Abstract
One disadvantage of vesicular stomatitis virus G (VSV-G) pseudotyped lentivirus vectors for clinical application is inactivation of the vector by human serum complement. To prevent this, monomethoxypoly(ethylene) glycol was conjugated to a VSV-G-human immunodeficiency virus vector expressing Escherichia coli beta-galactosidase. The modification did not affect transduction efficiency in vitro and protected the vector from inactivation in complement-active human and mouse sera. Blood from mice dosed intravenously with either the unmodified or the PEGylated virus particles was assayed for active vector by a limiting-dilution assay to evaluate transduction efficiency and for p24, an indicator of the total number of virus particles present. PEGylation extended the circulation half-life of active vector by a factor of 5 and reduced the rate of vector inactivation in the serum by a factor of 1,000. Pharmacokinetic profiles for the total number of virus particles present in the circulation were unaffected by PEGylation. Modification of the vector with poly(ethylene) glycol significantly enhanced transduction efficiency in the bone marrow and in the spleen 14 days after systemic administration of the virus. These results, in concert with the pharmacokinetic profiles, indicate that PEGylation does protect the virus from inactivation in the serum and, as a result, improves the transduction efficiency of VSV-G pseudotyped lentivirus vectors in susceptible organs in vivo.
Lentiviruses are a family of retroviruses that includes human immunodeficiency virus types 1 and 2 (HIV-1 and HIV-2), simian immunodeficiency virus, feline immunodeficiency virus, and bovine immunodeficiency and equine infectious anemia viruses. Gene delivery using HIV-based vectors was first described in 1990 (35, 38) and was later optimized by Naldini and colleagues (31). Since then, lentiviruses have generated great interest as vectors for gene therapy. In terms of gene delivery, they have all the conventional attributes of other retrovirus vectors, including stable integration of the transgene into the target cell genome. Toxicity associated with lentivirus vector gene transfer remains low, as they do not appear to trigger detectable immune or inflammatory responses (33, 36, 47). However, their major attribute is the ability to transduce slowly dividing and postmitotic cells of many tissues, including the retina, respiratory epithelium, brain, kidney, muscle, and liver (18, 24, 28, 30, 43, 45, 50).
Despite these advantages over other virus vectors for gene delivery, there are several drawbacks associated with lentiviruses which limit further testing in large-animal models, a prerequisite for clinical evaluation. These include limited virus tropism and inability to target gene expression to specific cell types, inactivation in the presence of serum, and high susceptibility to disruption by shear forces encountered during concentration by ultracentrifugation and multiple freeze-thaw cycles (23). Pseudotyping, a process in which the natural envelope proteins of the virus are replaced with surface glycoproteins from a variety of other viruses, has addressed and significantly mitigated some of these problems.
Early pseudotyping experiments employing the rhabdovirus envelope protein from vesicular stomatitis virus G (VSV-G) demonstrated that the use of this protein rapidly broadened the tropism of the virus (2, 31). This heterologous envelope protein also conferred previously unobtainable robust physical stability on the virus-like particles, allowing them to be concentrated and stored for increased efficiency (7). However, use of VSV-G pseudotyped vectors in vivo continues to be hampered by an innate immune response directed against the virus particles. This effect is largely mediated through the classical complement pathway (8, 14, 40). Several groups have found that improper posttranslational processing of the viral envelope by (α1-3)galactosyltransferase in the packaging cell line is largely responsible for precipitating antibody-mediated activation of complement (39, 41, 42). Others have found the lentivirus vectors pseudotyped with the VSV-G glycoprotein to be inherently sensitive to complement inactivation regardless of the type of producer cell line employed, indicating that this effect may be due to a complex series of unknown mechanisms (14).
Serum inactivation of VSV-G pseudotyped lentivirus vectors is a significant barrier to the development of these otherwise highly efficient vectors for in vivo gene delivery. A rapid method for covalent attachment of activated monomethoxypoly(ethylene) glycol to free lysine groups on the protein capsids of adenovirus vectors has been developed (12). This process did not significantly compromise viral transduction efficiency and blunted the immune response against virus capsid proteins (9, 10). In addition, poly(ethylene) glycol (PEG) conjugation protected the virus from the immune response in preimmunized animals, which allowed significant gene expression upon readministration. Given this information, we hypothesized that PEGylation of VSV-G pseudotyped lentivirus might effectively protect the vector from inactivation in serum.
The primary goal of this study was to develop a PEGylation process for a VSV-G pseudotyped lentivirus vector to enhance the stability of the virus particles in the presence of serum in vitro and in vivo. A recombinant VSV-G pseudotyped HIV-based vector encoding the Escherichia coli beta-galactosidase transgene was conjugated with monomethoxypoly(ethylene) glycol activated by succinimidyl succinate. The physical properties of the PEG-vector conjugates were determined by several methods. The stability of the PEGylated vector was compared to that of the unmodified virus in human serum inactivation assays. We also compared the pharmacokinetic profiles of the active and inactive forms of the unmodified and PEGylated VSV-G pseudotyped HIV vectors after intravenous injection in vivo. As a final test of vector stability in the presence of serum, the biodistribution pattern of each vector was assessed. These results indicate that a PEGylated VSV-G pseudotyped HIV-based vector is resistant to serum inactivation and transduces susceptible tissue with improved efficiency following systemic administration.
MATERIALS AND METHODS
Production of conjugated lentivirus vectors.
The helper packaging construct pCMVΔR8.2, encoding the HIV helper function; the transfer vector pHxLacZWP (51), encoding E. coli beta-galactosidase; and the plasmid encoding the VSV-G envelope, pMD.G, were mixed in a 1:2:3 molar ratio and used to transfect 293T cells by the calcium phosphate precipitation method (BD Biosciences Clontech, Palo Alto, Calif.) as described previously (27). Following the protocol provided by the manufacturer, 180 μg of the endotoxin-free DNA mixture was added to each 150-mm-diameter plate of 293T cells. Forty-four hours after transfection, fresh medium was added to each plate, and the virus was harvested 16 h later. Cell supernatant containing virus from 20 150-mm-diameter plates was concentrated by ultracentrifugation at 104,000 × g for 2 h at 4°C in an SW28 rotor (Beckman, Fullerton, Calif.). The virus was resuspended in 600 μl of 100 mM potassium phosphate-buffered saline (pH 7.4) and stored at −80°C until it was used. Virus stocks were screened for the presence of replication-competent lentivirus by monitoring p24 antigen expression (15) in the culture medium of transduced 293T cells for 30 days. In all cases, p24 was undetectable once the input vector had been eliminated from the culture.
The protein contents of lentivirus preparations were determined by a microplate assay with DC protein assay reagents with bovine serum albumin as a standard (Bio-Rad, Hercules, Calif.). Monomethoxypoly(ethylene) glycol activated by succinimidyl succinate was obtained from Sigma Chemicals (St. Louis, Mo.). Ten micrograms of the polymer was added for each microgram of protein present in each preparation. Conjugation reactions were performed as described previously (12). All conjugation reactions were performed at 25°C with gentle agitation. The reactions were stopped by the addition of 10× l-lysine (Sigma Chemicals) with respect to the amount of PEG added. Unreacted PEG, excess lysine, and reaction by-products were eliminated by buffer exchange over a Micro-Bio Spin P-30 chromatography column (Bio-Rad) equilibrated with 100 mM potassium phosphate-buffered saline (pH 7.4). During each reaction, a separate aliquot of virus was agitated at room temperature in the absence of monomethoxypoly(ethylene) glycol activated by succinimidyl succinate and processed in the same manner as the conjugated virus. This preparation was used to determine if loss of viral titer, if it occurred, was due to the PEGylation process or to incubation at room temperature and processing, and it served as the unPEGylated control for all studies outlined below. All experiments involving the production and functional analysis of replication-incompetent VSV-G-HIV pseudotyped vectors were performed under biosafety level 2+ containment as approved by the Institutional Biosafety Committees of both the Wistar Institute and The University of Texas at Austin.
Characterization of PEGylated VSV-G pseudotyped HIV-based vector. (i) CZE.
