Parkinson’s disease cybrids containing an nt2 neuron-like nuclear background. Mitochondrion. 2008;8:219–28. doi: 10.1016/j.mito.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Etminan M, Gill SS, Samii A. Intake of vitamin E, vitamin C, and carotenoids and the risk of Parkinson’s disease: a meta-analysis. Lancet Neurol. 2005;4:362–5. doi: 10.1016/S1474-4422(05)70097-1. [DOI] [PubMed] [Google Scholar]
- Fahn S. A pilot trial of high-dose alpha-tocopherol and ascorbate in early Parkinson’s disease. Ann Neurol. 1992;32(Suppl.):S128–32. doi: 10.1002/ana.410320722. [DOI] [PubMed] [Google Scholar]
- Fahn S, Cohen G. The oxidant stress hypothesis in Parkinson’s disease: evidence supporting it. Ann Neurol. 1992;32:804–12. doi: 10.1002/ana.410320616. [DOI] [PubMed] [Google Scholar]
- Fall CP, Bennett JP., Jr MPP+ induced SH-SY5Y apoptosis is potentiated by cyclosporin A and inhibited by aristolochic acid. Brain Res. 1998;811:143–6. doi: 10.1016/s0006-8993(98)00879-8. [DOI] [PubMed] [Google Scholar]
- Faust K, Gehrke S, Yang Y, Yang L, Beal F, Lu B. Neuroprotective effects of compounds with antioxidant and anti-inflammatory properties in a Drosophila model of Parkinson’s disease. BMC Neurosci. 2009;10:109. doi: 10.1186/1471-2202-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes HB, Baimbridge KG, Church J, Hayden MR, Raymond LA. Mitochondrial sensitivity and altered calcium handling underlie enhanced NMDA-induced apoptosis in YAC128 model of Huntington’s disease. J Neurosci. 2007;27:13614–23. doi: 10.1523/JNEUROSCI.3455-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante KL, Shefner J, Zhang H, Betensky R, O’Brien M, Yu H, et al. Tolerance of high-dose (3,000 mg/day) coenzyme Q10 in ALS. Neurology. 2005;65:1834–6. doi: 10.1212/01.wnl.0000187070.35365.d7. [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, Andreassen OA, Dedeoglu A, Ferrante KL, Jenkins BG, Hersch SM, et al. Therapeutic effects of coenzyme Q10 and remacemide in transgenic mouse models of Huntington’s disease. J Neurosci. 2002;22:1592–9. doi: 10.1523/JNEUROSCI.22-05-01592.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flierl A, Jackson C, Cottrell B, Murdock D, Seibel P, Wallace DC. Targeted delivery of DNA to the mitochondrial compartment via import sequence-conjugated peptide nucleic acid. Mol Ther. 2003;7:550–7. doi: 10.1016/s1525-0016(03)00037-6. [DOI] [PubMed] [Google Scholar]
- Fontaine MA, Geddes JW, Banks A, Butterfield DA. Effect of exogenous and endogenous antioxidants on 3-nitropionic acid-induced in vivo oxidative stress and striatal lesions: insights into Huntington’s disease. J Neurochem. 2000;75:1709–15. doi: 10.1046/j.1471-4159.2000.0751709.x. [DOI] [PubMed] [Google Scholar]
- Forte M, Gold BG, Marracci G, Chaudhary P, Basso E, Johnsen D, et al. Cyclophilin D inactivation protects axons in experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis. Proc Natl Acad Sci USA. 2007;104:7558–63. doi: 10.1073/pnas.0702228104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi S, Wood-Kaczmar A, Yao Z, Plun-Favreau H, Deas E, Klupsch K, et al. PINK1-associated Parkinson’s disease is caused by neuronal vulnerability to calcium-induced cell death. Mol Cell. 2009;33:627–38. doi: 10.1016/j.molcel.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellerich FN, Gizatullina Z, Nguyen HP, Trumbeckaite S, Vielhaber S, Seppet E, et al. Impaired regulation of brain mitochondria by extramitochondrial Ca2+ in transgenic Huntington disease rats. J Biol Chem. 2008;283:30715–24. doi: 10.1074/jbc.M709555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BG, Voda J, Yu X, McKeon G, Bourdette DN. FK506 and a nonimmunosuppressant derivative reduce axonal and myelin damage in experimental autoimmune encephalomyelitis: neuroimmunophilin ligand-mediated neuroprotection in a model of multiple sclerosis. J Neurosci Res. 2004;77:367–77. doi: 10.1002/jnr.20165. [DOI] [PubMed] [Google Scholar]
- Gordeeva AV, Zvyagilskaya RA, Labas YA. Cross-talk between reactive oxygen species and calcium in living cells. Biochemistry (Mosc) 2003;68:1077–80. doi: 10.1023/a:1026398310003. [DOI] [PubMed] [Google Scholar]
- Graham D, Huynh NN, Hamilton CA, Beattie E, Smith RA, Cocheme HM, et al. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension. 2009;54:322–8. doi: 10.1161/HYPERTENSIONAHA.109.130351. [DOI] [PubMed] [Google Scholar]
- Green PS, Gridley KE, Simpkins JW. Estradiol protects against beta-amyloid (25-35)-induced toxicity in SK-N-SH human neuroblastoma cells. Neurosci Lett. 1996;218:165–8. doi: 10.1016/s0304-3940(96)13148-7. [DOI] [PubMed] [Google Scholar]
- Grivennikova VG, Vinogradov AD. Generation of superoxide by the mitochondrial Complex I. Biochim Biophys Acta. 2006;1757:553–61. doi: 10.1016/j.bbabio.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Gu G, Reyes PE, Golden GT, Woltjer RL, Hulette C, Montine TJ, et al. Mitochondrial DNA deletions/rearrangements in Parkinson disease and related neurodegenerative disorders. J Neuropathol Exp Neurol. 2002;61:634–9. doi: 10.1093/jnen/61.7.634. [DOI] [PubMed] [Google Scholar]
- Gu M, Cooper JM, Taanman JW, Schapira AH. Mitochondrial DNA transmission of the mitochondrial defect in Parkinson’s disease. Ann Neurol. 1998;44:177–86. doi: 10.1002/ana.410440207. [DOI] [PubMed] [Google Scholar]
- Gu M, Gash MT, Mann VM, Javoy-Agid F, Cooper JM, Schapira AH. Mitochondrial defect in Huntington’s disease caudate nucleus. Ann Neurol. 1996;39:385–9. doi: 10.1002/ana.410390317. [DOI] [PubMed] [Google Scholar]
