Abstract
Objective
To measure interferon (IFN) inducible chemokines in plasma of patients with systemic sclerosis (SSc) and investigate their correlation with disease severity.
Methods
We examined the correlation of IFN-inducible chemokines, IFNγ-inducible protein-10 (IP-10/CXCL10), IFN-inducible T cell alpha chemoattractant (I-TAC/CXCL11), and monocyte chemoattractant protein-1 (MCP-1/CCL2) with the IFN gene expression signature. We generated an IFN-inducible chemokine score with the correlated chemokines, IP-10 and I-TAC and compared it in 266 SSc patients enrolled in the GENISOS cohort to that of 97 matched controls. Subsequently, the correlation between the baseline IFN-inducible chemokine score and markers of disease severity was assessed. Finally, the course of IFN-inducible chemokine score over time was examined.
Results
The plasma IFN-inducible chemokine score correlated with the IFN gene expression signature and this score was higher in SSc patients. It also was associated with the absence of anti–RNA polymerase III antibodies, presence of anti–U1 ribonucleoprotein antibodies (RNP), but not with disease duration, type, or other autoantibodies. The chemokine scores correlated with concomitantly obtained muscle, skin and lung components of the Medsger Severity Index, as well as, FVC, DLco, creatine kinase. Its association with disease severity was independent of anti-RNP or other potential confounders (age, gender, ethnicity, disease duration, and treatment with immunosuppressive agents). Finally, there was not a significant change in the IFN-inducible chemokine score over time.
Conclusions
The IFN-inducible chemokine score is a stable serological marker of more severe subtype of SSc and may be useful for risk stratification regardless of disease type or duration.
INTRODUCTION
Immune dysregulation has been proposed as an important contributor to the pathogenesis of systemic sclerosis (SSc or Scleroderma). Several groups reported that peripheral blood cells (PBCs) from patients with SSc demonstrate dysregulation of interferon (IFN)–inducible gene (1–5). In a previous large global gene expression study, we have demonstrated that the IFN signature in patients with SSc is similar to that seen in patients with systemic lupus erythmatosus (SLE) (6) studies reported that in SLE, a serum chemokine composite score correlated tightly with the IFN gene expression signature (7;8). In a small study of 30 patients with SLE, this serum chemokine composite score also showed a higher correlation with disease activity measures than the IFN gene expression score (7). Similar observations also have been made in patients with dermatomyositis (9). Of note, although these chemokines can be induced by IFN, it is likely that the IFN inducible chemokines are not exclusively regulated by type I or type II IFN. The course of SSc is highly variable ranging from stable, mild involvement to progressive disease leading to wide-spread fibrosis of skin and internal organs. Currently available demographic and clinical parameters are not sufficient to classify SSc patients with varying degrees of disease severity.
In this study, we hypothesized that plasma IFN-inducible chemokines correlate with the IFN gene signature in SSc and are associated with disease severity. We first examined the correlation of IFNγ-inducible protein-10 (IP-10/CXCL10), IFN-inducible T cell alpha chemoattractant (I-TAC/CXCL11), and monocyte chemoattractant protein-1 (MCP-1/CCL2) with the IFN gene signature because these chemokines have been shown to correlate with the IFN gene signature and disease severity in SLE (7;8) and dermatomyositis (9). We then investigated the association of our IFN inducible chemokine score with clinical features of SSc at the cross-sectional level. Finally, the longitudinal changes in IFN-inducible chemokine levels and their correlation with clinical disease parameters were examined.
PATIENTS AND METHODS
Study participants
All patients with SSc met the 1980 American College of Rheumatology (ACR; formerly, the American Rheumatism Association) preliminary criteria for the classification of SSc (10) or had three out of five CREST (Calcinosis, Raynaud’s phenomenon, Esophageal dysmotility, Sclerodactyly, and Telangiectasia with presence of sclerodactyly being mandatory).
The selection criteria for study subjects used for calculation of IFN gene expression signature were described in our previous publication (6). The study subjects used for plasma chemokine level measurement and chemokine/clinical data correlation were recruited from the prospective outcome Genetics versus Environment in Scleroderma Outcome Study (GENISOS) (n=266). The unaffected control subjects had no history of autoimmune diseases and were matched by age, sex, and ethnicity to patients with SSc (n=97). In addition to baseline samples, a follow-up plasma sample was available in 63 patients. All study subjects provided written informed consent, and the study was approved by the institutional review boards of all participating centers.
Plasma chemokine measurements and IFN-indncible chemokine score calculation
We measured chemokine levels in plasma of patients enrolled in the GENISOS and the unaffected controls. Plasma was collected using ethylenediamine tetraacetic acid blood collection tube and stored at −80°C until analysis. Plasma samples had not undergone more than two thaw-freeze cycles before chemokine level determination. Chemokine levels were determined by ELISA using electrochemiluminescent multiplex assays (Meso Scale Discovery, Gaithersburg, MD, USA) (11). Each sample was run in duplicates. The plasma levels of IP-10 and I-TAC were determined in all 266 patients and follow-up samples, while levels of MCP-1 were only measured in a subgroup of patients with concomitant microarray/qPCR data. The relative levels of IP-10 and I-TAC were used for calculation of IFN-inducible chemokine score following a normalization method by Bauer et al. (7). Specifically, concentration values were normalized according to the 95th percentile level across all samples and the normalized values were summed up to obtain the IFN-inducible chemokine score.
IFN gene expression signature calculation
We calculated the Microarray IFN score based on the levels of the 43 IFN-inducible transcripts that were differentially expressed in patients with SSc compared to unaffected controls in our previous global gene expression study (6). The cumulative Microarray IFN score was calculated as previously described (6;12).