PEGylated and unPEGylated VSV-G pseudotyped lentivirus vectors were characterized by capillary zone electrophoresis (CZE) using a PACE 5000 system (Beckman Coulter, Fullerton, Calif.) with an untreated 50-μm (internal diameter) by 27-cm capillary. The temperature of the capillary was controlled at 22°C. A preliminary 2-min wash in 1 N NaOH and a second wash in running buffer (50 mM sodium phosphate buffer, pH 7.0, 10 mM sodium chloride) were performed prior to sample analysis. Reverse polarity at 12.5 kV was used for the separations, and the detector signal at 214 nm was recorded and processed by the PACE station software package.
(ii) Enzyme-linked immunosorbent assay (ELISA).
VSV-G pseudotyped lentivirus was conjugated to biotinylated monomethoxypoly(ethylene) glycol activated by succinimidyl succinate (Shearwater Corp., Huntsville, Ala.) under the conditions described above. Unreacted PEG was removed from these samples by dialysis (Slide-a-Lyzer dialysis cassettes; 10,000-mW cutoff; Pierce, Rockford, Ill.) against 100 mM potassium phosphate-buffered saline (pH 7.4) for 6 h. Small aliquots of virus were diluted to various particle concentrations predetermined by a viral-titer assay. These were added to Streptawell streptavidin-coated 96-well assay plates (Roche Applied Science, Indianapolis, Ind.) with standard samples of specific concentrations of PEG-biotin for a period of 1 h at 37°C. Sample and standard dilutions were performed in Tris-buffered saline (pH 7.4) containing 0.1% bovine serum albumin (Sigma Chemicals). After 1 h, the plate was washed (Tris-buffered saline, 0.1% bovine serum albumin, 0.05% Tween 20) and incubated with an anti-biotin hydrogen peroxide oxioreductase Fab antibody (Roche) at a 1:1,000 dilution in sample dilution buffer for an additional hour at 37°C. After the plate was washed, a solution of 2,2′-azino-di-[3-ethylbenzthiazoline sulfonate (6)] diammonium salt (Roche) was added, and optical densities were read at 405 nm on an SLT Rainbow microplate reader equipped with WinSelect data analysis software (Tecan USA, Research Triangle Park, N.C.).
(iii) Particle size analysis.
The particle size distributions of unmodified and PEGylated lentiviral preparations were determined as described elsewhere (1). Briefly, particle size was determined using a DynaPro LSR laser light-scattering device (Protein Solutions, Lakewood, N.J.) and detection system. Regularization histograms and assignment of hydrodynamic radius values to various subpopulations within the sample were calculated using either Dynamics or DynaLS software (Protein Solutions).
(iv) Partitioning assays.
Partition coefficients of native and PEGylated viruses were determined as described previously (12). Partitioning assays were performed at 25°C in single microcentrifuge tubes containing 1 g of a two-phase system of 4.75% (wt/wt) PEG 8000 (Sigma Chemicals) and 4.75% dextran T500 (Amersham Biosciences, Piscataway, N.J.) in 0.15 M NaCl containing 0.01 M sodium phosphate buffer, pH 6.8. Lentivirus and PEGylated lentivirus were incorporated into the system by replacing 0.1 g of the water used to prepare the phases with 0.1 g of virus in coupling buffer. Samples were mixed 30 to 40 times by inversion and left to settle under gravity until complete separation of the phases was achieved. Aliquots from the top and bottom phases were analyzed for protein concentration with Bio-Rad protein assay reagents with bovine serum albumin as a standard. The partition coefficient (K) is determined by the ratio between the protein concentrations in the top and bottom phases.
(v) Viral-titer assay.
Titers of PEGylated and unPEGylated VSV-G pseudotyped lentiviruses were determined by serial dilution on 293T cells seeded at a density of 1.5 × 104/well in 12-well plates (Falcon; BD Biosciences, Medford, Mass.) in the presence of 8 μg of Polybrene (Sigma-Aldrich, St. Louis, Mo.)/ml. The virus was diluted in serum-free Dulbecco's modified medium (Cellgro; Mediatech, Herndon, Va.) containing Polybrene. Dilutions of virus were added to cell monolayers in a volume of 100 μl for 2 h. After that time, 1 ml of Dulbecco's modified Eagle's medium containing 10% heat-inactivated fetal bovine serum (FBS) (BioWhittaker, Walkersville, Md.) was added to each well, and infection was allowed to continue for 24 h. Beta-galactosidase expression was determined by X-Gal (5-bromo-4-chloro-3-indoyl-β-d-galactopyranoside) staining according to a standard protocol (11). The viral titer was determined by counting positively stained cells in an average of 20 microscope fields at ×100 magnification and extrapolating this average over the surface area of the well as described previously (11).
(vi) Neutralization assay.
Two C57BL/6 mice were dosed with 2.4 × 107 transducing units (TU) of unmodified VSV-G-HIV by subcutaneous injection. Thirty days after the initial administration, each animal was given a second booster of virus (2.3 × 107 TU). The animals were euthanized 28 days later, and blood was collected by intracardiac puncture. Unmodified and PEGylated VGV-G- HIV preparations were incubated in the presence of heat-inactivated sera from immunized and nonimmunized mice for 30 min at room temperature with gentle shaking. One hundred-microliter aliquots (equivalent to a multiplicity of infection of 40) were applied to 293T cells in triplicate. Two hours later, the virus was replaced with complete medium. Twenty-four hours after infection, the medium was removed, the cells were washed with PBS, and gene expression was assessed by X-Gal staining.
(vii) In vitro serum inactivation assays.
Human sera were collected from three healthy human volunteers. Murine serum samples were taken from three C57BL/6 mice (Taconic, Germantown, N.Y.). An aliquot of each sample was incubated at 56°C for 1 h to inactivate complement. Each sample was tested for complement activity with an EZ complement CH50 diagnostic kit (Diamedix Corp., Miami, Fla.). This assay measures the ability of the test sample to lyse 50% of a standardized suspension of sheep erythrocytes sensitized by an anti-sheep erythrocyte antibody with respect to a reference serum with a known CH50 value. Samples with CH50 values of 0 to 100 are considered to have low or absent levels of complement. Samples with values of 101 to 300 are considered to have normal complement, and those with a value of 301 or greater are considered to have high levels of active complement. Equal amounts of active virus (PEGylated or unPEGylated VSV-G-HIV) were used to prepare test samples. Each virus was diluted fivefold in normal human serum, heat-inactivated human serum (HIS), or inactivated fetal bovine serum (FBS) (BioWhittaker) as a control to a total volume of 100 μl. Control samples were diluted in a similar manner in sodium phosphate-buffered saline (PBS) (pH 7.4; Sigma Aldrich). Each sample was incubated for 1 h at 37°C. Then all samples were serially diluted and assessed for viral titer on 293T cells as described above. The data are reported as the number of transduced cells obtained from each treatment group relative to that obtained from virus incubated in PBS.
(viii) Animal studies.
All procedures were approved by the Institutional Animal Care and Use Committees of The University of Texas at Austin.
Intraocular injection.
Thirty CD-1 mice (five per treatment group; Taconic Laboratories) were anesthetized by intraperitoneal injection of a 1:1 mixture of ketamine (Fort Dodge Animal Health, Overland Park, Kans.) and xylazine (Sigma). Subretinal injections (1 μl) of either PEGylated or unPEGylated VSV-G-HIV or PBS were performed as described previously (4) with two doses of virus (1.7 × 107 and 1.7 × 108 TU/ml). The same individual performed all the surgical procedures to minimize variability in injection technique. All animals were sacrificed 7 days after injection, and one eye was enucleated, fixed in 4% paraformaldehyde, transferred to 30% sucrose for 3 to 4 h, and frozen in optimal-cutting-temperature compound (Fisher Scientific, Pittsburgh, Pa.). For each eye, 10-μm-thick serial sections were cut and stained for beta-galactosidase expression as described previously (21) and counterstained with hematoxylin (Biochemical Sciences, Inc., Swedesboro, N.J.). The remaining eye was placed in 500 μl of lysis buffer (provided with the Beta-Galactosidase Enzyme Assay System; Promega, Madison, Wis.) and homogenized with a disposable tissue grinder (Kendall, Mansfield, Mass.). Samples were then centrifuged at 2,000 × g for 10 min at 4°C. The supernatants were incubated with the assay buffer (Promega) for 30 min at 37°C. The reaction was quenched with 1 M sodium carbonate. Values are reported as relative light units (RLU) obtained at 420 nm and were normalized with respect to protein content as determined with Bio-Rad protein assay reagents with bovine serum albumin as a standard.