- Guo S, Bezard E, Zhao B. Protective effect of green tea polyphenols on the SH-SY5Y cells against 6-OHDA induced apoptosis through ROS-NO pathway. Free Radic Biol Med. 2005;39:682–95. doi: 10.1016/j.freeradbiomed.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Gurel A, Coskun O, Armutcu F, Kanter M, Ozen OA. Vitamin E against oxidative damage caused by formaldehyde in frontal cortex and hippocampus: biochemical and histological studies. J Chem Neuroanat. 2005;29:173–8. doi: 10.1016/j.jchemneu.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Gutzmann H, Hadler D. Sustained efficacy and safety of idebenone in the treatment of Alzheimer’s disease: update on a 2-year double-blind multicentre study. J Neural Transm Suppl. 1998;54:301–10. doi: 10.1007/978-3-7091-7508-8_30. [DOI] [PubMed] [Google Scholar]
- Haas RH, Nasirian F, Nakano K, Ward D, Pay M, Hill R, et al. Low platelet mitochondrial complex I and complex II/III activity in early untreated Parkinson’s disease. Ann Neurol. 1995;37:714–22. doi: 10.1002/ana.410370604. [DOI] [PubMed] [Google Scholar]
- Haas RH, Parikh S, Falk MJ, Saneto RP, Wolf NI, Darin N, et al. Mitochondrial disease: a practical approach for primary care physicians. Pediatrics. 2007;120:1326–33. doi: 10.1542/peds.2007-0391. [DOI] [PubMed] [Google Scholar]
- Halestrap AP. Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem Soc Trans. 2006;34:232–7. doi: 10.1042/BST20060232. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Griffiths EJ, Connern CP. Mitochondrial calcium handling and oxidative stress. Biochem Soc Trans. 1993;21:353–8. doi: 10.1042/bst0210353. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Woodfield KY, Connern CP. Oxidative stress, thiol reagents, and membrane potential modulate the mitochondrial permeability transition by affecting nucleotide binding to the adenine nucleotide translocase. J Biol Chem. 1997;272:3346–54. doi: 10.1074/jbc.272.6.3346. [DOI] [PubMed] [Google Scholar]
- Hanagasi HA, Ayribas D, Baysal K, Emre M. Mitochondrial complex I and II/III, and IV activities in familial and sporadic Parkinson’s disease. Int J Neurosci. 2005;115:479–93. doi: 10.1080/00207450590523017. [DOI] [PubMed] [Google Scholar]
- Harrison FE, Hosseini AH, McDonald MP, May JM. Vitamin C reduces spatial learning deficits in middle-aged and very old APP/PSEN1 transgenic and wild-type mice. Pharmacol Biochem Behav. 2009;93:443–50. doi: 10.1016/j.pbb.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Yoshida M, Yamato M, Ide T, Wu Z, Ochi-Shindou M, et al. Reverse of age-dependent memory impairment and mitochondrial DNA damage in microglia by an overexpression of human mitochondrial transcription factor a in mice. J Neurosci. 2008;28:8624–34. doi: 10.1523/JNEUROSCI.1957-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervias I, Beal MF, Manfredi G. Mitochondrial dysfunction and amyotrophic lateral sclerosis. Muscle Nerve. 2006;33:598–608. doi: 10.1002/mus.20489. [DOI] [PubMed] [Google Scholar]
- Higgins CM, Jung C, Xu Z. ALS-associated mutant SOD1G93A causes mitochondrial vacuolation by expansion of the intermembrane space and by involvement of SOD1 aggregation and peroxisomes. BMC Neurosci. 2003;4:16. doi: 10.1186/1471-2202-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano M, Nishigaki Y, Marti R. Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): a disease of two genomes. Neurologist. 2004;10:8–17. doi: 10.1097/01.nrl.0000106919.06469.04. [DOI] [PubMed] [Google Scholar]
- Hong WK, Han EH, Kim DG, Ahn JY, Park JS, Han BG. Amyloid-beta-peptide reduces the expression level of mitochondrial cytochrome oxidase subunits. Neurochem Res. 2007;32:1483–8. doi: 10.1007/s11064-007-9336-7. [DOI] [PubMed] [Google Scholar]
- Hollenbeck PJ, Saxton WMJ. The axonal transport of mitochondria. Cell Sci. 2005;118(December (1):5411–9. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath TL, Diano S, Leranth C, Garcia-Segura LM, Cowley MA, Shanabrough M, et al. Coenzyme Q induces nigral mitochondrial uncoupling and prevents dopamine cell loss in a primate model of Parkinson’s disease. Endocrinology. 2003;144:2757–60. doi: 10.1210/en.2003-0163. [DOI] [PubMed] [Google Scholar]
- Howell N, Elson JL, Chinnery PF, Turnbull DM. mtDNA mutations and common neurodegenerative disorders. Trends Genet. 2005;21:583–6. doi: 10.1016/j.tig.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Hurt EC, Muller U, Schatz G. The first twelve amino acids of a yeast mitochondrial outer membrane protein can direct a nuclear-coded cytochrome oxidase subunit to the mitochondrial inner membrane. EMBO J. 1985;4:3509–18. doi: 10.1002/j.1460-2075.1985.tb04110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchin TP, Heath PR, Pearson RC, Sinclair AJ. Mitochondrial DNA mutations in Alzheimer’s disease. Biochem Biophys Res Commun. 1997;241:221–5. doi: 10.1006/bbrc.1997.7793. [DOI] [PubMed] [Google Scholar]
- Ishige K, Schubert D, Sagara Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic Biol Med. 2001;30:433–46. doi: 10.1016/s0891-5849(00)00498-6. [DOI] [PubMed] [Google Scholar]
