Abstract
Objective
Medicare Part D provides formulary protections for antipsychotics, but does not exempt these drugs from cost-sharing. We investigated the impact of Part D coverage on antipsychotic drug spending, adherence, and clinical outcomes among beneficiaries with varying indications for use.
Methods
We conducted a historical cohort study of Medicare Advantage beneficiaries who received antipsychotic drugs, with diagnoses of schizophrenia or bipolar disorder, or no mental health diagnoses (N=10,190). Half had a coverage gap; half had no gap because of low-income subsidies. Using fixed effects regression models, we examined changes in spending and adherence as beneficiaries experienced cost-sharing increases after reaching the gap. We examined changes in hospitalizations and emergency department visits using proportional hazard models.
Results
Across all diagnostic groups, monthly total antipsychotic spending decreased with cost-sharing increases in the gap compared with those with no gap (e.g., schizophrenia: −$123 [−$138, −$108]), and out-of-pocket spending increased (e.g., schizophrenia: $104 [$98, $110]). Adherence similarly decreased, with the largest declines among those with schizophrenia (−20.6 percentage points [−22.3, −18.9] in proportion of days covered). Among beneficiaries with schizophrenia and bipolar disorder, hospitalizations and emergency department visit rates increased with cost-sharing increases (e.g., schizophrenia: HR=1.32 [1.06, 1.65] for all hospitalizations), but did not among subjects without mental health diagnoses. Clinical event rates did not change among beneficiaries with low income subsidies without gaps.
Conclusions
There is evidence of interruptions in antipsychotic use attributable to Part D cost-sharing. Adverse events increased among beneficiaries with approved indications for use, but not among beneficiaries without such indications.
Keywords: Medicare, antipsychotics, benefit design
Introduction
There is an urgent need to identify insurance benefit designs that promote efficiency while maintaining or improving quality of care, especially for those with severe and persistent mental disorders. The design of Medicare Part D prescription drug benefits has significant implications for patients requiring chronic and high cost drug therapy, such as those prescribed antipsychotics for serious mental illness. In order to ensure access to the range of potential therapeutic agents, Medicare designated antipsychotics as one of six protected therapeutic drug classes, meaning that all drugs within the class must be included on Part D plan formularies. These protected classes, however, are subject to the substantial and complex cost-sharing requirements included in many Part D plans, which could have the unintended consequence of limiting access to these drugs for some patients. We do not know, however, whether these Part D benefit design features provide access to necessary drugs for the vulnerable populations they are intended to protect, including beneficiaries with serious mental illness who require antipsychotic drug therapy.1
Use of and spending on antipsychotic drugs has grown rapidly over the last decade.2,3 In 2009, Medicare Part D spending on antipsychotics was $5.9 billion, which was second only to antihyperlipidemics in total spending.4 Although strong clinical evidence supports the use of these drugs for conditions such as schizophrenia and bipolar disorder, there is also considerable off-label use for unapproved or controversial indications.5–10 The Part D program uses cost-sharing to control drug spending, but does not differentiate cost-sharing requirements by clinical indication.
Existing studies suggest that Part D cost-sharing, including the standard coverage gap that begins after beneficiaries reach an annual spending threshold ($2,930 in 2012), is associated with reductions in use of some clinically necessary medications.11–14 Few studies have, however, examined the effects of Part D cost-sharing across a range of higher and lower value indications, especially within the protected drug classes, and there is limited evidence on downstream clinical outcomes. Worse clinical outcomes and increased service utilization could, increase spending in Medicare Parts A and B and offset the savings in Part D associated with cost-sharing. Although the Patient Protection and Affordable Care Act (ACA) includes a provision to phase out the most controversial form of Part D cost-sharing, i.e., the coverage gap, by 2020, the future of this provision remains uncertain given budgetary pressures.15 Even if the gap is closed, substantial cost-sharing will remain in Part D plans in the form of copayments and coinsurance. These cost-sharing structures require that beneficiaries pay relatively more for brand name versus generic products, but do not differentiate on the basis of clinical indication.
The purpose of this study was to examine the effect of Part D coverage on beneficiaries receiving antipsychotic drug therapy. We compared beneficiaries who have a coverage gap with those receiving a low income subsidy (LIS) and, as a result, do not face a gap. We focused on three groups as examples that vary in whether antipsychotic treatment is central to evidence-based care. These are schizophrenia where antipsychotics are necessary; bipolar disorder, where antipsychotics can be necessary, but other therapeutic options exist; and cases where there is no clear indication for antipsychotic use (i.e., beneficiaries with no mental health diagnoses). We assessed changes in antipsychotic spending, adherence, and clinical outcomes associated with Part D cost-sharing, and examined variations across these groups. We hypothesized that Part D cost-sharing would be associated with decreases in adherence to antipsychotic drug therapy across all groups, but that the adverse clinical effects would be greatest among those with approved indications for use and fewer therapeutic alternatives. This information can help inform improvements in Part D coverage policies, as well as efforts to define drug coverage for essential health benefits created in the ACA.
Methods
Setting
This study includes beneficiaries enrolled in Medicare Advantage prescription drug plans offered by a national plan sponsor; this sponsor offered a range of Medicare Advantage plans, including health maintenance organizations, preferred provider organizations, and private fee-for-service plans, in many geographic regions. This study uses Part D Event files (e.g., drugs dispensed, date, and days supply); beneficiary information (e.g., low income subsidy status, risk scores); and medical claims (e.g., diagnoses, hospitalizations, emergency department visits) 2006–2007. The Kaiser Foundation Research Institute Institutional Review Board approved the study.
