Abstract
Signal transduction networks in mammalian cells, comprised of a limited set of interacting biochemical pathways, are accessed by various growth factor and cytokine receptors to elicit distinct cell responses. This raises the question as to how specificity of the stimulus-response relationship is encoded at the molecular level. It has been proposed that specificity arises not simply from the activation of unique signalling pathways but also from quantitative differences in the activation and regulation of shared, receptor-proximal signalling proteins. To address such hypotheses, data sets with greater precision and coverage of experimental conditions will need to be acquired, and rigorous frameworks that codify and parameterise the inherently nonlinear relationships among signalling activities will need to be developed. Here we apply a systematic approach combining quantitative measurements and mathematical modelling to compare the signalling networks accessed by fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF) receptors in mouse fibroblasts, in which the extracellular signal-regulated kinase (ERK) cascade is activated by Ras- and phosphoinositide 3-kinase (PI3K)-dependent pathways. We show that while the FGF stimulation of PI3K signalling is relatively weak, this deficiency is compensated for by a more potent, Ras-dependent activation of ERK. Thus, as the modelling would predict, the ERK pathway is activated to a greater extent in cells co-stimulated with FGF and PDGF, relative to the saturated levels achieved with either ligand alone. It is envisioned that similar approaches will prove valuable in the elucidation of quantitative differences among other, closely related receptor signalling networks.
Keywords: mitogen-activated protein kinase, growth factor, crosstalk, kinetic analysis, computational biology
INTRODUCTION
Mammalian cells respond to a diverse variety of growth factors, cytokines, and hormones, which are generally recognized through ligation of specific cell surface receptors. Whereas a cell’s receptor repertoire determines the subset of chemical signals to which it can respond, those receptors typically plug into a common set of conserved signalling pathways; a question of longstanding interest, then, is how different stimuli or combinations of stimuli might elicit distinct responses [1]. Several conceptual and theoretical models of how such specificity is achieved have been offered. For one, specificity might be encoded by the interactions of individual receptor molecules with other cellular proteins. In the case of receptor tyrosine kinases, which include the receptors for various extracellular growth factors, ligand binding results in self-phosphorylation of tyrosine residues in the receptor cytoplasmic domain [2] and interactions with multiple Src homology 2 domain- and phosphotyrosine binding domain-containing enzymes and protein adaptors [3]. Thus, the phosphorylation stoichiometries of the various receptor sites and their binding affinities for cytoplasmic proteins quantitatively influence the relative activation levels of different signalling pathways, which additionally offers an explanation for how the same stimulus can elicit different responses depending on its concentration. Specificity might also be encoded by the kinetics of activation. In one popular conceptual model, it was proposed that the signalling outcome depends on whether an upstream pathway is activated with sustained versus transient kinetics [4]. Theoretical models have supplemented this concept with the notion that the initial rate of activation, rapid versus slow, might be selective for triggering different downstream pathways [5, 6].
The use of mathematical models complements the traditional, pathway-oriented (epistasis) approach by readily incorporating nonlinearities that commonly arise as a consequence of inter-pathway crosstalk and feedback/feed-forward loops. To the extent that they are trained on quantitative data, such models are capable of generating quantitative predictions. The concepts of signalling specificity outlined above highlight the need for systematic measurements characterizing the nonlinear input-output relationships in signal transduction networks, accounting for magnitude, sensitivity, and kinetics. Whereas early investigations sought to establish the relative potencies of growth factors in the activation [7, 8] and desensitization [9] of certain signalling pathways, very few studies have been designed so as to vary the stimulation dose and time in conjunction with molecular perturbations of the network. This has been rectified to varying extents in recent years through data-driven mathematical modelling, which has been successfully applied to the analysis of cells stimulated with multiple growth factors and cytokines [10–14]; however, a more concerted data acquisition effort is needed to bring this approach to the level of predicting network dynamics for a variety of stimuli in common cellular backgrounds, which will be important if we are to understand and predict naturally occurring and interventional modes of cell regulation [15, 16].
In this work, we build upon systematic analyses of the platelet-derived growth factor (PDGF) receptor signalling network in mouse fibroblasts, resulting in activation of the classical Raf → MEK → extracellular signal-regulated kinase (ERK) cascade, a master regulator of cell fate [17, 18]. Our previous studies focused on the dynamic contributions of the canonical, Ras-dependent pathway and non-canonical, phosphoinositide 3-kinase (PI3K)-dependent crosstalk [19] as well as regulation of the network by multiple, ERK-dependent negative feedback loops [20]. Here, we offer a quantitative data set of fibroblast growth factor (FGF) receptor-mediated signalling readouts in the same cells and analyses directed towards reconciling the FGF and PDGF receptor networks with a unified kinetic model. One major difference is that FGF is a relatively weak stimulus for the PI3K pathway in these cells, and thus ERK activation is primarily Ras-dependent in this context. We further show that the potencies of Ras-dependent signalling to MEK/ERK mediated by FGF and PDGF receptors cannot be explained by differences in the amounts of Ras-GTP generated on a whole-cell basis; Ras activated in response to FGF is more potent. It is speculated that FGF and PDGF receptor signalling components are differentially localized and thus activate different pools of membrane-associated Ras.
EXPERIMENTAL
Reagents
All tissue culture reagents were from Invitrogen (Carlsbad, CA). Human recombinant PDGF-BB and murine recombinant FGF-2 were purchased from Peprotech (Rocky Hill, NJ). Antibodies against total ERK1/2 and phospho-specific antibodies against Akt pSer473, ERK pThr202/pTyr204, and MEK pSer217/pSer221 were from Cell Signaling Technology (Beverly, MA); antibodies against Ras (Y13-259, agarose-conjugated) and Akt-1/2 N terminus were from Santa Cruz Biotechnology (Santa Cruz, CA). The pharmacological inhibitors LY294002 and PD098059 were from Calbiochem (San Diego, CA) and were pre-incubated with the cells for 30–60 minutes prior to growth factor stimulation. The choice of these inhibitors follows conditions used previously [19]; the specificity of LY294002 treatment was checked using the LY303511 compound, and although PD098059 suffers from lack of solubility and potency, its specificity is well characterized [21]. The siGENOME siRNA reagents and siGENOME SMARTpool siRNAs against ERK1 (GeneID: 26417) and ERK2 (GeneID: 26413) and siGENOME Non-Targeting siRNA Pool #2 were purchased from Dharmacon (Lafayette, CO). Unless otherwise noted, all other reagents were from Sigma-Aldrich.
Cell culture and siRNA transfection
NIH 3T3 fibroblasts (American Type Culture Collection, Manassas, VA) were cultured at 37°C, 5% CO2 in Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum, 2 mM L-glutamine, and the antibiotics penicillin and streptomycin. Where applicable, NIH 3T3 cells were serially infected with retrovirus bearing empty vector or S17N H-Ras and selected using puromycin prior to each experiment, as described previously [19, 22]. NIH 3T3 cells were transfected with siRNAs according to the manufacturer’s protocol and incubated for three days prior to the experiment.
