Abstract
Background
Cranial radiotherapy (CRT) is a known risk factor for neurocognitive impairment in survivors of childhood cancer and may increase risk for mild cognitive impairment and dementia in adulthood.
Methods
We performed a cross-sectional evaluation of survivors of childhood acute lymphoblastic leukemia (ALL) treated with 18 Gy (n = 127) or 24 Gy (n = 138) CRT. Impairment (age-adjusted score >1 standard deviation below expected mean, two-sided exact binomial test) on the Wechsler Memory Scale IV (WMS-IV) was measured. A subset of survivors (n = 85) completed structural and functional neuroimaging.
Results
Survivors who received 24 Gy, but not 18 Gy, CRT had impairment in immediate (impairment rate = 33.8%, 95% confidence interval [CI] = 25.9% to 42.4%; P < .001) and delayed memory (impairment rate = 30.2%, 95% CI = 22.6% to 38.6%; P < .001). The mean score for long-term narrative memory among survivors who received 24 Gy CRT was equivalent to that for individuals older than 69 years. Impaired immediate memory was associated with smaller right (P = .02) and left (P = .008) temporal lobe volumes, and impaired delayed memory was associated with thinner parietal and frontal cortices. Lower hippocampal volumes and increased functional magnetic resonance imaging activation were observed with memory impairment. Reduced cognitive status (Brief Cognitive Status Exam from the WMS-IV) was identified after 24 Gy (18.5%, 95% CI = 12.4% to 26.1%; P < .001), but not 18 Gy (8.7%, 95% CI = 4.4% to 15.0%; P = .11), CRT, suggesting a dose–response effect. Employment rates were equivalent (63.8% for 24 Gy CRT and 63.0% for 18 Gy CRT).
Conclusions
Adult survivors who received 24 Gy CRT had reduced cognitive status and memory, with reduced integrity in neuroanatomical regions essential in memory formation, consistent with early onset mild cognitive impairment.
Survival rates for most childhood cancers have improved over the last four decades. In the modern era of cancer treatment, more than 80% of those diagnosed with a pediatric malignancy will become 5-year survivors (1). This progress has resulted in a growing population of adult survivors of childhood cancer, with current estimates that one in every 640 young adults aged between 20 and 39 years is a survivor of a pediatric malignancy (2).
Survivors exposed to cranial radiotherapy (CRT) represent a subpopulation at particularly high-risk for long-term morbidity. It is established that within the first 5 to 10 years after CRT survivors of childhood acute lymphoblastic leukemia (ALL) are at increased risk for deficits in neurocognitive skills, including attention, working memory, and processing speed (3,4). Yet, it is unknown whether survivors, as they age, will demonstrate early onset of age-related memory problems or dementia. Thus, aging adult survivors of childhood ALL provide a “sentinel” population, representing the first large group of survivors exposed to CRT to reach the middle decades of life.
As survivors age, global brain injury from early CRT may reduce cognitive reserve, placing them at risk for early onset dementia or memory impairment (5,6). However, the prevalence of dementia and memory impairment in aging adult survivors of childhood ALL has not previously been established. Our objective was to estimate the prevalence of memory impairment; mild cognitive impairment (MCI), a state of cognitive function intermediate between the changes seen in aging and those fulfilling the criteria for dementia (7); and dementia in this population. We also wanted to determine whether neuroanatomical characteristics of dementia (eg, reduced hippocampal volume with increased activation and thinner parietal and frontal cortices) were associated with this memory impairment.
Methods
Study Population
We identified 443 survivors (Supplementary Figure 1, available online) who were treated for childhood ALL at St. Jude Children’s Research Hospital with CRT before age 16 years and who were at least 25 years of age at the time of follow-up and eligible for evaluation in the St. Jude Lifetime Cohort Study [SJLIFE, study methodology previously published (8,9)]. Exclusion criteria for this report included history of craniotomy, ventriculoperitoneal shunt placement, subsequent central nervous system neoplasm, traumatic brain injury, previous genetic diagnosis with known association with neurocognitive impairment (eg, Down syndrome), or current receipt of anticancer therapy. The study protocol was approved by the St. Jude Children’s Research Hospital Institutional Review Board, and all participants provided written informed consent.
Procedures
Neurocognitive testing was conducted during a 2-hour session using the Wechsler Memory Scale IV (10), including four composite memory domains (immediate, delayed, auditory, and visual memory) derived from eight specific subtests (logical, design, verbal paired associates, and visual reproduction, each of which had immediate and delayed recall measures); the Brief Cognitive Status Exam; and the Wechsler Abbreviated Scale of Intelligence (11). Order of testing was standardized, and survivors’ schedules were adjusted to limit impact from fatigue and extraneous factors.
Survivors were invited to participate in structural and functional assessment with magnetic resonance imaging (MRI) on a 3T Siemens Trio MR (Siemens Medical Systems, Malvern, PA). Three-dimensional T1-weighted, T2-weighted, and FLAIR-weighted imaging sets were acquired, registered to the ICBM average 152 T2 atlas aligned in Talairach space, resampled to a 1-mm isotropic resolution, intensity corrected (12), and segmented by tissue class (13,14). White matter, gray matter, and cerebrospinal fluid volumes were assessed for frontal, parietal, occipital, and temporal lobes. Diffusion tensor imaging (DTI) was acquired with 12 noncollinear, noncoplanar diffusion gradient directions to calculate the diffusion tensor for each voxel. Voxelwise tensor calculations were performed with the DTI toolkit under SPM8 (http://www.fil.ion.ucl.ac.uk/spm/), and parameter maps of fractional anisotropy, apparent diffusion coefficient, and radial and axial diffusivity were generated. After registering the parametric maps to the atlas space, average values for each parameter within the segmented white matter regions were assessed for each lobe. The T1-weighted MRI set was further processed with the FreeSurfer software (http://surfer.nmr.mgh.harvard.edu/, Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA) to assess cortical thickness within selected regions and hippocampal volumes (15). During task-specific functional MRI, patients performed an auditory cued-recall memory task validated to predict memory loss in patients at risk for Alzheimer’s disease (16). Image acquisition was conducted as previously reported and analyzed with SPM5 software (http://www.fil.ion.ucl.ac.uk/spm/) (17).