We also calculated the IFN quantitative PCR (qPCR) score by calculating a composite score of relative transcript levels of three IFN inducible genes (STAT1, IFI6, and IFIT3) measured by qPCR analysis as previously described (6).
Of note, some of the genes included in the IFN-inducible transcript and qPCR scores such as STAT1 can be induced by type I as well as type II IFNs.
Clinical Outcome Measures
The disease duration was calculated using two different starting points: 1-The onset of disease from the first non-Raynaud’s phenomenon; 2- The onset of disease from the first symptom attributable to SSc (Raynaud’s or non-Raynaud’s phenomenon). Autoantibodies, including anti− RNA polymerase III antibodies (ARA), anti−Ul ribonucleoprotein antibodies (RNP), anticentromere antibodies (ACA), antitopoisomerase antibodies (ATA), were detected in all SSc serum samples at the laboratories of the UTHSC-H Division of Rheumatology. Briefly, antinuclear antibodies and ACAs were detected by indirect immunofluorescence using HEp-2 cell substrates (Antibodies, Davis, CA). ATA, anti-Ro, and RNP antibodies were determined by passive immunodiffusion against calf thymus extract (Inova Diagnostics, San Diego, CA). ARA testing was performed by enzyme-linked immunosorbent assay (MBL, Nagoya, Japan). For the association analysis with SSc-related antibodies, we excluded patients with more than one SSc related-antibody (n=7) and patients with anti-Ro positivity (n=10). The anti-Ro positive patients were excluded because these antibodies are not SSc specific and have been linked to IFN inducible gene expression signature (6).
Medsger Severity Index (MSI), including skin, muscle, gastrointestinal tract, lung, heart and kidney components was captured prospectively and was utilized for the assessment of clinical severity (13;14). Furthermore, the concomitantly collected percent predicted forced vital capacity (FVC), diffusing capacity (DLCO), modified Rodnan Skin Score (mRSS) (15), and creatine kinase (CK) were used as additional surrogates for severity of interstitial lung disease (ILD) (16), skin, and muscle involvement, respectively. Medication information was also collected prospectively. Patients on immunosuppressive agents at the time of blood draw (exception: prednisone equivalent dose ≤ 5 mg per day or hydroxychloroquine) were categorized as treated with immunosuppressive agents. Concomitantly obtained complete blood count was also available in all baseline samples.
Statistical analysis
We first examined the correlation of the IFN-inducible chemokine score with the IFN microarray and qPCR scores by Spearman’s rank order test. The non-parametric analytic approach was chosen to the small sample size (n=24). Subsequently, we compared the log transformed chemokine levels between 266 SSc patients enrolled in the GENISOS cohort and 97 unaffected controls using Student’s t test. We did not observe significant deviations from normal distribution for log transformed plasma chemokine levels based on D’Agostino tests for normality. The associations of log transformed plasma chemokine levels with the clinical manifestations of SSc were investigated by the univariable linear regression and Pearson’s correlation. Multivariable linear regression models were also constructed to adjust for potential confounders. First, the association of disease subtypes (independent variable) with chemokine levels (outcome variable) was examined after adjustment for potential confounding demographic variables and treatment with immunosuppressive agents. Subsequently, the correlation of chemokine levels (independent variable) with disease severity (outcome variable) was examined after adjustment for potential demographic confounders, disease duration, and treatment with immunosuppressive agents. The first-order interaction term between the treatment status with immunosuppressive agents and the investigated independent variables did not yield significant results. Therefore, the patients were not subgrouped according to treatment status and this variable was included as potential confounder in the multivariable models.
We also performed Bonferroni’s correction for multiple comparison based on three independent comparisons (MCP-1, ITAC, IP-10) in the gene expression/chemokine analysis and two independent comparisons (ITAC and IP-10) for the SSc subtype and severity comparisons. Next, the association of the baseline IFN inducible chemokine levels with percent change in mRSS and FVC between the baseline and follow-up visit at year 1 ([level baseline− Level year 1]/Level baseline) was examined by linear regression to investigate the predictive significance of the chemokine composite score for short-term change in skin involvement and ILD.
The longitudinal comparison of log-transformed IFN-inducible chemokine levels was performed by paired t-test. Furthermore, the percent change in chemokines ([levelbaseline− Levelfollow-up]/Levelbaseline) was correlated with percent change in FVC and mRSS by linear regression and Pearson’s correlation. Two-sided p values less than 0.05 were considered significant. The analyses were performed using the STATA/SE 11.2 statistical program (StataCorp, College Station, TX).
RESULTS
Correlation of plasma IFN-inducible chemokine levels with IFN transcript signature
We examined 24 patients with SSc by global gene expression profiling, qPCR, and multiplex chemokine assays using concomitantly obtained whole blood RNA and plasma samples. The demographic and clinical characteristics of these patients are shown in Table S1. None of these 24 patients were treated with immunosuppressive agents at the time of blood draw. These 24 patients were a subgroup of our large whole blood gene expression study (6) from whom a concomitantly collected plasma sample was available. The correlations of the investigated chemokines with the microarray and qPCR IFN scores are shown in Table 1. Plasma IP-10 and I-TAC correlated significantly with both the microarray IFN score (p=0.007, Rho = 0.536, and p=0.0l, Ho = 0.518, respectively) and the qPCR IFN score (p=0.003, Rho = 0.581, p=0.009, Rho = 0.521, respectively). However, no significant correlation was detected between MCP-1 and the microarray IFN score (p = 0.27, Rho = 0.234) or the IFN qPCR score (p = 0.126, Rho = 0.321). The composite chemokine score of IP-10 and I-TAC correlated stronger with the microarray IFN score (Rho = 0.612) and the IFN qPCR score (Rho = 0.620) than the individual IP-10 and I-TAC chemokine levels. Addition of MCP-1 to the above composite chemokine score weakened the correlation. Therefore, only IP-10 and I-TAC were utilized for the IFN-inducible chemokine score. Exclusion of the 4 patients with late SSc (disease duration longer than 5 years from the first non-Raynaud’s symptom − there samples were obtained on follow-up visits in the GENISOS cohort) did not change the above correlations (Supplement Table S2).