Kinetic studies.
Groups of 10 mice (C57BL/6; Taconic Laboratories) were injected via the tail vein with 2.1 × 107 TU of either unmodified or PEGylated virus. Blood was collected from the retro-orbital plexus on a rotating schedule from three animals within a treatment group (in order to prevent extensive depletion of blood volume) at the following time points: 10 and 30 s; 1, 5, 10, 15, 20, 30, and 45 min; and 1, 2, 4, 6, 8, and 12 h. No more than five samples were taken from a given animal during the study period. Upon the fifth blood draw, the animals were euthanized, and the remaining blood was collected by intracardiac puncture. The titer of circulating virus was determined by serial dilution of whole blood on 293T cells as described above. p24 levels were determined from serum by ELISA (Perkin-Elmer Life Sciences, Boston, Mass.) after acidification treatment for disruption of immune complexes according to the manufacturer's protocol.
Biodistribution studies.
Groups of four C57BL/6 mice were injected via the tail vein with 2.1 × 107 TU of either unmodified or PEGylated virus. Fourteen days after injection, the animals were euthanized and organs were harvested in the following order: gonads, muscle, intestine, kidney, liver, spleen, lung, heart, and lymph nodes. A portion of each organ was snap frozen in liquid nitrogen for PCR analysis. Blood was collected by intacardiac puncture, and bone marrow was collected in PBS from the femur and tibia of each animal. Both were quick frozen for PCR analysis.
DNA extraction and biodistribution by real-time quantitative TaqMan PCR.
Genomic DNA was extracted from each tissue using a DNeasy tissue kit (Qiagen, Valencia, Calif.) according to the manufacturer's instructions. Viral cDNA was quantitated by TaqMan PCR in a reaction well containing a 50-μl total reaction volume. The TaqMan probe for detection of integrated pHxLacZWP from animal tissues was labeled with the fluorescent reporter dye 6FAM at the 5′ end and the quencher dye TAMRA at the 3′ end (6FAM-AGCTCTCTCGACGCAGGACTCGGC-TAMRA). The sequences of the TaqMan primer sets for amplification of the region encompassing the packaging signal of pHxLacZWP are sense, 5′-TGAAAGCGAAAGGGAACCA-3′, and antisense, 5′-CCGTGCGCGCTTCAG-3′). Each reaction mixture contained 100 ng of genomic DNA, 0.9 nM each primer, 0.2 nM labeled probe, and 25 μl of TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, Calif.). All reactions were set up in MicroAmp Optical 96-well reaction plates (Applied Biosystems). The amplification conditions were 55°C for 2 min and 95°C for 10 min, followed by 95°C for 15 s and 60°C for 1 min and repeated for 50 cycles. Data were collected from the GeneAmp PCR System 9600 (Applied Biosystems) and transferred directly to a Macintosh 7100 computer for analysis using Sequence Detection Software (Applied Biosystems). Unknown samples, standards, template controls, and DNA template negative controls were all run in triplicate. The frequencies of unknown samples were interpolated from a standard curve derived from the simultaneous amplification of linearized pHxLacZWP plasmid ranging from 0 to 108 copies. The average copy number for each sample was calculated, and all results were reported as the number of copies per 100 ng of total DNA.
Pharmacokinetic analysis.
Pharmacokinetic parameters were determined using standard model-independent methods (16) with the assistance of a nonlinear regression computer program (WinNonlin Professional version 4.0; Pharsight Corp., Mountain View, Calif.). Areas under the concentration-time curve (AUC) were estimated by nonlinear regression and extrapolated to infinity. The half-life (t1/2) was calculated as the quotient of the natural log of 2 divided by the terminal slope of the concentration-versus-time profile. Clearance, a pharmacokinetic term that, in the context of the data presented here, represents the rate at which vector is inactivated, was calculated as the dose administered divided by the AUC for the respective vector preparation.
Data analysis.
SuperANOVA (Abacus Concepts, Berkley, Calif.) was used to perform a one-way analysis of variance with a Bonferonni-Dunn all-means comparison posthoc analysis to determine differences between individual groups. Differences were determined to be significant when the probability of chance explaining the results was reduced to <5% (P < 0.05).
RESULTS
Production and physical characterization of PEGylated VSV-G pseudotyped HIV-1-based vector.
Conjugation of PEG to therapeutic proteins, enzymes, and recombinant viral vectors generally results in some loss of bioactivity (6, 49). An optimized PEGylation process for recombinant adenovirus vectors that minimized loss of transduction efficiency while affording protection against the immune response has been described (9, 10, 12). Based upon this information, we resuspended concentrated VSV-G pseudotyped lentivirus vectors in 100 mM potassium phosphate-buffered saline (pH 7.4). Activated monomethoxypoly(ethylene) glycol was added at a concentration 10 times that of the total protein concentration of the virus preparation. The preparations were stirred gently at room temperature. Samples were analyzed 1 and 2 h after the start of the reaction. At these time points, the transduction efficiency was compared to the original viral titer. After 1 h, the titer of the PEGylated preparation dropped slightly from 1.01 × 108 to 9.53 × 107 TU/ml. The titer fell to 8.42 × 107 TU/ml after a 2-h reaction period. These losses were similar to those detected in control preparations subjected to agitation in the absence of PEG at room temperature.
In order to assess the extent to which the VSV-G proteins were modified in this time frame, an ELISA was developed using activated PEG labeled with biotin to determine the approximate number of PEG molecules attached to each virus particle (34). The only difference between these and the initial conjugation reactions was the presence of the biotin label on the activated polymer. Reactions were performed as described above for either 1 or 2 h. Excess PEG was removed from each preparation by dialysis, and the number of molecules of PEG added to the virus was determined using a reference standard curve of biotin-PEG. After 1 h, there were ∼3,000 molecules of PEG coupled to each virus particle. Extending the reaction time for another hour did not seem to produce a marked increase in the number of PEG molecules associated with the vector (3,100 molecules/particle). Considering that there are ∼216 copies of the VSV-G protein on a single particle and that there are 30 lysine residues associated with each copy (5, 29), ∼6,480 PEG chains at most can be coupled to each virus envelope. Our results suggest that only half of these sites are linked to PEG molecules.
Based upon ELISA results and transduction efficiency data, we selected a 1-h conjugation process for all vectors employed in the studies outlined here. The average initial viral titer was in the range of 1.75 × 108 to 2.1 × 108 TU/ml. After the PEGylation process, the titer was (1.06 ± 0.15) × 108 TU/ml, which was similar to the titer of unmodified virus agitated at room temperature in the absence of PEG [(1.02 ± 0.25) × 108 TU/ml]. The average number of PEG molecules conjugated to each virus particle in the preparations employed in the studies outlined here was 3,000 ± 280.
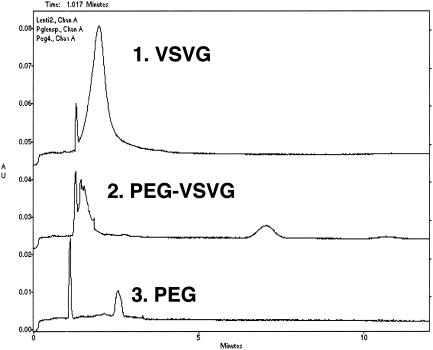
We also monitored the PEGylation process by whole-virus CZE with the rationale that the covalent attachment of PEG to the viral capsid shields the surface charge associated with the virus and confers a more neutral mobility to the virus particle. CZE analysis served as an indicator that the vector was significantly altered by the conjugation process. CZE was performed on all viral preparations after termination and clean up of the reaction. Under optimized conditions, unmodified VSV-G pseudotyped lentivirus has a major peak at 2 min (Fig. 1, peak 1). The PEGylated vector was detected as a major peak at 7 min (peak 2), indicating that the PEGylation process significantly altered the surface charge of the vector. A solution of free PEG in sample buffer produced a peak at 2.5 min (peak 3). This peak was not detected in any of the PEGylated preparations, indicating that desalting and buffer exchange effectively removed excess unreacted PEG. The peak at ∼1 min was present in all samples and a blank buffer control (not shown) and was determined to be a system peak.