Medical record abstraction was performed to capture exposure data, including cumulative doses of chemotherapy exposure, surgical procedures, and radiation treatment (whole-brain dose). All participants completed comprehensive questionnaires covering the following domains: health history and status, social and demographic factors, health behaviors, and psychosocial history. Comprehensive medical assessments were also conducted as part of the SJLIFE cohort protocol (8).
Statistical Analyses
Descriptive statistics for the entire eligible cohort and participants, stratified by CRT dose (24 Gy and 18 Gy), and for the subset of survivors evaluated by MRI are provided in Table 1. Age-adjusted z scores for the four composite memory domains and eight memory subtests were calculated based on comparison with normal data with ages grouped as specified in the scoring manual (10). Impairment for each memory test was defined as a z score more than 1 standard deviation (SD) below the normal mean of zero (18–20). Exact binomial tests were used to compare memory test impairment rates with 15% (1 SD) in the normal population, and Fisher exact tests were used to compare impairment rates between the two CRT dose groups. Multiple logistic regression models were developed to investigate the relationship between impairment and treatment factors (cumulative intrathecal and intravenous methotrexate and CRT doses). The models were adjusted for time from CRT, age at CRT, and sex. The small number of minority participants precluded analysis by race/ethnicity. Comparison of structural and functional neuroimaging measures between impaired and unimpaired survivors for both immediate and delayed memory function were assessed using two sample t tests. A P value of less than .05 was considered statistically significant, and all statistical tests were two-sided.
Table 1.
Survivor demographic and treatment characteristics*
| Characteristic | Eligible | Evaluated | 24 Gy CRT | 18 Gy CRT | P† | Evaluated by MRI |
|---|---|---|---|---|---|---|
| n = 443 | n = 265 | n = 138 | n = 127 | n = 85 | ||
| Demographics, No. (%) | ||||||
| Sex | ||||||
| Female | 219 (49.4) | 137 (51.7) | 70 (50.7) | 67 (52.8) | .74 | 44 (51.8) |
| Male | 224 (50.6) | 128 (48.3) | 68 (49.3) | 60 (47.2) | 41 (48.2) | |
| Race | ||||||
| White | 403 (91.0) | 241 (90.9) | 130 (94.2) | 111 (87.4) | .05 | 78 (91.8) |
| Other | 40 (9.0) | 24 (9.1) | 8 (5.8) | 16 (12.6) | 7 (8.2) | |
| Highest grade | ||||||
| College graduate | — | 90 (34.0) | 37 (26.8) | 53 (41.7) | .01 | 31 (36.5) |
| Non–college graduate | — | 172 (64.9) | 100 (72.5) | 72 (56.7) | 52 (61.2) | |
| Current employment | — | |||||
| Full time | — | 168 (63.4) | 88 (63.8) | 80 (63.0) | .97 | 59 (69.4) |
| Part time or unemployed | — | 95 (35.9) | 50 (36.2) | 45 (35.4) | 25 (29.4) | |
| Current age, y, mean (SD) | 37.4 (6.5) | 37.1 (6.6) | 40.9 (5.8) | 33.0 (4.6) | <.001 | 36.7 (6.4) |
| Treatment characteristics, mean (SD) | ||||||
| IT MTX, mg/m2‡ | 143.0 (111.0) | 143.0 (113.0) | 101.0 (114.0) | 189.0 (94.1) | <.001 | 158.0 (131.0) |
| IV MTX, g/m2‡ | 2.6 (3.1) | 2.9 (3.5) | 2.1 (1.5) | 3.7 (4.6) | <.001 | 2.2 (3.1) |
| HD IV MTX, g/m2‡ | 1.6 (3.2) | 1.9 (3.7) | 0.4 (1.7) | 3.5 (4.5) | <.001 | 1.6 (3.4) |
| Age at CRT, y | 6.7 (3.9) | 6.9 (4.0) | 6.4 (4.1) | 7.3 (3.9) | .05 | 6.6 (4.1) |
| Time from CRT, y | 29.1 (6.6) | 30.3 (6.5) | 34.5 (4.7) | 25.6 (4.9) | <0.001 | 30.1 (6.2) |
| CRT dose, No. (%) | ||||||
| 24 Gy | 244 (55.1) | 138 (52.1) | — | — | 36 (42.4) | |
| 18 Gy | 199 (44.9) | 127 (47.9) | — | — | 49 (57.7) | |
* CRT = cranial radiotherapy; HD = high-dose; IT = intrathecal; IV = intravenous; MRI = magnetic resonance imaging; MTX = methotrexate; SD = standard deviation.
† P values are two-sided and based on χ2 test or two-sample t test for comparing the differences between the two radiation dose groups across demographic and treatment characteristics.
‡ Cumulative doses listed for IT MTX, IV MTX, and HD IV MTX calculated separately.
Results
Survivor Characteristics
Of 443 eligible survivors, 265 (60%) completed the memory evaluation, of whom 85 also completed the MRI examination (Table 1). The median age at evaluation was 36 years (range = 25–54 years), and median time from CRT was 30 years (range = 15–46 years). CRT exposure was evenly distributed between high- (24 Gy, n = 138) and low-dose exposure (18 Gy, n = 127). Ninety-five percent of participants also received intrathecal methotrexate, whereas 75% received intravenous methotrexate exposure. There was no difference in the distribution of CRT dose (P = .12) or demographic characteristics between participants and nonparticipants. Because of the evolution of treatment strategies over the years, not surprisingly, patients treated with 24 Gy CRT were older with a longer time since CRT at evaluation and received lower doses of methotrexate than those treated with 18 Gy CRT (Table 1).
