Abstract
Leishmaniasis is a worldwide parasitic disease, caused by monoflagellate parasites of the genus Leishmania. In the search for more effective agents against these parasites, the identification of molecular targets has been attempted to ensure the efficiency of drugs and to avoid collateral damages on the host's cells. In this work, we have investigated some of the mechanisms of action of a group of natural sesquiterpene lactones that are effective against Leishmania mexicana mexicana promastigotes. We first observed that the antiproliferative effect of mexicanin I (Mxc), dehydroleucodine (DhL), psilostachyin (Psi), and, at lesser extent, psilostachyin C (Psi C) is blocked by 1.5 mM reduced glutathione. The reducing agent was also able to reverse the early effect of the compounds, suggesting that lactones may react with intracellular sulfhydryl groups. Moreover, we have shown that all the sesquiterpene lactones, except Psi C, significantly decreased the endogenous concentration of glutathione within the parasite. Consistent with these findings, the active sesquiterpene lactones increased between 2.7 and 5.4 times the generation of ROS by parasites. These results indicate that the induction of oxidative stress is at least one of the mechanisms of action of DhL, Mxc, and Psi on parasites while Psi C would act by another mechanism.
1. Introduction
Leishmaniasis is a parasitic disease caused by flagellated parasites of the genus Leishmania and transmitted by phlebotomine sandflies. These parasites exhibit a heteroxenous life cycle, alternating between intracellular amastigotes in the mammalian cells and flagellate promastigotes in the vector.
Leishmaniasis affects about 12 million people worldwide and, according to the World Health Organization (WHO), 2 million of new cases occur annually and 350 million people are considered at risk of contracting leishmaniasis [1]. The clinical forms of the disease depend on the species of Leishmania involved and include local infections of the skin, subcutaneous tissue, and regional lymphatic nodes (cutaneous leishmaniasis); metastatic infections of the oronasal mucosa (mucocutaneous leishmaniasis); and disseminated infection involving visceral organs (visceral leishmaniasis) [2].
Leishmaniasis is distributed worldwide with foci of infection in Central and South America, Southern Europe, North and East Africa, the Middle East and India [3]. In Argentina, this parasitosis affects the northern region of the country with an incidence that has increased over the last two decades [4].
Current drugs used to treat leishmaniasis include pentavalent antimonials, pentamidine, and amphotericin B, which induce serious toxic effects on patients. Parasite resistance to these drugs has also been described. New formulations, such as liposomal amphotericin B and other drugs (miltefosine, paromomycin), have serious drawbacks such as parenteral route of administration, duration of the treatment, teratogenic effects, toxicity, and cost of treatment, which limit their use in endemic areas [5]. Therefore, there is an urgent need for novel candidates to treat this parasitic disease.
Sesquiterpene lactones, a group of natural compounds characteristic of the Asteraceae family, have been pointed out as good candidates for antiprotozoal therapy since many of them are active against trypanosomatids [6–8]. Moreover, we have previously described the trypanocidal and leishmanicidal activity of natural sesquiterpene lactones isolated from Argentinean Asteraceae species [9–16].
One of the most important aspects in antiprotozoal drug discovery is to determine the mechanism of action of the potential candidates and to identify the possible molecular targets upon which these compounds act. Among other mechanisms, it is presumed that sesquiterpene lactones could exert their leishmanicidal activity by the generation of an oxidative environment within the parasite [17, 18]. The particular defense mechanism against oxidative stress in trypanosomatids makes parasites susceptible to these kinds of compounds.
In this sense, the aim of the present work was to evaluate the possible effect of four bioactive sesquiterpene lactones: dehydroleucodine (DhL); mexicanin I (Mxc). psilostachyin (Psi), and psilostachyin C (Psi C) on the defense mechanism of Leishmania mexicana mexicana against oxidative stress.
2. Materials and Methods
2.1. Compounds
Mexicanin I (Mxc) was isolated from the aerial parts of Gaillardia megapotamica and dehydroleucodine (DhL) was isolated from Artemisia douglasiana as previously described [19]. Psilostachyin (Psi) and psilostachyin C (PsiC) have been isolated from Ambrosia tenuifolia and A. scabra, respectively [11, 13].