Table 1.
Correlation of plasma IFN inducible chemokines with the IFN inducible transcript signature
| Chemokines | Correlation with IFN array score |
Correlation with IFN qPCR score |
||||
|---|---|---|---|---|---|---|
| p | pc* | Rho | p | pc* | Rho | |
| IP-10 | 0.007 | 0.021 | 0.536 | 0.003 | 0.009 | 0.581 |
| I-TAC | 0.010 | 0.030 | 0.518 | 0.009 | 0.027 | 0.521 |
| MCP-1 | 0.27 | 0.81 | 0.234 | 0.126 | 0.378 | 0.321 |
| IP-10 & I-TAC | 0.0015 | 0.0045 | 0.612 | 0.001 | 0.003 | 0.620 |
| IP-10 &I-TAC&MCP-1 | 0.0198 | 0.0594 | 0.472 | 0.010 | 0.030 | 0.515 |
pc: p value corrected for multiple comparison
Comparison of IFN inducible chemokine levels in SSc patients and unaffected controls
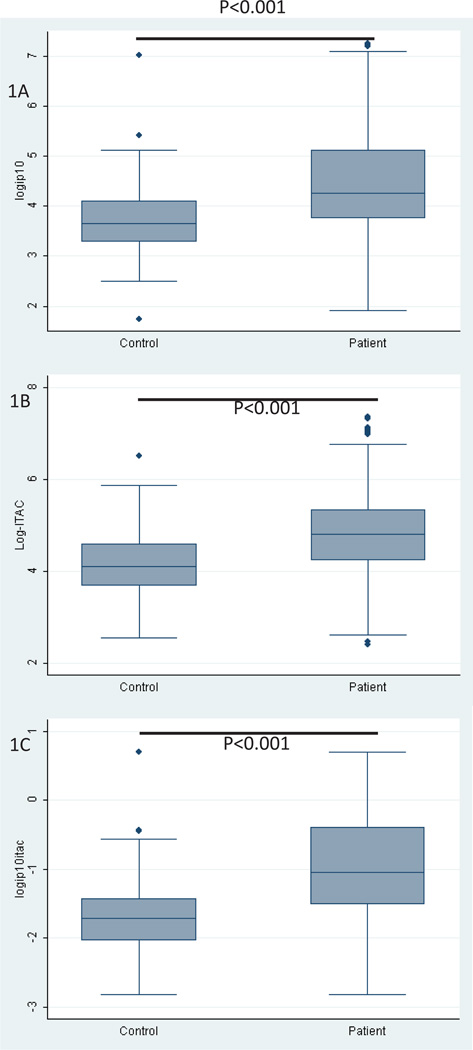
The IFN-inducible chemokine scores were determined in all baseline plasma samples of patients enrolled in the GENISOS cohort (n=266) and 97 matched controls. The demographic and clinical characteristics of the patients and control subjects at the time of blood draw are shown in Table 2. Patients with SSc had higher circulating levels of IP-10 (p < 0.0001), I-TAC (p < 0.0001) than their age, gender and ethnicity-matched unaffected controls (n=97) (Figure 1). The IFN-inducible chemokine score was also higher in patients with SSc (p < 0.0001). In this analysis, 39.2% of patients had a positive IFN-inducible chemokine score when this composite score was dichotomized based on its 95th percentile level in unaffected controls.
Table 2.
Study subject characteristics
| Characteristic | GENISOS Cohort | Control Subjects |
|---|---|---|
| Gender, female | 221 (83%) | 78 (80%) |
| Age at the time of first study visit, mean (SD) | 48.6(13.5) | 48 (12.7) |
| Ethnicity | ||
| Caucasian | 125 (47%) | 48 (49%) |
| African American | 54 (20%) | 17(18%) |
| Latinos | 77 (29%) | 27 (29%) |
| Diffuse cutaneous involvement | 156(59%) | |
| *Disease duration 1, mean (SD) | 2.5(1.6) | |
| **Disease duration 2, mean (SD) | 4.5 (5.4) | |
| ACA | 32 (12%) | |
| ATA | 49(18%) | |
| ARA | 61 (23%) | |
| RNP | 30(11%) | |
| Treatment with immunosuppressive agents | 82 (32%) |
Abbreviations: SD: Standard deviation; ACA: Anti-centromere antibodies; ATA: Anti-topoisomerase antibodies; ARA: Anti-RNA polymerase III antibodies (ARA); RNP: Anti-Ul ribonucleoprotein antibodies.
Disease duration 1 was calculated from the onset of the first non-Raynaud’s phenomenon.
Disease duration 2 was calculated from the onset of the first symptom attributable to SSc including Raynaud’s phenomenon.
Figure 1.
Comparison of IFN inducible chemokine levels in SSc patients and unaffected controls. (A) IP-10 (B) I-TAC (C) IFN-inducible chemokine score in patients with SSc and unaffected controls. Each box represents the 25th to 75th percentiles. The line inside the box represents the median.