Lysate preparation and biochemical assays
Cells were serum-starved for 4 hours prior to stimulation. Detergent lysates were prepared for quantitative immunoblotting, and immunoblots were performed using enhanced chemiluminescence, as described previously [19, 20]. Blots comparing lysates prepared on the same day, representing either different inhibitor treatments or different cell variants and respective control conditions, were performed in parallel and exposed at the same time. The BioRad Fluor S-Max system, which gives a linear response with respect to light output, was used, and band intensity was quantified using local background subtraction. To determine the amounts of Ras-bound GTP (from all Ras isoforms), eluted from anti-Ras immunoprecipitates (Y13-259, agarose-conjugated), the coupled nucleoside-5’-diphosphate kinase/luciferase assay was performed as described previously [19, 22]. Both types of data were first normalized by an appropriate loading control and then further normalized to evaluate the consistency of relative trends across independent experiments, according to the procedures described in detail previously [19].
Kinetic models and computational analysis
The mathematical models and analyses applied in this work are described in detail in Supplementary Text S1. Our previously published model of the PDGF receptor network [20] was modified by removing the feedback modulation of ERK phosphatase expression levels; as previously shown in that work, this simplification substantially reduces the number of model parameters without affecting the fit to the PDGF data set. The model of FGF receptor signalling was adapted from this model with phenomenological equations for the kinetics of FGF receptor-mediated recruitment of Ras guanine nucleotide exchange factor and PI3K activities. SBML files encoding the modified PDGF receptor network model and the FGF receptor network model are provided in the Supplementary Material.
The parameter estimation approach used has been described in detail previously [20, 23] and is reviewed in Supplementary Text S1. Briefly, it uses a Monte Carlo/simulated annealing-based algorithm to generate a large (n = 10,000) ensemble of “good” parameter sets rather than one “best” fit. After compiling the ensemble, the model output is recalculated for each parameter set, and at each time point, an ensemble mean and standard deviation are calculated.
RESULTS
The FGF receptor signalling network in mouse fibroblasts features relatively weak stimulation of the PI3K/Akt pathway and predominantly Ras-dependent activation of the ERK cascade
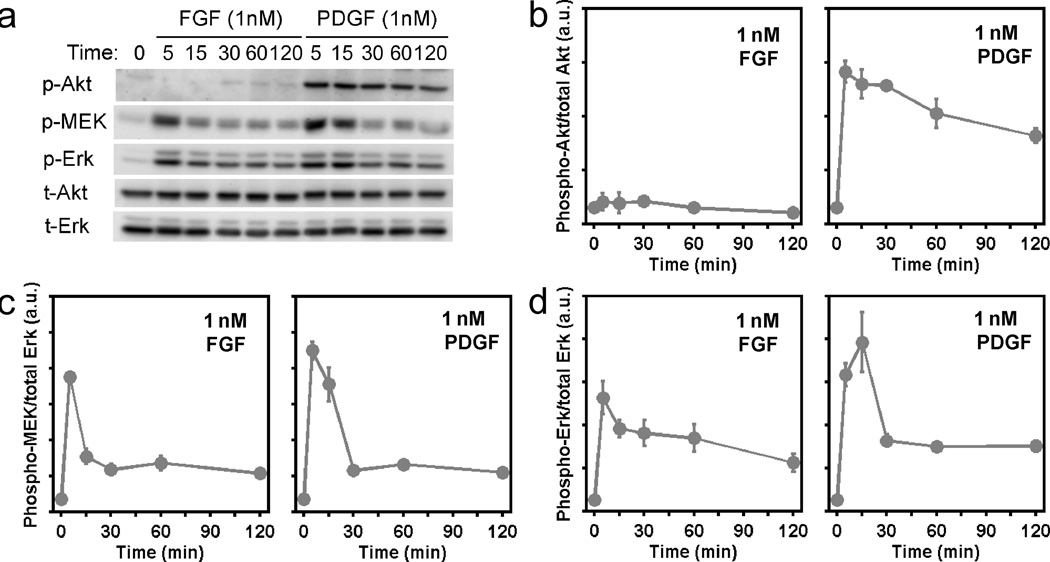
In quantitative studies carried out in NIH 3T3 mouse fibroblasts, we previously showed that PDGF receptor-mediated signalling to ERK is channeled through two distinct pathways: the canonical, Ras-dependent pathway and an equally if not more important pathway involving PI3K-dependent crosstalk. As expected, we find that FGF also stimulates activation of the ERK pathway in these cells, assessed quantitatively at the level of MEK1/2 and ERK1/2 phosphorylation (Fig. 1a). The response was found to be saturated at concentrations of FGF-2 well below 1 nM (Supplementary Fig. S1). In contrast, FGF only weakly stimulates PI3K-dependent phosphorylation of Akt (Fig. 1a&b). Despite this disparity in PI3K signalling, maximal FGF and PDGF stimulation yield quantitatively similar plateau levels (i.e., for time points ≥ 30 minutes) of MEK phosphorylation (Fig. 1c), and of ERK phosphorylation (Fig. 1d), measured in side-by-side experiments. The time courses of FGF-stimulated MEK and ERK phosphorylation do, however, exhibit lower peak values than those in PDGF-stimulated cells. Thus, for FGF in relation to PDGF, it would seem that PI3K-dependent signalling is contributing far less to the activation of MEK and, conversely, that Ras-dependent signalling is contributing more.
Figure 1. Quantitative comparison of FGF and PDGF receptor-mediated signalling in mouse fibroblasts under maximal stimulation conditions.
a. NIH 3T3 fibroblasts were maximally stimulated with FGF-2 or PDGF-BB (both 1 nM) in side-by-side experiments, and phosphorylation time courses of Akt1/2/3 (p-Akt), MEK1/2 (p-MEK), and ERK1/2 (p-Erk) were measured by quantitative immunoblotting alongside total Akt (t-Akt) and total ERK (t-Erk) as loading controls. The blots shown are representative of 6 independent experiments. b–d. Quantitative comparison of FGF- and PDGF-stimulated Akt (b), MEK (c), and ERK (d) phosphorylation kinetics. Values are normalized as previously described and are reported as mean ± s.e.m. in arbitrary units (n = 6).