2.2. Parasites
Axenic cultures of Leishmania mexicana mexicana promastigotes were grown in Diamond's liquid medium (0.106 M NaCl, 29 mM KH2PO4, 23 mM K2HPO4, 12.5 g/L tryptone, 12.5 g/L tryptose, and 12.5 g/L yeast extract, adjusted to pH 7.2) supplemented with 75 μM hemine, 75 IU/mL penicillin, 75 μg/mL streptomycin, and 20% fetal bovine serum at 25°C.
2.3. Treatments
Leishmania mexicana mexicana promastigotes (2 × 106 parasites) were incubated with 0.5 μg/mL of Mxc, Psi, or Psi C or 2.5 μg/mL of DhL, at 25°C, either in the presence or in the absence of 1.5 mM glutathione (GSH). The concentrations used for each compound were those corresponding to each IC50, as previously determined (data not shown). Aliquots of the parasites were collected every 24 h and counted in a Neubauer hemocytometer [16]. In other experiments, parasites were preincubated with the compounds for 30 min and the reducing agent was then added. Alternatively, the lactones were withdrawn after incubation for 1 h and before adding GSH. Controls were carried out in the presence of DMSO (less than 0.05%) which was used to dissolve the compounds.
2.4. Measurement of ROS
The fluorescent probe, H2DCFDA, was used to measure the intracellular generation of ROS, according to Duranteau et al. [20]. Briefly, parasites (1 × 106 cells) were previously treated with the lactones (10 μg/mL of each sesquiterpene lactone for 3 h) and then incubated with 10 μM of the probe for 1 h at room temperature in the dark. The fluorescence intensity of H2DCFDA was measured at 507 nm excitation and 538 nm emission wavelengths. To validate the assay, generation of ROS by 4 mM H2O2 was used as a positive control.
2.5. Measurement of Reduced Glutathione
Endogenous GSH was measured in parasite lysates by using 5,5′-dithiobis-2-nitrobenzoic acid (DTNB), according to Beutler et al. [21]. Briefly, parasites (1 × 107 cells/mL) were previously incubated with the lactones (10 μg/mL) for 3 h at 25°C then pelleted, lysed with 200 μL lysis solution (10% EDTA, 0.5% Triton X-100 in bidistilled water) during 30 min, and centrifuged at 12,000 ×g. Supernatants were mixed with 300 μL of solution P (0.2 M HPO3, 5 mM EDTA, and 5.1 M NaCl) and centrifuged again at 12,000 ×g. Supernatants were mixed with 800 μL of 0.3 M Na2HPO4, and 200 μL of DTNB (in 1% sodium citrate). Absorbances were then measured in a spectrophotometer at 412 nm, and the concentration of GSH was derived from a standard curve.
2.6. Statistical Analysis
Results are presented as mean ± SD. The level of statistical significance was determined by using one-way analysis of variance (ANOVA) followed by Dunnett's multiple comparisons test.
3. Results and Discussion
We had previously reported the antileishmanial activity of Mxc, DhL, Psi, and Psi C (Figure 1) [9–16]. The common functional group α-methylene-γ-lactone present in the sesquiterpene lactones is believed to be responsible for their antiprotozoal activity. However, the presence of other alkylating groups such as α,β-unsaturated cyclopentenones and other factors, such as lipophilicity, molecular geometry, and chemical environment, may also influence their bioactivity [22].
Figure 1.
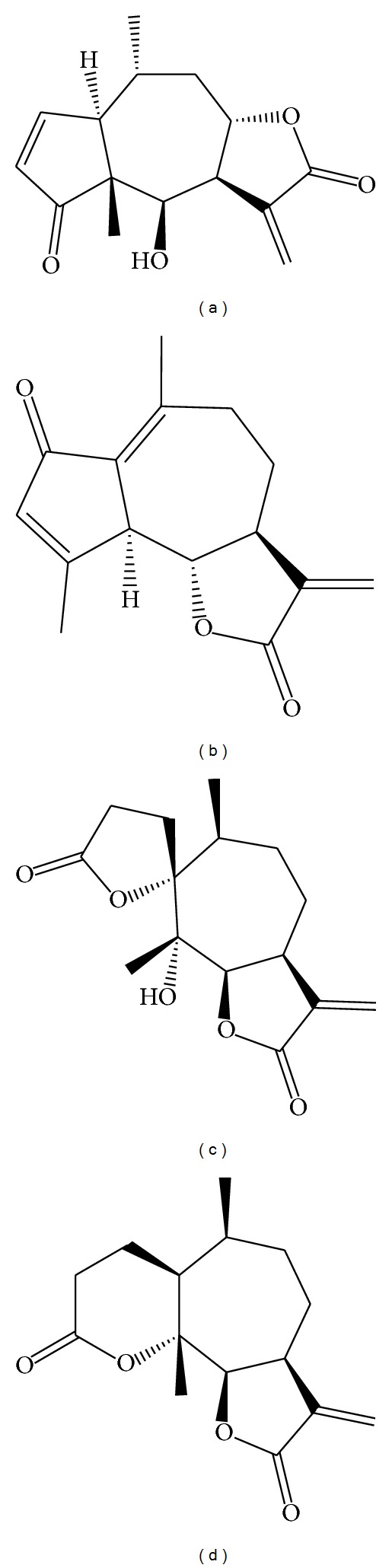
Chemical structures of the sesquiterpene lactone: mexicanin I (a), dehydroleucodine (b), psilostachyin (c), and psilostachyin C (d).