- Jacobs HT, Fernandez-Ayala DJ, Manjiry S, Kemppainen E, Toivonen JM, O’Dell KM. Mitochondrial disease in flies. Biochim Biophys Acta. 2004;1659:190–6. doi: 10.1016/j.bbabio.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Jaiswal MK, Zech WD, Goos M, Leutbecher C, Ferri A, Zippelius A, et al. Impairment of mitochondrial calcium handling in a mtSOD1 cell culture model of motoneuron disease. BMC Neurosci. 2009;10:64. doi: 10.1186/1471-2202-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AM, Sharpley MS, Manas AR, Frerman FE, Hirst J, Smith RA, et al. Interaction of the mitochondria-targeted antioxidant MitoQ with phospholipid bilayers and ubiquinone oxidoreductases. J Biol Chem. 2007;282:14708–18. doi: 10.1074/jbc.M611463200. [DOI] [PubMed] [Google Scholar]
- Jauslin ML, Meier T, Smith RA, Murphy MP. Mitochondria-targeted antioxidants protect Friedreich Ataxia fibroblasts from endogenous oxidative stress more effectively than untargeted antioxidants. FASEB J. 2003;17:1972–4. doi: 10.1096/fj.03-0240fje. [DOI] [PubMed] [Google Scholar]
- Kang HC, Kwon JW, Lee YM, Kim HD, Lee HJ, Hahn SH. Nonspecific mitochondrial disease with epilepsy in children: diagnostic approaches and epileptic phenotypes. Childs Nerv Syst. 2007;23:1301–7. doi: 10.1007/s00381-007-0369-7. [DOI] [PubMed] [Google Scholar]
- Kantrow SP, Tatro LG, Piantadosi CA. Oxidative stress and adenine nucleotide control of mitochondrial permeability transition. Free Radic Biol Med. 2000;28:251–60. doi: 10.1016/s0891-5849(99)00238-5. [DOI] [PubMed] [Google Scholar]
- Kasiviswanathan R, Longley MJ, Chan SS, Copeland WC. Disease mutations in the human mitochondrial DNA polymerase thumb subdomain impart severe defects in mitochondrial DNA replication. J Biol Chem. 2009;284:19501–10. doi: 10.1074/jbc.M109.011940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasparova S, Sumbalova Z, Bystricky P, Kucharska J, Liptaj T, Mlynarik V, et al. Effect of coenzyme Q10 and vitamin E on brain energy metabolism in the animal model of Huntington’s disease. Neurochem Int. 2006;48:93–9. doi: 10.1016/j.neuint.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Keep M, Elmer E, Fong KS, Csiszar K. Intrathecal cyclosporin prolongs survival of late-stage ALS mice. Brain Res. 2001;894:327–31. doi: 10.1016/s0006-8993(01)02012-1. [DOI] [PubMed] [Google Scholar]
- Kim SH, Vlkolinsky R, Cairns N, Lubec G. Decreased levels of complex III core protein 1 and complex V beta chain in brains from patients with Alzheimer’s disease and Down syndrome. Cell Mol Life Sci. 2000;57:1810–6. doi: 10.1007/PL00000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkinezos IG, Hernandez D, Bradley WG, Moraes CT. An ALS mouse model with a permeable blood-brain barrier benefits from systemic cyclosporine A treatment. J Neurochem. 2004;88:821–6. doi: 10.1046/j.1471-4159.2003.02181.x. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Bergeron C, Rajput A, Dozic S, Mastrogiacomo F, Chang LJ, et al. Brain cytochrome oxidase in Alzheimer’s disease. J Neurochem. 1992;59:776–9. doi: 10.1111/j.1471-4159.1992.tb09439.x. [DOI] [PubMed] [Google Scholar]
- Kontush A, Mann U, Arlt S, Ujeyl A, Luhrs C, Muller-Thomsen T, et al. Influence of vitamin E and C supplementation on lipoprotein oxidation in patients with Alzheimer’s disease. Free Radic Biol Med. 2001;31:345–54. doi: 10.1016/s0891-5849(01)00595-0. [DOI] [PubMed] [Google Scholar]
- Krieg AM. Immune effects and mechanisms of action of CpG motifs. Vaccine. 2000;19:618–22. doi: 10.1016/s0264-410x(00)00249-8. [DOI] [PubMed] [Google Scholar]
- Kruman II, Pedersen WA, Springer JE, Mattson MP. ALS-linked Cu/Zn-SOD mutation increases vulnerability of motor neurons to excitotoxicity by a mechanism involving increased oxidative stress and perturbed calcium homeostasis. Exp Neurol. 1999;160:28–39. doi: 10.1006/exnr.1999.7190. [DOI] [PubMed] [Google Scholar]
- Kumar P, Kumar A. Neuroprotective effect of cyclosporine and FK506 against 3-nitropropionic acid induced cognitive dysfunction and glutathione redox in rat: possible role of nitric oxide. Neurosci Res. 2009;63:302–14. doi: 10.1016/j.neures.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Kurzer MS, Xu X. Dietary phytoestrogens. Annu Rev Nutr. 1997;17:353–81. doi: 10.1146/annurev.nutr.17.1.353. [DOI] [PubMed] [Google Scholar]
- Lazarou M, Thorburn DR, Ryan MT, McKenzie M. Assembly of mitochondrial complex I and defects in disease. Biochim Biophys Acta. 2009;1793:78–88. doi: 10.1016/j.bbamcr.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Leo S, Szabadkai G, Rizzuto R. The mitochondrial antioxidants MitoE(2) and MitoQ(10) increase mitochondrial Ca(2+) load upon cell stimulation by inhibiting Ca(2+) efflux from the organelle. Ann NY Acad Sci. 2008;1147:264–74. doi: 10.1196/annals.1427.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung AW, Halestrap AP. Recent progress in elucidating the molecular mechanism of the mitochondrial permeability transition pore. Biochim Biophys Acta. 2008;1777:946–52. doi: 10.1016/j.bbabio.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Levites Y, Weinreb O, Maor G, Youdim MB, Mandel S. Green tea polyphenol (−)-epigallocatechin-3-gallate prevents N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic neurodegeneration. J Neurochem. 2001;78:1073–82. doi: 10.1046/j.1471-4159.2001.00490.x. [DOI] [PubMed] [Google Scholar]
- Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119(6):873–87. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Lim D, Fedrizzi L, Tartari M, Zuccato C, Cattaneo E, Brini M, et al. Calcium homeostasis and mitochondrial dysfunction in striatal neurons of Huntington disease. J Biol Chem. 2008;283:5780–9. doi: 10.1074/jbc.M704704200. [DOI] [PubMed] [Google Scholar]