FIG. 1.
Comparison of capillary electropherograms of unmodified VSV-G pseudotyped lentivirus (VSVG) (1), PEGylated VSV-G pseudotyped lentivirus (PEG-VSVG) (2), and a control solution of monomethoxypoly(ethylene) glycol (10 mg/ml) activated by succinimidyl succinate (PEG) (3). Virus samples were diluted 1:2 with separation buffer (50 mM sodium phosphate-buffered saline, 10 mM sodium chloride, pH 7.0) prior to analysis. The samples were run on a PACE 5000 system with a 5-s injection time and a separation voltage of 12.5 kV at 22°C. AU, absorbance units.
Several additional biophysical tests were performed to confirm that activated PEG molecules were successfully conjugated to the surface of the lentivirus and that different lots of virus had similar characteristics. Partitioning the viral preparations in an aqueous two-phase system and calculation of partition coefficients (K) demonstrated that PEGylation significantly modified the viral particles. The K values shifted from 1.1 ± 0.08 for unmodified preparations to 1.4 ± 0.02 for PEGylated vector. Dynamic light scattering also revealed that PEGylation changed the hydrodynamic radius of the vector. Unmodified virus particles had an average hydrodynamic radius of 166.2 ± 32.7 nm, while that of the modified vectors was 223.4 ± 26.4 nm.
PEGylated VSV-G-HIV complexes are protected from neutralization by immune serum.
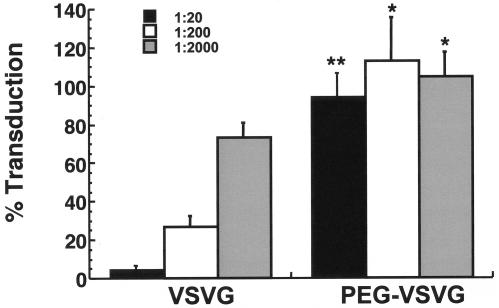
PEGylated and unPEGylated preparations were added to 293T cells in the presence of mouse serum containing neutralizing antibodies against the unmodified vector. The transduction levels were compared to that of the same preparation incubated in heat-inactivated nonimmune serum. A 1:20 dilution of immune serum reduced the transduction efficiency of the unmodified vector (VSVG) by 94.7%, while the transduction efficiency of the PEGylated vector (PEG-VSVG) was slightly diminished (Fig. 2). The transduction efficiency of the native virus was reduced by 72.8 and 26.4% after incubation with a 1:200 and a 1:2,000 dilution of the same serum, respectively. The transduction efficiencies of the PEGylated preparations were not significantly compromised in the presence of immune serum at these dilutions.
FIG. 2.
Transduction efficiency of PEGylated VSV-G lentivirus is not compromised in the presence of neutralizing antibodies in vitro. Both VSV-G pseudotyped lentivirus (VSVG) and PEGylated virus (PEG-VSVG) at a final concentration of 6.0 × 106 TU/ml were incubated in culture medium containing heat-inactivated immune serum from C57BL/6 mice at dilutions of 1:20, 1:200, and 1:2,000 at room temperature for 30 min and added to 293T cells. The transduction levels are reported as the ratio of positive cells from virus incubated with immune serum to the number of cells transduced by the virus in the presence of heat-inactivated nonimmune serum. The data reflect the results from triplicate samples in two separate experiments. Error bars reflect the standard deviation of the data. *, P < 0.05; **, P ≤ 0.001 (Student's t test).
Effect of PEGylation on in vivo performance of VSV-G pseudotyped HIV-1-based vectors.
In order to determine if the PEGylation process compromised viral transduction efficiency in vivo, we selected a target that can be transduced by the virus, is readily accessible for vector delivery, and is considered to be immune privileged to minimize potential complement inactivation of the virus. Lentiviral vectors have been employed as gene transfer vectors for long-term gene expression in the eye (22, 30, 45). The subretinal space and the anterior chamber of the eye are considered to be immune-privileged regions where there is a partial to complete loss of antigen-specific immune responses (44, 46). Given this information and previous experience with administration of viral vectors to the eye (3), we compared the in vivo transduction efficiencies of unmodified and PEGylated VSV-G pseudotyped HIV-based vectors 7 days after subretinal injection in CD-1 mice (Fig. 3).
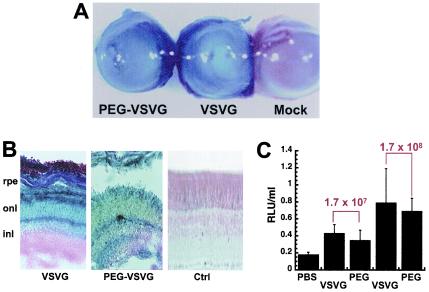
FIG. 3.
PEGylation does not compromise transduction efficiency of VSV-G-HIV in vivo. (A) Photomicrograph of whole eyes demonstrating grossly positive staining for beta-galactosidase expression from animals treated with PEGylated lentivirus (PEG-VSVG) and unmodified virus (VSVG) at a concentration of 1.7 × 108 TU/ml and the absence of gene expression after injection of PBS (Mock; negative control). (magnification, ×100). (B) Histological sections indicate that transduced cells from eyes treated with native virus (VSVG) are located predominantly in the outer nuclear layer (onl), while those from eyes treated with the PEGylated virus (PEG-VSVG) are located throughout the retinal pigment epithelium (rpe), the outer nuclear later (onl), and the inner nuclear layer (inl) compared to sections from animals dosed with saline (Ctrl) (magnification, ×200). (C) Quantitative analysis of beta-galactosidase gene expression in eyes 7 days after treatment with either unmodified virus (VSVG), PEGylated virus (PEG) at two different doses (1.7 × 107 and 1.7 × 108 TU/ml), or PBS. The data are reported as the average number of RLU per milliliter of homogenate from five animals per treatment group and were normalized for protein content. The error bars represent the standard deviations of the data.
Unmodified and PEGylated preparations produced similar levels of transgene expression upon gross observation of whole eyes after histochemical staining for beta-galactosidase (Fig. 3A). Evaluation of transgene expression in whole mounts and sections revealed that the unmodified virus (VSV-G) transduced cells in both the retinal pigment epithelium and the outer nuclear layer (Fig. 3B). The PEGylated VSV-G pseudotyped vector (PEG-VSVG) transduced cells in both of these regions with comparable efficiencies. In addition, the inner nuclear layer was found to be partially positive for beta-galactosidase expression compared to sections from animals dosed with saline (Fig. 3B). Transgene expression was also quantitated by chemiluminescence from animals given a dose of either 1.7 × 107 or 1.7 × 108 TU of the native or the PEGylated virus/ml (Fig. 3C). There was a slight but not statistically significant difference between the activity in samples from animals treated with either 1.7 × 107 or 1.7 × 108 TU of unmodified virus/ml (VSVG, 0.43 ± 0.09 and 0.79 ± 0.3 RLU/ml) and samples from animals given the same doses of PEGylated vector (PEG; 0.35 ± 0.10 and 0.70 ± 0.15 RLU/ml; P = 0.06 [Student's t test]). Transgene expression in all treatment groups was significantly higher than that detected in animals treated with PBS (0.18 ± 0.02 RLU/ml; P = 0.01).
Effect of PEGylation on inactivation of VSV-G pseudotyped HIV-1-based vector by human and mouse sera.