In this work we have corroborated the antiproliferative effect of the four lactones on L. mexicana mexicana promastigotes and we have demonstrated that this effect was blocked by 1.5 mM GSH (Figure 2). As these lactones are nonpolar molecules they could easily pass through the parasite's plasmalemma. The blocking effect of GSH might be due to the transformation of the compounds into derivatives unable to traverse the plasmalemma. However, it is more likely that the compounds interfere with the intracellular concentration of GSH, as the antiproliferative effect of lactones can be reversed by GSH when the reducing agent is added 30 min after incubation with the compounds or 1 h after incubation followed by withdrawal of the lactones (Figure 3). In addition, it was observed that DhL, Mxc, and Psi, but not Psi C, reduced the concentration of endogenous GSH (Figure 4).
Figure 2.
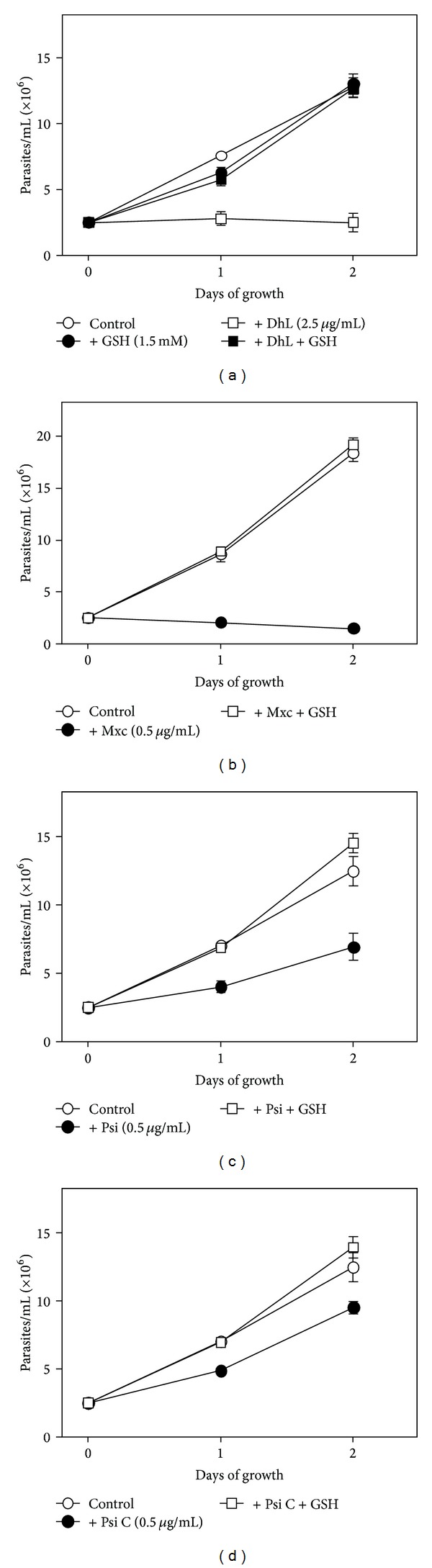
The effect of sesquiterpene lactones on the growth of L. mexicana mexicana is blocked by adding of GSH. Parasites were incubated with the lactones; dehydroleucodine (DhL) (a), mexicanin I (Mxc) (b), psilostachyin (Psi) (c), or psilostachyin C (Psi C) (d) in the presence or in the absence of 1.5 mM glutathione (GSH), as indicated in the figure. Parasite counts were done daily. Glutathione alone did not affect the parasite growth (a).