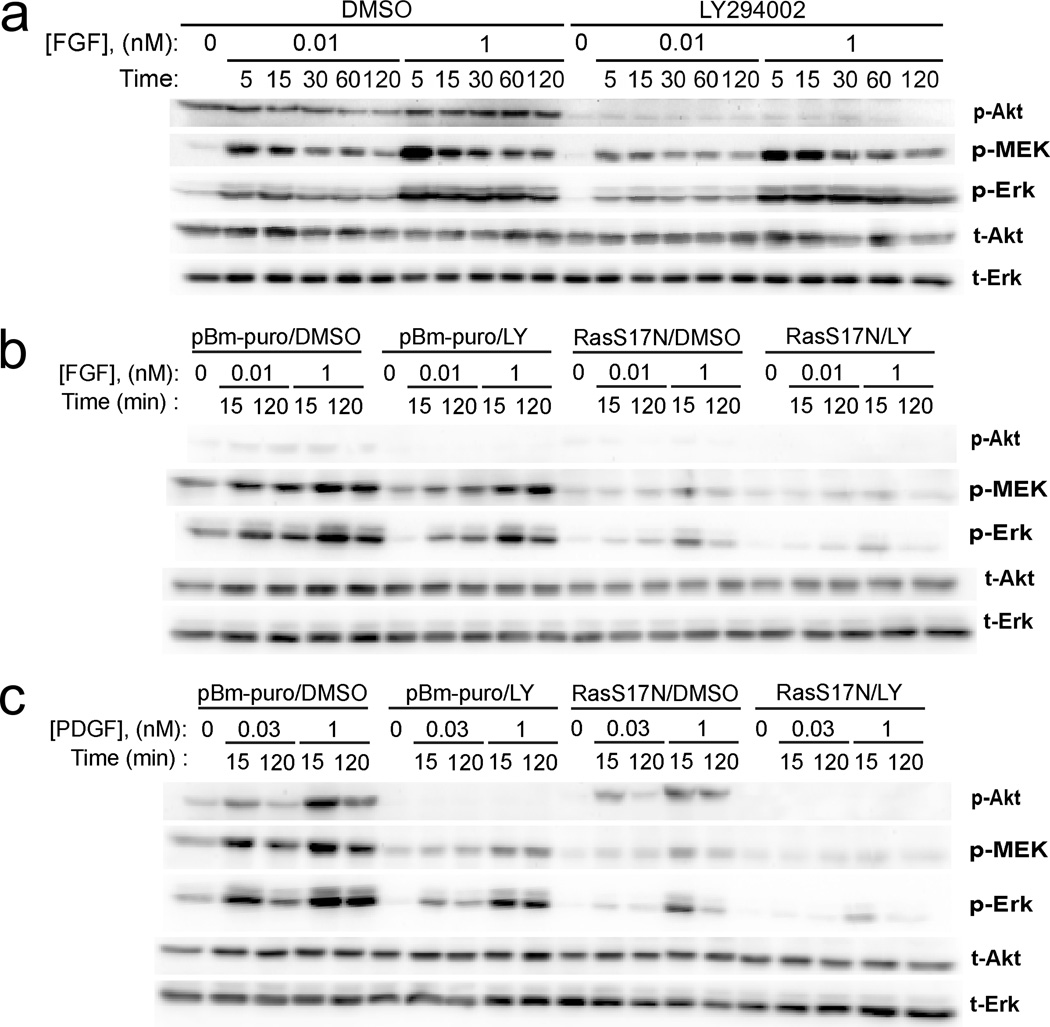
In support of this hypothesis, we found that inhibition of PI3K using LY294002 only modestly affects MEK and ERK phosphorylation stimulated by high (1 nM) or subsaturating (0.01 nM) doses of FGF (Fig. 2a&b), whereas PI3K inhibition reduces PDGF-stimulated MEK/ERK phosphorylation substantially (Fig. 2c and refs. [19, 20]). Accordingly, blocking receptor-mediated activation of endogenous Ras through stable expression of dominant-negative S17N H-Ras has a more dramatic effect on FGF-stimulated MEK/ERK phosphorylation; in response to either ligand, simultaneous blockade of both Ras- and PI3K-dependent signalling ablates MEK and ERK phosphorylation almost completely (Fig. 2b&c). Quantification of peak phospho-ERK/total ERK ratios (1 nM FGF or PDGF stimulation for 15 minutes) showed that PI3K inhibition reduced ERK phosphorylation by only 13 ± 7% in FGF-stimulated cells, as compared with 58 ± 3% in PDGF-stimulated cells, whereas Ras inhibition reduced ERK phosphorylation by 66 ± 7% and 42 ± 11% in FGF- and PDGF-stimulated cells, respectively (mean ± s.e.m.).
Figure 2. FGF-stimulated MEK/ERK phosphorylation is predominantly Ras-dependent.
a. Phosphorylation time courses of Akt1/2/3 (p-Akt), MEK1/2 (p-MEK), and ERK1/2 (p-Erk) in FGF-2-stimulated NIH 3T3 fibroblasts were measured by quantitative immunoblotting alongside total Akt (t-Akt) and total ERK (t-Erk) as loading controls. Cells in which PI3K was inhibited (100 µM LY294002) are compared with cells incubated with 0.2% DMSO vehicle control. The blots shown are representative of 6 independent experiments. b. NIH 3T3 fibroblasts were infected with retrovirus produced from empty vector or vector with dominant-negative (S17N) H-Ras, pretreated with either DMSO control or LY294002, then stimulated with FGF-2 (dose and time indicated). The panel of immunoblotting readouts is the same as in a, and the blots shown are representative of three independent experiments. c. Same as b except that the cells were stimulated with PDGF-BB (dose and time indicated). Note that, whereas the grayscale intensity of the p-Akt blot in a clearly shows the difference between lanes under FGF stimulation, those in b and c were acquired under the same conditions and thus reflect the difference between FGF and PDGF stimulation, as in Fig. 1a.
It is established, largely through the use of GTPase-defective mutants of Ras, that PI3K is an effector of Ras [24]; however, activated PDGF receptors are far more potent in mobilizing PI3K recruitment. We previously showed that expression of dominant-negative Ras modestly reduces PDGF-stimulated PI3K/Akt signalling in our cells, but only under select conditions of low PDGF doses and an early time point [22]. Otherwise, canvassing a range of PDGF concentrations and time points, we have not resolved any significant effect [19]. These observations are corroborated by the present, independent measurements of PDGF-stimulated Akt phosphorylation (Fig. 2c). Although the blot shown exhibits a noticeably reduced band intensity for S17N Ras-expressing cells stimulated with 1 nM PDGF for 15 minutes, quantification across biological replicates indicated that the estimated reduction of Akt phosphorylation (normalized by loading control) is a mere 9 ± 17% under those conditions (mean ± s.e.m.). Under FGF stimulation, it is plausible that the Ras-PI3K link plays a more prominent role, but the low level of Akt phosphorylation elicited by FGF in our cells does not allow for a definitive analysis of this possibility.
Computational analysis of Ras- and PI3K-dependent activation of the ERK cascade in the FGF receptor signalling network
We previously formulated a coarse-grained kinetic model of the PDGF receptor signalling network, accounting for Ras- and PI3K-dependent signalling to ERK and multiple negative feedback mechanisms [20]; as explained in detail in the Supplementary Material, Text S1, a related model with 44 adjustable parameters was formulated for the FGF receptor network. As in our prior work, the approach here is not designed to identify a single set of “best” parameter values, for it has been argued that the parameters in kinetic models of even moderate scope are not identifiable [25]. Rather, we seek to collect a large ensemble of parameter sets that perform almost equally well in fitting the data. With such an ensemble, one may then use the model to generate predictions and estimate their uncertainty [20].
