Abstract
The purpose of this paper is to systematically review the experimental and human studies on obesogenic chemicals and their mechanisms of action to provide a comprehensive view on the multifactorial aspects of obesity. The literatures were searched in available databases. The relevant papers were selected in three phases. After quality assessment, two reviewers extracted the data while another checked their extracted data. In this review, we summarized information regarding environmental chemicals that can be associated with obesity. Most evidence comes from experimental and laboratory studies; however a growing number of human studies also support the role of obesogenic chemicals. The current evidence proposes that the systemic responses to exposure to environmental factors could potentially increase the risk of excess weight. The effects of exposure to these chemicals are of crucial importance during developmental phases of life, when preprogramming for an adipogenic outcome may occur. By considering the adverse transgenerational effects of obesogen chemicals on human health, the global obesity epidemic should be considered as a multifactorial complex disorder necessitating the emphasis of public health interventions for environmental protection.
1. Introduction
Obesity is becoming a human health crisis at individual and public health levels. It has numerous adverse health effects and is considered as one of the main predisposing factors for the emerging epidemic of noncommunicable diseases [1]. Nowadays, overweight and obesity are growing in populations with different levels of economic situation. It is estimated that by continuing the actual trend, the global prevalence rate of 33.0% for overweight and obesity among adult population (1.3 billion people) in 2005 would reach up to 57.8% (3.3 billion people) by 2030 [2]. The World Health Organization included excess weight, with a prevalence higher than undernutrition, as one of the top 10 health risks worldwide [3].
The rise in the incidence in obesity matches the rise in the use and distribution of industrial chemicals that may have a role in development of obesity. In her interesting review in 2002, Baillie-Hamilton postulated a role for chemical toxins in the etiology of obesity by presenting the coincidence of the obesity epidemic with the noticeable increase of industrial chemicals in the environment over the past four decades. An accumulating body of evidence suggests that substances as endocrine-disrupting chemicals (EDCs) may be linked to the obesity epidemic [4]. EDCs are chemicals that alter the normal functioning of hormones and other signaling molecules in the body [5]. Additional studies proposed the existence of chemicals termed “obesogens,” molecules with adverse effects on lipid metabolism and adipogenesis, and in turn resulting in obesity [6, 7].
The environmental obesogen hypothesis suggests that prenatal or early-life exposure to certain substances as EDCs may predispose exposed individuals to increased fat mass and excess weight. It is suggested that exposure to obesogens can modify the epigenome of multipotent stromal stem cells, biasing them to the adipocyte lineage at the expense of bone. Hence, humans exposed to obesogens during early life might have an altered stem cell compartment, already preprogrammed for an adipogenic outcome [8].
The list of chemicals studied as possible obesogens continues to grow and includes diethylstilbestrol (DES), bisphenol A (BPA), phthalates, organotins, polybrominated diphenyl ethers (PBDEs), polyfluoroalkyl chemicals (PFCs), organochlorine (OC) pesticides, and polychlorinated biphenyls (PCBs) and some solvents caused weight gain, and it is proposed that these chemicals were interfering with weight homeostasis by changing weight-controlling hormones, modifying sensitivity to neurotransmitters, or altering the sympathetic nervous system activity [9].
The purpose of this paper is to systematically review the experimental and human studies on obesogenic chemicals and their mechanisms of action to provide a comprehensive view on underlying mechanisms and the multifactorial aspects of obesity for clinicians and public health stakeholders.
2. Methods
2.1. Literature Search Strategy
Relevant literature reporting the environmental obesogens was identified through electronic search of MEDLINE, PubMed, ISI Web of Science, and Scopus/Embase with no time or language restrictions. The literature search was conducted during January and February 2013. We searched the databases using the following strategy: for Scopus/Embase we used the Emtree Thesaurus terms; for PubMed search, we considered Medical Subheading (MeSH) words, and for other databases we used keywords (text words). For PubMed search, we used (“endocrine disruptors” [MeSH] OR “endocrine disrupting chemicals” OR “obesogen” [mh] OR “Polychlorinated Biphenyls” [MeSH] OR “Hydrocarbons, Chlorinated” [MeSH] OR “Dioxins” [MeSH] OR “Polybrominated Biphenyls” [MeSH] OR “Carbon Tetrachloride” [MeSH] OR “Organothiophosphorus Compounds” [MeSH] OR “phthalic acid” [Substance Name] OR “Phthalic Acids” [MeSH] OR “Organotin Compounds” [MeSH] OR “bisphenol A” [Substance Name] AND ((“obesity” [mh] OR “overweight” [mh] OR “excess weight” [mh] OR “body mass index” [mh] OR “weight gain” [mh] OR “adipogenesis” [mh] OR “adipose tissue” [mh] OR “fat deposition” [mh]) AND (publisher[sb] OR “in process” [sb]).