Figure 3.
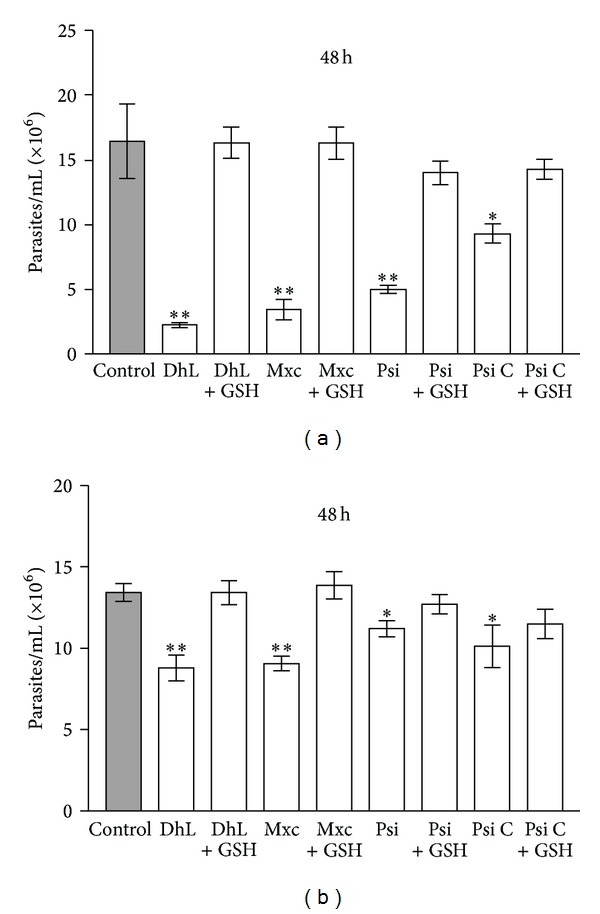
The effect of glutathione (GSH) on the number of parasites preincubated with 0.5 μg/mL mexicanin I (Mxc), psilostachyin (Psi) or psilostachyin C (Psi C) or with 2.5 μg/mL of dehydroleucodine (DhL), for 30 min (a) or preincubated 1 h with the lactones and followed by withdrawal of the compounds before adding the reducing agent (b). Bars represent the means of parasite concentration ± SD from three independent experiments. (∗∗) and (∗) indicate significant differences with the control (P < 0.01 and P < 0.05 resp.).
Figure 4.
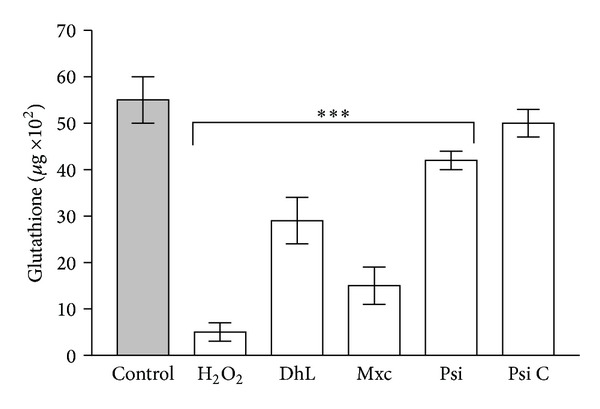
Concentration of endogenous glutathione in the parasites after treatment with 10 μg/mL dehydroleucodine (DhL), mexicanin I (Mxc), psilostachyin (Psi), or psilostachyin C (Psi C), as described in materials and methods. (∗∗∗): significant differences with the control (P < 0.02). H2O2 (5 mM) was used as positive control.
On the other hand, treatment with DhL, Mxc, or Psi, but not Psi C, induced a significant increase of ROS in L. mexicana promastigotes (Figure 5).
Figure 5.
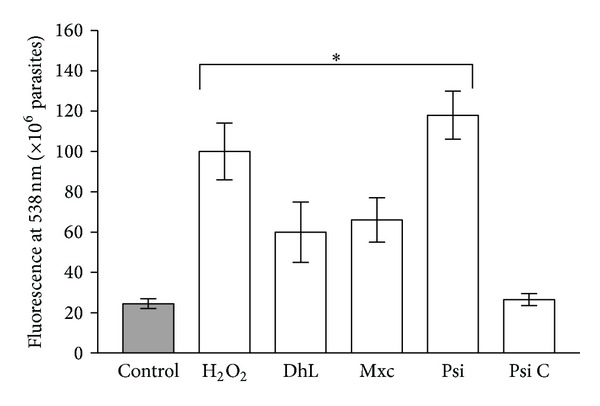
Generation of ROS by the parasites after treatment with 10 μg/mL of mexicanin I (Mxc), dehydroleucodine (DhL), psilostachyin (Psi), and psilostachyin C (Psi C). Values are expressed as units of fluorescence emitted by the probe at 538 nm. Bars represent the means of fluorescence ± SD from three independent experiments. (∗): significant differences with the control (P < 0.05).