Association of disease subtypes with plasma IFN inducible chemokines
Table 3 shows the univariable and multivariable associations of disease subtypes with IFN-inducible chemokines in 266 SSc patients enrolled in the GENISOS cohort. After adjustment for potential confounders (age at enrollment, gender, ethnicity, and treatment with immunosuppressive agents) in the multivariable model, we found that neither disease type (limited/diffuse) nor duration correlated with IP-10, I-TAC or the IFN-inducible chemokine score. RNP antibodies correlated with higher levels of IP-10, I-TAC and the IFN-inducible chemokine score (p < 0.001, p = 0.018, p < 0.001, respectively). ARA antibodies correlated with lower plasma levels of I-TAC and the IFN-inducible chemokine score (p = 0.004 and p = 0.003, respectively). The other SSc-related antibodies did not correlate with the investigated chemokines. ARA antibodies were negatively associated with IFN-inducible chemokine score even after patients with RNP antibodies were excluded from the analysis (p=0.05). The inclusion of patients with two SSc-related autoantibodies (n=7) or anti-Ro antibodies (n=10) did not change the above observed significant associations with RNP and ARA (data not shown).
Table 3.
Association of disease subtypes with plasma IFN inducible chemokines
| IP-10 | ITAC | IFN-inducibleChemokine Score | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pu | pm | Mean diff (95 CI) | pu | pm | Mean diff (95 CI) | pu | pm | pmc¶ | Mean diff (95 CI) | |
| DD1 | 0.126 | 0.204 | −0.05 (−0.14, 0.03) | 0.080 | 0.150 | −0.06 (−0.14, 0.02) | 0.045 | 0.088 | 0.176 | −0.06 (−0.13, 0.01) |
| DD2 | 0.470 | 0.482 | −0.01 (−0.03, 0.02) | 0.521 | 0.587 | 0.01 (−0.02, 0.03) | 0.590 | 0.655 | 1.000 | 0.005 (−0.02, 0.03) |
| DT | 0.060 | 0.090 | −0.23 (−0.50, 0.04) | 0.826 | 0.857 | 0.02 (−0.24, 0.29) | 0.339 | 0.575 | 1.000 | −0.06 (−0.29, 0.16) |
| ACA | 0219 | 0.318 | −0.21 (−0.62, 0.20) | 0.730 | 0.583 | 0.11 (−0.29, 0.51) | 0.989 | 0.764 | 1.000 | 0.05 (−0.29, 0.39) |
| ATA | 0.548 | 0.258 | −0.21 (−0.57, 0.15) | 0.604 | 0.796 | 0.05 (−0.31, 0.40) | 0.827 | 0.6821 | 1.000 | −0.08 (−0.38, 0.22) |
| ARA | 0.219 | 0.096 | −0.27 (−0.58, 0.05) | 0.010 | 0.004 | −0.44 (−0.73, –0.15) | 0.011 | 0.003 | 0.006 | −0.39 (−0.64, –0.13) |
| RNP | <0.001 | <0.001 | 0.83 (0.38, 1.27) | 0.041 | 0.018 | 0.52(0.09, 0.96) | 0.001 | <0.001 | <0.001 | 0.66 (0.30, 1.03) |
| Fibrillin | 0.776 | 0.635 | 0.11 (−0.38, 0.57) | 0.799 | 0.738 | 0.08 (−0.37, 0.52) | 0.775 | 0.875 | 1.000 | 0.03 (−0.35, 0.41) |
Abbreviations: DD1: Disease duration calculated from the onset of the first non-Raynaud’s phenomenon; DD2: Disease duration calculated from the onset of the first SSc symptom including the Raynaud’s phenomenon; DT: Disease type; ACA: Anti-centromere antibodies; ATA Anti-topoisomerase antibodies; ARA: Anti–RNA polymerase III antibodies (ARA); RNP: Anti-Ul ribonucleoprotein antibodies; pu : p value from univariable model; pm : p value from multivariable model after adjustment for age at enrollment, gender, ethnicity, and treatment with immunosuppressive agents; pm¶ : p value from multivariable model with multiple comparison correction.
Correlation of IFN inducible chemokines with disease severity
Table 4 shows the univariable and multivariable associations of IFN-inducible chemokines with disease severity in 266 SSc patients. IP-10 correlated with higher mRSS (p=0.014), while I-TAC levels were associated with lower FVC (p = 0.003), DLco (p = 0.002), and higher creatine kinase (p<0.001).
Table 4.
