Abstract
Because dietary fats provide an important source of energy in the newborn, the efficient digestion of dietary fats is critical to their well-being. Despite the importance of dietary fat digestion, newborns have a deficiency of pancreatic triglyceride lipase, the predominant digestive lipase in adults. The efficient dietary fat digestion in newborns suggests that other lipases must compensate for the lack of pancreatic triglyceride lipase. In this study, we test the hypothesis that breast milk, pancreatic carboxyl ester lipase (CEL), or both contribute to dietary fat digestion in the newborn. To test this hypothesis, we determined the amount and composition of fecal fat in wild-type and CEL-deficient newborns nursed by either wild-type or CEL-deficient dams. We tested all genetic permutations of the nursing pairs. An interaction between the genotype of the dam and of the pup determined the amount of fecal fat (P < 0.001). Fecal fat was highest in CEL-deficient pups nursed by CEL-deficient dams. Furthermore, only the feces from the CEL-deficient pups nursed by CEL-deficient dams contained undigested lipids. Even with increased fecal fats, the CEL-deficient pups had normal weight gain. Our results demonstrate that CEL contributes significantly to dietary triglyceride digestion whether it originates from mother’s milk or pancreatic secretions. However, only the absence of both mother’s milk and pancreatic CEL produces fat maldigestion. The absence of a single CEL source makes no difference in the efficiency of dietary fat absorption.
Introduction
The digestion of triglycerides and other dietary fats commences in the stomach where preduodenal lipases, gastric lipase or lingual lipase, release ~15% of the fatty acids from triglycerides (1). The emulsion particles move from the stomach into the duodenum and mix with digestive enzymes secreted by pancreatic acinar cells. The key duodenal lipase is colipase-dependent pancreatic triglyceride lipase (PTL)3 in children and adults, as evidenced by the marked fat malabsorption in patients with isolated congenital pancreatic triglyceride lipase deficiency (2,3). Human newborns have a deficiency of pancreatic triglyceride lipase. Yet, they do not have significant steatorrhea when fed either breast milk or formula despite consuming 3- to 5-fold more fat per kg body weight than adults consume (4). Similarly, newborn rodents also do not express PTL and have efficient dietary fat absorption even though fat constitutes 60% of the calories in rodent breast milk (5,6). Thus, human and rodent newborns must have compensatory mechanisms for the intestinal digestion of dietary fats.
Pancreatic lipase related protein 2 (PLRP2) is a likely candidate for this role. Rat and mouse newborns as well as human newborns express PLRP2, which shares 65% amino acid sequence identity with pancreatic triglyceride lipase (7,8). PLRP2-deficient mice provide definitive evidence that PLRP2 participates in fat digestion in suckling mice (9). PLRP2-deficient suckling mice have significant dietary fat malabsorption and their feces contain both triglycerides and diglycerides, indicating that PLRP2 cleaves these dietary fats. Consequently, PLRP2-deficient newborns gain weight at a slower rate than do wild-type littermates and weigh 20% less than wild-type littermates at weaning (9). These findings confirmed the hypothesis that PLRP2 contributes to dietary fat digestion in newborns.
The additional observation that feces from PLRP2-deficient pups also contain fatty acids indicates that other lipases contribute to triglyceride digestion in the newborn. One lipase that could potentially influence triglyceride digestion is carboxyl ester lipase (CEL). In humans and mice, both mammary tissue and pancreas express CEL. Indirect evidence implicates breast milk CEL in fat digestion (10). Breast milk CEL activity has been detected in the duodenum of nursing babies, indicating that it survives passage through the stomach (11,12). In another study, kittens fed a commercial formula gained weight at about one-half the rate of nursed littermates (13). Normal weight gain was restored after supplementing the formula with CEL.
Several other observations argue against a significant role for breast milk CEL in suckling newborns. First, premature and newborn infants have lower bile salt concentrations than those found in adults (14–16). The lower concentration decreases the activity of breast milk CEL, which requires bile salts for activity. Second, formula-fed infants have only small increases in fecal fat compared with breast-fed infants despite having no breast milk CEL (17). Although the participation of breast milk CEL in fat digestion has been frequently proposed, little direct evidence supports an essential requirement for this lipase.