The generation of free radicals in Leishmania by the sesquiterpene lactones would be deleterious for trypanosomatids, as the regulation of oxidative stress is crucial for parasite survival. It is known that sesquiterpene lactones react with sulfhydryl groups by the Michael-type addition and therefore could act by inhibiting the activity of enzymes that are vital against oxidative stress (e.g., trypanothione reductase) [17]. This situation could lead to an increase in the level of reactive oxygen species and to parasite damage via the generation of an oxidative burst by a deregulation of the redox balance within the parasite [23]. However, a direct interaction of the compounds with GSH or trypanothione should not be ruled out.
The decrease in the concentration of glutathione within the parasites induced by the sesquiterpene lactones Mxc, DhL, and Psi would lead to an enhancement in the production of reactive oxygen species. These results are in accordance with ROS production and the in vitro leishmanicidal activity, with psilostachyin C being the less active compound against L. mexicana. Given that sesquiterpene lactones can also induce GSH depletion and ROS generation in certain mammalian cells (e.g., tumor cells) [24], these compounds should be improved before use as therapeutic agents against Leishmania.
One vital step in the process of drug development is the identification of the molecular target/s of such drugs. Taking into consideration the data obtained, we can suggest that Mxc, DhL, and Psi were able to affect the defense mechanism against oxidative stress in L. mexicana. This mechanism could be related to inhibition of key enzymes that maintain redox balance in the parasite.
This study must be complemented by further investigations on amastigotes forms of Leishmania and on in vivo models of leishmaniasis.
Authors' Contribution
Patricia Barrera and Valeria P. Sülsen contributed equally to this work.
Acknowledgments
This research was supported in part by Grant PIP 01540 (Consejo Nacional de Investigaciones Científicas y Técnicas) and UBACYT 20020110200114 and 20020100100201.
References
- 1.WHO. Control of leishmaniases. World Health Organization. Technical Report Series. 2010;(949) http://www.who.int/leishmaniasis/en/ [PubMed]
- 2.Khaw M, Panosian CB. Human antiprotozoal therapy: past, present, and future. Clinical Microbiology Reviews. 1995;8(3):427–439. doi: 10.1128/cmr.8.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Croft SL, Sundar S, Fairlamb AH. Drug resistance in leishmaniasis. Clinical Microbiology Reviews. 2006;19(1):111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salomón O, Acardi S, Liotta D, et al. Epidemiological aspects of cutaneous leishmaniasis in the Iguazú falls area of Argentina. Acta Tropica. 2009;109(1):5–11. doi: 10.1016/j.actatropica.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 5.DNDi. Drugs for Neglected Diseases Initiative. Leishmaniasis. Current treatments. 2013, http://www.dndi.org/diseases-projects/diseases/vl/current-treatment.html.