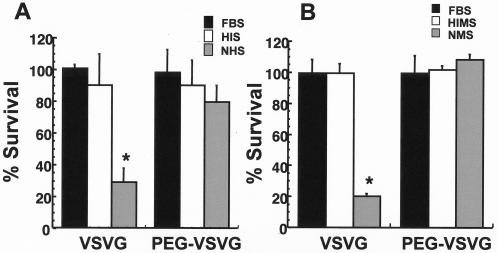
To compare the sensitivities of unmodified and PEGylated VSV-G pseudotyped HIV-based vectors to inactivation in human serum, serum samples were collected from three healthy human volunteers. All samples contained normal levels of active complement (average CH50, 239.6 ± 13.6), while complement activity was low in heat-inactivated samples (CH50,44.2 ± 13.2) as determined by a complement CH50 diagnostic assay. Heat-inactivated fetal bovine serum was selected as a control. These samples had an average CH50 value of 21.6 ± 5.2, indicating that complement activity was also low. The vector was subjected to 80% serum inactivation assays, which consist of evaluating viral infectivity following a 1:5 dilution of vector in serum as described by DePolo et al. (13). The titer of unmodified VSV-G vector was largely unaffected after incubation in either HIS or FBS (Fig. 4A). The infectivity of the PEGylated vector (PEG-VSVG) was not significantly affected after exposure to HIS and FBS. In the presence of normal, complement-active human serum (normal human serum), the titer of the unmodified preparation was reduced to 29.3%± 8.1% of the original concentration. In contrast, the titer of the PEGylated preparation was reduced to 79.6%± 10.1% of the original concentration by normal human serum.
FIG. 4.
PEGylation protects VSV-G-HIV from inactivation in the presence of human serum (A) and sera from C57BL/6 mice (B). Percent survival is the ratio of the titer of the virus after incubation in the presence of serum to the titer of virus incubated in saline. The data represent results obtained from three different lots of serum (mouse and human), and the error bars represent the standard deviations of the data. FBS, control; HIS, heat-inactivated human serum; NHS, normal human serum; HIMS, heat-inactivated mouse serum; NMS, normal mouse serum. *, P ≤ 0.05 (Student's t test).
In an effort to select an animal model in which to test the effect of PEGylation on the in vivo activity of VSV-G pseudotyped HIV-based vectors, we also screened serum from C57BL/6 mice for virus inactivation using the same assay as for the human serum. Incubation with heat-inactivated mouse serum or FBS did not significantly affect the infectivity of either the unmodified or PEGylated preparations (Fig. 4B). After incubation in normal mouse serum, the viral titer of the unmodified vector dropped to 20.4% ± 1.6% of its original concentration, while the titer of the PEGylated preparation was similar to that detected after incubation in control complement-inactivated mouse serum or FBS.
Effect of PEGylation on circulation half-life of VSV-G pseudotyped HIV-1-based vector after a single intravenous dose.
The data in Fig. 4 suggest that serum from C57BL/6 mice can inactivate VSV-G pseudotyped HIV-based vectors at a rate similar to that seen in human serum. Thus, it was of interest to determine if the in vitro results reflected sensitivity of the vector to inactivation in vivo. C57BL/6 mice were given a single intravenous dose of either PEGylated or unmodified VSV-G pseudotyped vector expressing the E. coli beta-galactosidase transgene at a concentration of 2.1 × 108 TU/ml. Whole blood was collected for 12 h, serially diluted in culture medium, and added to 293T monolayers to determine the viral titer soon after collection. These data would allow us to determine the kinetic parameters of active vector in the circulation. Serum was also processed and assessed by ELISA for the presence of the HIV major structural core protein, p24. These data were used to determine the presence of both active and inactivated vector in the serum. Discrepancies between the kinetics of active vector and the total number of virus particles present would allow us to characterize the susceptibility of the native and PEGylated vectors to inactivation by serum components in vivo.
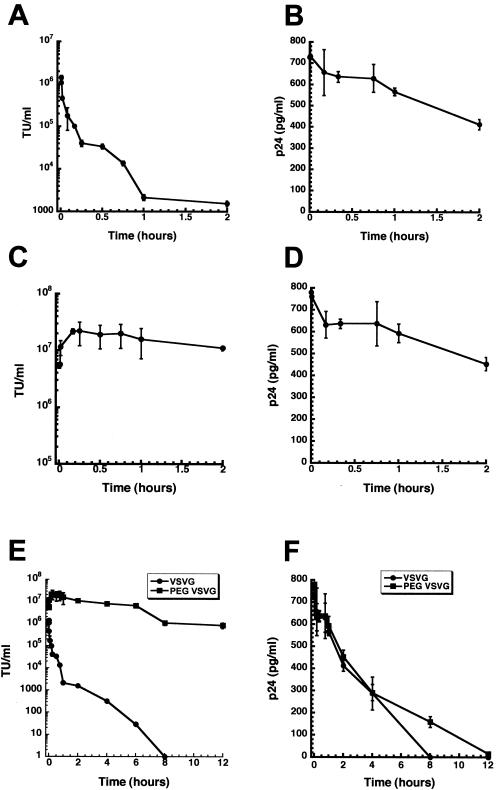
Thirty seconds after administration of the unmodified vector, a peak active-vector concentration of (1.44 ± 0.15) × 106 TU/ml was detected (Fig. 5A). The blood concentration of active, unmodified vector exhibited apparently steady first-order kinetics, as it rapidly declined by ∼2 log units to (1.38 ± 0.18) × 104 TU/ml 45 min after injection. The vascular concentration of active vector fell an additional 3 log units to 29.3 ± 4.6 TU/ml 6 h after injection (Fig. 5E). Active vector could not be detected in samples collected 8 h after injection. The calculated half-life, apparent volume of distribution, and clearance rate of active, unmodified vector were 0.55 h, 72.4 ml, and 277.2 ml/h, respectively (Table 1).
FIG. 5.
Pharmacokinetic analysis of unmodified VSV-G-HIV and PEGylated VSV-G-HIV in C57BL/6 mice. Equal titers (2.1 × 107 TU) of native or PEGylated VSV-G-HIV expressing E. coli beta-galactosidase were administered by tail vein injection to C57BL/6 mice. The viral titers (in TU per milliliter) of unmodified VSV-G-HIV (A) and PEGylated virus (C) were determined by serial dilution of whole blood and infection of 293T cells. The p24 levels of unmodified virus (B) and PEGylated VSV-G-HIV (D) were determined from serum by ELISA. (E) Twelve-hour kinetic profile of circulating infectious virus in C57BL/6 mice after intravenous injection of either PEGylated VSV-G pseudotyped HIV (PEG VSVG) or unmodified vector (VSVG). (F) Extended kinetic profile of circulating HIV p24 protein in C57BL/6 mice. Each data point reflects the average concentration obtained from three animals within each treatment group. The error bars represent the standard deviations of the data.
TABLE 1.
Pharmacokinetic parameters from concentration versus time curves of animals after a single intravenous dose of unmodified and PEGylated VSV-G pseudotyped lentiviral vectors
| Parametera | Treatment group
|
|||
|---|---|---|---|---|
| VSV-G
|
VSV-G-PEG
|
|||
| Viral titer | p24 | Viral titer | p24 | |
| Cmax | 1.4 × 106 | 734.0 | 2.21 × 107 | 780.0 |
| tmax (h) | 0.008 | 0.008 | 0.25 | 0.003 |
| t1/2 (h) | 0.55 | 4.65 | 2.84 | 3.73 |
| AUC0-∞ | 7.83 × 104 | 4,312 | 8.45 × 107 | 3,694 |
| CL (ml/h) | 277.2 | NDb | 0.257 | ND |
| Vss (ml) | 72.4 | ND | 1.04 | ND |
Area units for viral titer and p24 versus time curves are TU·h/ml and pg·h/ml, respectively. CL, systemic clearance rate; Vss, apparent volume of distribution at steady state.
ND, not determined.
A peak concentration in blood of (2.2 ± 0.5) × 107 TU/ml of active vector/ml was detected in mice dosed with the PEGylated preparation 20 min after injection (Fig. 5C). The concentration in blood declined to (1.1 ± 0.1) × 107 TU/ml 2 h after injection and dropped by an additional log unit to (1.01 ± 0.2) × 106 TU/ml 12 h after injection (Fig. 5E). PEGylation increased the calculated half-life of the vector by a factor of 5 to 2.84 h (Table 1). The clearance rate of active vector was reduced by a factor of 1,000 to 0.257 ml/h by the PEGylation process. The apparent volume of distribution was also reduced to 1.04 ml. Despite the differences between the pharmacokinetic parameters of the vascular concentrations of active PEGylated and unPEGylated vectors, the half-life and peak concentrations were remarkably similar for the total number of viral particles present in the systemic circulation (Fig. 5B, D, and F and Table 1). Thus, the rapid decline of active, unmodified vector and the prolonged distribution phase of the PEGylated virus suggest that PEGylation protects VSV-G pseudotyped HIV-based vectors from inactivation by serum in vivo.