Memory Impairment
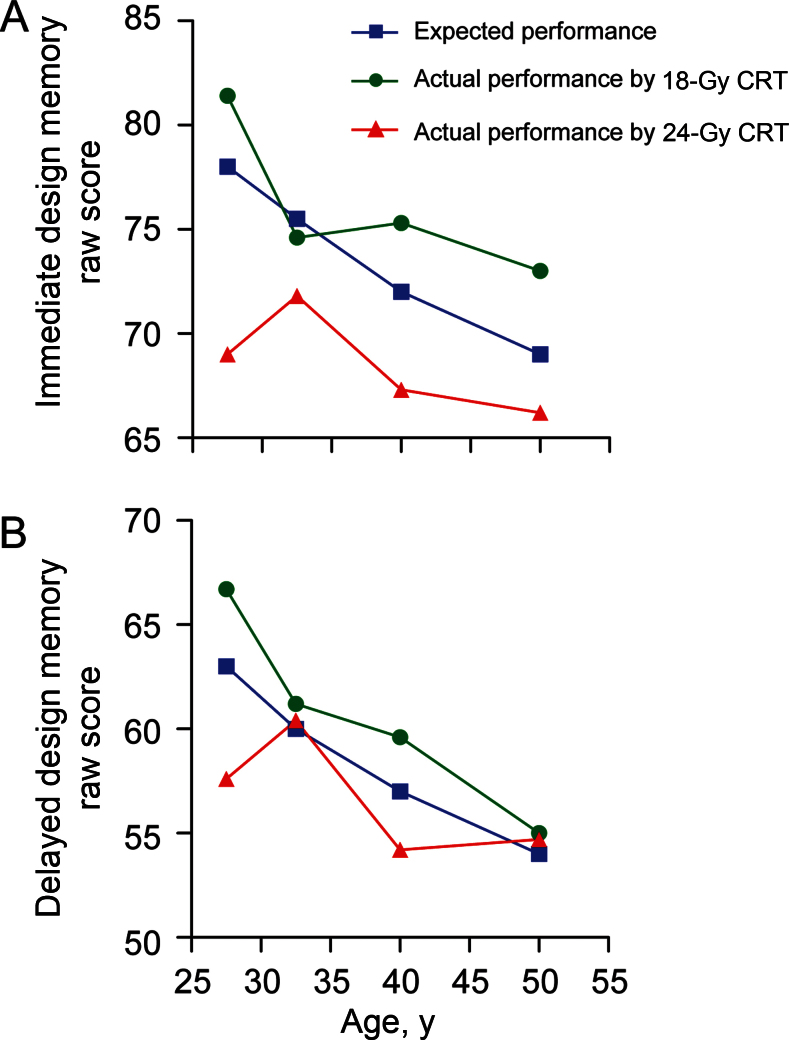
Compared with age-adjusted normative rates, survivors who received 24 Gy CRT had increased impairment on domains assessing immediate memory (impairment rate = 33.8%, 95% confidence interval [CI] = 25.9% to 42.4%; P <.001) and delayed memory (impairment rate = 30.2%, 95% CI = 22.6% to 38.6%; P < .001) (Table 2), whereas no increase was seen after 18 Gy CRT. There was no evidence for increased impairment based on age at evaluation (Supplementary Table 2, available online). On subtests evaluating logical memory (ie, narrative story recall) and design memory, higher CRT dose (24 vs 18 Gy) was associated with a higher prevalence of delayed memory impairment (logical: 28.3% vs 11.9%, P = .001; designs: 13.2% vs 3.2%, P = .003). However, no statistically significant CRT dose response was identified for immediate logical and design memory. The mean raw score for delayed logical memory among survivors who received 24 Gy CRT was equivalent to the mean score of adults older than 69 years in the general population (Table 3). Similarly, 24 Gy CRT survivors at a mean current age of 41 years had performance that would be expected in the 55 to 64 year age range in the general population for verbal paired associations and visual reproduction. Survivors who received 24 Gy CRT also demonstrated accelerated aging on the design memory test (Figure 1).
Table 2.