- 6.Schmidt T, Khalid S, Romanha A, et al. The potential of secondary metabolites from plants as drugs or leads against protozoan neglected diseases—part I. Current Medicinal Chemistry. 2012;19(14):2128–2175. doi: 10.2174/092986712800229023. [DOI] [PubMed] [Google Scholar]
- 7.Salem MM, Werbovetz KA. Natural products from plants as drug candidates and lead compounds against leishmaniasis and trypanosomiasis. Current Medicinal Chemistry. 2006;13(21):2571–2598. doi: 10.2174/092986706778201611. [DOI] [PubMed] [Google Scholar]
- 8.Polonio T, Efferth T. Leishmaniasis: drug resistance and natural products. International Journal of Molecular Medicine. 2008;22(3):277–286. [PubMed] [Google Scholar]
- 9.Lozano E, Barrera P, Salinas R, et al. Sesquiterpene lactones and the diterpene 5-epi-icetexone affect the intracellular and extracellular stages of Trypanosoma cruzi . Parasitology International. 2012;61(4):p. 628. doi: 10.1016/j.parint.2012.06.005. 633. [DOI] [PubMed] [Google Scholar]
- 10.Sülsen V, Gutierrez Yappu D, Laurella L, et al. In vitro antiplasmodial activity of sesquiterpene lactones from Ambrosia tenuifolia . Evidence-Based Complementary and Alternative Medicine. 2011;2011:4 pages. doi: 10.1155/2011/352938.352938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sülsen VP, Frank FM, Cazorla SI, et al. Psilostachyin C: a natural compound with trypanocidal activity. International Journal of Antimicrobial Agents. 2011;37(6):536–543. doi: 10.1016/j.ijantimicag.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Sülsen V, Barrera P, Muschietti L, Martino V, Sosa M. Antiproliferative effect and ultrastructural alterations induced by psilostachyin on Trypanosoma cruzi . Molecules. 2010;15(1):545–553. doi: 10.3390/molecules15010545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sülsen VP, Frank FM, Cazorla SI, et al. Trypanocidal and leishmanicidal activities of sesquiterpene lactones from Ambrosia tenuifolia Sprengel (Asteraceae) Antimicrobial Agents and Chemotherapy. 2008;52(7):2415–2419. doi: 10.1128/AAC.01630-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrera PA, Jimenez-Ortiz V, Tonn C, Giordano O, Galanti N, Sosa MA. Natural sesquiterpene lactones are active against Leishmania mexicana . Journal of Parasitology. 2008;94(5):1143–1149. doi: 10.1645/GE-1501.1. [DOI] [PubMed] [Google Scholar]
- 15.Jimenez-Ortiz V, Brengio SD, Giordano O, et al. The trypanocidal effect of sesquiterpene lactones helenalin and mexicanin on cultured epimastigotes. Journal of Parasitology. 2005;91(1):170–174. doi: 10.1645/GE-3373. [DOI] [PubMed] [Google Scholar]
- 16.Brengio SD, Belmonte SA, Guerreiro E, Giordano OS, Pietrobon EO, Sosa MA. The sesquiterpene lactone dehydroleucodine (DhL) affects the growth of cultured epimastigotes of Trypanosoma cruzi . Journal of Parasitology. 2000;86(2):407–412. doi: 10.1645/0022-3395(2000)086[0407:TSLDDA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 17.Chawla B, Madhubala R. Drug targets in Leishmania. Journal of Parasitic Diseases. 2010;34(1):1–13. doi: 10.1007/s12639-010-0006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cavalli A, Bolognesi ML. Neglected tropical diseases: multi-target-directed ligands in the search for novel lead candidates against Trypanosoma and Leishmania . Journal of Medicinal Chemistry. 2009;52(23):7339–7359. doi: 10.1021/jm9004835. [DOI] [PubMed] [Google Scholar]
- 19.Giordano OS, Guerreiro E, Pestchanker MJ, Guzman J, Pastor D, Guardia T. The gastric cytoprotective effect of several sesquiterpene lactones. Journal of Natural Products. 1990;53(4):803–809. doi: 10.1021/np50070a004. [DOI] [PubMed] [Google Scholar]
- 20.Duranteau J, Chandel NS, Kulisz A, Shao Z, Schumacker PT. Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. The Journal of Biological Chemistry. 1998;273(19):11619–11624. doi: 10.1074/jbc.273.19.11619. [DOI] [PubMed] [Google Scholar]
- 21.Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. The Journal of Laboratory and Clinical Medicine. 1963;61:882–888. [PubMed] [Google Scholar]
- 22.Schmidt TJ, Nour AMM, Khalid SA, Kaiser M, Brun R. Quantitative structure—antiprotozoal activity relationships of sesquiterpene lactones. Molecules. 2009;14(6):2062–2076. doi: 10.3390/molecules14062062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaturvedi D. Sesquiterpene lactones: structural diversity and their biological activities. In: Tiwari V, Mishra B, editors. Opportunity, Challenge and Scope of Natural Products in Medicinal Chemistry. Kerala, India: Research Signpost; 2011. pp. 313–334. [Google Scholar]
- 24.Mathema VB, Koh Y, Thakuri BC, Sillanpää M. Parthenolide, a sesquiterpene lactone, expresses multiple anti-cancer and anti-inflammatory activities. Inflammation. 2012;35:560–565. doi: 10.1007/s10753-011-9346-0. [DOI] [PubMed] [Google Scholar]