Study Population
The study includes non-institutionalized beneficiaries with baseline antipsychotic use, i.e., at least one antipsychotic dispensed in 2006, and at least one month of enrollment in 2007. We identified three mutually exclusive cohorts: 1) schizophrenia (ICD-9-CM 295.X); 2) bipolar disorder (ICD-9-CM 296.0, 296.1, 296.4–296.8, 301.11, 301.13); and 3) no mental health diagnoses.16–18 We required subjects to have at least one inpatient or at least two outpatient diagnoses in 2006 or 2007 for schizophrenia or bipolar disorder; subjects with both schizophrenia and bipolar disorder diagnoses were classified in the schizophrenia group. We excluded beneficiaries with other mental health diagnoses (e.g., depression, anxiety) without concurrent diagnoses of schizophrenia or bipolar disorder to focus on populations with more definitive clinical evidence regarding the appropriateness of antipsychotic use. We categorized remaining subjects without any of these indications as having no mental health diagnoses. We also excluded beneficiaries with dementia diagnoses because of the complexity in determining more versus less appropriate antipsychotic use in dementia using claims data.19,20 The list of excluded diagnoses (ICD-9-CM codes) is available in the online appendix (Table A1).
We examined outcomes in 2007 and allowed beneficiaries to leave the cohort due to disenrollment or death. We included individual Medicare Advantage beneficiaries enrolled in plans with a full coverage gap between $2,400 in total drug spending and $3,850 in out-of-pocket spending in 2007. We also included beneficiaries receiving low income subsidies (LIS) with a full premium subsidy; these subsidies reduce standard cost-sharing requirements and eliminate the gap.
Drug Spending and Adherence
We examined monthly total and out-of-pocket spending on all Part D drugs and for antipsychotics in 2007. Total drug spending includes the drug acquisition cost and dispensing fees. Out-of-pocket costs include copayments and the full price during uncovered periods. To measure monthly adherence to antipsychotics, we calculated the proportion of days covered (PDC) by summing the days supply of any antipsychotic dispensed in each month, allowing for carry-over of remaining supply, and dividing by the total number of days in the month.
Clinical Events
We identified hospitalizations and emergency department (ED) visits from health plan claims. We used the Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Project Clinical Classifications software to identify events for mental health and substance use disorder diagnoses.21 This system classifies events based on ICD-9-CM diagnoses into 15 general categories of mental health conditions. We assessed time to each hospitalization or ED visit for all diagnoses, and for mental health and non-mental health diagnoses, separately.
Analyses
We focused on differences in outcomes in months before and after beneficiaries reached the coverage gap threshold of $2,400 in total drug spending. We examined these differences among beneficiaries with a gap who reached it and a comparison group of LIS beneficiaries with similar drug spending (i.e., >$2,400), but who did not experience a gap in coverage, i.e., a difference-in-difference approach, to account for secular changes in our outcomes. To examine changes in drug spending and adherence (PDC), we used linear fixed effects regression models (xtreg, fe in Stata 10) to account for time invariant differences between beneficiaries with and without a gap. We focused on the time period beginning 30 days after reaching the gap threshold, after which beneficiaries would have been more likely to have exhausted any existing drug supply from fills dispensed at or before the point of reaching the gap. The models included indicators for reaching the gap threshold and interactions with an indicator for having a gap versus no gap. We also modeled monthly changes in adherence before and after reaching the gap threshold by including monthly indicators and interactions with the gap indicator. Time invariant covariates, such as age, gender, and baseline risk-score, drop out of the model. Because subjects face little cost-sharing during the catastrophic coverage period, we censored observations during the catastrophic coverage period (i.e., after cumulative out-of-pocket spending reached $3,850) for subjects who reached it; otherwise, subjects were followed until the end of the year.
To examine time to repeated clinical events we used the Andersen-Gill (A-G) extension of the Cox model.22 The A-G Cox model allows for the analysis of time to repeated events (such as hospitalizations or ED visits) accounting for correlation in repeated outcomes within individuals. For each diagnostic group, we analyzed the gap and LIS groups separately; these models included a time-varying indicator for reaching the gap threshold, as defined above. We also adjusted for gender, age (40–64 years old, 65–74, 75+ vs. <40), and the Part D (RxHCC) risk score, which is a summary score calibrated to predict current year drug spending based on prior year diagnoses. We adjusted confidence intervals for multiple comparisons using the Bonferroni correction.
Results
A substantial number of subjects were under 65 years old because they qualified for Medicare due to a disability (Table 1). The proportion of younger, disabled beneficiaries was greater among LIS with no gap versus non-LIS beneficiaries with a gap, and among beneficiaries with schizophrenia or bipolar disorder versus no mental health diagnoses. Mean comorbidity levels followed a similar pattern, as measured by Part D risk scores. Overall, 47% of beneficiaries with a gap reached it, and 11% exited and reached the catastrophic coverage period; the frequency varied across diagnostic groups.
Table 1.