2.2. Study Selection and Eligibility Criteria
Duplicates were removed; the relevant papers were selected in three phases. In the first and second phases, titles and abstracts of papers were screened and irrelevant papers were excluded. In the last phase, the full text of recruited papers was explored intensely to select only relevant papers. For any additional pertinent studies, the reference list of all reviews and relevant papers was screened as well. All these three screening phases were done by two independent reviewers (FJ and PP). In the next step, the eligibility of relevant papers was checked. Discrepancies were resolved by consultation and consensus.
2.3. Quality Assessment
Identification of main findings of studies was conducted on a case-by-case basis and included consideration of any statistical analyses that might have been conducted, consistency of the general pattern across exposure groups.
2.4. Data Extraction and Abstraction
The required information that was extracted from all eligible papers was as follows: (i) general characteristics of the study (first author's name, publication year, study year, study design) (ii) characteristics of the chemical, (iii) reason for using the chemical, (iv) suggested obesogen mechanism, and (v) adverse effects on humans or animals.
Two reviewers (FJ and PP) extracted the data while another (RK) checked their extracted data.
3. Results
The flowchart of our study selection is presented in Figure 1. We found that actually many environmental obesogens are identified; they are mainly classified as chemical simulators of metabolic hormones or brain neurotransmitters [10, 11]. Several experimental studies reported the association of exposure to some environmental chemicals with obesity. Bisphenol A [12–15], tributyltin (TBT) [16, 17], nonylphenol [18, 19] and genistein [20, 21], phatalate [22], perfluoroalkyl compounds (PFCs) [23], and perfluorooctanoic acid (PFOA) [24] are some of the obesogen chemicals described by experimental studies. The major environmental obesogen chemicals are presented in Table 1.
Figure 1.
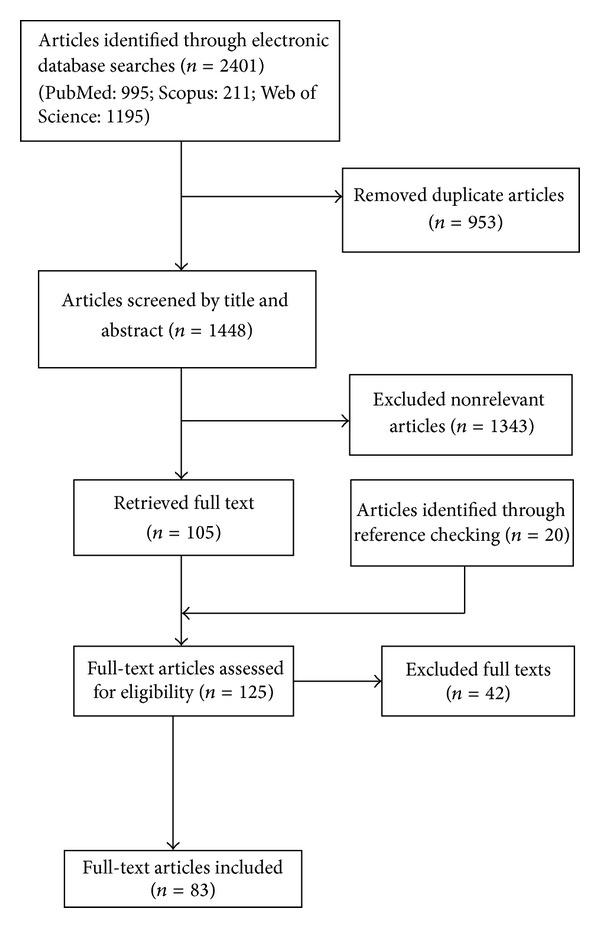
Flowchart of study selection.
Table 1.