Memory and cognitive impairment in adult survivors of childhood acute lymphoblastic leukemia by cranial radiotherapy dose
| 24 Gy n = 138 | 18 Gy n = 127 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Domain | No. | Mean (SD)* | Impaired | P† | No. | Mean (SD)* | Impaired | P† | P‡ |
| Specific ability | No. (%; 95% CI) | No. (%; 95% CI) | |||||||
| Wechsler memory domains | |||||||||
| Immediate | 136 | −0.64 (0.97) | 46 (33.8; 25.9 to 42.4) | <.001 | 126 | 0.04 (1.02) | 22 (17.5; 11.2 to 25.2) | .51 | .003 |
| Delayed | 136 | −0.56 (0.99) | 41 (30.2; 22.6 to 38.6) | <.001 | 126 | 0.05 (1.02) | 19 (15.1; 9.3 to 22.5) | 1.0 | .005 |
| Auditory | 137 | −0.56 (1.05) | 42 (30.7; 23.1 to 39.1) | <.001 | 126 | 0.06 (0.97) | 21 (16.7; 10.6 to 24.3) | .67 | .009 |
| Visual | 136 | −0.51 (0.87) | 29 (21.3; 14.8 to 29.2) | .06 | 126 | 0.07 (0.98) | 17 (13.5; 8.1 to 20.7) | .75 | .11 |
| Memory subtests | |||||||||
| Logical memory– immediate | 138 | −0.62 (1.00) | 32 (23.2; 16.4 to 31.1) | .01 | 127 | 0.14 (1.04) | 19 (15.0; 9.2 to 22.4) | 1.0 | .12 |
| Logical memory–delayed | 138 | −0.67 (1.08) | 39 (28.3; 20.9 to 36.6) | <.001 | 126 | 0.23 (1.01) | 15 (11.9; 6.8 to 18.9) | .40 | .001 |
| Design memory–immediate | 136 | −0.30 (0.81) | 20 (14.7; 9.2 to 21.8) | 1.0 | 126 | 0.11 (0.96) | 11 (8.7; 4.4 to 15.1) | .05 | .18 |
| Design memory–delayed | 136 | −0.18 (0.79) | 18 (13.2; 8.0 to 20.1) | .67 | 126 | 0.13 (0.88) | 4 (3.2; 0.9 to 7.9) | <.001 | .003 |
| Verbal paired association–immediate | 137 | −0.34 (0.94) | 28 (20.4; 14.0 to 28.2) | .11 | 126 | 0.00 (0.92) | 13 (10.3; 5.6 to 17.0) | .17 | .03 |
| Verbal paired association–delayed | 137 | −0.23 (1.03) | 26 (19.0; 12.8 to 26.6) | .24 | 126 | 0.15 (0.91) | 12 (9.5; 5.0 to 16.0) | .10 | .04 |
| Visual reproduction–immediate | 137 | −0.59 (1.05) | 37 (27.0; 19.8 to 35.2) | <.001 | 127 | 0.08 (0.94) | 16 (12.6; 7.4 to 19.6) | .54 | .004 |
| Visual reproduction–delayed | 137 | −0.55 (0.88) | 33 (24.1; 17.2 to 32.1) | .007 | 127 | 0.07 (0.99) | 14 (11.0; 6.2 to 17.8) | .25 | .006 |
| Brief Cognitive Status Exam | |||||||||
| Reduced status§ | 135 | 25 (18.5; 12.4 to 26.1) | <.001 | 127 | 11 (8.7; 4.4 to 15.0) | .11 | |||
| Brief Cognitive Status Exam | No. (%) | No. (%) | |||||||
| Average | 77 (55.8) | 88 (69.3) | |||||||
| Low average | 21 (15.2) | 21 (16.5) | |||||||
| Borderline | 12 (8.7) | 7 (5.5) | |||||||
| Low | 16 (11.6) | 11 (8.7) | |||||||
| Very low | 9 (6.5) | 0 (0) | |||||||
* Mean and standard deviation (SD) represented in age-adjusted z scores, referenced to nationally representative norms. CI = confidence interval.
† P value based on two-sided exact one sample bionomial test for comparing the impairment rate to the 15% impairment (>1 SD) in general population.
‡ P value based on two-sided Fisher exact test for comparing the impairment between 24 Gy and 18 Gy cranial radiotheraphy groups.
§ Reduced cognitive status defined as low or very low on the Brief Cognitive Status Exam and the P value is based on exact one sample bionomial test for comparing the reduced congnitive rate to 5% rate in the general population.
Table 3.
Mean raw scores for memory subtests among 265 survivors at a median age of 36 years compared with expected age range in the general population by cranial radiotheraphy dose exposure
| Memory subtests | No. | 18 Gy (mean age = 33.0 years) | No. | 24 Gy (mean age = 40.9 years) | ||
|---|---|---|---|---|---|---|
| Mean raw score | Expected age, y | Mean raw score | Expected age, y | |||
| Logical memory–immediate | 127 | 24.4 | 20–69 | 138 | 21.2 | >69 |
| Logical memory–delayed | 126 | 20.9 | 45–54 | 138 | 17.4 | >69 |
| Design memory–immediate | 126 | 76.8 | 24–34 | 136 | 67.6 | 45–54 |
| Design memory–delayed | 136 | 62.3 | 25–34 | 136 | 55.0 | 35–54 |
| Verbal paired association– immediate | 126 | 35.0 | 30–34 | 137 | 29.6 | 55–64 |
| Verbal paired association–delayed | 126 | 11.6 | 25–44 | 137 | 9.6 | 55–64 |
| Visual reproduction–immediate | 127 | 36.6 | 35–44 | 137 | 33.1 | 55–64 |
| Visual reproduction–delayed | 127 | 28.7 | 30–44 | 137 | 21.2 | 55–64 |
Figure 1.
Immediate (A) and delayed (B) design memory mean raw scores by age group for survivors by cranial radiotherapy (CRT) dose compared with expected performance.
On multivariable analysis (Table 4), 24 Gy CRT (vs 18 Gy CRT) was associated with a twofold increased risk for impaired immediate memory (odds ratio [OR] = 2.78, 95 % CI = 1.22 to 6.34) and delayed memory (OR = 2.21, 95% CI = 0.90 to 5.42), although the latter did not achieve statistical significance. Age at CRT, time from CRT, and cumulative intrathecal or intravenous methotrexate exposure were not statistically significantly associated with either immediate or delayed memory deficits. Among memory subtests, even after adjustment for other factors, 24 Gy CRT remained associated with increased impairment in delayed but not immediate design memory (Table 4).
Table 4.