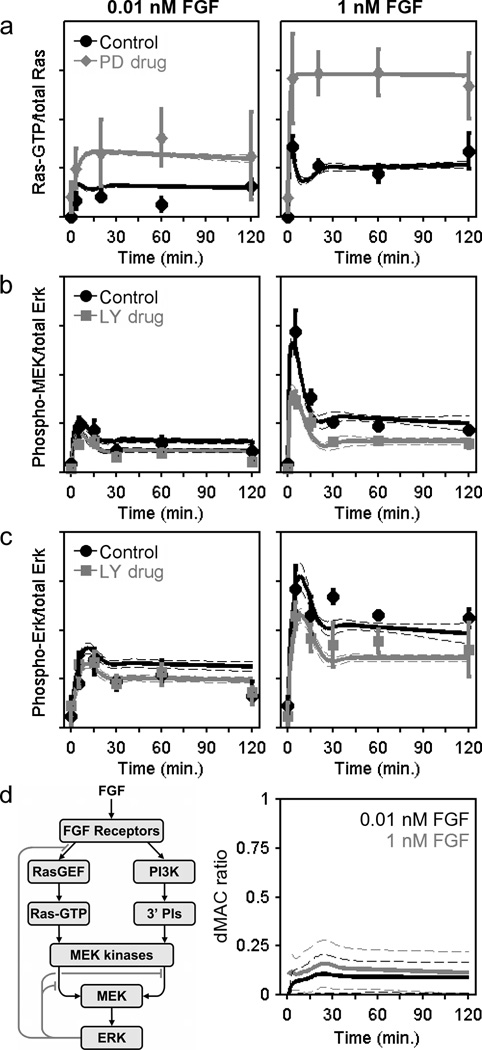
The global fit of the model, which identifies a large ensemble of suitable parameter sets, is shown alongside the available kinetic data (Fig. 3a–c and Supplementary Fig. S2) and allows for further analysis and predictions of a quantitative nature. For example, we computed the dynamic MEK activation comparator (dMAC), which quantitatively relates the contributions of the PI3K- and Ras-dependent inputs to MEK as a function of time; we find this analysis to be useful because the kinetics of FGF- and PDGF-stimulated MEK phosphorylation are subject to strong negative feedback adaptation, confounding a straightforward comparison. Across the spectrum of PDGF doses assessed previously (0.03–1 nM), the estimated dMAC values level out in the range of 1.5–2 [20]. In other words, in the PDGF receptor signalling network, a dMAC value greater than 1 indicates that PI3K-dependent crosstalk is somewhat more potent than the Ras-dependent pathway in activating MEK. By comparison, although a careful quantification of the MEK/ERK phosphorylation data reveals a subtle effect of PI3K inhibition (Fig. 3b&c), the estimated dMAC for the FGF receptor network plateaus at ≈ 0.1 (Fig. 3d), quantifying the extent to which PI3K-dependent crosstalk plays a subordinate role in this cell/receptor context.
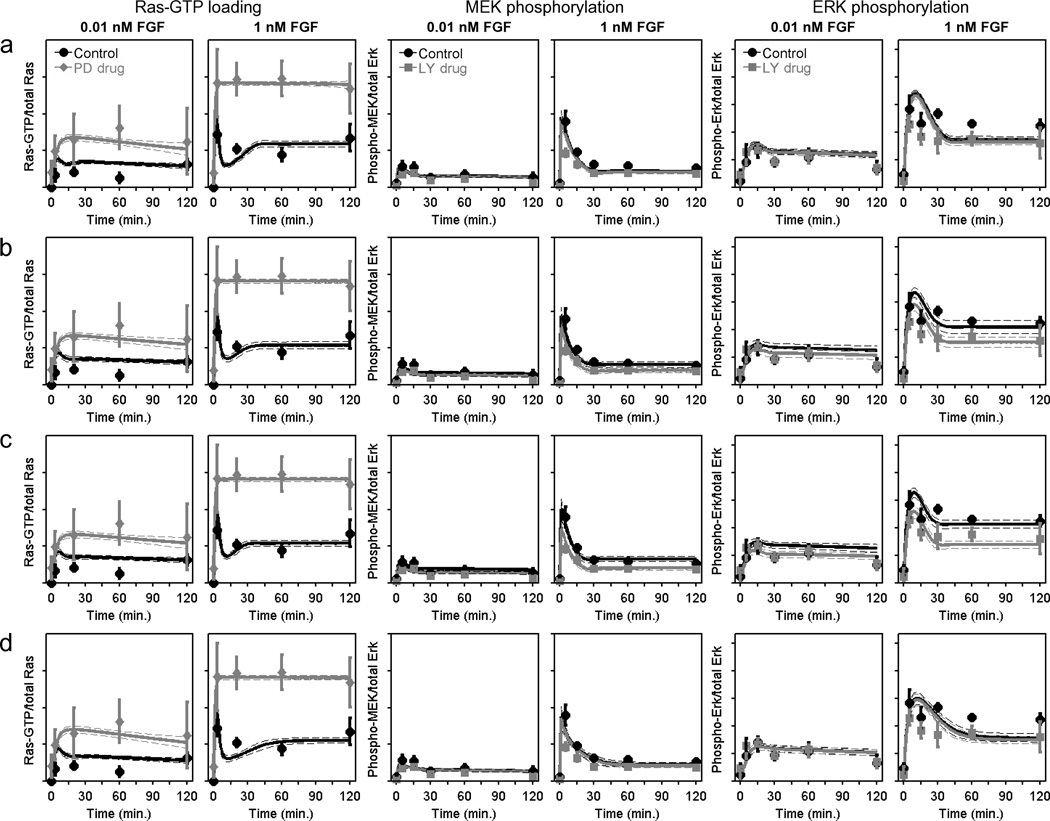
Figure 3. Kinetic model of the FGF receptor signalling network.
a–c. A simulated annealing algorithm was used to align the kinetic model (Supplementary Text S1) to the data as indicated, thus collecting an ensemble of parameter sets that fit the data set well. Solid curves are ensemble means, and the dashed curves are mean ± s.d. (n = 10,000). The data used to constrain the model, reported as normalized mean ± s.e.m. (n = 3). Ras-GTP measurements (a) were acquired for NIH 3T3 cells with MEK inhibited (50 µM PD098059) versus DMSO control (basal level subtracted). MEK (b) and ERK (c) phosphorylation kinetics were measured with PI3K inhibited versus DMSO control (representative blots shown in Fig. 2a). d. The schematic shows the basic structure of the model with Ras- and PI3K-dependent pathways to MEK/ERK and feedback desensitization of Ras-GEF and MEK kinase activities. The dynamic MEK activation comparator (dMAC; Supplementary Text S1) quantitatively compares the contributions of the two MEK activation pathways as a function of time (gray, 1 nM FGF; black, 0.01 nM FGF).