Correlation of plasma IFN inducible chemokines with disease severity
| IP-10 | I-TAC | IP-10 & I-TAC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cor | pu | pm† | pmc¶ | Cor | pu | pm† | pmc¶ | Cor | pu | pm† | pmc‡ | |
| FVC | −0.08 | 0.272 | 0.427 | 0.854 | −0.21 | 0.003 | 0.005 | 0.010 | −0.17 | 0.013 | 0.024 | 0.048 |
| DLCO | −0.10 | 0.160 | 0.038 | 0.076 | −0.21 | 0.002 | <0.001 | <.001 | −0.18 | 0.008 | 0.001 | 0.002 |
| CK | 0.12 | 0.077 | 0.189 | 0.378 | 0.23 | <0.001 | 0.001 | 0.002 | 0.21 | 0.002 | 0.004 | 0.008 |
| mRSS | 0.16 | 0.014 | 0.027 | 0.054 | 0.04 | 0.502 | 0.582 | 1 | 0.1 | 0.125 | 0.161 | 0.322 |
| Skin* | 0.16 | 0.014 | 0.013 | 0.026 | 0.04 | 0.542 | 0.412 | 0.824 | 0.12 | 0.073 | 0.043 | 0.086 |
| Muscle* | 0.19 | 0.003 | 0.004 | 0.008 | 0.15 | 0.02 | 0.032 | 0.064 | 0.18 | 0.006 | 0.011 | 0.022 |
| GI* | 0.02 | 0.779 | 0.656 | 1 | 0.07 | 0.29 | 0.191 | 0.382 | 0.06 | 0.38 | 0.270 | 0.540 |
| Lung* | 0.08 | 0.207 | 0.106 | 0.212 | 0.15 | 0.018 | 0.008 | 0.016 | 0.15 | 0.021 | 0.009 | 0.018 |
| Heart* | 0.04 | 0.552 | 0.381 | 0.762 | 0.02 | 0.811 | 0.600 | 1 | 0.04 | 0.565 | 0.377 | 0.754 |
| Kidney* | −0.07 | 0.257 | 0.144 | 0.288 | 0.03 | 0.604 | 0.768 | 1 | 0.01 | 0.878 | 0.876 | 1.000 |
| Joint* | 0.13 | 0.051 | 0.030 | 0.060 | 0.06 | 0.340 | 0.327 | 0.654 | 0.10 | 0.106 | 0.080 | 0.160 |
Components of Medsger Severity Index.
Multivariate model after adjustment for age at enrollment, gender, ethnicity, disease duration, and treatment with immunosuppressive agents
Abbreviations: FVC: Percent predicted forced vital capacity; DLco: Percent predicted diffusing capacity; mRSS: modified Rodnan Skin Score; CK: Creatine kinase. Cor: Correlation coefficient; pu: p value from univariable model; pm† : p value from multivariable model after adjustment for age at enrollment, gender, ethnicity, disease duration, and treatment with immunosuppressive agents; pm‡: p value from multivariable model with multiple comparison correction.
The IFN-inducible chemokine score correlated with lower FVC (p = 0.013) and DLco (p = 0.002) and higher creatine kinase (p=0.004).
As shown in Table 4, the association with components of Medsger Severity Index paralleled the above findings. Specifically, the IFN inducible chemokine score was associated with muscle (p=0.006) and lung (p=0.021) components of Severity Index. Furthermore, the composite score showed a trend for association with the skin component of Severity Index (p=0.073).
Adjustment for age at enrollment, gender, ethnicity, disease duration, and treatment with immunosuppressive agents, as well as correction for multiple comparison did not change the above observed associations (Table 4).
After exclusion of patients with RNP antibodies, all above correlations remained significant (data not shown) and the correlation between the IFN-inducible chemokine score and higher mRSS also became significant (r=0.16, p=0.018). Furthermore, a subgroup analysis based on the disease type (limited or diffuse) did not show a more significant association in any of the subgroups than the overall cohort for the association with the mRSS.
We further investigated whether the IFN inducible chemokine score is associated with interstitial lung disease (ILD). The association of the composite score with a combined outcome of increased reticular markings on chest X-ray, rales on physical exam, or FVC<70% was examined which has been shown to predict presence of ILD related changes on high resolution chest CT (17). Higher IFN composite score was associated with this combined outcome (p=0.048). Furthermore, higher IFN composite score was associated with severe restrictive lung disease defined as FVC<50% (p=0.045).
The baseline IFN inducible chemokine levels were associated with lower lymphocyte count (r=− 0.19, p=0.004) but not with total white cell count (p=0.59), hematocrit (p=0.586), or platelet count (p=0.313). The negative association with lymphocyte count was independent of age at enrollment, gender, ethnicity, disease duration, and treatment with immunosuppressive agents in the multivariable model (p=0.008).
Neither baseline IFN inducible chemokine levels nor IP10, ITAC individually correlated significantly with the short-term change in FVC or mRSS.
Progression of IFN-inducible chemokine score over time and its clinical correlations
Follow-up plasma sample was available in 63 patients with a mean (±SD) time-in-study of 3.1 (1.2) years. Neither IP-10 nor I-TAC changed significantly over time (p=0.977 and p=0.512, respectively). The change in IP-10 and I-TAC did not correlate significantly with change in mRSS, creatine kinase or FVC (data not shown). Similarly, the IFN-inducible chemokine score did not change significantly over time (p=0.621). As expected, the baseline IFN-inducible chemokine score correlated significantly with its levels on the follow-up visits (r=0.39, p=0.002).
We found no correlation between change in the IFN-inducible chemokine score and FVC or creatine kinase over time (r= −0.08, p=0.598; r=−0.06, 0.719, respectively), although there was a trend for correlation between change in the IFN-inducible chemokine score and change in mRSS (r=0.23, p=0.084).
We next excluded all patients who were on immunosuppressive agents either at the baseline and/or follow-up visit in order to remove the confounding effect of treatment. In the remaining 35 patients, the IFN-inducible chemokine score did not change significantly over time (p=0.662) and its follow-up levels correlated significantly with its baseline values (r=0.48, p=0.003). Moreover, change in the IFN-inducible chemokine score did not correlate with change in FVC (r=−0.16 p=0.348), creatine kinase (r=−0.31, p=0.119) or mRSS (r=0.16, p=0.406).
DISCUSSION
We developed composite plasma IFN-inducible chemokine score that correlated with the IFN gene expression signature and the disease severity in SSc. To our knowledge, this is the first report of plasma IFN-inducible chemokines correlating with the IFN gene expression signature in SSc. Furthermore, availability of plasma samples in the GENISOS cohort enabled us to examine the correlation of the IFN-inducible chemokine score with subtypes and severity of SSc in a large and well-characterized patient population with early disease. We were able to demonstrate for the first time, an association between the IFN-inducible chemokine score and the severity of lung and muscle involvement, and confirmed previous observations that linked IFN activity to severity of skin involvement in SSc (3; 18).