Multivariable regression models predicting memory impairment among 265 survivors*
| Variable | Immediate memory | Delayed memory | Immediate design subtest | Delayed design subtest | Immediate logical memory subtest | Delayed logical memory subtest | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P† | OR (95% CI) | P† | OR (95% CI) | P† | OR (95% CI) | P† | OR (95% CI) | P† | OR (95% CI) | P† | |
| Time from CRT | 0.99 (0.92 to 1.06) | .70 | 1.00 (0.93 to 1.08) | .90 | 1.01 (0.92 to 1.11) | .77 | 0.97 (0.88 to 1.08) | .63 | 1.00 (0.92 to 1.08) | .92 | 1.02 (0.95 to 1.1) | .62 |
| Age at CRT | 0.94 (0.87 to 1.01) | .10 | 0.97 (0.89 to 1.04) | .38 | 0.98 (0.89 to 1.09) | .75 | 1.02 (0.91 to 1.14) | .76 | 0.96 (0.88 to 1.05) | .35 | 1.00 (0.92 to 1.08) | .93 |
| Sex | ||||||||||||
| Female | 0.79 (0.45 to 1.4) | .42 | 0.71 (0.39 to 1.29) | .26 | 0.75 (0.35 to 1.6) | .46 | 0.87 (0.36 to 2.15) | .77 | 0.48 (0.25 to 0.9) | .02 | 0.85 (0.46 to 1.57 | .61 |
| Male | 1.00 (referent) | 1.00 (referent) | 1.00 | 1.00 (referent) | 1.00 | 1.00 (referent) | 1.00 | 1.00 (referent) | 1.00 | 1.00 (referent) | ||
| IT methotrexate | ||||||||||||
| Yes | 1.43 (0.31 to 6.69) | .65 | 3.96 (0.43 to 36.2) | .22 | 1.77 (0.17 to 18.0) | .63 | 0.25 (0.03 to 2.07) | .20 | 3.19 (0.34 to 30.0) | .31 | 4.50 (0.49 to 41.2) | .18 |
| No | 1.00 (referent) | 1.00 | 1.00 (referent) | 1.00 | 1.00 (referent) | 1.00 | 1.00 (referent) | 1.00 | 1.00 (referent) | 1.00 | 1.00 (referent) | |
| IV methotrexate | ||||||||||||
| Yes | 0.69 (0.34 to 1.40) | .31 | 1.22 (0.56 to 2.67) | .62 | 1.20 (0.44 to 3.27) | .73 | 2.19 (0.52 to 9.31) | .29 | 1.26 (0.56 to 2.85) | .57 | 1.07 (0.47 to 2.41) | .88 |
| No | 1.00 (referent) | 1.00 | 1.00 (referent) | 1.00 | 1.00 (referent) | 1.00 | 1.00 (referent) | 1.00 | 1.00 (referent) | 1.00 | 1.00 (referent) | |
| CRT dose | ||||||||||||
| 24 Gy | 2.78 (1.22 to 6.34) | .02 | 2.21 (0.9 to 5.42) | .08 | 1.53 (0.49 to 4.79) | .47 | 5.40 (1.25 to 23.4) | .02 | 1.66 (0.65 to 4.25) | .29 | 2.50 (0.98 to 6.4) | .06 |
| 18 Gy | 1.00 (referent) | 1.00 | 1.00 (referent) | 1.00 | 1.00 (referent) | 1.00 | 1.00 (referent) | 1.00 | 1.00 (referent) | 1.00 | 1.00 (referent) | |
* For immediate, delayed, immediate design and delayed design memory, three patients were excluded from analysis because of missing data. CI = confidence interval; CRT = cranial radiotherapy; IT = intrathecal; IV = intravenous; OR = odds ratio.
† P value is based on two-sided Wald’s χ2 test for the multiple logistic regression model.
Reduced cognitive status, defined as low or very low on the BCSE, was identified after 24 Gy CRT (18.5%, 95% CI = 12.4% to 26.1%; P < .001), but not 18 Gy CRT (8.7%, 95% CI = 4.4% to 15.0%; P = .11), suggesting a CRT dose–response effect (Table 2). However, current employment rates were equivalent (63.8% in 24 Gy CRT and 63.0% in 18 Gy CRT) in both CRT dose groups, suggesting no difference in functional status. Multivariable analyses identified that both immediate memory (OR = 3.1, 95% CI = 1.3 to 7.5) and delayed memory impairment (OR = 4.3, 95% CI = 1.7 to 10.5), but not full-scale IQ, was associated with cognitive impairment.
Structural and Functional Neuroimaging
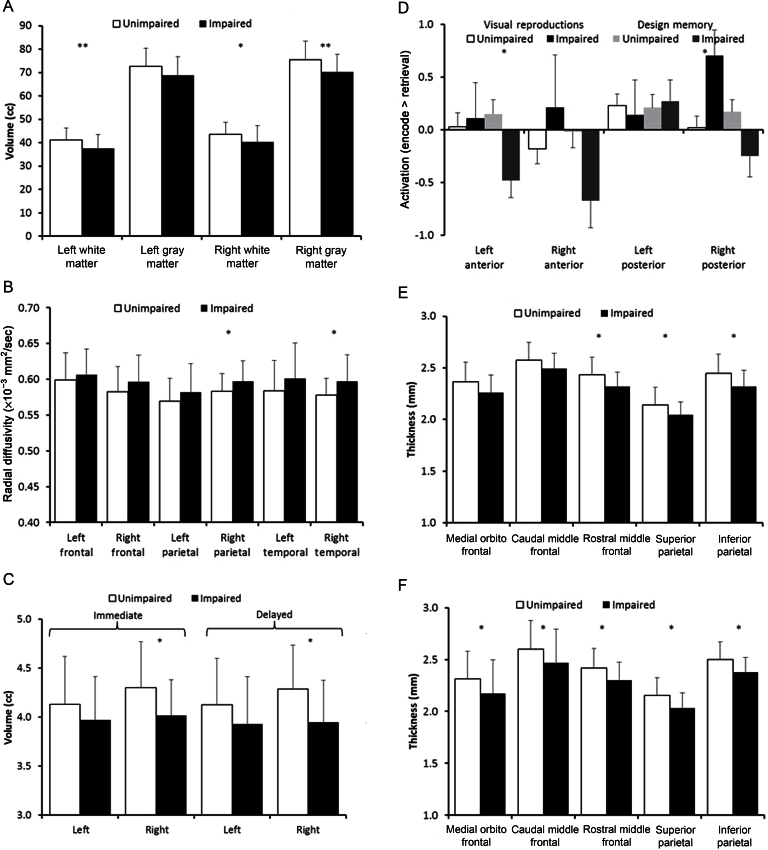
Impaired immediate memory was associated with smaller right (P = .02) and left (P = .008) temporal lobe white matter volumes on MRI structural imaging assessment (Figure 2A) and with increased radial diffusivity, an inverse measure of white matter integrity, in the right parietal (P = .04) and temporal lobes (P = .03) on diffusion tensor imaging (Figure 2B). Smaller right hippocampal volumes (Figure 2C) were associated with impaired immediate (P = .02) and delayed memory (P = .01). On functional MRI, activation (retrieval > encoding) in the left anterior hippocampus was associated with design memory impairment for all patients (P = .005) and within the 24 Gy (P = .04) (Figure 2D) but not 18 Gy population. Activation (encoding > retrieval) in the right posterior hippocampus was associated with visual reproduction impairment after 24 Gy CRT (P = .03) (Figure 2D). Impaired delayed memory was associated with thinner bilateral parietal and frontal cortices (Figure 2, E and F).