Effect of PEGylation on the biodistribution of VSV-G pseudotyped HIV-based vectors after a single intravenous dose.
Because the difference between the pharmacokinetic profiles of the two vectors was so dramatic, a final study was performed to determine how PEGylation dictates the biodistribution of virus in a whole-animal model. C57BL/6 mice were injected via the tail vein with 2.1 × 107 TU of either unmodified or PEGylated vector. Fourteen days after injection, organs were harvested for histology and TaqMan PCR analysis. A sixfold (P = 0.03) and threefold (P = 0.0008) increase in the viral genome copy number was detected by TaqMan PCR in the bone marrow and spleen, respectively, from animals given the PEGylated vector in contrast to samples from animals dosed with unmodified vector (Table 2). These results, in concert with the pharmacokinetic profiles, suggest that PEGylation does protect the vector from inactivation in the serum and, as a result, facilitates transduction of susceptible target tissues.
TABLE 2.
Tissue distribution of PEGylated and unPEGylated VSV-G pseudotyped lentivirus in mice 14 days after systemic administration as determined by TaqMan PCRa
| Tissue | VSV-G | VSV-G-PEG |
|---|---|---|
| Blood | 0.13 ± 0.12 | 1.0 ± 0.9 |
| Gonad | 0.25 ± 0.10 | 0 |
| Muscle | 0 | 1.3 ± 0.4 |
| Bone marrow | 7.9 ± 1.0 | 45.4 ± 7.9 |
| Intestine | 0.15 ± 0.14 | 0.45 ± 0.3 |
| Kidney | 0.05 ± 0.4 | 0.4 ± 0.1 |
| Spleen | 29.9 ± 1.8 | 88.5 ± 8.8 |
| Liver | 10.7 ± 1.7 | 39.6 ± 21.4 |
| Lung | 0.8 ± 0.6 | 5.6 ± 4.3 |
| Heart | 0.2 ± 0.1 | 0.2 ± 0.1 |
| Lymph node | 0.6 ± 0.5 | 1.9 ± 0.6 |
Data are reported as the average copy number detected per 100 ng of genomic DNA ± the standard error of the mean for four animals per treatment group. All samples from mice dosed with saline (vehicle control) had a mean of 0.6 ± 0.7 copy/100 ng of total DNA, which was defined as background. Signals that were 3 standard deviations above background were considered positive (>2.7 copies/100 ng of total DNA).
Serum transaminase levels at the time of necropsy were also used to assess the toxicity associated with systemic administration of the PEGylated and unPEGylated vectors. The serum alanine transaminase (ALT) levels of animals dosed with the unmodified VSV-G pseudotyped vector were significantly higher than those of animals treated with saline (420 ± 59 versus 44 ± 0.7 U/liter; P = 0.01). Similar results were seen for aspartate transaminase (AST) levels (751 ± 137 versus 36.5 ± 2.8 U/liter). Animals treated with the PEGylated vector had ALT and AST levels that were significantly lower than those of animals dosed with the unmodified virus (ALT, 184.5 ± 66.9 U/liter; AST, 320 ± 73.7 U/liter; P = 0.03) but markedly higher than those of animals given vehicle controls.
DISCUSSION
While lentivirus-mediated gene therapy continues to be a promising mode of treatment for various clinical applications, there is limited information about the toxicity of and immune reaction to these vectors (48). In addition, the biodistribution properties and systemic effects of these viruses have not been studied in detail. One of the most significant barriers to targeting genes in vivo after systemic administration of the gene delivery vector is undesirable interaction of the vector with blood components. The opsonization of foreign particles with plasma proteins represents one of the first steps in the natural process of removal of foreign particles by the innate immune system. Several groups have shown that retroviral vectors are highly susceptible to this effect, which leads to significantly reduced circulation times in vivo after intravenous administration (8, 14, 42). Hydrophilic polymers, such as PEG, have been coupled to several nonviral vectors to protect them from plasma components and to extend their circulation time in the blood (32, 52).
In the studies outlined here, a method for conjugation of monomethoxypoly(ethylene) glycol activated by succinimidyl succinate was developed based upon previous experience with PEGylation of adenovirus vectors (12). Results of biophysical tests indicated that the PEGylation process linked PEG molecules to ∼50% of potential sites on the HIV vector. Analysis of the PEGylated preparations by CZE suggests that other proteins are also associated with the vector preparation, as the broad peak detected after the 1-min system peak in the PEGylated preparation can be attributed to a mixture of FBS and free protein present in the preparation (Fig. 1). In addition, only a limited number of the lysine residues included in the VSV-G protein may be exposed and available for conjugation. Thus, our estimate of the level of PEGylation is likely to be an overestimate. Other analytical methodologies are being evaluated to accurately determine the number of PEG molecules associated with each virus particle.
PEGylation altered the physical characteristics of the vector but did not significantly compromise transduction efficiency. In addition, it minimized virus neutralization by anti-VSV-G-HIV vector antibodies in vitro (Fig. 2), suggesting that readministration of the vector may be facilitated. We also found that the polymer effectively protected the vector from inactivation by human serum in vitro (Fig. 4). This was of particular interest, as our results with the unmodified vector were in accordance with those of previous studies (8, 14, 42). As shown previously, the present study also indicates that recombinant HIV-based vectors are rapidly inactivated in mouse serum in vitro (20) (Fig. 4).
To date it is not clear if the in vitro serum stability of recombinant virus vectors for gene therapy is correlated with the in vivo situation. The studies outlined here suggest that stability of the vector in vivo is correlated with in vitro observations. Indeed, the unmodified vector was found to be highly susceptible to inactivation in serum in a whole-animal model after systemic administration (Fig. 5). The decline in titer followed first-order clearance kinetics with a t1/2 of ∼33 min (Table 1). This is significantly longer than that of similar vectors reported in a chimpanzee model (15 to 20 min) and in a macaque model (5 min) (13) but is similar to that reported from mathematical modeling of HIV-1 clearance in a model of clinical infection (30 min) (53). In addition, the long-term kinetic data for active, unmodified vector (Fig. 5E) suggests that there may be two distinct virus-like particle populations present in a given preparation. One is highly susceptible to inactivation in serum and is responsible for the rapid decline in concentrations in serum during the first hour after administration. The other, more robust population is responsible for the steady decline over the remaining 7-h period. We have routinely noticed the presence of two distinct virus subpopulations in our lentivirus preparations when assessing physical-stability characteristics (unpublished data), and this has also been observed by others (20). While this phenomenon was not detected in the PEGylated preparations, it must be considered in the development and kinetic analysis of recombinant viral vectors administered by the systemic route.
It is also noteworthy that the p24 core protein had a significantly longer half-life (4.65 h) than that calculated by limiting dilution for active, circulating, unmodified virus. The maximal concentration of active, unmodified virus detected in less than 1 min after administration was a fraction of the total dose administered (1.4 × 106 TU/ml), while that for the p24 protein detected at the same time was similar to what was in the total dose. These results suggest that the unmodified virus was present for a significant amount of time in the circulation, but in an inactive state. Several possible explanations for the initial rapid decrease in the circulating vector concentration include inactivation and aggregation in serum; trapping in locations such as the lung, spleen, and kidney; and binding to serum proteins or receptors. The apparent volume of distribution at steady state for the unmodified virus was 72.4 ml, ∼40 times the blood volume of the mouse (26). This suggests that the unmodified virus could have experienced significant protein binding, as drugs which exhibit volume-of-distribution values which exceed the physiological blood volume undergo extensive binding to serum proteins and extravascular tissues (17).
The pharmacokinetic parameters of active, PEGylated, VSV-G pseudotyped vector were drastically different from those of the unmodified vector. The average peak serum concentration of 2.2 × 107 TU/ml, representative of the total dose administered, was detected in mice dosed with the PEGylated vector. This, and the fact that the circulation half-life was extended beyond that of the unmodified vector by a factor of 5, suggests that PEGylation achieved and maintained high levels of functional vector in the circulation. Since the vector was administered as a rapid intravenous bolus, the increase in concentration for the first 20 min represents an apparent distribution phase that is likely the result of a delay in distribution into the central vasculature. This phenomenon was distinctly different from what was seen with the unmodified virus and is characteristic of PEGylated molecules (19, 37). It is also important to note that the elimination half-life for the p24 protein was fairly close to that in the viral-titer data, which suggests that the majority of the virus detected in the serum over the 12-h period was functional.