- Lim KM, Kim HH, Bae ON, Noh JY, Kim KY, Kim SH, et al. Inhibition of platelet aggregation by 1-methyl-4-phenyl pyridinium ion (MPP+) through ATP depletion: evidence for the reduced platelet activities in Parkinson’s disease. Platelets. 2009;20:163–70. doi: 10.1080/09537100902721746. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–95. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Littarru GP, Tiano L. Bioenergetic and antioxidant properties of coenzyme Q10: recent developments. Mol Biotechnol. 2007;37:31–7. doi: 10.1007/s12033-007-0052-y. [DOI] [PubMed] [Google Scholar]
- Liu J, Ames BN. Reducing mitochondrial decay with mitochondrial nutrients to delay and treat cognitive dysfunction, Alzheimer’s disease, and Parkinson’s disease. Nutr Neurosci. 2005;8:67–89. doi: 10.1080/10284150500047161. [DOI] [PubMed] [Google Scholar]
- Liu Y, Fiskum G, Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem. 2002;80:780–7. doi: 10.1046/j.0022-3042.2002.00744.x. [DOI] [PubMed] [Google Scholar]
- Logroscino G, Marder K, Cote L, Tang MX, Shea S, Mayeux R. Dietary lipids and antioxidants in Parkinson’s disease: a population-based, case–control study. Ann Neurol. 1996;39:89–94. doi: 10.1002/ana.410390113. [DOI] [PubMed] [Google Scholar]
- Longnecker MP, Kamel F, Umbach DM, Munsat TL, Shefner JM, Lansdell LW, et al. Dietary intake of calcium, magnesium and antioxidants in relation to risk of amyotrophic lateral sclerosis. Neuroepidemiology. 2000;19:210–6. doi: 10.1159/000026258. [DOI] [PubMed] [Google Scholar]
- Luchowska E, Luchowski P, Wielosz M, Turski WA, Urbanska EM. FK506 attenuates 1-methyl-4-phenylpyridinium- and 3-nitropropionic acid-evoked inhibition of kynurenic acid synthesis in rat cortical slices. Acta Neurobiol Exp (Wars) 2003;63:101–8. doi: 10.55782/ane-2003-1459. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Tang MX, Shea S, Mayeux R. Antioxidant vitamin intake and risk of Alzheimer disease. Arch Neurol. 2003;60:203–8. doi: 10.1001/archneur.60.2.203. [DOI] [PubMed] [Google Scholar]
- Luft R, Ikkos D, Palmieri G, Ernster L, Afzelius B. A case of severe hypermetabolism of nonthyroid origin with a defect in the maintenance of mitochondrial respiratory control: a correlated clinical, biochemical, and morphological study. J Clin Invest. 1962;41:1776–804. doi: 10.1172/JCI104637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, et al. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science. 2004;304:448–52. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- Marella M, Seo BB, Nakamaru-Ogiso E, Greenamyre JT, Matsuno-Yagi A, Yagi T. Protection by the NDI1 gene against neurodegeneration in a rotenone rat model of Parkinson’s disease. PLoS One. 2008;3:e1433. doi: 10.1371/journal.pone.0001433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Kleiner I, Gabrilovac J, Bradvica M, Vidovic T, Cerovski B, Fumic K, et al. Leber’s hereditary optic neuroretinopathy (LHON) associated with mitochondrial DNA point mutation G11778A in two Croatian families. Coll Antropol. 2006;30:171–4. [PubMed] [Google Scholar]
- Martin LJ, Gertz B, Pan Y, Price AC, Molkentin JD, Chang Q. The mitochondrial permeability transition pore in motor neurons: involvement in the pathobiology of ALS mice. Exp Neurol. 2009;218:333–46. doi: 10.1016/j.expneurol.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews RT, Yang L, Browne S, Baik M, Beal MF. Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuroprotective effects. Proc Natl Acad Sci USA. 1998;95:8892–7. doi: 10.1073/pnas.95.15.8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiazzi M, D’Aurelio M, Gajewski CD, Martushova K, Kiaei M, Beal MF, et al. Mutated human SOD1 causes dysfunction of oxidative phosphorylation in mitochondria of transgenic mice. J Biol Chem. 2002;277:29626–33. doi: 10.1074/jbc.M203065200. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Mitochondrial regulation of neuronal plasticity. Neurochem Res. 2007;32:707–15. doi: 10.1007/s11064-006-9170-3. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Partin J, Begley JG. Amyloid beta-peptide induces apoptosis-related events in synapses and dendrites. Brain Res. 1998;807:167–76. doi: 10.1016/s0006-8993(98)00763-x. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Rydel RE, Lieberburg I, Smith-Swintosky VL. Altered calcium signaling and neuronal injury: stroke and Alzheimer’s disease as examples. Ann NY Acad Sci. 1993;679:1–21. doi: 10.1111/j.1749-6632.1993.tb18285.x. [DOI] [PubMed] [Google Scholar]
- McFarland R, Taylor RW, Turnbull DM. The neurology of mitochondrial DNA disease. Lancet Neurol. 2002;1:343–51. doi: 10.1016/s1474-4422(02)00159-x. [DOI] [PubMed] [Google Scholar]
- McFarland R, Taylor RW, Turnbull DM. Mitochondrial disease—its impact, etiology, and pathology. Curr Top Dev Biol. 2007;77:113–55. doi: 10.1016/S0070-2153(06)77005-3. [DOI] [PubMed] [Google Scholar]
- McIntosh LJ, Trush MA, Troncoso JC. Increased susceptibility of Alzheimer’s disease temporal cortex to oxygen free radical-mediated processes. Free Radic Biol Med. 1997;23:183–90. doi: 10.1016/s0891-5849(96)00573-4. [DOI] [PubMed] [Google Scholar]
- McKenzie M, Liolitsa D, Hanna MG. Mitochondrial disease: mutations and mechanisms. Neurochem Res. 2004;29:589–600. doi: 10.1023/b:nere.0000014829.42364.dd. [DOI] [PubMed] [Google Scholar]