Summary of main obesogen chemicals and their health effects.
| Chemicals | 1st author | St.yr | Ge.Loc | Sample size | Study population | Characteristics | Uses | Mechanisms | Human effects | Animal effects |
|---|---|---|---|---|---|---|---|---|---|---|
| Phytoestrogens (genistein and daidzein) |
Miriam J. J. de Kleijn [21] 2002 | 1971 | USA | 5209 | 30–59 y | Included in various food and food supplements, in particular soy product | High doses inhibited adipose deposition but at low doses similar to those found in Western and Eastern diets, in soy milk, or in food supplements containing soy, it induced adipose tissue deposition especially in males | |||
|
| ||||||||||
| Perfluorooctanoic acid (PFOA) | Frank D. Gilliland [24] | 1985–1989 | USA | 115 | Perfluorooctanoic acid (PFOA) increases PPAR-dependent lipid mobilization, fatty acid oxidation, and adipose tissue atrophy during periods of experimental exposure. PFOA probably exerts anorexigenic effects through a central hypothalamic mechanism that triggers a decrease in food intake in adult rodents | |||||
|
| ||||||||||
| Perfluoroalkyl compounds (PFCs) | Sakr,CarineJ [23] 2007 | 2007 | 1025 | Agonists for one or more of the PPARs, providing a mechanistic link to disturbed lipid and steroid metabolism | ||||||
|
| ||||||||||
| Phtalates | Elizabeth E Hatch [22] 2008 | 1999–2002 | USA | 4369 participants | 6–80 y | As plasticizers and stabilizers in a variety of plastics. They are found in industrial paints and solvents but also in cosmetics, perfumes, and medicines | PPARα, PPARγ, ER, and peptidergic hormones | |||
|
| ||||||||||
| Nonylphenol | Mei-Lien Chen [19] 2009 | 2008 | Taiwan | 960 | Primary and junior high schools | |||||
|
| ||||||||||
| Organotins, as tributyltin chloride (TBT) and bis (triphenyltin) oxide | Zhenghong Zuo [17] 2011 | 2009 | China | 32 mice | Mice, aged 21 days and weighing 10.5–13.5 g | Tetravalent tin compounds with a variety of mono-, di-, tri-, or tetrasubstituted organic functional groups | Antifouling agents in paints for marine shipping and for a variety of other uses | PPARg and RXR have been shown to disrupt normal development and homeostatic controls over adipogenesis and energy balance. suggested an inhibition of adipogenesis in the 3T3-L1 cells(1) TBT stimulates adipocytes differentiation in vitro and increases adipose mass in vivo in the 3T3-L1 cells | In-utero studies, showed TBT to accumulate lipids in adipose, testis, and liver tissues in neonate mice. and increasing epididymal adipose mass in adult mice | |
|
| ||||||||||
| Bisphenol A (BPA) | He-xing Wang1 [15] 2012 | 2011 | China | 360 | 8–15 y | BPA is a small (228 Da) molecule which is used as a monomer in polymerization reaction to produce polycarbonate plastics | Used in food and water containers baby bottles, lining of food and beverage metal cans, medical tubing, epoxy resins, and dental fillings | BPA mimics the actions of E2 on blood glucose homeostasis via two pathways: a rapid pathway involving ncmER and a prolonged pathway involving ER. It inhibits adiponectin release and stimulates release of IL-6 and TNFα. mouse triggers 3T3-L1 cells (fibroblasts that can differentiate into adipocytes) to differentiate into adipocytes. Suppression of adiponectin and increased IL-6 and TNFα | There has been no information on BPA effects on human adipocytes | Mice treated with low doses of E2 or BPA showed rapid increases in insulin release and reduced plasma glucose. High dose for 15 d reduction in body weight. 3 m did not alter body weight and fat depot |
Diverse mechanisms are explained for obesogen chemicals; mainly they have disruptive effects on homeostasis of energy balance, glucose and lipid metabolism, and control of adipogenesis. A summary of the underlying mechanisms of these substances is reported in Table 2.
Table 2.