We observed a negative association with ARA antibodies independent of other potential confounding factors. In our previous gene expression study (6), we did not observe an association between ARA and IFN signature because we were underpowered as only 15 patients with these antibodies were investigated in that study. The other gene expression studies have not investigated an association of the IFN signature with ARA (1;4;5;19). A negative association of ARA with IFN chemokine score indicates the dysregulation of IFN pathways are less likely to play a pathogenic role in this autoantibody subgroup of SSc. Of interest, ARA is highly associated with extensive skin involvement but severe ILD is infrequent in this subgroup. In agreement with published data(20), 82% of patients with ARA had diffuse cutaneous involvement, but only 2% of them had severe restrictive lung disease in the GENISOS cohort. Further studies are needed to examine whether lack of IFN activation contributes to the observed dissociation between fibrosis in skin and pulmonary tissue in this subgroup of SSc. ARA antibodies are also associated with scleroderma renal crisis. Of note, we did not observe an association of IFN-inducible chemokine scores with severity of renal involvement in our cohort. In addition, we confirmed previous observations that RNP antibodies are associated with increased IFN activity in patients with SSc (6;19). Confirming findings of previous studies, we did not find an association of IFN-inducible chemokine score with disease type (limited versus diffuse) (l;4–6) or duration (1;4;6).
The easier accessibility of plasma samples compared to peripheral blood RNA samples enabled us to correlate the IFN-inducible chemokines with clinical features in a large, well characterized cohort patients with early SSc. The IFN-inducible chemokine score correlated with concomitant severity of skin, lung, and muscle involvement. This correlation was independent of potential demographic confounders, treatment with immunosuppressive agents, and disease duration Notably, the observed association with markers of clinical severity remained significant even after exclusion of patients with RNP antibodies. This indicates that the association of IFN-inducible chemokines with more severe forms of SSc is not mainly driven by patients have features of mixed connective tissue disease. Our findings also confirmed previous studies indicating that transcripts of IFN inducible genes correlate with severity of skin disease in patients with SSc (3;18). However, this is the first study to show an association between IFN-inducible chemokine score and the severity of lung and muscle disease. This association with severity of lung involvement is especially important as this disease manifestation is the primary cause of SSc-related mortality (21;22). However, the baseline chemokine composite score did not predict short term change in FVC in the present study although future longitudinal studies with combined analysis of longitudinal measurements (serially obtained FVCs) and survival data are needed to investigate the predictive significance of the composite score for long-term progression of SSc-ILD. Our findings provide further support for deleterious effects of IFN in SSc. The development of SSc has been reported in patients undergoing IFNα treatment (23;24). Furthermore, a randomized, placebo-controlled trial of subcutaneous IFNα in patients with early SSc showed that treatment with IFNα resulted in worsening lung function and a trend toward skin deterioration (25). The potential role of IFN in the pathogenesis of SSc has led to an ongoing phase I study of an anti-IFNα monoclonal antibody,sifalimumab, for treatment of this disease. The IFN-inducible chemokine score developed in this study might be helpful in identifying SSc patients who would benefit from this treatment modality.
Our IFN-inducible chemokine score was comprised of IP-10 and I-TAC, ligands for the receptor CXC receptor-3 (CXCR3). An elevation in I-TAC levels in the plasma of patients with SSc is a novel observation. In agreement with our findings, higher levels of IP-10 have been shown previously in patients with SSc compared to unaffected controls (19;26;27). Higher IP-10 levels were associated with presence of interstitial lung disease, defined as presence of ground glass and/or interstitial fibrosis detected by high resolution chest CT. However, the correlation of IP-10 with the severity of ILD (FVC) has not been investigated previously. Type I and II IFNs can stimulate overlapping series of genes including IP-10 and I-TAC (28). Therefore, we cannot discern whether the observed association of IFN inducible chemokines with the disease severity is driven type I or type II IFNs. Furthermore, it is likely that these chemokines are not exclusively induced by IFN because the redundancies in biological pathways enable induction of key chemokines by several upstream molecules. For example, Weckerle et al. have recently shown moderate cross-sectional correlation between Tumor Necrosis Factor- a levels and IFN-α serum activity in patients with SLE (29).
In agreement with our findings, IP-10 and I-TAC levels correlated with the IFN gene signature in patients with SLE (7) and dermatomyositis (9). However, MCP-1 did not correlate with the IFN gene signature in our study contrary to observations in SLE (7) and dermatomyositis (9). It is possible MCP-1 levels in patients with SSc are mainly regulated by molecules involved in other pathways. In fact, MCP-1 was recently identified as a key IL-13 regulated cytokine in a fibrotic murine model as well as skin biopsy samples of patients with SSc (30).
The longitudinal examination of IFN-inducible chemokines indicated that they do not significantly change over time and that the chemokine levels in baseline and follow-up samples correlate with each other. Furthermore, the changes in chemokine levels did not correlate with progression of FVC (severity of ILD). This suggests that the IFN-inducible chemokine score is a marker for more severe subtype of SSc but not a dynamic measure of disease activity for SSc-related ILD. This notion was also supported by the fact that the chemokine score at the cross-sectional level did not correlate with disease duration. However, we observed a trend for correlation between change in the IFN-inducible chemokine score and mRSS. This weak correlation was not confirmed in the subgroup of patients not treated with immunosuppressive agents. Based on the current study, we cannot exclude a weak correlation between change in the IFN-inducible chemokine score and skin involvement. Larger longitudinal studies are needed to investigate this possibility. Of note, fibrotic processes in SSc are often irreversible, thus it is more difficult to identify dynamic markers of disease activity in this disease than other autoimmune diseases such as SLE or dermatomyositis. The investigated disease severity markers such as FVC and mRSS reflect disease damage (irreversible) as well as disease activity (reversible). Nevertheless, we believe stable markers of disease severity are important for identifying patients that may benefit from more aggressive monitoring and treatment.