Computational analysis of the FGF receptor signalling network accurately predicts the influence of ERK-dependent negative feedback loops
One of the key regulatory features of the ERK signalling network is negative feedback adaptation, which impinges both upstream and downstream of Ras. Parsing the different mechanisms of concurrent feedback regulation is another level of analysis that calls for a kinetic modelling approach. In the quantitative fit of the model, feedback regulation upstream of Ras is determined by comparing the Ras-GTP loading data obtained in the presence versus absence of a MEK inhibitor (Fig. 3a); with the magnitude of the upstream feedback constrained, the feedback downstream of Ras is left to account for the residual desensitization of the pathway resulting in transient MEK phosphorylation kinetics (Fig. 3b).
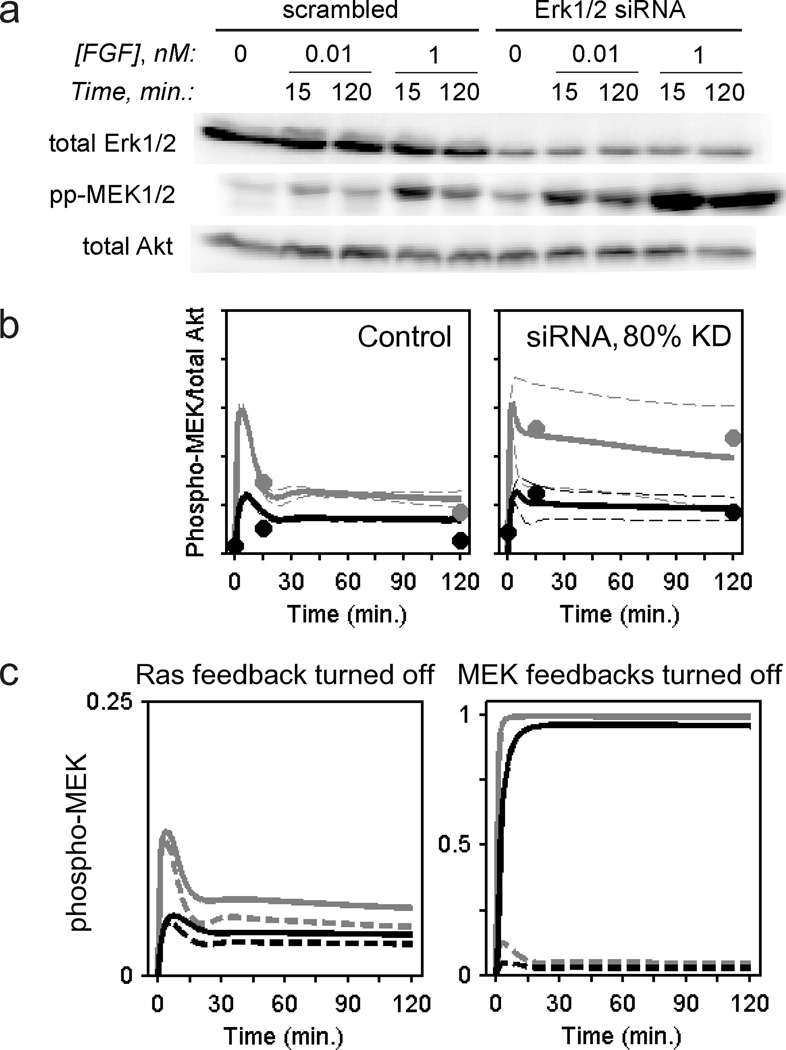
To test the validity of this model, we evaluated its ability to predict a priori the effect of simultaneous siRNA knockdown of ERK1 and ERK2 (≈ 80% reduction in total ERK1/2, as estimated by immunoblotting), which relieves the aforementioned negative feedback loops; accordingly, MEK phosphorylation is markedly enhanced relative to the control (Fig. 4a). In the corresponding model prediction, there is an expected degree of variability across the ensemble of parameter sets, as shown by large standard deviations; however, as a central estimate, the ensemble mean is almost perfectly aligned with the data (Fig. 4b). Given the size of the ensemble (n = 10,000), the standard error of the mean is only 1% of the standard deviation.
Figure 4. siRNA knockdown of ERK1 and ERK2 enhances FGF-stimulated MEK phosphorylation, as quantitatively predicted by the current model.
a. NIH 3T3 cells were transfected with siRNAs directed against ERK1 and ERK2; their pan-ERK expression and FGF-stimulated MEK1/2 phosphorylation were measured by quantitative immunoblotting in parallel with a scrambled siRNA control. Total Akt (as a loading control) was also assessed. The results are representative of two independent experiments. b. The quantified results from a are overlaid with a priori kinetic model predictions of MEK phosphorylation kinetics, assuming 80% knockdown of ERK in the model; solid curves represent ensemble means, and dashed curves are mean ± s.d. (n = 10,000). FGF concentrations are: gray, 1 nM; black, 0.01 nM. c. Feedback desensitization of MEK kinase activities is the dominant mode of ERK self-regulation. Model predictions (ensemble means) of MEK phosphorylation are shown (gray, 1 nM FGF; black, 0.01 nM FGF). Solid curves are hypothetical scenarios in which one of the two layers of ERK-dependent feedback is selectively turned off, as indicated. The dashed curves are with all ERK-dependent feedback loops intact.
To assess the relative contributions of the two layers of negative feedback, we selectively turned off one or the other type of feedback in the FGF receptor network model. Turning off the upstream feedback enhances MEK phosphorylation in the model only modestly in comparison with silencing the downstream feedback, which results in nearly stoichiometric phosphorylation of MEK (Fig. 4c). These results strongly suggest that negative feedback impinging downstream of Ras and upstream of MEK plays a dominant role in regulating the pathway, consistent with our previous analysis of PDGF receptor-mediated signalling [20].
A single model encompassing the PDGF and FGF receptor networks reveals quantitative differences in the propagation of Ras/ERK signalling
Having demonstrated that kinetic models of the FGF and PDGF receptor signalling networks with similar mathematical structures are capable of generating good fits to all available data and a certain degree of predictive power, we sought to identify minimal parametric requirements that reconcile the two network models. That is, for those processes not directly affected by receptor-level interactions (those which are downstream of Ras-GTP and 3’ phosphoinositide accumulation), we assessed whether or not a common set of model parameters can simultaneously capture FGF- and PDGF-stimulated signalling kinetics. If not, we wish to identify which alterations of the parameters (differences between the two networks as stimulated by FGF versus PDGF) allow a good fit of both data sets.