Summary of mechanisms suggested for main obesogen chemicals.
| Mechanism | Acting by | Chemicals |
|---|---|---|
| Metabolic sensors | PPAR, RXR, TR | TBT, TPT, PFCs, phthalate |
| Sex steroid dysregulations | CYP19, ER, AR | TBT, TPT, phthalate, BPA, alkylphenol, phytoestrogen, DES |
| Central integration of energy balance | PH, HPA, EC, NE | TBT, TPT, phthalate, BPA, alkylphenol, phytoestrogen, SSRI, typical antidepressant, atypical antipsychotic |
| Metabolic point | GR signaling(11_HSD), HPT | TBT, TPT, PBDEs, Dithiocarbamates, Glycyrrhetinic acid,TZD |
TBT: tributyltin; TPT: triphenyltin; BPA: bisphenol A; PFCs: perfluoroalkyl compounds; PBDEs: polybrominated diphenyl ethers; DES: diethylstilbestrol; SSRI: selective serotoninreuptake inhibitor; TZD: thiazolidinediones; NE: neuroendocrine effects; PH: peptidergic hormones; EC: endocannabinoid; HPT: hypothalamus-pituitary-thyroid; HPA: hypothalamus-pituitary-adrenal.; TR: thyroid hormone receptor; PPAR: peroxisome proliferator activated receptors; RXR: 9-cis retinoic acid receptor; ER: estrogen receptors; AR: androgen receptors.
Conflicting results are reported about the effects of obesogen chemicals in human studies. The concentrations of many industrial obesogen chemicals are found to be high in general population [25]. For instance, some studies examined the obesogenic effects of phthalates, which are esters mainly added to plastics to increase their flexibility, transparency, durability, and longevity. Cross-sectional data from the National Health and Nutrition Examination Survey (NHANES) in the USA found significant associations between several phthalates metabolites (monobenzyl phthalate (MBzP), mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), and mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP)) and measures of abdominal obesity and insulin resistance in men but not in women [26]. A cross-sectional study on 90 girls aged 6–8 years found slightly higher concentrations of some phthalate metabolites as monoethyl phthalate (MEP), mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), and mono-n-butyl phthalate (MBP) among overweight girls than in their other counterparts; however the difference was not statistically significant [27]. Some epidemiologic studies documented the obesogenic effects of some environmental chemicals, as PCB [28] and BPA [29], whereas such effects are conflicting for some other chemicals as organochlorine pesticides [30–32].
Phytoestrogens, notably soy products, have beneficial health effects and are added to several food and food supplements. However some studies suggested that they may act as obesogen chemicals. Genistein is one the mostly used phytoestrogens in the human diet, and by its estrogenic activity, it has favorable effects for regulating the homeostasis of lipids and carbohydrates [33]. Though its beneficial effects in inhibiting fat deposition in the adipose tissue are considered to be obtained at high pharmacological doses, its low doses in foods are found to increase adiposity and mild peripheral insulin resistance particularly in males [34].
4. Discussion
In this review, we summarized information regarding environmental chemicals that can be associated with obesity. Most evidence comes from experimental and laboratory studies; however a growing number of human studies also support the role of obesogen chemicals.
Chemicals as heavy metals, some solvents, pesticides, BPA, organophosphates, phthalates, PCB, PBBs, and many other substances are documented to cause weight gain. These chemicals interfere with weight and lipid homeostasis by various mechanisms related to weight-controlling hormones, activity of the sympathetic nervous system, and sensitivity to neurotransmitters.
Exposure to these chemicals varies in different age groups; their effects during fetal and infancy periods may be irreversible and long-lasting for adulthood. Even exposure to low doses of EDCs during critical times of differentiation can change the developmental programming and may result in obesity [35]. Barker's hypothesis on the effects of intrauterine growth on fetal programming and fetal origins of adult diseases is well documented [36, 37]; however, other characteristics as later growth spurt and environmental factors are considered to influence this programming. Exposure to environmental chemicals with endocrine-disrupting activities in early life may result in everlasting adverse health effects [38]. Such health consequences may become apparent not only in childhood, but also in adulthood [5], and even in succeeding generations [39]. Transgenerational effects may be because of mutations as well as because of factors regulating gene expression [5]. Our findings support the role of obesogens, as chemicals with disruptive effects on fat homeostasis and various weight controlling mechanisms, in programming the development of excess weight from early life. Although all obesogen chemicals are not yet identified, and their detailed mechanisms of action remain to be explored, generally it is assumed that exposure to different doses of these environmental chemicals in various periods of life from fetal to adult period interacts with some endocrine signaling mechanisms and in turn leads to obesity.
EDCs act by diverse mechanisms; accumulating body of evidence supports that these chemicals disrupt some epigenetic, structural, and functional mechanisms, which control energy homeostasis, lipid metabolism, appetite regulation, and adipogenesis [40–44].