Figure 2.
Structural and functional neuroimaging measures were compared between impaired (black bars) and unimpaired (white bars) survivors for both immediate and delayed memory function using two sample, two-sided t tests. All survivors are included in analyses (A, B, C, E, and F), and only survivors that had received 24 Gy CRT are shown in (D). Statistically significant differences between groups were identified by a single asterisk (*) for P less than.05 and double asterisks (**) for P less than.01. Bars represent mean values displayed with one standard deviation error bars. A) Plots of bilateral volumes of gray matter and white matter in the temporal lobes categorized by impairment in immediate memory. B) Plots of bilateral diffusion tensor imaging measures of radial diffusivity for frontal, parietal, and temporal lobes categorized by impairment in immediate memory. C) Plots of bilateral hippocampal volumes categorized by impairment in immediate memory (left) and delayed memory (right). D) Plots of bilateral functional MRI activation in anterior and posterior regions of the hippocampus with greater activation for retrieval than for encoding. Patients were categorized by two different criteria. A first criterion was impaired (black bars) or unimpaired (white bars) on neurocognitive subtests of visual reproduction. A second criterion was impaired (dark gray bars) or unimpaired (light gray bars) on neurocognitive subtests of design memory. E, F) Plots of cortical thickness for a priori selected regions of frontal (medial orbitofrontal, caudal middle frontal, rostral middle frontal) and parietal (superior and inferior) regions on left (E) and right (F) categorized by impairment in delayed memory.
Discussion
As with other neurocognitive abilities, memory functions tend to decline with older age. MCI typically represents a state in which memory has declined beyond what is believed to be acceptable for aging, and dementia refers to the state of decline in multiple cognitive domains of sufficient severity to affect function. Young children who sustain mild, focal brain injury have a high capacity for adaptation because of neuronal plasticity, although children with diffuse brain injury, exemplified by moderate to severe traumatic brain injury, are at increased risk for continued functional limitations and early onset of dementia (21,22). Like traumatic brain injury, survivors of childhood cancer treated with whole-brain CRT have sustained not focal but diffuse injury at a young age and may be at increased risk for early-onset memory loss and dementia. Specific aspects of delayed memory more commonly associated with aging and dementia have not been routinely assessed among survivors treated with CRT. Among studies that reported memory assessment in the first 5 to 10 years from CRT, evidence is equivocal regarding deficits in delayed memory (23–25). However, we now identify that at a median age of 41 years, adult survivors of childhood cancer who received 24 Gy CRT have delayed recall of narrative events similar to the general population older than 69 years. Furthermore, these survivors function at a level one to two decades older than their chronologic age in their ability to recall verbal associations and to reproduce visual designs and patterns. These delayed memory findings suggest that survivors of childhood cancer who received 24 Gy CRT are experiencing early onset of cognitive aging.
Survivors who received 18 Gy CRT had no statistically significant impairment in immediate or delayed memory in the time interval studied. Those who received 24 Gy showed twice the rate of impairment relative to lower-dose CRT. Additionally, it appears that delayed memory may be more affected by CRT dose than immediate memory. Rates of impairment in delayed logical (narrative) and design memory are statistically significantly greater with increasing CRT dose, whereas impairments in immediate logical and design memory do not increase. Thus, an increase in CRT dose may selectively increase risk for delayed memory deficits compared with immediate recall, a pattern consistent with normal aging, but at a median age of 36 years. Thus, it is possible that CRT exposure to the developing central nervous system may result in reduced cognitive reserve that, over time, manifests as early onset of memory impairment. It will be important to continue to monitor memory performance in those who received 18 Gy CRT to determine whether decline becomes apparent over a longer postirradiation interval. Fortunately, the SJLIFE cohort provides a platform for longitudinal reassessment of this population across the lifespan.
In the general population, impairment in memory and reduced cognitive function may exist without evidence for frank dementia. In this study, impairment in cognitive status did not seem to manifest as a measurable change in functional status as employment rates did not differ between the two dose groups. This suggests that, rather than frank dementia, deficits in middle adulthood may be more consistent with MCI, a state of cognitive function intermediate between the changes seen in aging and those fulfilling the criteria for dementia (7). Typically with MCI, patients and their families are aware of the increasing forgetfulness; however, functional activities are still intact. Longitudinal studies have shown that persons with MCI are at increased risk for the development of frank dementia (26). Thus, continued longitudinal follow-up of this sentinel population is essential. Finally, multivariable models suggest this reduced cognitive function is not a product of established global cognitive injury (full-scale IQ) but rather is associated primarily with memory impairment.