Study Population Characteristics in 2007
| Schizophrenia | Bipolar Disorder | No MH Diagnosis | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Standard Gap | No Gap (LIS) | Standard Gap | No Gap (LIS) | Standard Gap | No Gap (LIS) | |
| Total N | 1,672 | 2,234 | 1,847 | 1,599 | 1,535 | 1,303 |
|
| ||||||
| Age: <65 | 68% | 85% | 68% | 89% | 31% | 63% |
| 65–69 | 13% | 7% | 14% | 5% | 16% | 9% |
| 70–74 | 8% | 4% | 9% | 3% | 15% | 8% |
| 75–79 | 6% | 2% | 5% | 2% | 15% | 8% |
| 80+ | 5% | 2% | 4% | 1% | 24% | 12% |
|
| ||||||
| Gender: Female | 52% | 50% | 66% | 70% | 54% | 52% |
|
| ||||||
| Reached coverage gap threshold in 2007 ($2,400 in total drug spending) | 47% | 75% | 58% | 82% | 33% | 41% |
|
| ||||||
| Reached catastrophic coverage threshold in 2007 ($3,850 in True out-of- pocket costs) ** | 11% | 40% | 13% | 50% | 7% | 13% |
|
| ||||||
| PDC>80% in Q1 2007 | 54.7% | 67.1% | 37.6% | 55.3% | 40.7% | 46.2% |
|
| ||||||
| Mean PDC in Q1 2007 (SD) | 68.13 (37.22) | 77.98 (32.44) | 52.28 (40.60) | 68.38 (37.73) | 51.62 (42.58) | 60.54 (38.78) |
|
| ||||||
| Mean Comorbidity (RxHCC) score (SD) | 1.54 (0.50) | 1.78 (0.59) | 1.34 (0.43) | 1.54 (0.50) | 1.06 (0.40) | 1.29 (0.49) |
True out-of-pocket costs include out-of-pocket costs covered by Medicare’s Low Income Subsidy (LIS)
Antipsychotic Spending
For beneficiaries with a gap compared with LIS beneficiaries with no gap, total Part D and antipsychotic spending decreased after reaching the gap threshold, and the magnitude varied by indication: e.g., antipsychotic spending for schizophrenia: −$123, 95% confidence interval [−$138, −$108]; bipolar disorder: −$93 [−$105, −$82]; and no mental health diagnosis: −$36 [−$48, −$24] (Table 2). Monthly out-of-pocket spending on antipsychotics increased for beneficiaries with a gap who reached it compared with those with no gap: schizophrenia: $104 [$98, $110]; bipolar disorder: $64 [$59, $69]; and no mental health diagnosis: $57 [$51, $63].
Table 2.
Changes in Monthly Part D and Antipsychotic Spending and Adherence After Reaching the Gap Threshold
| Standard Gap | No Gap (LIS) | Gap – No Gap | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Schizophrenia | Diff | 95% CI | Diff | 95% CI | Diff | 95% CI |
| Total Part D spending ($) | −171 | (−187, −155) | 14 | (3, 26) | −186 | (−205, −166) |
| Total antipsychotic spending ($) | −116 | (−128, −104) | 6 | (−2, 15) | −123 | (−138, −108) |
| Out-of-pocket Part D spending ($) | 235 | (229, 242) | 2 | (−3, 6) | 234 | (226, 242) |
| Out-of-pocket antipsychotic spending ($) | 104 | (99, 109) | 0 | (−3, 4) | 104 | (98, 110) |
| Proportion of days covered (percentage points) | −19.8 | (−21.2, −18.4) | 0.9 | (−0.1, 1.8) | −20.6 | (−22.3, −18.9) |
|
| ||||||
| Bipolar disorder | Diff | 95% CI | Diff | 95% CI | Diff | 95% CI |
|
| ||||||
| Total Part D spending ($) | −170 | (−184, −156) | 27 | (14, 40) | −197 | (−216, −178) |
| Total antipsychotic spending ($) | −86 | (−95, −78) | 7 | (−1, 15) | −93 | (−105, −82) |
| Out-of-pocket Part D spending ($) | 216 | (210, 223) | 2 | (−4, 8) | 214 | (206, 223) |
| Out-of-pocket antipsychotic spending ($) | 64 | (61, 68) | 0 | (−3, 3) | 64 | (59, 69) |
| Proportion of days covered (percentage points) | −20.5 | (−21.9, −19.2) | −2.4 | (−3.8, −1.1) | −18.1 | (−20.0, −16.2) |
|
| ||||||
| No Mental Health Diagnosis | Diff | 95% CI | Diff | 95% CI | Diff | 95% CI |
|
| ||||||
| Total Part D spending ($) | −108 | (−125, −91) | 20 | (4, 37) | −128 | (−152, −105) |
| Total antipsychotic spending ($) | −32 | (−41, −24) | 3 | (−5, 12) | −36 | (−48, −24) |
| Out-of-pocket Part D spending ($) | 227 | (219, 236) | 1 | (−8, 9) | 227 | (215, 239) |
| Out-of-pocket antipsychotic spending ($) | 57 | (53, 61) | −1 | (−5, 4) | 57 | (51, 63) |
| Proportion of days covered (percentage points) | −13.7 | (−15.5, −12.0) | −2.8 | (−4.5, −1.0) | −11.0 | (−13.4, −8.5) |
Notes: To examine changes in drug spending and adherence (PDC), we used linear fixed effects regression models (xtreg, fe in Stata 10). Because the average days supply of an antipsychotic prescription was 30 days, we examined separately the first month after reaching the gap (transition period), and ≥ 31 days after reaching the gap; these tables report the post-transition period, after beneficiaries would have been more likely to have exhausted any existing drug supply from fills dispensed prior to or at the point of reaching the gap. The models included indicators for these two gap periods and interactions between these indicators and an indicator for having a coverage gap vs. no gap due to the LIS. We censored outcomes during the catastrophic coverage period for subjects who reached it. Mean values of the outcomes during the pre-gap period are available in the online appendix (A2).