Chemical obesogens are considered to function through various factors as leptin, ghrelin, melanocyte-stimulating hormones, neuropeptide Y, amphetamine-regulated transcript, agouti-related protein, and cocaine, as well as through inhibiting aromatases as the P450 family members (CYP19 and CYP3A1) [42–44] or through modifying the expression of various receptors for steroid hormones, retinoic X, peroxisome proliferator-activated, and glucocorticoids [45]. The exposure to obesogen chemicals may influence the steroid hormone receptors or may change serum levels of metabolic hormones or may influence nuclear receptor signaling pathways in preadipocytes, which would result in adipocyte differentiation and a tendency to excess weight [44, 45].
The systemic reactions to exposure to environmental chemical factors can potentially increase the risk for obesity-related health effects, as metabolic syndrome, insulin resistance [46], prediabetes, diabetes, oxidative stress [47], prehypertension [48], hypertension, and nonalcoholic fatty liver diseases [49] even in the pediatric age group.
Even in the pediatric age group, environmental chemicals can influence oxidative stress and proinflammatory cytokines [46, 47, 50], which in turn would initiate the second hit suggested in the “two-hit hypothesis” [51, 52] for the progression of fatty liver to metabolic syndrome and diabetes.
The other aspect of the influences of environmental factors on obesity and its health consequences can be the impact of these chemicals on intrauterine growth retardation, low birth weight, and prematurity [53–55], which are documented as predisposing factors for obesity and adult chronic diseases.
Whether the results of laboratory models can be generalized to health hazards in humans remain to be determined, but a growing number of epidemiologic studies also suggest a link between exposure to environmental chemicals with obesity. However, it should be considered that in many human studies, weight gain has not been an endpoint in the original proposal, and excess weight has been reported as an adverse effect.
Environmental factors have diverse health effects [47–50, 56–58]. Although rapid changes in lifestyle habits, along with increased energy intake and decreased energy expenditure, are considered as the main causes of excess weight, but by considering the rapid escalating trend of obesity in various age groups and in populations with different lifestyle habits and diverse socioeconomic levels, it is obvious that it is simple-minded to consider only these two factors responsible for this expanding global problem; the role of other environmental determinants as obesogen chemicals is being proposed in this regard.
4.1. Study Limitations
Most studies included in this review have been observational and cross-sectional. Large-scale longitudinal studies with long-term followup are necessary to document the clinical importance of exposure to environmental chemicals.
5. Conclusion
The current evidence proposes that the systemic responses to exposure to environmental factors, notably during developmental phases of life, could potentially increase the risk of excess weight. By taking into account the current knowledge on the adverse transgenerational effects of obesogen chemicals on human health, the global obesity epidemic should be considered as a multifactorial complex disorder necessitating the emphasis of public health interventions for environmental protection.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. Journal of the American Medical Association. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Stevens GA, Singh GM, Lu Y, et al. National, regional, and global trends in adult overweight and obesity prevalences. Population Health Metrics. 2012;10(1, article 22) doi: 10.1186/1478-7954-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Consultation W. Obesity: preventing and managing the global epidemic. World Health Organization Technical Report Series. 2000;(894) [PubMed]
- 4.Baillie-Hamilton PF. Chemical toxins: a hypothesis to explain the global obesity epidemic. The Journal of Alternative and Complementary Medicine. 2002;8(2):185–192. doi: 10.1089/107555302317371479. [DOI] [PubMed] [Google Scholar]
- 5.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, et al. Endocrine-disrupting chemicals: an endocrine society scientific statement. Endocrine Reviews. 2009;30(4):293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grün F, Blumberg B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology. 2006;147(6, supplement):S50–S55. doi: 10.1210/en.2005-1129. [DOI] [PubMed] [Google Scholar]