Survivors with memory and cognitive impairment demonstrated reduced integrity on structural and functional neuroimaging in anatomical regions established as essential for memory formation. The formation of new memories is mediated through the temporal lobes, including hippocampal and parahippocampal structures, whereas long-term recall requires additional processing in frontal and parietal lobes. Reduction in cortical volume and thickness, as well as white matter volume and integrity, among survivors with memory impairments lends supporting evidence for early onset of age-related changes or perhaps MCI. Although indices of radial diffusivity in the unimpaired survivors were approximately normal for age (27), the survivors with impaired short-term memory demonstrated statistically significantly elevated diffusivity in the temporal–parietal memory network, which is consistent with patterns seen in patients with MCI (28). Furthermore, these increased diffusivity measures are more indicative of an older population (29). Similar to older subjects with cognitive impairment, these childhood cancer survivors with impaired delayed memory displayed atypical cortical thinning of the medial orbito-frontal and parietal regions (30). Hippocampal volumes were approximately age appropriate on the left but displayed pronounced atrophy on the right, effectively eliminating the typical bilateral asymmetry (31). Greater atrophy of the right hippocampus has been demonstrated in healthy older subjects that later develop profound neurocognitive impairments in memory (30,32). Functional MRI during the cued-recall memory task showed elevated hippocampal activation in patients with memory impairment. A similar pattern of compensatory hippocampal engagement during this task was reported for older adults with increased genetic risk for Alzheimer’s disease, and altered hippocampal activation was predictive of subsequent progression to dementia (16). Taken together, the structural and functional neuroimaging phenotype displayed by these survivors is most consistent with early aging and increased risk for memory impairment.
The strengths of this study include the large population of aging adult survivors with detailed ascertainment of their cancer therapy, the ability to compare populations based on CRT dose, the relatively long interval from CRT exposure, and use of a dedicated testing battery specifically validated and normalized for memory assessment in the general population. When interpreting the findings, study limitations should be also considered. We were not able on this cross-sectional evaluation to identify whether memory deficits increase with time from CRT. Such a relationship is essential to determine whether these memory findings are simply the result of the initial, static neurocognitive injury or, as hypothesized, are progressive with aging. Second, there is a risk of participation bias because survivors with perceived memory problems may be more likely to participate in the formal evaluation, which would result in overestimation of rates. However, there were no obvious differences between participants and nonparticipants based on demographic or radiation-related factors. Third, it is important to note that in the evolution of ALL therapy, prophylactic CRT doses were reduced over time to limit neurocognitive injury; thus survivors who received 24 Gy CRT in an earlier treatment era also had an older chronologic age. However, the memory battery scores are age-adjusted to allow reasonable direct comparison for a dose–response effect despite the age differential.
In summary, we identified early onset of memory and cognitive impairment associated with CRT dose in survivors of childhood ALL still at a relatively young age. Structural and functional neuroimaging provides biologic plausibility that impairments are associated with neural pathways established as essential for memory formation. For certain delayed memory domains, survivors function at a cognitive age two and three decades beyond their chronological age. This evidence should result in revision of Long-term Follow-up Guidelines from the Children’s Oncology Group to include evaluation of memory measures in aging adult survivors (33). Although modern therapies for ALL have reduced or eliminated the use of CRT in front-line therapy, there are estimated to be more than 50 000 survivors of ALL in the United States, most of whom received CRT, who may benefit from early screening and diagnosis of MCI (2). Additionally, although impairment in other cognitive domains after 18 Gy exposure has been established (34), in this cross-sectional assessment 18 Gy CRT resulted in minimal impairment in memory. Continued follow-up of this population is needed to determine their true risk for memory impairment as they age. Future studies should determine the longitudinal trajectory of memory loss and monitor for frank dementia as this population ages, and if longitudinal memory loss is confirmed, intervention strategies to slow that loss, such as physical activity interventions, should be considered.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health (R21 CA138988; GTA, principal investigator). Support to St. Jude Children’s Research Hospital was also provided by the Cancer Center Support (CORE) grant (CA 21765, R. Gilbertson, principal investigator) and ALSAC.
Supplementary Material
GTA had full access to the data in the study and had final responsibility for the decision to submit for publication. All authors contributed to the conception and design of the study and commented on the final draft of the report. Analyses were performed by NZ and DS. WER, AS, RJO, CMH, NS, MJK, and KRK contributed to the collection of data. GTA, WER, RCP, RJO, CMH, MJK, LK, C-HP, MMH, LLR, and KRK contributed to the interpretation of data and intellectual content.
RCP received funding from Pfizer, Inc and Janssen Alzheimer Immunotherapy (chair, Data Monitoring Committee), Elan Pharmaceuticals (consultant), GE Healthcare (consultant), Novartis, Inc (one CME lecture). All other authors have no relevant conflicts of interest to report. This study was completely independent of any pharmaceutical company or other commercial interest. The study sponsors had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
The abstract was presented orally at the American Society for Hematology annual meeting in December 2012.