Adherence
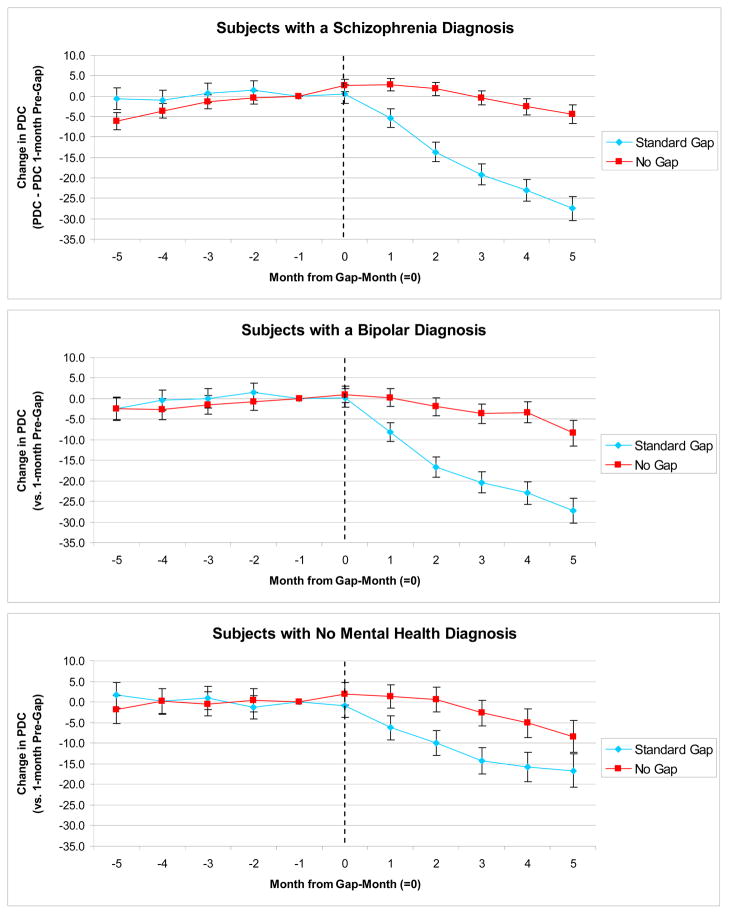
Among beneficiaries with a gap who reached it, baseline (pre-gap) levels of adherence were highest among beneficiaries with schizophrenia (PDC=78.5%) compared with beneficiaries with bipolar disorder (59.2%) and without mental health diagnoses (63.2%) (mean pre-gap spending and adherence levels are available in the online appendix Table A2). On average, the proportion of days covered by antipsychotics decreased for beneficiaries with a gap vs. no gap after reaching the gap threshold by 20.6 percentage points [−22.3, −18.9] for beneficiaries with schizophrenia, 18.1 percentage points [−20.0, −16.2] for beneficiaries with bipolar disorder, and 11.0 percentage points [−13.4, −8.5] for beneficiaries without mental health diagnoses (Table 2). Monthly differences in proportion of days covered for beneficiaries with a gap versus no gap in each diagnostic group, for the five months before and after reaching the gap threshold, mirror the above findings and illustrate that drug use prior to reaching the gap threshold was stable (Figure 1).
Figure 1. Changes in Adherence to Antipsychotics Before and After Reaching the Gap Threshold.
Notes: We used linear fixed effects regression models (xtreg, fe in Stata 10) to examine changes in the monthly PDC (proportion of days covered) by any antipsychotic in the five months before and after reaching the coverage gap threshold ($2,400 in total drug spending); changes are relative to the month prior to reaching the coverage gap (month -1). Beneficiaries with no gap receive the low income subsidy (LIS) that reduces cost-sharing and eliminates the gap.
Clinical Events
Among beneficiaries with schizophrenia or bipolar disorder with a gap, there was an increase in hospitalizations overall after experiencing cost-sharing increases in the gap, largely attributable to increases in mental health hospitalizations (e.g., HR=1.32, 99.5% CI [1.06, 1.65] and HR=1.29 [1.02, 1.64], respectively, for beneficiaries with schizophrenia) (Table 3). Similarly, among beneficiaries with bipolar disorder, ED visits overall and for mental health diagnoses also increased significantly after reaching the gap (e.g., HR=1.17 [1.00, 1.37] and HR=1.35 [1.10, 1.66], respectively). Hospitalizations and ED visits did not increase among subjects without mental health diagnoses or among LIS beneficiaries with no gap.
Table 3.