To address these questions, a series of four model variations were formulated and globally fit to the available data for FGF and PDGF; care was taken to co-normalize the two data sets based on the side-by-side measurements shown in Fig. 1. The model variations focus on differences in the phosphorylation kinetics of MEK (which, according to our model, could reflect the levels or/and potencies of the MEK kinase activities mobilized by Ras- and PI3K-dependent signalling) and of ERK (Supplementary Text S1; Table 1). In the least complicated Variation 1, MEK and ERK phosphorylation kinetics as stimulated by FGF and PDGF are identical; that is, the two receptor networks differ only in their propensities for generating Ras-GTP and 3’ phosphoinositides. Although Variation 1 is most satisfying from the standpoint of Occam’s razor, it fails to accurately capture the kinetics of FGF-stimulated MEK/ERK phosphorylation. For the saturating dose of 1 nM FGF-2, the model cannot distinguish the MEK and ERK phosphorylation kinetics in PI3K-inhibited versus control cells (Fig. 5a), as reflected in the corresponding error metrics (Table 1). By comparison, Variation 2 allows MEK phosphorylation kinetics to vary between FGF and PDGF and offers a demonstrably better fit (Fig. 5b), which is somewhat further improved upon by allowing both MEK and ERK phosphorylation kinetics to vary (Variation 3; Fig. 5c). Compared with Variation 1, the fit is not as much improved if only ERK phosphorylation kinetics are varied (Variation 4; Fig. 5d). The corresponding fits of these model variations to archival PDGF data are compared in Supplementary Fig. S3 and show that Variations 1 and 4 do not allow an optimal fit to the MEK and ERK phosphorylation kinetics for LY294002-treated cells. Because these two models are constrained such that maximal FGF and PDGF receptor signalling yield identical Ras-dependent contributions upstream of MEK, they are forced into a compromise: peak MEK and ERK phosphorylation levels in PI3K-inhibited cells are forced to be too high for FGF-stimulated cells and too low for PDGF-stimulated cells.
TABLE 1. Comparison of models encompassing FGF and PDGF receptor signalling networks.
The various model variations differ according to which level(s) of the MEK kinase → MEK → ERK pathway are allowed to have different kinetic parameters in FGF- versus PDGF-stimulated cells (Supplementary Text S1). The sum of squared deviations (SSD) for each readout (Ras, MEK, ERK; N is the number of measurements fit) serves as a relative error metric and is reported as the mean ± s.d. for the 10,000 parameter sets in each ensemble.
| FGF only | Variation 1: Same parameters |
Variation 2: Distinct MEK activation |
Variation 3: Distinct MEK, ERK activation |
Variation 4: Distinct ERK activation |
|
|---|---|---|---|---|---|
| SSDRas (FGF, N = 18) | 1.50±0.09 | 2.07±0.19 | 2.27±0.30 | 2.29±0.28 | 2.22±0.23 |
| SSDMEK (FGF, N = 22) | 0.32±0.07 | 0.61±0.09 | 0.46±0.17 | 0.33±0.10 | 0.70±0.11 |
| SSDERK (FGF, N = 30) | 0.62±0.07 | 1.59±0.11 | 1.32±0.14 | 1.16±0.11 | 1.57±0.14 |
| SSDRas (PDGF, N = 21) | — | 1.65±0.21 | 1.60±0.24 | 1.64±0.23 | 1.65±0.23 |
| SSDMEK (PDGF, N = 84) | — | 2.81±0.20 | 2.24±0.78 | 2.19±0.21 | 2.40±0.21 |
| SSDERK (PDGF, N = 104) | — | 3.60±0.13 | 3.29±0.20 | 3.14±0.15 | 3.50±0.17 |
Figure 5. To reconcile the kinetic models of FGF and PDGF receptor signalling networks, Ras activated in response to FGF stimulation must be more potent in propagating activation of MEK.
A kinetic model encompassing both FGF and PDGF receptor-mediated signalling was formulated with four variations that allowed different levels of the MEK kinase → MEK → ERK cascade to differ between the two receptor networks (Supplementary Text S1). Fits of the FGF-stimulated Ras-GTP loading, MEK phosphorylation, and ERK phosphorylation data are displayed as in Fig. 3a–c for the FGF only model (see also Supplementary Figs. S1 and S2 and Table 1). a. Variation 1: All common parameters the same. b. Variation 2: Distinct MEK activation kinetics; enzymatic parameters of the MEK kinase activities were allowed to have different values for FGF versus PDGF stimulation. c. Variation 3: Distinct MEK and ERK activation kinetics; enzymatic parameters of the MEK kinase and MEK activities were allowed to have different values for FGF versus PDGF stimulation. d. Variation 4: Distinct ERK activation kinetics.
The interpretation of these results taken together is as follows. Relative to maximal PDGF stimulation conditions, FGF generates a comparable level of Ras-GTP but far less PI3K-dependent signalling (on a whole-cell basis), yet the two ligands yield similar levels of MEK phosphorylation at steady state; accordingly, with PI3K inhibited, FGF stimulates higher levels of MEK and ERK phosphorylation than PDGF. Therefore, in the context of the model, the Ras-GTP stimulated by FGF must be more potent in activating MEK phosphorylation. By comparison, the kinetics of ERK phosphorylation by MEK are apparently consistent between the two networks.
Co-stimulation with FGF and PDGF activates higher levels of MEK and ERK phosphorylation, consistent with computational analysis
To further test the basic conclusions derived from computational analysis, we measured the magnitude of MEK/ERK phosphorylation stimulated in response to co-stimulation with maximal doses of FGF and PDGF, in parallel with each growth factor alone. PDGF receptor-mediated signalling in this cell background is characterized by saturation of both Ras and PI3K activation at submaximal receptor ligation (spare receptor effect); therefore, if it were the case that FGF receptors access Ras-dependent signalling with equal or less potency, co-stimulation with both ligands would fail to activate MEK and ERK beyond the maximum level stimulated by PDGF. Instead, the analysis presented here suggests that co-stimulation would combine more potent Ras-dependent signalling mediated by FGF receptors with full mobilization of PI3K-dependent signalling mediated by PDGF receptors to yield greater activation of MEK and ERK. As shown in Fig. 6, this prediction was confirmed. Whereas the kinetics of MEK and ERK phosphorylation stimulated by maximal FGF versus PDGF stimulation are consistent with those measured independently (Fig. 1c), the sum of normalized MEK levels in response to co-stimulation (times of 5, 15, and 120 minutes) is significantly higher than those of FGF and PDGF only (p = 0.016 and 0.038, paired t-tests, n = 4), with ERK phosphorylation following suit (Fig. 6).
Figure 6. MEK/ERK activation co-stimulated by FGF and PDGF exceeds the saturated levels stimulated by either ligand alone.