References
- 1. Howlader NNA, Krapcho M, Neyman N. et al. , eds. SEER Cancer Statistics Review 1975–2008. Bethesda, MD: National Cancer Institute; 2011. [Google Scholar]
- 2. Mariotto AB, Rowland JH, Yabroff KR, et al. Long-term survivors of childhood cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1033–1040 [DOI] [PubMed] [Google Scholar]
- 3. Fletcher JM, Copeland DR. Neurobehavioral effects of central nervous system prophylactic treatment of cancer in children. J Clin Exp Neuropsychol. 1988;10(4):495–537 [DOI] [PubMed] [Google Scholar]
- 4. Langer T, Martus P, Ottensmeier H, Hertzberg H, Beck JD, Meier W. CNS late-effects after ALL therapy in childhood. Part III: neuropsychological performance in long-term survivors of childhood ALL: impairments of concentration, attention, and memory. Med Pediatr Oncol. 2002;38(5):320–328 [DOI] [PubMed] [Google Scholar]
- 5. Edelstein K, D’Agostino N, Bernstein LJ, et al. Long-term neurocognitive outcomes in young adult survivors of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2011;33(6):450–458 [DOI] [PubMed] [Google Scholar]
- 6. Richardson RB. Ionizing radiation and aging: rejuvenating an old idea. Aging. 2009;1(11):887–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Petersen RC. Clinical practice. Mild cognitive impairment. N Engl J Med. 2011;364(23):2227–2234 [DOI] [PubMed] [Google Scholar]
- 8. Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort Study. Pediatr Blood Cancer. 2011;56(5):825–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ojha RP, Oancea SC, Ness KK, et al. Assessment of potential bias from non-participation in a dynamic clinical cohort of long-term childhood cancer survivors: results from the St. Jude lifetime cohort study. Pediatr Blood Cancer. 2012;60(5):856–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wechsler D. WMS-IV: Administration and Scoring Manual. San Antonio, TX: Pearson; 2009. [Google Scholar]
- 11. Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 12. Ji Q, Glass JO, Reddick WE. A novel, fast entropy-minimization algorithm for bias field correction in MR images. Magn Reson Imaging. 2007;25(2):259–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reddick WE, Glass JO, Langston JW, Helton KJ. Quantitative MRI assessment of leukoencephalopathy. Magn Reson Med. 2002;47(5):912–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Glass JO, Reddick WE, Reeves C, Pui CH. Improving the segmentation of therapy-induced leukoencephalopathy in children with acute lymphoblastic leukemia using a priori information and a gradient magnitude threshold. Magn Reson Med. 2004;52(6):1336–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sowell ER, Peterson BS, Kan E, et al. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex. 2007;17(7):1550–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bookheimer SY, Strojwas MH, Cohen MS, et al. Patterns of brain activation in people at risk for Alzheimer’s disease. N Engl J Med. 2000;343(7):450–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zou P, Helton KJ, Smeltzer M, et al. Hemodynamic responses to visual stimulation in children with sickle cell anemia. Brain Imaging Behav. 2011;5(4):295–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mulhern RK, Fairclough D, Ochs J. A prospective comparison of neuropsychologic performance of children surviving leukemia who received 18 Gy, 24 Gy, or no cranial irradiation. J Clin Oncol. 1991;9(8):1348–1356 [DOI] [PubMed] [Google Scholar]
- 19. Krull KR, Sabin ND, Reddick WE, et al. Neurocognitive function and CNS integrity in adult survivors of childhood hodgkin lymphoma. J Clin Oncol. 2012;30(29):3618–3624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Conklin HM, Krull KR, Reddick WE, Pei D, Cheng C, Pui CH. Cognitive outcomes following contemporary treatment without cranial irradiation for childhood acute lymphoblastic leukemia. J Natl Cancer Inst. 2012;104(18):1386–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stiles J, Reilly J, Paul B, Moses P. Cognitive development following early brain injury: evidence for neural adaptation. Trends Cogn Sci. 2005;9(3): 136–143 [DOI] [PubMed] [Google Scholar]
- 22. Kiraly M, Kiraly SJ. Traumatic brain injury and delayed sequelae: a review—traumatic brain injury and mild traumatic brain injury (concussion) are precursors to later-onset brain disorders, including early-onset dementia. Sci World J. 2007. Nov 12; 7:1768–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hill DE, Ciesielski KT, Sethre-Hofstad L, Duncan MH, Lorenzi M. Visual and verbal short-term memory deficits in childhood leukemia survivors after intrathecal chemotherapy. J Pediatr Psychol. 1997;22(6):861–870 [DOI] [PubMed] [Google Scholar]
- 24. Schatz J, Kramer JH, Ablin A, Matthay KK. Processing speed, working memory, and IQ: a developmental model of cognitive deficits following cranial radiation therapy. Neuropsychology. 2000;14(2):189–200 [DOI] [PubMed] [Google Scholar]
- 25. Butler RW, Hill JM, Steinherz PG, Meyers PA, Finlay JL. Neuropsychologic effects of cranial irradiation, intrathecal methotrexate, and systemic methotrexate in childhood cancer. J Clin Oncol. 1994;12(12):2621–2629 [DOI] [PubMed] [Google Scholar]
- 26. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194 [DOI] [PubMed] [Google Scholar]
- 27. Metwalli NS, Benatar M, Nair G, Usher S, Hu X, Carew JD. Utility of axial and radial diffusivity from diffusion tensor MRI as markers of neurodegeneration in amyotrophic lateral sclerosis. Brain Res. 2010. Aug 12;1348:156–164 [DOI] [PubMed] [Google Scholar]
- 28. Grambaite R, Reinvang I, Selnes P, et al. Pre-dementia memory impairment is associated with white matter tract affection. J Int Neuropsychol Soc. 2011;17(1):143–153 [DOI] [PubMed] [Google Scholar]
- 29. Bartzokis G, Lu PH, Heydari P, et al. Multimodal magnetic resonance imaging assessment of white matter aging trajectories over the lifespan of healthy individuals. Biol Psych. 2012; 72(12): 1026–1034 [DOI] [PubMed] [Google Scholar]
- 30. Tondelli M, Wilcock GK, Nichelli P, De Jager CA, Jenkinson M, Zamboni G. Structural MRI changes detectable up to ten years before clinical Alzheimer’s disease. Neurobiol Aging. 2012;33(4):825.e25–36 [DOI] [PubMed] [Google Scholar]
- 31. Schuff N, Tosun D, Insel PS, et al. Nonlinear time course of brain volume loss in cognitively normal and impaired elders. Neurobiol Aging. 2012;33(5):845–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nickl-Jockschat T, Kleiman A, Schulz JB, et al. Neuroanatomic changes and their association with cognitive decline in mild cognitive impairment: a meta-analysis. Brain Struct Funct. 2012;217(1):115–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: the Children’s Oncology Group long-term follow-up guidelines from the Children’s Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22(24):4979–4990 [DOI] [PubMed] [Google Scholar]
- 34. Spiegler BJ, Kennedy K, Maze R, et al. Comparison of long-term neurocognitive outcomes in young children with acute lymphoblastic leukemia treated with cranial radiation or high-dose or very high-dose intravenous methotrexate. J Clin Oncol. 2006;24(24):3858–3864 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.