Changes in Clinical Event Rates After Reaching the Gap Threshold
| Standard Gap | No Gap (LIS) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Schizophrenia | Rate* | HR | 99.5% CI | Rate* | HR | 99.5% CI |
| All Hospitalizations | 68.7 | 1.32 | (1.06, 1.65) | 82.5 | 1.10 | (0.91, 1.32) |
| MH Hospitalizations | 61.0 | 1.29 | (1.02, 1.64) | 73.0 | 1.17 | (0.96, 1.42) |
| Non-MH Hospitalizations | 7.7 | 1.58 | (0.81 3.08) | 9.5 | 0.69 | (0.41 1.18) |
|
| ||||||
| All ED visits | 131.7 | 1.14 | (0.97, 1.34) | 192.3 | 1.04 | (0.92, 1.17) |
| MH ED visits | 85.6 | 1.13 | (0.92,1.38) | 114.6 | 1.06 | (0.91, 1.24) |
| Non-MH ED visits | 46.1 | 1.16 | (0.88 1.53) | 77.7 | 1.00 | (0.83 1.21) |
|
| ||||||
| Bipolar disorder | Rate* | HR | 99.5% CI | Rate* | HR | 99.5% CI |
|
| ||||||
| All Hospitalizations | 61.1 | 1.45 | (1.16, 1.82) | 67.3 | 0.98 | (0.76, 1.26) |
| MH Hospitalizations | 47.3 | 1.52 | (1.18, 1.97) | 54.7 | 0.99 | (0.75, 1.30) |
| Non-MH Hospitalizations | 13.8 | 1.22 | (0.76 1.97) | 12.5 | 0.96 | (0.53 1.72) |
|
| ||||||
| All ED visits | 128.9 | 1.17 | (1.00, 1.37) | 217.6 | 0.96 | (0.84, 1.10) |
| MH ED visits | 71.3 | 1.35 | (1.10, 1.66) | 107.0 | 0.89 | (0.74, 1.08) |
| Non-MH ED visits | 57.6 | 0.98 | (0.77 1.24) | 110.6 | 1.03 | (0.85 1.24) |
|
| ||||||
| No Mental Health Diagnosis | Rate* | HR | 99.5% CI | Rate* | HR | 99.5% CI |
|
| ||||||
| Non-MH Hospitalizations | 19.7 | 0.92 | (0.51, 1.66) | 22.4 | 1.21 | (0.70, 2.10) |
| Non-MH ED visits | 38.9 | 1.08 | (0.73 1.61) | 72.6 | 0.83 | (0.61 1.15) |
Unadjusted annual rate in 2007 per 100 patients
Notes: To examine time to hospitalizations and emergency department (ED) visits we used the Anderson-Gill extension of the Cox model; we fit separate models for the Gap and No Gap groups. These models include a time-varying indicator for being in the first 30 days from the reaching the gap threshold (transition period) and being >=31 days from the gap threshold; the models also adjust for gender, age, and the Part D (RxHCC) risk score. These tables present hazard ratios (HR) for being >=31 days from reaching the gap threshold vs. pre-gap threshold. In sensitivity analyses we adjusted for the CMS-HCC risk scores, which predict spending in Parts A and B, instead of the RxHCC score; conclusions were the same.
Discussion
We studied the effects of Part D cost-sharing on drug spending, adherence, and clinical events, among beneficiaries receiving antipsychotic drugs for a range of indications. Total drug spending decreased as cost-sharing increased in the gap, in part because of reduced use of antipsychotics. Although use decreased across all groups, the largest declines were among beneficiaries for whom antipsychotic use most likely represented medically necessary, evidence-based care, i.e., those with schizophrenia and bipolar disorder. Indicators of clinical harm associated with interruptions in therapy were greatest for these populations as well. Importantly, the Part D program attempted to protect access to antipsychotics by requiring inclusion on plan formularies; failure to consider other benefit design factors, namely cost-sharing, resulted in poor beneficiary access.
The reductions in drug use, particularly among beneficiaries with severe and persistent mental disorders, raise questions about the effects of these coverage policies on health outcomes and net medical spending.23 Previous work has found increases in hospitalizations and other medical costs associated with reductions in antipsychotic drug adherence among patients with schizophrenia and bipolar disorder.16,24–26 Consistent with this literature, we found that mental health hospitalizations increased for beneficiaries with schizophrenia and bipolar disorder during the gap. Rates of mental health emergency department visits also increased significantly for beneficiaries with bipolar disorder. In contrast, we did not find similar evidence of increased clinical events among beneficiaries with unclear indications for antipsychotics (i.e., those with no mental health diagnoses), or among LIS beneficiaries who did not face a gap.
Preliminary estimates of changes in net medical spending during the gap, based on average unit costs, suggest that increases in hospitalization and emergency department spending could offset savings in pharmacy costs associated with the coverage gap among beneficiaries with schizophrenia and bipolar disorder, but not among those without mental health diagnoses (online appendix Table A3). There could also be cumulative effects associated with poor adherence not captured in these estimates, especially to the extent that changes in disorder control lead to worsening functional and economic status, such as with employment loss.