Our study was conducted in a multiethnic cohort that increased the generalizability of our findings across investigated ethnic groups. Furthermore, the enrollment of only patients with early disease decreased the likelihood that our results are influenced by survival bias. The careful prospective collection of demographic and medical data including medication regimen also enabled us to adjust for several potential confounding variables.
The current study has some limitations. The GENISOS cohort is based on three tertiary care centers in Texas which partially explains the high proportion of patients with diffuse cutaneous diseases in this study. While the IFN-inducible chemokines have been linked to disease severity in SSc in our study, their actual contribution to various disease manifestations of SSc needs to be explored in future mechanistic studies. Furthermore, the longitudinal study could be conducted only in a subgroup of patients with available repeat plasma samples. In addition, the lack of a validated disease activity scale in SSc has hampered identification of biomarkers that track dynamic changes in disease activity. For example, FVC and mRSS both reflect disease activity as well as disease damage, because fibrotic changes in the lung and skin are partially irreversible.
In summary, the composite score of IP-10 and I-TAC correlates with the IFN gene expression signature in SSc. This IFN-inducible chemokine score correlates with severity of lung, skin, and muscle involvement even after adjustment for potential demographic and clinical confounders. Furthermore, the IFN-inducible chemokine score does not change significantly over time, suggesting that this composite score can serve as a stable maker for more severe subtype of SSc. This finding may lead to more effective and focused monitoring and treatment of patients with SSc.
Acknowledgments
Grant Funding:
This study was supported by the National Institute of Health (NIH)/NIAMS R01-AR055258 (Mayes); NIH/NIAMS K23 AR061436 (Assassi); Scleroderma Family Registry and DNA Repository N01 AR2251, R01 AR055258 (Mayes); NIH T32 AR052283 (Reveille); University Clinic Research Center Grants: M01 RR00073 (UTMB) and M01 RR01346 (UTHSC-SA); NIH Clinical and Translational Sciences Award UL1 RR024148 and TL1 RR024147 from the National Center for Research Resources.
Footnotes
The authors have no relevant financial disclosures.
This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process which may lead to differences between this version and the Version of Record.
Reference List
- 1.York MR, Nagai T, Mangini AJ, Lemaire R, van Seventer JM, Lafyatis R. A macrophage marker, Siglec-1, is increased on circulating monocytes in patients with systemic sclerosis and induced by type I interferons and toll-like receptor agonists. Arthritis Rheum. 2007;56(3):1010–1020. doi: 10.1002/art.22382. [DOI] [PubMed] [Google Scholar]
- 2.Tan FK, Zhou X, Mayes MD, Gourh P, Guo X, Marcum C, et al. Signatures of differentially regulated interferon gene expression and vasculotrophism in the peripheral blood cells of systemic sclerosis patients. Rheumatology (Oxford) 2006;45(6):694–702. doi: 10.1093/rheumatology/kei244. [DOI] [PubMed] [Google Scholar]
- 3.Higgs BW, Liu Z, White B, Zhu W, White Wl, Morehouse C, et al. Patients with systemic lupus erythematosus, myositis, rheumatoid arthritis and scleroderma share activation of a common type I interferon pathway. Ann Rheum Dis. 2011;7011:2029–2036. doi: 10.1136/ard.2011.150326. [DOI] [PubMed] [Google Scholar]
- 4.Duan H, Fleming J, Pritchard DK, Amon LM, Xue J, Arnett HA, et al. Combined analysis of monocyte and lymphocyte messenger RNA expression with serum protein profiles in patients with scleroderma. Arthritis Rheum. 2008;58(5):1465–1474. doi: 10.1002/art.23451. [DOI] [PubMed] [Google Scholar]
- 5.Bos CL, van Baarsen LG, Timmer TC, Overbeek MJ, Basoski NM, Rustenburg F, et al. Molecular subtypes of systemic sclerosis in association with anti-centromere antibodies and digital ulcers. Genes Immun. 2009 doi: 10.1038/gene.2008.98. [DOI] [PubMed] [Google Scholar]
- 6.Assassi S, Mayes MD, Arnett FC, Gourh P, Agarwal SK, McNearney TA, et al. Systemic sclerosis and lupus: points in an interferon-mediated continuum. Arthritis Rheum. 2010;62(2):589–598. doi: 10.1002/art.27224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauer JW, Baechler EC, Petri M, Batliwalla FM, Crawford D, Ortmann WA, et al. Elevated serum levels of interferon-regulated chemokines are biomarkers for active human systemic lupus erythematosus. PLoS Med. 2006;3(12):e491. doi: 10.1371/journal.pmed.0030491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauer JW, Petri M, Batliwalla FM, Koeuth T, Wilson J, Slattery C, et al. Interferon-regulated chemokines as biomarkers of systemic lupus erythematosus disease activity: a validation study. Arthritis Rheum. 2009;60(10):3098–3107. doi: 10.1002/art.24803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilgic H, Ytterberg SR, Amin S, McNallan KT, Wilson JC, Koeuth T, et al. lnterleukin-6 and type I interferon-regulated genes and chemokines mark disease activity in dermatomyositis. Arthritis Rheum. 2009;60(11):3436–3446. doi: 10.1002/art.24936. [DOI] [PubMed] [Google Scholar]