- 7.Tabb MM, Blumberg B. New modes of action for endocrine-disrupting chemicals. Molecular Endocrinology. 2006;20(3):475–482. doi: 10.1210/me.2004-0513. [DOI] [PubMed] [Google Scholar]
- 8.Janesick A, Blumberg B. Endocrine disrupting chemicals and the developmental programming of adipogenesis and obesity. Birth Defects Research C. 2011;93(1):34–50. doi: 10.1002/bdrc.20197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatch EE, Nelson JW, Stahlhut RW, Webster TF. Association of endocrine disruptors and obesity: perspectives from epidemiological studies. International Journal of Andrology. 2010;33(2):324–331. doi: 10.1111/j.1365-2605.2009.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grün F, Blumberg B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology. 2006;147(6):S50–S55. doi: 10.1210/en.2005-1129. [DOI] [PubMed] [Google Scholar]
- 11.Grün F, Blumberg B. Minireview: the case for obesogens. Molecular Endocrinology. 2009;23(8):1127–1134. doi: 10.1210/me.2008-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masuno H, Iwanami J, Kidani T, Sakayama K, Honda K. Bisphenol A accelerates terminal differentiation of 3T3-L1 cells into adipocytes through the phosphatidylinositol 3-kinase pathway. Toxicological Sciences. 2005;84(2):319–327. doi: 10.1093/toxsci/kfi088. [DOI] [PubMed] [Google Scholar]
- 13.Masuno H, Kidani T, Sekiya K, et al. Bisphenol A in combination with insulin can accelerate the conversion of 3T3-L1 fibroblasts to adipocytes. Journal of Lipid Research. 2002;43(5):676–684. [PubMed] [Google Scholar]
- 14.Sakurai A, Toyoda S, Masuda M, Sakakibara M. Removal of bisphenol A by peroxidase-catalyzed reaction using culture broth of Coprinus cinereus. Journal of Chemical Engineering of Japan. 2004;37(2):137–142. [Google Scholar]
- 15.Wang H-X, Zhou Y, Tang C-X, Wu J-G, Chen Y, Jiang Q-W. Association between bisphenol A exposure and body mass index in Chinese school children: a cross-sectional study. Environmental Health. 2012;11(1, article 1) doi: 10.1186/1476-069X-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inadera H, Shimomura A. Environmental chemical tributyltin augments adipocyte differentiation. Toxicology Letters. 2005;159(3):226–234. doi: 10.1016/j.toxlet.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Zuo Z, Chen S, Wu T, et al. Tributyltin causes obesity and hepatic steatosis in male mice. Environmental Toxicology. 2011;26(1):79–85. doi: 10.1002/tox.20531. [DOI] [PubMed] [Google Scholar]
- 18.Masuno H, Okamoto S, Iwanami J, et al. Effect of 4-nonylphenol on cell proliferation and adipocyte formation in cultures of fully differentiated 3T3-L1 cells. Toxicological Sciences. 2003;75(2):314–320. doi: 10.1093/toxsci/kfg203. [DOI] [PubMed] [Google Scholar]
- 19.Chen M-L, Lee H-Y, Chuang H-Y, Guo B-R, Mao IF. Association between nonylphenol exposure and development of secondary sexual characteristics. Chemosphere. 2009;76(7):927–931. doi: 10.1016/j.chemosphere.2009.04.054. [DOI] [PubMed] [Google Scholar]
- 20.Dang Z-C, Audinot V, Papapoulos SE, Boutin JA, Löwik CWGM. Peroxisome proliferator-activated receptor γ (PPARγ) as a molecular target for the soy phytoestrogen genistein. The Journal of Biological Chemistry. 2003;278(2):962–967. doi: 10.1074/jbc.M209483200. [DOI] [PubMed] [Google Scholar]
- 21.de Kleijn MJJ, van der Schouw YT, Wilson PWF, Grobbee DE, Jacques PF. Dietary intake of phytoestrogens is associated with a favorable metabolic cardiovascular risk profile in postmenopausal U. S. women: the Framingham study. Journal of Nutrition. 2002;132(2):276–282. doi: 10.1093/jn/132.2.276. [DOI] [PubMed] [Google Scholar]
- 22.Hatch EE, Nelson JW, Qureshi MM, et al. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999–2002. Environmental Health. 2008;7, article 27 doi: 10.1186/1476-069X-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakr CJ, Kreckmann KH, Green JW, Gillies PJ, Reynolds JL, Leonard RC. Cross-sectional study of lipids and liver enzymes related to a serum biomarker of exposure (ammonium perfluorooctanoate or APFO) as part of a general health survey in a cohort of occupationally exposed workers. Journal of Occupational and Environmental Medicine. 2007;49(10):1086–1096. doi: 10.1097/JOM.0b013e318156eca3. [DOI] [PubMed] [Google Scholar]
- 24.Gilliland FD, Mandel JS. Serum perfluorooctanoic acid and hepatic enzymes, lipoproteins, and cholesterol: a study of occupationally exposed men. American Journal of Industrial Medicine. 1998;29(5):560–568. doi: 10.1002/(SICI)1097-0274(199605)29:5<560::AID-AJIM17>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 25.Curtis K, Wilding B. Is it in us? Chemical contamination in our bodies. A Report from the Body Burden Work Group & Commonweal Biomonitoring Resources Center, USA, 2008, http://www.isitinus.org.