There also were increases in adverse clinical events among beneficiaries with bipolar disorder who had other, often less expensive, therapeutic options (e.g., lithium, anticonvulsants). In supplemental analyses (online appendix Table A4), the increases in cost-sharing because of the coverage gap were also associated with decreased use of the potential substitutes for antipsychotics, on average. In some cases, the alternatives could have been previously poorly tolerated, or antipsychotics could have been prescribed in combination with a mood stabilizer and/or anticonvulsant when individual drug regimens were not adequately effective.27,28 Such determinations are difficult to make using claims data and to incorporate into the design of insurance benefits. Beneficiaries may have also had limited awareness of these alternatives, or limited ability to pay for all of their medications during the gap.29 Thus, greater access protections are needed, particularly in clinical areas with substantial treatment response heterogeneity or high levels of patient vulnerability.
Taken together, these findings suggest that greater cost sharing for specific patients likely advance neither efficiency nor quality and safety goals of the Part D program. Starting 2011, Medicare beneficiaries began receiving a 50% discount for brand name drugs during the gap, as mandated by the ACA; generic drugs were covered at 7%. Additional coverage will be phased in until 2020, after which, beneficiaries will pay 25% of the total cost. These decreases in out-of-pocket costs could mitigate some cost-related reductions in use across all indications, and adverse clinical effects could be less, especially among those with strong indications for antipsychotic drug therapy. Beneficiaries requiring high cost drug therapy, such as atypical antipsychotics, will, however, continue to face substantial out-of-pocket costs throughout the gap phase-out period, and even after its closure considerable cost-sharing will remain in Part D plans. In addition, given fiscal pressures, some proposals would repeal the closure of the coverage gap;15 closing the gap is estimated to cost $42.6 billion dollars between 2010 and 2019.30,31
Our findings suggest that cost-sharing creates powerful patient incentives that need to be better aligned with clinical goals and evidence. Neither the current Part D formulary protections nor cost-sharing requirements account for the diagnostic indication associated with the prescription. These questions about what care is covered and who pays what portion of the cost of that care represent fundamental issues in the design of health insurance, and have important implications for determining essential health benefits.32 Newer approaches, such as value-based insurance design, attempt to align patient costs with clinical need.33,34 Eliminating cost-sharing could be desirable for patients with clear, evidence-based indications for drug use, such as antipsychotic drug therapy for patients with schizophrenia or bipolar disorder. Alternatively high cost-sharing levels could be effective for reducing spending with few or even decreasing adverse effects if applied to uncertain or dubious indications, particularly in cases the costs of therapy are substantial or where serious side effects are associated with use. For example, use of atypical antipsychotics is associated with metabolic side effects, such as weight gain, increased risk of diabetes, and hyperlipidemia.9,10,35–37 More thoughtful clinical tailoring of benefit designs could help preserve access to necessary care, discourage less necessary care, and also could be less expensive than alternatives such as broadly filling in the coverage gap.
This study focused on a single drug class; effects could vary in other drug classes, e.g., with a different mix of indications, costs, or therapeutic options. Nevertheless, antipsychotics represent an important class from a policy perspective given their status as a protected class and the high levels of spending on these drugs by Medicare. Generic versions of several second generation antipsychotics are becoming available now, which could mitigate the adverse effects of higher cost-sharing for some beneficiaries. However, given the high level of therapeutic tailoring in these populations, the need to protect access to a wide range of therapeutic options will persist.
This study was conducted among beneficiaries enrolled in Medicare Advantage plans and may not generalize to beneficiaries in stand-alone Part D plans; however, these plans were located in multiple geographic areas and included a range of delivery systems. Utilization management requirements could also vary across plans; Medicare Advantage (MA) plans have greater incentives to structure benefits to minimize utilization and spending in Parts A and B compared with stand-alone plans. In these MA plans, there were no prior authorization or step therapy requirements for antipsychotics in 2007; thus findings from this study isolate the effects of cost-sharing in the absence of other utilization restrictions.38,39 Adherence was measured using dispensing data. While this method has been validated in previous studies,40–42 it can be less sensitive over shorter follow-up periods (e.g., those that are shorter than the average days supply for a dispensed medication). Thus, we conducted sensitivity analyses of adherence limited to subjects with at least 90-days of follow-up after reaching the gap threshold; results were the same.
This was a non-randomized study and there are likely to be unmeasured differences between beneficiaries with and without a gap due to the low income subsidies. To mitigate these concerns, we focused on fixed effects analyses that are robust to time-stable confounders, the type most likely to be present in this study, and focus on relative changes within each group before and after the gap threshold within a relatively short time frame. Nevertheless, differences between the groups could still bias our results. For example, LIS beneficiaries are more likely to qualify for Medicare due to permanent disabilities and also have higher comorbidity risk scores and drug spending. Factors such as greater polypharmacy, functional limitations, and disease severity could contribute to differences in proclivity to adhere to medications or of experiencing adverse clinical events compared with non-LIS beneficiaries. These differences, however, are likely to bias our analyses toward finding fewer differences between LIS and non-LIS beneficiaries. Importantly, we found similar trends in adherence (Figure 1) and spending (not shown) in months prior to the gap threshold in the two groups. In addition, we conducted sensitivity analyses in which we stratified LIS and non-LIS beneficiaries by disability status and found similar effects across the disabled and aged populations.
In conclusion, Part D cost-sharing was associated with reductions in use of antipsychotic drugs, one of six drug classes receiving special formulary protections under Part D. For beneficiaries with clear diagnostic indications for these drugs, mental health-related hospitalizations and emergency department visits also increased. While the ACA closes the coverage gap by 2020, beneficiaries will continue to face substantial cost-sharing through copayments and coinsurance in most Part D plans. Future benefit designs should account for out-of-pocket costs in the consideration of access and align these costs better with clinical goals.