MEK (Left) and ERK (Right) phosphorylation were quantified as in Fig. 1 for cells stimulated with 1 nM FGF-2 (circles), 1 nM PDGF-BB (squares), or 1 nM FGF-2 + 1 nM PDGF-BB (Costim.; triangles) for the indicated times. Values are normalized as previously described and are reported as mean ± s.e.m. in arbitrary units (n = 4).
DISCUSSION
Even structurally related receptors in the same cellular background should be expected to mediate the activation of intracellular signalling networks with distinct magnitudes and kinetics of protein phosphorylation. Unraveling the mechanisms that give rise to this level of complexity will be important if we are to understand how different cell types integrate information about various stimuli and how to perturb those processes to affect cell behaviour. In the present comparison of FGF and PDGF receptor-mediated signalling in mouse fibroblasts, quantitative analysis and modelling were used to elucidate both marked and subtle differences between the two networks.
Their major point of divergence lies in the activation of PI3K signalling, with PDGF stimulating this pathway maximally and FGF weakly so. Activated PDGF receptors directly engage the regulatory subunits of type IA PI3Ks with high avidity and specificity [26, 27], and so it is likely that potent activation of PI3K signalling is a general feature of PDGF receptor signalling. In contrast, FGF receptors generally rely upon the scaffold proteins FRS2 and Gab1 for PI3K recruitment [28, 29], and therefore one might expect PI3K signalling to vary according to the expression levels of FRS2, Gab1, and/or other accessory proteins.
The less obvious difference between the two networks in our cells lies in their common, Ras-dependent pathway to MEK/ERK activation. Whereas both growth factors maximally stimulate Ras-GTP loading and MEK/ERK phosphorylation to similar extents, the level of MEK/ERK phosphorylation elicited by PDGF stimulation relies more on PI3K-dependent signalling. Our kinetic models encompassing both receptor networks reconcile those quantitative measurements by allowing Ras-GTP generated in response to FGF to yield higher MEK kinase activity, although differential access to MEK phosphatases or differences in feedback regulation provide alternative mechanistic explanations. How might such differences be encoded? The answer, we speculate, lies in the localization of the receptors. Mathematical formulations of the standard type formulated here, which are most appropriate for modelling population data [23], do not readily account for such effects. Depending on the experimental context, growth factors are capable of stimulating Ras-GTP loading and downstream signalling not only at the plasma membrane but also from internal membranes associated with early endosomes, Golgi apparatus, and endoplasmic reticulum [30–32]. At the plasma membrane, Ras-GTP might be enriched in the vicinity of activated receptors [33] and in membrane microdomains in a Ras isoform-dependent manner [34–36]. What is clear from these and other indications is that the Ras-GTP level measured on a whole-cell basis only tells part of the story. We find it plausible that differential localization of receptors along with their intracellular binding partners results in activation of distinct Ras pools that vary in their propagation of signalling through MEK. An alternative explanation is that a third, FGF-stimulated pathway, not accounted for here, synergizes with Ras to more potently activate MEK kinases.
This study was intended as a test case for more systematic comparisons of signalling networks that, analyzed no further than the level of molecular connectivity/network structure, might be considered quite similar. In our view it also illustrates the various levels of quantitative scrutiny required, none higher than the level of mathematical model formulation and experimental validation, to achieve different levels of network characterization. The comparable levels of Ras/ERK signalling and the marked disparity in PI3K signalling elicited by FGF and PDGF stimulation in our cells might readily have been ascertained from a screen of the sort carried out in recent years [37]; however, the full picture in the context of pathways that converge upon MEK and ERK phosphorylation and the feedback regulation of those pathways would have been glossed over. Hence, the challenge will be how to sensibly apply more systematic approaches across an array of cellular contexts.
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to acknowledge Shoeb Ahmed for performing feasibility studies related to this work.
FUNDING
This work was supported through a grant from the National Institutes of Health (GM088987).
Abbreviations used
- dMAC
dynamic MEK activation comparator
- ERK
extracellular signal-regulated kinase
- FGF
fibroblast growth factor
- PDGF
platelet-derived growth factor
- PI3K
phosphoinositide 3-kinase
- SSD
sum of squared deviations
Footnotes
AUTHOR CONTRIBUTION
Murat Cirit designed and performed all laboratory and computational experiments. Jason M. Haugh obtained grant support, designed and supervised the study, and wrote the paper. Both authors analysed results and contributed to the editing of the paper.
REFERENCES
- 1.Bray D. Protein molecules as computational elements in living cells. Nature. 1995;376:307–312. doi: 10.1038/376307a0. [DOI] [PubMed] [Google Scholar]
- 2.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pawson T. Specificity in signal transduction: from phosphotyrosine-SH2 domain interactions to complex cellular systems. Cell. 2004;116:191–203. doi: 10.1016/s0092-8674(03)01077-8. [DOI] [PubMed] [Google Scholar]
- 4.Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-related kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 5.Komarova NL, Zou XF, Nie Q, Bardwell L. A theoretical framework for specificity in cell signaling. Mol. Syst. Biol. 2005;1 doi: 10.1038/msb4100031. Art. No. 2005.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behar M, Dohlman HG, Elston TC. Kinetic insulation as an effective mechanism for achieving pathway specificity in intracellular signaling networks. Proc. Natl. Acad. Sci. U.S.A. 2007;104:16146–16151. doi: 10.1073/pnas.0703894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson TR, Stephens LR, Hawkins PT. Receptor specificity of growth factor-stimulated synthesis of 3-phosphorylated inositol lipids in Swiss 3T3 cells. J. Biol. Chem. 1992;267:16627–16636. [PubMed] [Google Scholar]
- 8.Osterop APRM, Medema RH, van der Zon GCM, Bos JL, Moller W, Maassen JA. Epidermal-growth-factor-receptors generate Ras-GTP more efficiently than insulin receptors. Eur. J. Biochem. 1993;212:477–482. doi: 10.1111/j.1432-1033.1993.tb17684.x. [DOI] [PubMed] [Google Scholar]