- 26.Stahlhut RW, van Wijngaarden E, Dye TD, Cook S, Swan SH. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environmental Health Perspectives. 2007;115(6):876–882. doi: 10.1289/ehp.9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolff MS, Teitelbaum SL, Windham G, et al. Pilot study of urinary biomarkers of phytoestrogens, phthalates, and phenols in girls. Environmental Health Perspectives. 2007;115(1):116–121. doi: 10.1289/ehp.9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goncharov A, Haase RF, Santiago-Rivera A, et al. High serum PCBs are associated with elevation of serum lipids and cardiovascular disease in a Native American population. Environmental Research. 2008;106(2):226–239. doi: 10.1016/j.envres.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeuchi T, Tsutsumi O, Ikezuki Y, Takai Y, Taketani Y. Positive relationship between androgen and the endocrine disruptor, bisphenol A, in normal women and women with ovarian dysfunction. Endocrine Journal. 2004;51(2):165–169. doi: 10.1507/endocrj.51.165. [DOI] [PubMed] [Google Scholar]
- 30.Hue O, Marcotte J, Berrigan F, et al. Plasma concentration of organochlorine compounds is associated with age and not obesity. Chemosphere. 2007;67(7):1463–1467. doi: 10.1016/j.chemosphere.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 31.Pelletier C, Doucet E, Imbeault P, Tremblay A. Association between weight loss-induced changes in plasma organochlorine concentrations, serum T3 concentration, and resting metabolic rate. Toxicological Sciences. 2002;67(1):46–51. doi: 10.1093/toxsci/67.1.46. [DOI] [PubMed] [Google Scholar]
- 32.Lee D-H, Lee I-K, Jin S-H, Steffes M, Jacobs DR. Association between serum concentrations of persistent organic pollutants and insulin resistance among nondiabetic adults: results from the National Health and Nutrition Examination Survey 1999–2002. Diabetes Care. 2007;30(3):622–628. doi: 10.2337/dc06-2190. [DOI] [PubMed] [Google Scholar]
- 33.Park D, Huang T, Frishman WH. Phytoestrogens as cardioprotective agents. Cardiology in Review. 2005;13(1):13–17. doi: 10.1097/01.crd.0000126084.68791.32. [DOI] [PubMed] [Google Scholar]
- 34.Penza M, Montani C, Romani A, et al. Genistein affects adipose tissue deposition in a dose-dependent and gender-specific manner. Endocrinology. 2006;147(12):5740–5751. doi: 10.1210/en.2006-0365. [DOI] [PubMed] [Google Scholar]
- 35.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132(6):2087–2102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 36.Dover GJ. The Barker hypothesis: how pediatricans will diagnose and prevent common adult-onset diseases. Transactions of the American Clinical and Climatological Association. 2009;120:199–207. [PMC free article] [PubMed] [Google Scholar]
- 37.Gram IT, Jacobsen BK, Straume B, Arnesen E, Løchen ML, Lund E. Early origin of coronary heart disease. Earlier published work supports the ‘Barker hypothesis’. British Medical Journal. 1995;310(6992):p. 1468. doi: 10.1136/bmj.310.6992.1468b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newbold RR. Impact of environmental endocrine disrupting chemicals on the development of obesity. Hormones. 2010;9(3):206–217. doi: 10.14310/horm.2002.1271. [DOI] [PubMed] [Google Scholar]
- 39.Anway MD, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors. Endocrinology. 2006;147(6):S43–S49. doi: 10.1210/en.2005-1058. [DOI] [PubMed] [Google Scholar]
- 40.Crews D, McLachlan JA. Epigenetics, evolution, endocrine disruption, health, and disease. Endocrinology. 2006;147(6):S4–S10. doi: 10.1210/en.2005-1122. [DOI] [PubMed] [Google Scholar]
- 41.Hanson MA, Gluckman PD. Developmental origins of health and disease: new insights. Basic & Clinical Pharmacology & Toxicology. 2008;102(2):90–93. doi: 10.1111/j.1742-7843.2007.00186.x. [DOI] [PubMed] [Google Scholar]