Supplementary Material
Acknowledgments
Funding: The National Institute of Mental Health (5R01MH090284) provided the funding for this study.
References
- 1.Zhang Y, Donohue JM, Lave JR, O’Donnell G, Newhouse JP. The effect of Medicare Part D on drug and medical spending. N Engl J Med. 2009 Jul 2;361(1):52–61. doi: 10.1056/NEJMsa0807998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.IMS Institute for Healthcare Informatics. [Accessed Sept 7, 2012];The Use of Medicines in the United States: Review of 2010. 2011 http://www.imshealth.com/imshealth/Global/Content/IMS%20Institute/Documents/IHII_UseOfMed_report%20.pdf.
- 3.Stagnitti MN. MEPS (Medical Expenditure Panel Survey) Agency for Healthcare Research and Quality; Jan, 2010. Statistical Brief #275: Trends in Antipsychotics Purchases and Expenses for the U.S. Civilian Noninstitutionalized Population, 1997 and 2007. [Google Scholar]
- 4.Medicare Payment Advisory Commission. A Data Book: Healthcare Spending and the Medicare Program. Washington, D.C: Jun, 2011. [Google Scholar]
- 5.Comparative Effectiveness Review Summary Guides for Clinicians. Rockville MD: 2007. Off -Label Use of Atypical Antipsychotic Drugs: A Summary for Clinicians and Policymakers. [PubMed] [Google Scholar]
- 6.Alexander GC, Gallagher SA, Mascola A, Moloney RM, Stafford RS. Increasing off-label use of antipsychotic medications in the United States, 1995–2008. Pharmacoepidemiol Drug Saf. 2011 Feb;20(2):177–184. doi: 10.1002/pds.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eguale T, Buckeridge DL, Winslade NE, Benedetti A, Hanley JA, Tamblyn R. Drug, Patient, and Physician Characteristics Associated With Off-label Prescribing in Primary Care. Arch Intern Med. 2012 Apr 16; doi: 10.1001/archinternmed.2012.340. [DOI] [PubMed] [Google Scholar]
- 8.Leslie DL, Rosenheck R. Off-label use of antipsychotic medications in Medicaid. Am J Manag Care. 2012 Mar;18(3):e109–117. [PubMed] [Google Scholar]
- 9.Maglione M, Maher AR, Hu J, et al. Off-Label Use of Atypical Antipsychotics: An Update. Rockville MD: 2011. [PubMed] [Google Scholar]
- 10.Wine JN, Sanda C, Caballero J. Effects of quetiapine on sleep in nonpsychiatric and psychiatric conditions. Ann Pharmacother. 2009 Apr;43(4):707–713. doi: 10.1345/aph.1L320. [DOI] [PubMed] [Google Scholar]
- 11.Fung V, Mangione CM, Huang J, et al. Falling into the Coverage Gap: Part D Drug Costs and Adherence for Medicare Advantage Prescription Drug Plan Beneficiaries with Diabetes. Health Serv Res. 2009 doi: 10.1111/j.1475-6773.2009.01071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li P, McElligott S, Bergquist H, Schwartz JS, Doshi JA. Effect of the Medicare Part D coverage gap on medication use among patients with hypertension and hyperlipidemia. Ann Intern Med. 2012 Jun 5;156(11):776–784. W–263, W–264, W–265, W–266, W–267, W–268, W–269. doi: 10.7326/0003-4819-156-11-201206050-00004. [DOI] [PubMed] [Google Scholar]
- 13.Polinski JM, Donohue JM, Kilabuk E, Shrank WH. Medicare Part D’s effect on the under- and overuse of medications: a systematic review. J Am Geriatr Soc. 2011 Oct;59(10):1922–1933. doi: 10.1111/j.1532-5415.2011.03537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Baik SH, Zhou L, Reynolds CF, Lave JR. Effects of Medicare Part D coverage gap on medication and medical treatment among elderly beneficiaries with depression. Arch Gen Psychiatry. 2012 Jul;69(7):672–679. doi: 10.1001/archgenpsychiatry.2011.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Kaiser Family Foundation. Proposed Changes to Medicare in the “Path to Prosperity”: Overview and Key Questions. Washington, D.C: The Henry J. Kaiser Family Foundation; Apr, 2011. [Google Scholar]
- 16.Weiden PJ, Kozma C, Grogg A, Locklear J. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv. 2004 Aug;55(8):886–891. doi: 10.1176/appi.ps.55.8.886. [DOI] [PubMed] [Google Scholar]
- 17.Lu CY, Adams AS, Ross-Degnan D, et al. Association between prior authorization for medications and health service use by Medicaid patients with bipolar disorder. Psychiatr Serv. 2011 Feb;62(2):186–193. doi: 10.1176/appi.ps.62.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lurie N, Popkin M, Dysken M, Moscovice I, Finch M. Accuracy of diagnoses of schizophrenia in Medicaid claims. Hosp Community Psychiatry. 1992 Jan;43(1):69–71. doi: 10.1176/ps.43.1.69. [DOI] [PubMed] [Google Scholar]
- 19.American Psychiatric Association. Practice Guidelines for the Treatment of Patients with Alzheimer’s Disease and Other Dementias. Arlington, VA: Dec, 2007. [PubMed] [Google Scholar]
- 20.Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006 Oct 12;355(15):1525–1538. doi: 10.1056/NEJMoa061240. [DOI] [PubMed] [Google Scholar]
- 21. [Accessed June 1, 2009];Mental Health and Substance Abuse Clinical Classifications Software (CCS-MHSA) http://www.hcup-us.ahrq.gov/toolssoftware/mhsa/mhsa.jsp.