- Milakovic T, Quintanilla RA, Johnson GV. Mutant huntingtin expression induces mitochondrial calcium handling defects in clonal striatal cells: functional consequences. J Biol Chem. 2006;281:34785–95. doi: 10.1074/jbc.M603845200. [DOI] [PubMed] [Google Scholar]
- Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- Mironov SL, Ivannikov MV, Johansson M. [Ca2+]i signaling between mitochondria and endoplasmic reticulum in neurons is regulated by microtubules. From mitochondrial permeability transition pore to Ca2+-induced Ca2+ release. J Biol Chem. 2005;280:715–21. doi: 10.1074/jbc.M409819200. [DOI] [PubMed] [Google Scholar]
- Mohebbi N, Mihailova M, Wagner CA. The calcineurin inhibitor FK506 (tacrolimus) is associated with transient metabolic acidosis and altered expression of renal acid-base transport proteins. Am J Physiol Renal Physiol. 2009;297:F499–509. doi: 10.1152/ajprenal.90489.2008. [DOI] [PubMed] [Google Scholar]
- Morava E, van den Heuvel L, Hol F, de Vries MC, Hogeveen M, Rodenburg RJ, et al. Mitochondrial disease criteria: diagnostic applications in children. Neurology. 2006;67:1823–6. doi: 10.1212/01.wnl.0000244435.27645.54. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Santos MS, Sena C, Nunes E, Seica R, Oliveira CR. CoQ10 therapy attenuates amyloid beta-peptide toxicity in brain mitochondria isolated from aged diabetic rats. Exp Neurol. 2005;196:112–9. doi: 10.1016/j.expneurol.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Morris RL, Hollenbeck PJ. Axonal transport of mitochondria along microtubules and F-actin in living vertebrate neurons. J Cell Biol. 1995;131:1315–26. doi: 10.1083/jcb.131.5.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller FL, Liu Y, Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem. 2004;279:49064–73. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]
- Murata T, Ohtsuka C, Terayama Y. Increased mitochondrial oxidative damage and oxidative DNA damage contributes to the neurodegenerative process in sporadic amyotrophic lateral sclerosis. Free Radic Res. 2008;42:221–5. doi: 10.1080/10715760701877262. [DOI] [PubMed] [Google Scholar]
- Muratovska A, Lightowlers RN, Taylor RW, Wilce JA, Murphy MP. Targeting large molecules to mitochondria. Adv Drug Deliv Rev. 2001;49:189–98. doi: 10.1016/s0169-409x(01)00134-x. [DOI] [PubMed] [Google Scholar]
- Muravchick S. Clinical implications of mitochondrial disease. Adv Drug Deliv Rev. 2008;60:1553–60. doi: 10.1016/j.addr.2008.03.019. [DOI] [PubMed] [Google Scholar]
- Murphy JR. Protein engineering and design for drug delivery. Curr Opin Struct Biol. 1996;6:541–5. doi: 10.1016/s0959-440x(96)80121-7. [DOI] [PubMed] [Google Scholar]
- Murphy MP. Targeting lipophilic cations to mitochondria. Biochim Biophys Acta. 2008;1777:1028–31. doi: 10.1016/j.bbabio.2008.03.029. [DOI] [PubMed] [Google Scholar]
- Murphy MP, Smith RA. Drug delivery to mitochondria: the key to mitochondrial medicine. Adv Drug Deliv Rev. 2000;41:235–50. doi: 10.1016/s0169-409x(99)00069-1. [DOI] [PubMed] [Google Scholar]
- Mutisya EM, Bowling AC, Beal MF. Cortical cytochrome oxidase activity is reduced in Alzheimer’s disease. J Neurochem. 1994;63:2179–84. doi: 10.1046/j.1471-4159.1994.63062179.x. [DOI] [PubMed] [Google Scholar]
- Naderi J, Somayajulu-Nitu M, Mukerji A, Sharda P, Sikorska M, Borowy-Borowski H, et al. Water-soluble formulation of Coenzyme Q10 inhibits Bax-induced destabilization of mitochondria in mammalian cells. Apoptosis. 2006;11:1359–69. doi: 10.1007/s10495-006-8417-4. [DOI] [PubMed] [Google Scholar]
- Nanjo F, Goto K, Seto R, Suzuki M, Sakai M, Hara Y. Scavenging effects of tea catechins and their derivatives on 1,1-diphenyl-2-picrylhydrazyl radical. Free Radic Biol Med. 1996;21:895–902. doi: 10.1016/0891-5849(96)00237-7. [DOI] [PubMed] [Google Scholar]
- Nelson LM, Matkin C, Longstreth WT, Jr, McGuire V. Population-based case–control study of amyotrophic lateral sclerosis in western Washington State. II. Diet. Am J Epidemiol. 2000;151:164–73. doi: 10.1093/oxfordjournals.aje.a010184. [DOI] [PubMed] [Google Scholar]
- Noorda G, Hermans-Peters M, Smeitink J, van Achterberg T, Kemps H, Goverde W, et al. Mitochondrial disease: needs and problems of children, their parents and family. A systematic review and pilot study into the need for information of parents during the diagnostic phase. J Inherit Metab Dis. 2007;30:333–40. doi: 10.1007/s10545-007-0426-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyango IG, Lu J, Rodova M, Lezi E, Crafter AB, Swerdlow RH. Regulation of neuron mitochondrial biogenesis and relevance to brain health. Biochim Biophys Acta. 2010;1802(January (1)):228–34. doi: 10.1016/j.bbadis.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Panickar KS, Polansky MM, Anderson RA. Green tea polyphenols attenuate glial swelling and mitochondrial dysfunction following oxygen–glucose deprivation in cultures. Nutr Neurosci. 2009;12:105–13. doi: 10.1179/147683009X423300. [DOI] [PubMed] [Google Scholar]
- Panov AV, Lund S, Greenamyre JT. Ca2+-induced permeability transition in human lymphoblastoid cell mitochondria from normal and Huntington’s disease individuals. Mol Cell Biochem. 2005;269:143–52. doi: 10.1007/s11010-005-3454-9. [DOI] [PubMed] [Google Scholar]
- Paraskevas GP, Kapaki E, Libitaki G, Zournas C, Segditsa I, Papageorgiou C. Ascorbate in healthy subjects, amyotrophic lateral sclerosis and Alzheimer’s disease. Acta Neurol Scand. 1997;96:88–90. doi: 10.1111/j.1600-0404.1997.tb00245.x. [DOI] [PubMed] [Google Scholar]