- 10.Preliminary criteria for the classification of systemic sclerosis (scleroderma) Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23(5):581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 11.Gourh P, Arnett FC, Assassi S, Tan FK, Huang M, Diekman L, et al. Plasma cytokine profiles in systemic sclerosis: associations with autoantibody subsets and clinical manifestations. Arthritis Res Ther. 2009;11(5):R147. doi: 10.1186/ar2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci US A. 2003;100(5):2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medsger TA, Jr, Silman AJ, Steen VD, Black CM, Akesson A, Bacon PA, et al. A disease severity scale for systemic sclerosis: development and testing. J Rheumatol. 1999;26(10):2159–2167. [PubMed] [Google Scholar]
- 14.Bombardieri S, Medsger TA, Jr, Silman AJ, Valentini G. The assessment of the patient with systemic sclerosis. Introduction. Clin Exp Rheumatol. 2003;21(3 Suppl 29):S2–S4. [PubMed] [Google Scholar]
- 15.Clements P, Lachenbruch P, Siebold J, White B, Weiner S, Martin R, et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol. 1995;22(7):1281–1285. [PubMed] [Google Scholar]
- 16.Furst D, khanna D, Matucci-Cerinic M, Clements P, Steen V, Pope J, et al. Systemic sclerosis -continuing progress in developing clinical measures of response. J Rheumatol. 2007;34(5):1194–1200. [PubMed] [Google Scholar]
- 17.Steele R, Hudson M, Lo E, Baron M. Canadian Scleroderma Research Group. Clinical decision rule to predict the presence of interstitial lung disease in systemic sclerosis. Arthritis Care Res (Hoboken) 2012;64(4):519–524. doi: 10.1002/acr.21583. [DOI] [PubMed] [Google Scholar]
- 18.Farina G, Lafyatis D, Lemaire R, Lafyatis R. A four-gene biomarker predicts skin disease in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheum. 2010;62(2):580–588. doi: 10.1002/art.27220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eloranta ML, Franck-Larsson K, Lovgren T, Kalamajski S, Ronnblom A, Rubin K, et al. Type I interferon system activation and association with disease manifestations in systemic sclerosis. Ann Rheum Dis. 2010;69(7):1396–1402. doi: 10.1136/ard.2009.121400. [DOI] [PubMed] [Google Scholar]
- 20.Steen VD. Autoantibodies in systemic sclerosis. Semin Arthritis Rheum. 2005;35(l):35–42. doi: 10.1016/j.semarthrit.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Tyndall AJ, Bannert B, Vonk M, Airo P, Cozzi F, Carreira PE, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. 2010;69(10):1809–1815. doi: 10.1136/ard.2009.114264. [DOI] [PubMed] [Google Scholar]
- 22.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis. 2007;66(7):940–944. doi: 10.1136/ard.2006.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solans R, Bosch JA, Esteban I, Vilardell M. Systemic sclerosis developing in association with the use of interferon alpha therapy for chronic viral hepatitis. Clin Exp Rheumatol. 2004;22(5):625–628. [PubMed] [Google Scholar]
- 24.Beretta L, Caronni M, Vanoli M, Scorza R. Systemic sclerosis after interferon-alfa therapy for myeloproliferative disorders. Br J Dermatol. 2002;147(2):385–386. doi: 10.1046/j.1365-2133.2002.48901.x. [DOI] [PubMed] [Google Scholar]
- 25.Black CM, Silman AJ, Herrick Al, Denton CP, Wilson H, Newman J, et al. Interferon-alpha does not improve outcome at one year in patients with diffuse cutaneous scleroderma: results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 1999;42(2):299–305. doi: 10.1002/1529-0131(199902)42:2<299::AID-ANR12>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 26.Rabquer BJ, Tsou PS, Hou Y, Thirunavukkarasu E, Haines GK, III, Impens AJ, et al. Dysregulated expression of MIG/CXCL9, IP-10/CXCL10 and CXCL16 and their receptors in systemic sclerosis. Arthritis Res Ther. 2011;13(1):R18. doi: 10.1186/ar3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antonelli A, Ferri C, Fallahi P, Ferrari SM, Giuggioli D, Colaci M, et al. CXCL10 (alpha) and CCL2 (beta) chemokines in systemic sclerosis-a longitudinal study. Rheumatology (Oxford) 2008;47(1):45–49. doi: 10.1093/rheumatology/kem313. [DOI] [PubMed] [Google Scholar]
- 28.Waddell SJ, Popper SJ, Rubins KH, Griffiths MJ, Brown PO, Levin M, et al. Dissecting interferon-induced transcriptional programs in human peripheral blood cells. PLoS ONE. 2010;5(3):e9753. doi: 10.1371/journal.pone.0009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weckerle CE, Imbuka D, Franek BS, Kelly JA, Kumabe M, James JA, et al. Large scale analysis of tumor necrosis factor alpha levels in systemic lupus erythematosus. Arthritis Rheum. 2012 doi: 10.1002/art.34483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenblatt MB, Sargent JL, Farina G, Tsang K, Lafyatis R, Glimcher LH, et al. Interspecies Comparison of Human and Murine Scleroderma Reveals IL-13 and CCL2 as Disease Subset-Specific Targets. Am J Pathol. 2012;180(3):1080–1094. doi: 10.1016/j.ajpath.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]