TaqMan PCR analysis revealed that this last phenomenon translated into a marked increase in the transduction efficiency of the PEGylated vector in susceptible target organs, such as the spleen and bone marrow (36). However, gene transfer in the liver was not statistically different between animals treated with either vector due to the high variability associated with these samples (Table 2). PEGylation of an HIV vector pseudotyped with a different protein, such as the hepatotropic Ross River virus envelope (25), may offer a significant advantage for liver-directed gene therapy in the future.
An important objective of this study was to determine if conjugation of PEG to the VSV-G envelope protein of a VSV-G pseudotyped HIV-based vector would protect it from inactivation in human serum. A rapid method for PEGylation of the vector was developed which effectively preserved transduction efficiency in the presence of either human or mouse serum. This study provides a comprehensive analysis of the pharmacokinetic parameters of an intravenously injected recombinant virus vector in a mouse model by measuring both clinically active vector and the physical presence of virus particles. We also demonstrated that PEGylation helps maintain high levels of functional vector in the circulation. One consequence of this may be the possibility of optimizing gene transfer after systemic administration of a lentivirus-based vector while minimizing vector-associated toxicity by lowering the dose necessary for efficient gene transfer.
Acknowledgments
We thank Katie Gerding of The University of Texas at Austin for assistance with complement assays.
G.P.K. is the recipient of a fellowship from the Medical Research Council of Canada. This work was funded by a New Investigator grant from the American Foundation for Pharmaceutical Education and the Burroughs-Wellcome Fund (M.A.C.).
REFERENCES
- 1.Adadevoh, K., M. Croyle, D. Malarme, E. Bonfils, and M. A. Bowe. 2002. A short-term field use and shipping stability study of a wild type Ad5 adenoviral reference material. Bioprocessing 1:62-69. [Google Scholar]
- 2.Akkina, R. K., R. M. Walton, M. L. Chen, Q. X. Li, V. Planelles, and I. S. Chen. 1996. High-efficiency gene transfer into CD34+ cells with a human immunodeficiency virus type 1-based retroviral vector pseudotyped with vesicular stomatitis virus envelope glycoprotein G. J. Virol. 70:2581-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auricchio, A., G. Kobinger, V. Anand, M. Hildinger, E. O'Connor, A. Maguire, J. M. Wilson, and J. Bennett. 2001. Exchange of surface proteins impacts on viral vector cellular specificity and transduction characteristics: the retina as a model. Hum. Mol. Genet. 10:3075-3081. [DOI] [PubMed] [Google Scholar]
- 4.Bennett, J., D. Duan, J. F. Engelhardt, and A. M. Maguire. 1997. Real-time, noninvasive in vivo assessment of adeno-associated virus-mediated retinal transduction. Investig. Ophthalmol. Vis. Sci. 38:2857-2863. [PubMed] [Google Scholar]
- 5.Beyer, W. R., M. Westphal, W. Ostertag, and D. van Laer. 2002. Oncoretrovirus and lentivirus vectors pseudotyped with lymphocytic choriomeningitis glycoprotein: generation, concentration, and broad host range. J. Virol. 76:1488-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhadra, D., S. Bhadra, P. Jain, and N. K. Jain. 2002. PEGnology: a review of PEG-ylated systems. Pharmazie 57:5-29. [PubMed] [Google Scholar]
- 7.Burns, J. C., T. Friedmann, W. Driever, M. Burrascano, and J. K. Yee. 1993. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and non-mammalian cells. Proc. Natl. Acad. Sci. USA 90:8033-8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper, N. R., F. C. Jensen, R. M. Welsh, Jr., and M. B. Oldstone. 1975. Lysis of RNA tumor viruses by human serum: direct antibody-independent triggering of the classical complement pathway. J. Exp. Med. 144:970-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Croyle, M. A., N. Chirmule, Y. Zhang, and J. M. Wilson. 2002. PEGylation of first-generation recombinant adenoviruses achieves significant gene expression upon intravenous re-administration. Hum. Gene Ther. 13:1887-1900. [DOI] [PubMed] [Google Scholar]
- 10.Croyle, M. A., N. Chirmule, Y. Zhang, and J. M. Wilson. 2001. “Stealth” adenoviruses blunt cell mediated and humoral immune responses against the vector and allow for significant gene expression upon re-administration in the lung. J. Virol. 75:4792-4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Croyle, M. A., B. J. Roessler, B. L. Davidson, J. M. Hilfinger, and G. L. Amidon. 1998. Factors that influence stability of recombinant adenoviral preparations for human gene therapy. Pharm. Dev. Technol. 3:373-383. [DOI] [PubMed] [Google Scholar]
- 12.Croyle, M. A., Q. C. Yu, and J. M. Wilson. 2000. Development of a rapid method for the PEGylation of adenoviruses with enhanced transduction and improved stability under harsh storage conditions. Hum. Gene Ther. 11:1721-1730. [DOI] [PubMed] [Google Scholar]
- 13.DePolo, N. J., C. E. Harkleroad, M. Bodner, A. T. Watt, C. A. Anderson, J. S. Greengard, K. K. Murthy, T. W. Dubensky, and D. J. Jolly. 1999. The resistance of retroviral vectors produced from human cells to serum inactivation in vivo and in vitro is primate species dependent. J. Virol. 73:6708-6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DePolo, N. J., J. D. Reed, P. L. Sheridan, K. Townsend, S. L. Sauter, D. J. Jolly, and T. W. Dubensky. 2000. VSV-G pseudotyped lentiviral vector particles produced in human cells are inactivated by human serum. Mol. Ther. 2:218-222. [DOI] [PubMed] [Google Scholar]
- 15.Dull, T., R. Zufferey, M. Kelly, R. J. Mandel, M. Nguyen, D. Trono, and L. Naldini. 1998. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 72:8463-8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibaldi, M., and D. Perrier. 1982. Noncompartmental analysis based on statistical moment theory, p. 409-417. In Pharmacokinetics, nd ed., vol. 15. Marcel Dekker, New York, N.Y.
- 17.Gibaldi, M. and D. Perrier. 1982. Apparent volume of distribution, p. 199-219. In Pharmacokinetics, 2nd ed., vol. 15. Marcel Dekker, New York, N.Y.