- Park JS, Li YF, Bai Y. Yeast NDI1 improves oxidative phosphorylation capacity and increases protection against oxidative stress and cell death in cells carrying a Leber’s hereditary optic neuropathy mutation. Biochim Biophys Acta. 2007;1772:533–42. doi: 10.1016/j.bbadis.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A, Castilho RF, Hansson O, Wieloch T, Brundin P. Oxidative stress, mitochondrial permeability transition and activation of caspases in calcium ionophore A23187-induced death of cultured striatal neurons. Brain Res. 2000;857:20–9. doi: 10.1016/s0006-8993(99)02320-3. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–88. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- Petri S, Kiaei M, Damiano M, Hiller A, Wille E, Manfredi G, et al. Cell-permeable peptide antioxidants as a novel therapeutic approach in a mouse model of amyotrophic lateral sclerosis. J Neurochem. 2006;98:1141–8. doi: 10.1111/j.1471-4159.2006.04018.x. [DOI] [PubMed] [Google Scholar]
- Pham DQ, Plakogiannis R. Vitamin E supplementation in Alzheimer’s disease, Parkinson’s disease, tardive dyskinesia, and cataract: Part 2. Ann Pharmacother. 2005;39:2065–72. doi: 10.1345/aph.1G271. [DOI] [PubMed] [Google Scholar]
- Polidori MC, Mecocci P, Browne SE, Senin U, Beal MF. Oxidative damage to mitochondrial DNA in Huntington’s disease parietal cortex. Neurosci Lett. 1999;272:53–6. doi: 10.1016/s0304-3940(99)00578-9. [DOI] [PubMed] [Google Scholar]
- Pons R, De Vivo DC. Mitochondrial disease. Curr Treat Options Neurol. 2001;3:271–88. doi: 10.1007/s11940-001-0008-7. [DOI] [PubMed] [Google Scholar]
- Quintanilla RA, Johnson GV. Role of mitochondrial dysfunction in the pathogenesis of Huntington’s disease. Brain Res Bull. 2009;80(October (4-5)):242–7. doi: 10.1016/j.brainresbull.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH. Mitochondrial medicine for aging and neurodegenerative diseases. Neuromol Med. 2008;10:291–315. doi: 10.1007/s12017-008-8044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol Med. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Xu HW, Davey F, Taylor M, Aiton J, Coote P, et al. Endophilin I expression is increased in the brains of Alzheimer disease patients. J Biol Chem. 2008;283:5685–91. doi: 10.1074/jbc.M707932200. [DOI] [PubMed] [Google Scholar]
- Rezai-Zadeh K, Shytle D, Sun N, Mori T, Hou H, Jeanniton D, et al. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J Neurosci. 2005;25:8807–14. doi: 10.1523/JNEUROSCI.1521-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter G, Sonnenschein A, Grunewald T, Reichmann H, Janetzky B. Novel mitochondrial DNA mutations in Parkinson’s disease. J Neural Transm. 2002;109:721–9. doi: 10.1007/s007020200060. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Cuenca S, Cocheme HM, Logan A, Abakumova I, Prime TA, Rose C, et al. Consequences of long-term oral administration of the mitochondria-targeted antioxidant MitoQ to wild-type mice. Free Radic Biol Med. 2010;48:161–72. doi: 10.1016/j.freeradbiomed.2009.10.039. [DOI] [PubMed] [Google Scholar]
- Rosenstock TR, Carvalho AC, Jurkiewicz A, Frussa-Filho R, Smaili SS. Mitochondrial calcium, oxidative stress and apoptosis in a neurodegenerative disease model induced by 3-nitropropionic acid. J Neurochem. 2004;88:1220–8. doi: 10.1046/j.1471-4159.2003.02250.x. [DOI] [PubMed] [Google Scholar]
- Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N Engl J Med. 1997;336:1216–22. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- Schapira AH. Mitochondrial dysfunction in neurodegenerative disorders. Biochim Biophys Acta. 1998;1366:225–33. doi: 10.1016/s0005-2728(98)00115-7. [DOI] [PubMed] [Google Scholar]
- Senin U, Parnetti L, Barbagallo-Sangiorgi G, Bartorelli L, Bocola V, Capurso A, et al. Idebenone in senile dementia of Alzheimer type: a multicentre study. Arch Gerontol Geriatr. 1992;15:249–60. doi: 10.1016/0167-4943(92)90060-h. [DOI] [PubMed] [Google Scholar]
- Setkowicz Z, Caryk M, Szafraniec M, Mudzinska A, Janeczko K. Tacrolimus (FK506) and cyclosporin A reduce macrophage recruitment to the rat brain injured at perinatal and early postnatal periods. Neurol Res. 2009;31(December (10)):1060–7. doi: 10.1179/174313209X383295. [DOI] [PubMed] [Google Scholar]
- Sheehan JP, Swerdlow RH, Miller SW, Davis RE, Parks JK, Parker WD, et al. Calcium homeostasis and reactive oxygen species production in cells transformed by mitochondria from individuals with sporadic Alzheimer’s disease. J Neurosci. 1997a;17:4612–22. doi: 10.1523/JNEUROSCI.17-12-04612.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan JP, Swerdlow RH, Parker WD, Miller SW, Davis RE, Tuttle JB. Altered calcium homeostasis in cells transformed by mitochondria from individuals with Parkinson’s disease. J Neurochem. 1997b;68:1221–33. doi: 10.1046/j.1471-4159.1997.68031221.x. [DOI] [PubMed] [Google Scholar]
- Shoffner JM, Brown MD, Torroni A, Lott MT, Cabell MF, Mirra SS, et al. Mitochondrial DNA variants observed in Alzheimer disease and Parkinson disease patients. Genomics. 1993;17:171–84. doi: 10.1006/geno.1993.1299. [DOI] [PubMed] [Google Scholar]
- Shults CW, Flint Beal M, Song D, Fontaine D. Pilot trial of high dosages of coenzyme Q10 in patients with Parkinson’s disease. Exp Neurol. 2004;188:491–4. doi: 10.1016/j.expneurol.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Shults CW, Oakes D, Kieburtz K, Beal MF, Haas R, Plumb S, et al. Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol. 2002;59:1541–50. doi: 10.1001/archneur.59.10.1541. [DOI] [PubMed] [Google Scholar]