- 22.Fleming T, Harrington D. Counting Processes and Survival Analysis. New York: John Wiley & Sons; 1991. [Google Scholar]
- 23.Hsu J, Price M, Huang J, et al. Unintended Consequences of Caps on Medicare Drug Benefits. N Engl J Med. 2006 Jun 1;354(22):2349–2359. doi: 10.1056/NEJMsa054436. [DOI] [PubMed] [Google Scholar]
- 24.Gilmer TP, Dolder CR, Lacro JP, et al. Adherence to treatment with antipsychotic medication and health care costs among Medicaid beneficiaries with schizophrenia. Am J Psychiatry. 2004 Apr;161(4):692–699. doi: 10.1176/appi.ajp.161.4.692. [DOI] [PubMed] [Google Scholar]
- 25.Lage MJ, Hassan MK. The relationship between antipsychotic medication adherence and patient outcomes among individuals diagnosed with bipolar disorder: a retrospective study. Ann Gen Psychiatry. 2009;8:7. doi: 10.1186/1744-859X-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Law MR, Soumerai SB, Ross-Degnan D, Adams AS. A longitudinal study of medication nonadherence and hospitalization risk in schizophrenia. J Clin Psychiatry. 2008 Jan;69(1):47–53. doi: 10.4088/jcp.v69n0107. [DOI] [PubMed] [Google Scholar]
- 27.Bowden CL. Atypical antipsychotic augmentation of mood stabilizer therapy in bipolar disorder. J Clin Psychiatry. 2005;66 (Suppl 3):12–19. [PubMed] [Google Scholar]
- 28.Hirschfeld RM. Guideline Watch: Practice Guideline for the Treatment of Patients With Bipolar Disorder. 2. Arlington, VA: American Psychiatric Association; 2005. [Google Scholar]
- 29.Hsu J, Fung V, Price M, et al. Medicare beneficiaries’ knowledge of Part D prescription drug program benefits and responses to drug costs. JAMA. 2008 Apr 23;299(16):1929–1936. doi: 10.1001/jama.299.16.1929. [DOI] [PubMed] [Google Scholar]
- 30.Weaver C. Health Law Timeline: Closing the Medicare Drug Gap. NPR. 2010 http://www.npr.org/templates/story/story.php?storyId=125300979.
- 31.The Congressional Budget Office. H.R. 4872, Reconciliation Act of 2010. 2011 Oct 5;2010 [Google Scholar]
- 32.Institute of Medicine. Essential Health Benefits: Balancing Coverage and Cost. Washington, D.C: Oct 6, 2011. [Google Scholar]
- 33.Fendrick AM, Smith DG, Chernew ME, Shah SN. A benefit-based copay for prescription drugs: patient contribution based on total benefits, not drug acquisition cost. American Journal of Managed Care. 2001;7(9):861–867. [PubMed] [Google Scholar]
- 34.Lohr KN, Brook RH, Kamberg CJ, et al. Effect of cost-sharing on use of medically effective and less effective care. Med Care. 1986 Sep;24(9 Suppl):S31–S38. [Google Scholar]
- 35.Coe HV, Hong IS. Safety of low doses of quetiapine when used for insomnia. Ann Pharmacother. 2012 May;46(5):718–722. doi: 10.1345/aph.1Q697. [DOI] [PubMed] [Google Scholar]
- 36.Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19 (Suppl 1):1–93. doi: 10.2165/00023210-200519001-00001. [DOI] [PubMed] [Google Scholar]
- 37.Newcomer JW. Antipsychotic medications: metabolic and cardiovascular risk. J Clin Psychiatry. 2007;68 (Suppl 4):8–13. [PubMed] [Google Scholar]
- 38.Zhang Y, Adams AS, Ross-Degnan D, Zhang F, Soumerai SB. Effects of prior authorization on medication discontinuation among Medicaid beneficiaries with bipolar disorder. Psychiatr Serv. 2009 Apr;60(4):520–527. doi: 10.1176/ps.2009.60.4.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu CY, Soumerai SB, Ross-Degnan D, Zhang F, Adams AS. Unintended impacts of a Medicaid prior authorization policy on access to medications for bipolar illness. Med Care. 2010 Jan;48(1):4–9. doi: 10.1097/MLR.0b013e3181bd4c10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997 Jan;50(1):105–116. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 41.Valenstein M, Copeland LA, Blow FC, et al. Pharmacy data identify poorly adherent patients with schizophrenia at increased risk for admission. Med Care. 2002 Aug;40(8):630–639. doi: 10.1097/00005650-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 42.Svarstad BL, Shireman TI, Sweeney JK. Using drug claims data to assess the relationship of medication adherence with hospitalization and costs. Psychiatr Serv. 2001 Jun;52(6):805–811. doi: 10.1176/appi.ps.52.6.805. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.