- 42.Mühlhäusler BS, Adam CL, Marrocco EM, et al. Impact of glucose infusion on the structural and functional characteristics of adipose tissue and on hypothalamic gene expression for appetite regulatory neuropeptides in the sheep fetus during late gestation. The Journal of Physiology. 2005;565(1):185–195. doi: 10.1113/jphysiol.2004.079079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bouret SG, Simerly RB. Minireview: leptin and development of hypothalamic feeding circuits. Endocrinology. 2004;145(6):2621–2626. doi: 10.1210/en.2004-0231. [DOI] [PubMed] [Google Scholar]
- 44.Lebrethon M-C, Aganina A, Fournier M, Gérard A, Parent A-S, Bourguignon J-P. Effects of in vivo and in vitro administration of ghrelin, leptin and neuropeptide mediators on pulsatile gonadotrophin-releasing hormone secretion from male rat hypothalamus before and after puberty. Journal of Neuroendocrinology. 2007;19(3):181–188. doi: 10.1111/j.1365-2826.2006.01518.x. [DOI] [PubMed] [Google Scholar]
- 45.Grün F, Blumberg B. Perturbed nuclear receptor signaling by environmental obesogens as emerging factors in the obesity crisis. Reviews in Endocrine and Metabolic Disorders. 2007;8(2):161–171. doi: 10.1007/s11154-007-9049-x. [DOI] [PubMed] [Google Scholar]
- 46.Kelishadi R, Mirghaffari N, Poursafa P, Gidding SS. Lifestyle and environmental factors associated with inflammation, oxidative stress and insulin resistance in children. Atherosclerosis. 2009;203(1):311–319. doi: 10.1016/j.atherosclerosis.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 47.Brook RD, Rajagopalan S, Pope C, III, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 48.Kelishadi R, Poursafa P, Keramatian K. Overweight, air and noise pollution: universal risk factors for pediatric pre-hypertension. Journal of Research in Medical Sciences. 2011;16(9):1234–1250. [PMC free article] [PubMed] [Google Scholar]
- 49.Kelishadi R, Poursafa P. Obesity and air pollution: global risk factors for pediatric non-alcoholic fatty liver disease. Hepatitis Monthly. 2011;11(10):794–802. doi: 10.5812/kowsar.1735143X.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poursafa P, Kelishadi R. Air pollution, platelet activation and atherosclerosis. Inflammation and Allergy. 2010;9(5):387–392. doi: 10.2174/187152810793937982. [DOI] [PubMed] [Google Scholar]
- 51.Day CP, James OFW. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114(4):842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 52.Takahashi Y, Fukusato T. Pediatric nonalcoholic fatty liver disease: overview with emphasis on histology. World Journal of Gastroenterology. 2010;16(42):5280–5285. doi: 10.3748/wjg.v16.i42.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sinclair KD, Lea RG, Rees WD, Young LE. The developmental origins of health and disease: current theories and epigenetic mechanisms. Reproduction in Domestic Ruminants. 2007;6(1):425–443. doi: 10.5661/rdr-vi-425. [DOI] [PubMed] [Google Scholar]
- 54.Kelishadi R, Poursafa P. Air pollution and non-respiratory health hazards for children. Archives of Medical Science. 2010;6(4):483–495. doi: 10.5114/aoms.2010.14458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kerkhof GF, Breukhoven PE, Leunissen RW, Willemsen RH, Hokken-Koelega A. Does preterm birth influence cardiovascular risk in early adulthood? The Journal of Pediatrics. 2012;161(3):390–396. doi: 10.1016/j.jpeds.2012.03.048. [DOI] [PubMed] [Google Scholar]
- 56.Kargarfard M, Poursafa P, Rezanejad S, Mousavinasab F. Effects of exercise in polluted air on the aerobic power, serum lactate level and cell blood count of active individuals. International Journal of Preventive Medicine. 2011;2(3):145–150. [PMC free article] [PubMed] [Google Scholar]
- 57.Nabavi SM, Jafari B, Jalali MS, Nedjat S, Ashrafi K, Salahesh A. Environmental air pollution and acute cerebrovascular complications: an ecologic study in Tehran, Iran. International Journal of Preventive Medicine. 2012;3(10):723–729. [PMC free article] [PubMed] [Google Scholar]
- 58.Hatami H. Importance of water and water-borne diseases: on the occasion of the world water day. International Journal of Preventive Medicine. 2013;4(3):243–245. [PMC free article] [PubMed] [Google Scholar]