- Siklos L, Kuhnt U. Calcium accumulation by dendritic mitochondria declines along the apical dendrites of pyramidal neurons in area CA1 of guinea pig hippocampal slices. Neurosci Lett. 1994;173:131–4. doi: 10.1016/0304-3940(94)90166-x. [DOI] [PubMed] [Google Scholar]
- Siler-Marsiglio KI, Pan Q, Paiva M, Madorsky I, Khurana NC, Heaton MB. Mitochondrially targeted vitamin E and vitamin E mitigate ethanol-mediated effects on cerebellar granule cell antioxidant defense systems. Brain Res. 2005;1052:202–11. doi: 10.1016/j.brainres.2005.06.030. [DOI] [PubMed] [Google Scholar]
- Sim CH, Lio DS, Mok SS, Masters CL, Hill AF, Culvenor JG, et al. C-terminal truncation and Parkinson’s disease-associated mutations down-regulate the protein serine/threonine kinase activity of PTEN-induced kinase-1. Hum Mol Genet. 2006;15:3251–62. doi: 10.1093/hmg/ddl398. [DOI] [PubMed] [Google Scholar]
- Smith RA, Porteous CM, Coulter CV, Murphy MP. Selective targeting of an antioxidant to mitochondria. Eur J Biochem. 1999;263:709–16. doi: 10.1046/j.1432-1327.1999.00543.x. [DOI] [PubMed] [Google Scholar]
- Stuart JA, Hashiguchi K, Wilson DM, 3rd, Copeland WC, Souza-Pinto NC, Bohr VA. DNA base excision repair activities and pathway function in mitochondrial and cellular lysates from cells lacking mitochondrial DNA. Nucleic Acids Res. 2004;32:2181–92. doi: 10.1093/nar/gkh533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S, Yao Y, Uryu K, Yang H, Lee VM, Trojanowski JQ, et al. Early vitamin E supplementation in young but not aged mice reduces Abeta levels and amyloid deposition in a transgenic model of Alzheimer’s disease. FASEB J. 2004;18:323–5. doi: 10.1096/fj.03-0961fje. [DOI] [PubMed] [Google Scholar]
- Supinski GS, Murphy MP, Callahan LA. MitoQ administration prevents endotoxin-induced cardiac dysfunction. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1095–102. doi: 10.1152/ajpregu.90902.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh YJ, Lee JY, Choi KJ, Ko SR. Effects of selected ginsenosides on phorbol ester-induced expression of cyclooxygenase-2 and activation of NF-kappaB and ERK1/2 in mouse skin. Ann NY Acad Sci. 2002;973:396–401. doi: 10.1111/j.1749-6632.2002.tb04672.x. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH. Mitochondrial DNA-related mitochondrial dysfunction in neurodegenerative diseases. Arch Pathol Lab Med. 2002;126:271–80. doi: 10.5858/2002-126-0271-MDRMDI. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH. The neurodegenerative mitochondriopathies. J Alzheimers Dis. 2009 Jun; doi: 10.3233/JAD-2009-1095. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH, Golbe LI, Parks JK, Cassarino DS, Binder DR, Grawey AE, et al. Mitochondrial dysfunction in cybrid lines expressing mitochondrial genes from patients with progressive supranuclear palsy. J Neurochem. 2000;75:1681–4. doi: 10.1046/j.1471-4159.2000.0751681.x. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Parks JK, Davis JN, 2nd, Cassarino DS, Trimmer PA, Currie LJ, et al. Matrilineal inheritance of complex I dysfunction in a multigenerational Parkinson’s disease family. Ann Neurol. 1998;44:873–81. doi: 10.1002/ana.410440605. [DOI] [PubMed] [Google Scholar]
- Szeto HH. Cell-permeable, mitochondrial-targeted, peptide antioxidants. AAPS J. 2006;8:E277–83. doi: 10.1007/BF02854898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabrizi SJ, Workman J, Hart PE, Mangiarini L, Mahal A, Bates G, et al. Mitochondrial dysfunction and free radical damage in the Huntington R6/2 transgenic mouse. Ann Neurol. 2000;47:80–6. doi: 10.1002/1531-8249(200001)47:1<80::aid-ana13>3.3.co;2-b. [DOI] [PubMed] [Google Scholar]
- Takuma K, Fang F, Zhang W, Yan S, Fukuzaki E, Du H, et al. RAGE-mediated signaling contributes to intraneuronal transport of amyloid-beta and neuronal dysfunction. Proc Natl Acad Sci USA. 2009;106:20021–6. doi: 10.1073/pnas.0905686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuma K, Yao J, Huang J, Xu H, Chen X, Luddy J, et al. ABAD enhances Abeta-induced cell stress via mitochondrial dysfunction. FASEB J. 2005;19:597–8. doi: 10.1096/fj.04-2582fje. [DOI] [PubMed] [Google Scholar]
- Tasset I, Sanchez F, Tunez I. The molecular bases of Huntington’s disease: the role played by oxidative stress. Rev Neurol. 2009;49:424–9. [PubMed] [Google Scholar]
- Trimmer PA, Keeney PM, Borland MK, Simon FA, Almeida J, Swerdlow RH, et al. Mitochondrial abnormalities in cybrid cell models of sporadic Alzheimer’s disease worsen with passage in culture. Neurobiol Dis. 2004;15:29–39. doi: 10.1016/j.nbd.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Tsang AH, Chung KK. Oxidative and nitrosative stress in Parkinson’s disease. Biochim Biophys Acta. 2009;1792:643–50. doi: 10.1016/j.bbadis.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Turner C, Cooper JM, Schapira AH. Clinical correlates of mitochondrial function in Huntington’s disease muscle. Mov Disord. 2007;22:1715–21. doi: 10.1002/mds.21540. [DOI] [PubMed] [Google Scholar]
- Vali S, Mythri RB, Jagatha B, Padiadpu J, Ramanujan KS, Andersen JK, et al. Integrating glutathione metabolism and mitochondrial dysfunction with implications for Parkinson’s disease: a dynamic model. Neuroscience. 2007;149:917–30. doi: 10.1016/j.neuroscience.2007.08.028. [DOI] [PubMed] [Google Scholar]