- 18.Gusella, G. L., E. Fedorova, B. Hanss, D. Marras, M. E. Klotman, and P. E. Klotman. 2002. Lentiviral gene transduction of kidney. Hum. Gene Ther. 13:407-414. [DOI] [PubMed] [Google Scholar]
- 19.Harris, J. M., N. E. Martin, and M. Modi. 2001. PEGylation: a novel process for modifying pharmacokinetics. Clin. Pharmacokinet. 40:539-551. [DOI] [PubMed] [Google Scholar]
- 20.Higashikawa, F., and L.-J. Chang. 2001. Kinetic analyses of stability of simple and complex retroviral vectors. Virology 280:124-131. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman, L. M., A. M. Maguire, and J. Bennett. 1997. Cell-mediated immune response and stability of intraocular transgene expression after adenovirus-mediated delivery. Investig. Ophthalmol. Vis. Sci. 38:2224-2233. [PubMed] [Google Scholar]
- 22.Igarashi, T., K. Miyake, K. Kato, A. Watanabe, M. Ishizaki, K. Ohara, and T. Shimada. 2003. Lentivirus mediated expression of angiostatin efficiently inhibits neovascularization in a murine proliferative retinopathy model. Gene Ther. 10:219-226. [DOI] [PubMed] [Google Scholar]
- 23.Kafri, T. 2001. Lentivirus vectors: difficulties and hopes before clinical trials. Curr. Opin. Mol. Ther. 3:316-326. [PubMed] [Google Scholar]
- 24.Kafri, T., U. Blomer, D. A. Peterson, F. H. Gage, and I. M. Verma. 1997. Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nat. Genet. 17:314-317. [DOI] [PubMed] [Google Scholar]
- 25.Kang, Y., C. S. Stein, J. A. Heth, P. L. Sinn, A. K. Penisten, P. D. Staber, K. L. Ratliff, H. Shen, C. K. Barker, I. Martins, C. M. Sharkey, D. A. Sanders, P. B. McCray, Jr., and B. L. Davidson. 2002. In vivo gene transfer using a non-primate lentiviral vector pseudotyped with Ross River virus glycoproteins. J. Virol. 76:9378-9388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karali, T. T. 1995. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm. Drug Dispos. 16:351-380. [DOI] [PubMed] [Google Scholar]
- 27.Kobinger, G. P., D. J. Weiner, Q. C. Yu, and J. M. Wilson. 2001. Filovirus-pseudotyped lentiviral vector can efficiently and stably transduce airway epithelia in vivo. Nat. Biotechnol. 19:225-230. [DOI] [PubMed] [Google Scholar]
- 28.Lotery, A. J., T. A. Derksen, S. R. Russel, R. F. Mullins, S. Sauter, L. M. Affatigato, E. M. Stone, and B. L. Davidson. 2002. Gene transfer to the nonhuman primate retina with recombinant feline immunodeficiency virus vectors. Hum. Gene Ther. 13:689-696. [DOI] [PubMed] [Google Scholar]
- 29.Mayo, K., D. Huseby, J. McDermott, B. Arvidson, L. Findlay, and E. Barklis. 2003. Retrovirus capsid protein assembly arrangements. J. Mol. Biol. 325:225-237. [DOI] [PubMed] [Google Scholar]
- 30.Miyoshi, H., M. Takahashi, F. H. Gage, and I. M. Verma. 1997. Stable and efficient gene transfer into the retina using an HIV-based lentiviral vector. Proc. Natl. Acad. Sci. USA 94:10319-10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naldini, L., U. Blomer, P. Gallay, D. Ory, R. Mulligan, F. H. Gage, I. M. Verma, and D. Trono. 1996. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272:263-267. [DOI] [PubMed] [Google Scholar]
- 32.Ogris, M., S. Brunner, S. Schuller, R. Kircheis, and E. Wagner. 1999. PEGylated DNA/transferrin-PEI complexes: reduced interaction with blood components, extended circulation in blood and potential for systemic gene delivery. Gene Ther. 6:595-605. [DOI] [PubMed] [Google Scholar]
- 33.Ohashi, K., F. Park, and M. A. Kay. 2002. Role of hepatocyte direct hyperplasia in lentivirus-mediated liver transduction in vivo. Hum. Gene Ther. 13:653-663. [DOI] [PubMed] [Google Scholar]
- 34.O'Riordan, C., A. Lachapelle, C. Delgado, V. Parkes, S. C. Wadsworth, A. E. Smith, and G. E. Francis. 1999. PEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivo. Hum. Gene Ther. 10:1349-1358. [DOI] [PubMed] [Google Scholar]
- 35.Page, K. A., N. R. Landau, and D. R. Littman. 1990. Construction and use of a human immunodeficiency virus vector for analysis of virus infectivity. J. Virol. 64:5270-5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan, D., R. Gunther, W. Duan, S. Wendell, W. Kaemmerer, T. Kafri, I. M. Verma, and C. B. Whitley. 2002. Biodistribution and toxicity studies of VSVG-pseudotyped lentiviral vector after intravenous administration in mice with the observation of in vivo transduction of bone marrow. Mol. Ther. 6:19-29. [DOI] [PubMed] [Google Scholar]
- 37.Pepinsky, R. B., R. I. Shapiro, S. Wang, A. Chakraborty, A. Gill, D. J. Lepage, D. Wen, P. Rayhorn, G. S. Horan, F. R. Taylor, E. A. Garber, A. Galdes, and T. M. Engber. 2002. Long-acting forms of sonic hedgehog with improved pharmacokinetic and pharmacodynamic properties are efficacious in a nerve injury model. J. Pharm. Sci. 91:371-387. [DOI] [PubMed] [Google Scholar]
- 38.Poznansky, M., A. Lever, L. Bergeron, W. Haseltine, and J. Sodroski. 1991. Gene transfer into human lymphocytes by a defective human immunodeficiency virus type 1 vector. J. Virol. 65:532-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rigg, R. J., J. Chen, J. S. Dando, S. P. Forestell, I. Plavec, and E. Bohnlein. 1996. A novel human amphotropic packaging cell line: high titer, complement resistance and improved safety. Virology 218:290-295. [DOI] [PubMed] [Google Scholar]
- 40.Rother, R. P., W. L. Fodor, J. P. Springhorn, C. W. Birks, E. Setter, M. S. Sandrin, S. P. Squinto, and S. A. Rollins. 1995. A novel mechanism of retrovirus inactivation in human serum mediated by anti-alpha-galactosyl natural antibody. J. Exp. Med. 182:1345-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rother, R. P., and S. P. Squinto. 1996. The alpha-galactosyl epitope: a sugar coating that makes viruses unpalatable. Cell 86:185-188. [DOI] [PubMed] [Google Scholar]
- 42.Russell, D. W., M. S. Berger, and A. D. Miller. 1995. The effects of human serum and cerebral spinal fluid on retroviral vectors and packaging cell lines. Hum. Gene Ther. 6:635-641. [DOI] [PubMed] [Google Scholar]
- 43.Stein, C. S., and B. L. Davidson. 2002. Gene transfer to the brain using feline immunodeficiency virus-based lentivirus vectors. Methods Enzymol. 346:433-454. [DOI] [PubMed] [Google Scholar]
- 44.Streilein, J. W., S. Masli, M. Takeuchi, and T. Kezuka. 2002. The eye's view of antigen presentation. Hum. Immunol. 63:435-443. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi, M., H. Miyoshi, I. M. Verma, and F. H. Gage. 1999. Rescue from photoreceptor degeneration in the rd mouse by human immunodeficiency virus vector-mediated gene transfer. J. Virol. 73:7812-7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor, A. W. 1999. Ocular immunosuppressive microenvironment., p. 72-89. In J. W. Streilein (ed.), Immune response and the eye, vol. 73. Karger, Basel, Switzerland. [DOI] [PubMed]
- 47.VandenDriessche, T., L. Thorrez, L. Naldini, A. Follenzi, L. Moons, Z. Berneman, D. Collen, and M. K. L. Chuah. 2002. Lentiviral vectors containing the human immunodeficiency virus type-1 centro polypurine tract can efficiently transduce nondividing hepatocytes and antigen-presenting cells in vivo. Blood 100:813-822. [DOI] [PubMed] [Google Scholar]
- 48.Verdier, F., and J. Descotes. 1999. Preclinical safety evaluation of human gene therapy products. Toxicol. Sci. 47:9-15. [DOI] [PubMed] [Google Scholar]
- 49.Veronese, F. M. 2001. Peptide and protein PEGylation: a review of problems and solutions. Biomaterials 22:405-417. [DOI] [PubMed] [Google Scholar]
- 50.Wang, G., V. Slepushkin, J. Zabner, S. Keshavjee, J. C. Johnston, S. L. Sauter, D. J. Jolly, T. W. Dubensky, B. L. Davidson, and P. B. McCray. 1999. Feline immunodeficiency virus vectors persistently transduce nondividing airway epithelia and correct cystic fibrosis defect. J. Clin. Investig. 104:R55-R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watson, D. J., G. P. Kobinger, M. A. Passini, J. M. Wilson, and J. H. Wolfe. 2002. Targeted transduction patterns in the mouse brain by lentivirus vectors pseudotyped with VSV, Ebola, Mokola, LCMV, or MuLV envelope proteins. Mol. Ther. 5:528-537. [DOI] [PubMed] [Google Scholar]
- 52.Woodle, M. C., C. M. Engbers, and S. Zalipsky. 1999. New amphipathic polymer-lipid conjugates forming long-circulating reticuloendothelial system-evading liposomes. Bioconjug. Chem. 5:493-496. [DOI] [PubMed] [Google Scholar]
- 53.Zhang, L., P. J. Dailey, A. Gettie, J. Blanchard, and D. D. Ho. 2002. The liver is a major organ for clearing simian immunodeficiency virus in rhesus monkeys. J. Virol. 76:5271-5273. [DOI] [PMC free article] [PubMed] [Google Scholar]