- 9.Klarlund JK, Cherniak AD, Czech MP. Divergent mechanisms for homologous desensitization of p21ras by insulin and growth factors. J. Biol. Chem. 1995;270:23421–23428. doi: 10.1074/jbc.270.40.23421. [DOI] [PubMed] [Google Scholar]
- 10.Janes KA, Albeck JG, Gaudet S, Sorger PK, Lauffenburger DA, Yaffe MB. A systems model of signaling identifies a molecular basis set for cytokine-induced apoptosis. Science. 2005;310:1646–1653. doi: 10.1126/science.1116598. [DOI] [PubMed] [Google Scholar]
- 11.Chen WW, Schoeberl B, Jasper PJ, Niepel M, Nielsen UB, Lauffenburger DA, Sorger PK. Input-output behavior of ErbB signaling pathways as revealed by a mass action model trained against dynamic data. Mol. Syst. Biol. 2009;5 doi: 10.1038/msb.2008.74. article no. 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borisov N, Aksamitiene E, Kiyatkin A, Legewie S, Berkhout J, Maiwald T, Kaimachnikov NP, Timmer J, Hoek JB, Kholodenko BN. Systems-level interactions between insulin-EGF networks amplify mitogenic signaling. Mol. Syst. Biol. 2009;5 doi: 10.1038/msb.2009.19. Art. no. 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schoeberl B, Pace EA, Fitzgerald JB, Harms BD, Xu L, Nie L, Linggi B, Kalra A, Paragas V, Bukhalid R, Grantcharova V, Kohli N, West KA, Leszczyniecka M, Feldhaus MJ, Kudla AJ, Nielsen UB. Therapeutically targeting ErbB3: a key node in ligand-induced activation of the ErbB receptor-PI3K axis. Sci. Signal. 2009;2 doi: 10.1126/scisignal.2000352. Art. no. ra31. [DOI] [PubMed] [Google Scholar]
- 14.Nakakuki T, Birtwistle MR, Saeki Y, Yumoto N, Ide K, Nagashima T, Brusch L, Ogunnaike BA, Okada-Hatakeyama M, Kholodenko BN. Ligand-specific c-Fos expression emerges from the spatiotemporal control of ErbB network dynamics. Cell. 2010;141:884–896. doi: 10.1016/j.cell.2010.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iyengar R. Why we need quantitative dynamic models. Sci. Signal. 2009;2:eg3. doi: 10.1126/scisignal.264eg3. [DOI] [PubMed] [Google Scholar]
- 16.Kreeger PK, Lauffenburger DA. Cancer systems biology: a network modeling perspective. Carcinogenesis. 2010;31:2–8. doi: 10.1093/carcin/bgp261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meloche S, Pouysségur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26:3227–3239. doi: 10.1038/sj.onc.1210414. [DOI] [PubMed] [Google Scholar]
- 18.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 19.Wang C-C, Cirit M, Haugh JM. PI3K-dependent crosstalk interactions converge with Ras as quantifiable inputs integrated by Erk. Mol. Syst. Biol. 2009;5 doi: 10.1038/msb.2009.4. article no. 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cirit M, Wang C-C, Haugh JM. Systematic quantification of negative feedback mechanisms in the extracellular signal-regulated kinase (ERK) signaling network. J. Biol. Chem. 2010;285:36736–36744. doi: 10.1074/jbc.M110.148759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaur H, Park CS, Lewis JM, Haugh JM. Quantitative model of Ras/phosphoinositide 3-kinase signalling cross-talk based on co-operative molecular assembly. Biochem. J. 2006;393:235–243. doi: 10.1042/BJ20051022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haugh JM, Elston TC, Cirit M, Wang C-C, Hao N, Yildirim N. Jayaraman A, Hahn J. Methods in Bioengineering: Systems Analysis of Biological Networks. Artech House; 2009. Data-driven, mechanistic modeling of biochemical reaction networks; pp. 57–74. [Google Scholar]
- 24.Castellano E, Downward J. Role of RAS in the regulation of PI 3-kinase. Curr. Top. Microbiol. Immunol. 2010;346:143–169. doi: 10.1007/82_2010_56. [DOI] [PubMed] [Google Scholar]
- 25.Gutenkunst RN, Waterfall JJ, Casey FP, Brown KS, Myers CR, Sethna JP. Universally sloppy parameter sensitivities in systems biology models. PLoS Comput. Biol. 2007;3:e189. doi: 10.1371/journal.pcbi.0030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kazlauskas A, Cooper JA. Phosphorylation of the PDGF receptor β-subunit creates a tight binding site for phosphatidylinositol-3 kinase. Embo. J. 1990;9:3279–3286. doi: 10.1002/j.1460-2075.1990.tb07527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ottinger EA, Botfield MC, Shoelson SE. Tandem SH2 domains confer high specificity in tyrosine kinase signaling. J. Biol. Chem. 1998;273:729–735. doi: 10.1074/jbc.273.2.729. [DOI] [PubMed] [Google Scholar]
- 28.Hadari YR, Gotoh N, Kouhara H, Lax I, Schlessinger J. Critical role for the docking-protein FRS2 alpha in FGF receptor-mediated signal transduction pathways. Proc. Natl. Acad. Sci. U.S.A. 2001;98:8578–8583. doi: 10.1073/pnas.161259898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ong SH, Hadari YR, Gotoh N, Guy GR, Schlessinger J, Lax I. Stimulation of phosphatidylinositol 3-kinase by fibroblast growth factor receptors is mediated by coordinated recruitment of multiple docking proteins. Proc. Natl. Acad. Sci. U.S.A. 2001;98:6074–6079. doi: 10.1073/pnas.111114298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pol A, Calvo M, Enrich C. Isolated endosomes from quiescent rat liver contain the signal transduction machinery: differential distribution of activated Raf-1 and Mek in the endocytic compartment. FEBS Lett. 1998;441:34–38. doi: 10.1016/s0014-5793(98)01517-8. [DOI] [PubMed] [Google Scholar]
- 31.Haugh JM, Huang AC, Wiley HS, Wells A, Lauffenburger DA. Internalized epidermal growth factor receptors participate in the activation of p21ras in fibroblasts. J. Biol. Chem. 1999;274:34350–34360. doi: 10.1074/jbc.274.48.34350. [DOI] [PubMed] [Google Scholar]
- 32.Bivona TG, Philips MR. Ras pathway signaling on endomembranes. Curr. Opin. Cell Biol. 2003;15:136–142. doi: 10.1016/s0955-0674(03)00016-4. [DOI] [PubMed] [Google Scholar]
- 33.Monine MI, Haugh JM. Signal transduction at point-blank range: analysis of a spatial coupling mechanism for pathway crosstalk. Biophys. J. 2008;95:2172–2182. doi: 10.1529/biophysj.108.128892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murakoshi H, Iino R, Kobayashi T, Fujiwara T, Ohshima C, Yoshimura A, Kusumi A. Single-molecule imaging analysis of Ras activation in living cells. Proc. Natl. Acad. Sci. U.S.A. 2004;101:7317–7322. doi: 10.1073/pnas.0401354101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plowman SJ, Muncke C, Parton RG, Hancock JF. H-ras, K-ras, and inner plasma membrane raft proteins operate in nanoclusters with differential dependence on the actin cytoskeleton. Proc. Natl. Acad. Sci. U.S.A. 2005;102:15500–15505. doi: 10.1073/pnas.0504114102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harding A, Tian TH, Westbury E, Frische E, Hancock JF. Subcellular localization determines MAP kinase signal output. Curr. Biol. 2005;15:869–873. doi: 10.1016/j.cub.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 37.Natarajan M, Lin KM, Hsueh RC, Sternweis PC, Ranganathan R. A global analysis of cross-talk in a mammalian cellular signalling network. Nat. Cell Biol. 2006;8:571–580. doi: 10.1038/ncb1418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.