- Valla J, Schneider L, Niedzielko T, Coon KD, Caselli R, Sabbagh MN, et al. Impaired platelet mitochondrial activity in Alzheimer’s disease and mild cognitive impairment. Mitochondrion. 2006;6:323–30. doi: 10.1016/j.mito.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese M, Pandey M, Samanta A, Gangopadhyay PK, Mohanakumar KP. Reduced NADH coenzyme Q dehydrogenase activity in platelets of Parkinson’s disease, but not Parkinson plus patients, from an Indian population. J Neurol Sci. 2009;279:39–42. doi: 10.1016/j.jns.2008.12.021. [DOI] [PubMed] [Google Scholar]
- Wang H, Lim PJ, Karbowski M, Monteiro MJ. Effects of overexpression of huntingtin proteins on mitochondrial integrity. Hum Mol Genet. 2009a;18:737–52. doi: 10.1093/hmg/ddn404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Chou AH, Yeh TH, Li AH, Chen YL, Kuo YL, et al. PINK1 mutants associated with recessive Parkinson’s disease are defective in inhibiting mitochondrial release of cytochrome c. Neurobiol Dis. 2007;28:216–26. doi: 10.1016/j.nbd.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Wang X, Su B, Fujioka H, Zhu X. Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer’s disease patients. Am J Pathol. 2008a;173:470–82. doi: 10.2353/ajpath.2008.071208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, et al. Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci. 2009b;29:9090–103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, et al. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci USA. 2008b;105:19318–23. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber CA, Ernst ME. Antioxidants, supplements, and Parkinson’s disease. Ann Pharmacother. 2006;40:935–8. doi: 10.1345/aph.1G551. [DOI] [PubMed] [Google Scholar]
- Wiedemann FR, Manfredi G, Mawrin C, Beal MF, Schon EA. Mitochondrial DNA and respiratory chain function in spinal cords of ALS patients. J Neurochem. 2002;80:616–25. doi: 10.1046/j.0022-3042.2001.00731.x. [DOI] [PubMed] [Google Scholar]
- Yang JL, Weissman L, Bohr VA, Mattson MP. Mitochondrial DNA damage and repair in neurodegenerative disorders. DNA Repair (Amst) 2008a;7:1110–20. doi: 10.1016/j.dnarep.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Yang Y, Li G, Wang J, Yang ES. Coenzyme Q10 attenuates beta-amyloid pathology in the aged transgenic mice with Alzheimer presenilin 1 mutation. J Mol Neurosci. 2008b;34:165–71. doi: 10.1007/s12031-007-9033-7. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ouyang Y, Yang L, Beal MF, McQuibban A, Vogel H, et al. Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc Natl Acad Sci USA. 2008c;105:7070–5. doi: 10.1073/pnas.0711845105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Taylor M, Davey F, Ren Y, Aiton J, Coote P, et al. Interaction of amyloid binding alcohol dehydrogenase/Abeta mediates up-regulation of peroxiredoxin II in the brains of Alzheimer’s disease patients and a transgenic Alzheimer’s disease mouse model. Mol Cell Neurosci. 2007;35:377–82. doi: 10.1016/j.mcn.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Yapa SC. Detection of subclinical ascorbate deficiency in early Parkinson’s disease. Public Health. 1992;106:393–5. doi: 10.1016/s0033-3506(05)80188-x. [DOI] [PubMed] [Google Scholar]
- Yoshino H, Nakagawa-Hattori Y, Kondo T, Mizuno Y. Mitochondrial complex I and II activities of lymphocytes and platelets in Parkinson’s disease. J Neural Transm Park Dis Dement Sect. 1992;4:27–34. doi: 10.1007/BF02257619. [DOI] [PubMed] [Google Scholar]
- Young AJ, Johnson S, Steffens DC, Doraiswamy PM. Coenzyme Q10: a review of its promise as a neuroprotectant. CNS Spectr. 2007;12:62–8. doi: 10.1017/s1092852900020538. [DOI] [PubMed] [Google Scholar]
- Yu JT, Chang RC, Tan L. Calcium dysregulation in Alzheimer’s disease: from mechanisms to therapeutic opportunities. Prog Neurobiol. 2009;89(November (3)):240–55. doi: 10.1016/j.pneurobio.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Zaidi SM, Banu N. Antioxidant potential of vitamins A, E and C in modulating oxidative stress in rat brain. Clin Chim Acta. 2004;340:229–33. doi: 10.1016/j.cccn.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Zandi PP, Anthony JC, Khachaturian AS, Stone SV, Gustafson D, Tschanz JT, et al. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: the Cache County Study. Arch Neurol. 2004;61:82–8. doi: 10.1001/archneur.61.1.82. [DOI] [PubMed] [Google Scholar]
- Zhang SM, Hernan MA, Chen H, Spiegelman D, Willett WC, Ascherio A. Intakes of vitamins E and C, carotenoids, vitamin supplements, and PD risk. Neurology. 2002;59:1161–9. doi: 10.1212/01.wnl.0000028688.75881.12. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Zhao H, Steinberg GK, Yenari MA. Cellular and molecular events underlying ischemia-induced neuronal apoptosis. Drug News Perspect. 2003;16:497–503. doi: 10.1358/dnp.2003.16.8.829348. [DOI] [PubMed] [Google Scholar]
- Zubenko GS, Sauer P. SOD-1 activity and platelet membrane fluidity in Alzheimer’s disease. Biol Psychiatry. 1989;25:671–8. doi: 10.1016/0006-3223(89)90236-9. [DOI] [PubMed] [Google Scholar]
- Zullo SJ, Parks WT, Chloupkova M, Wei B, Weiner H, Fenton WA, et al. Stable transformation of CHO cells and human NARP cybrids confers oligomycin resistance (oli(r)) following transfer of a mitochondrial DNA-encoded oli(r) ATPase6 gene to the nuclear genome: a model system for mtDNA gene therapy. Rejuvenation Res. 2005;8:18–28. doi: 10.1089/rej.2005.8.18. [DOI] [PubMed] [Google Scholar]