Two groups have investigated neonatal fat absorption in CEL-deficient mice and reached opposite conclusions about the contribution of breast milk CEL to neonatal fat digestion (18,19). Based on the normal weight gain in pups nursed by CEL−/− dams, Weng et al. (19) concluded “that there was no fat malabsorption in the neonatal period.” Howles et al. (18) evaluated fat absorption in neonatal mice and reported increased levels of glycerol in the ileum of CEL−/− pups nursed by CEL−/− dams but not in CEL−/− pups nursed by CEL+/− dams and concluded that “CEL in breast milk supplies an essential lipolytic activity.” Neither group directly measured fecal fats nor did they demonstrate the presence of undigested or partially digested dietary fats in the feces. Also, neither group reported fat absorption in a wild-type pup nursed by a CEL−/− dam or in a CEL−/− pup nursed by a wild-type dam. These permutations are important to establish the contribution of breast milk CEL to triglyceride absorption in neonates. In this article, we report the systematic characterization of triglyceride digestion in CEL−/− mice to establish the contribution of pancreatic and mother’s milk CEL to fat digestion in the newborn.
Materials and Methods
Animals
We used CEL- and PLRP2-deficient mice in this study (9,18). The CEL-deficient mice on a C57/Bl6 background were a gift from Drs. Phillip Howles and David Hui of the University of Cincinnati School of Medicine (18). PLRP2-deficient mice were on a Balbc background. Animal care and use were in accordance with institutional Animal Research Care Committee guidelines and our animal protocol was approved by the institutional Animal Research Care Committee. Mice consumed a standard commercial diet and water ad libitum. JL Rat and Mouse/Auto 4F 5K54 rodent diet (LabDiet, PMI Nutrition International) containing crude protein (19%), crude fat (5.6%), crude fiber (4.2%), and ash (6.3%). Genotyping was done as previously described (9,18). For fostering the pups, the biological litter and nesting material was removed from the cage of the foster mother. The foster mother was handled by the technician prior to moving the fostered pups and their nesting material into the cage of the foster mother. Pup survival did not decrease with this method. The mice were killed by anesthesia with Nembutal (50 mg/kg intraperitoneal) and cervical dislocation and the pancreas was carefully removed for RNA isolation.
To create mice deficient in both PLRP2 and CEL, we first bred PLRP2−/− with CEL−/− mice to create PLRP2+/−/CEL+/− mice. We then bred PLRP2+/−/CEL+/− with PLRP2+/−/CEL+/− to obtain PLRP2−/−/CEL+/− and PLRP2+/−/CEL−/− mice and to potentially obtain PLRP2−/−/CEL−/− mice. The PLRP2−/−/CEL+/− mice were bred with PLRP2+/−/CEL−/− mice in an attempt to obtain PLRP2−/−/CEL−/−.
RNA methods
We isolated total RNA from pancreas by the TRIzol method following the manufacturer’s instructions (Invitrogen). RT-PCR was performed with oligo-dT priming using the RETROscript kit (Ambion). CEL cDNA was amplified using the Advantage cDNA PCR kit (Clontech). The sense primer was 5′-TGC GTG TCT GAA GAT CAC AGA-3′. The anti-sense primer was 5′-GGA TCA TCG GGA ATG AAG TC-3′. The product was separated by agarose gel electrophoresis and detected by ethidium bromide staining (20).
Lipid methods
We set up 4 different nursing paradigms. We tested CEL-deficient pups nursed by CEL-deficient dams, wild-type pups nursed by wild-type dams, CEL-deficient pups fostered and nursed by wild-type dams, and wild-type pups fostered and nursed by CEL-deficient dams. To obtain stool from suckling animals, 8-d-old pups were killed and the colon was removed (9). The feces were removed by extrusion from 5 to 9 pups and pooled for each individual sample. The feces were dried to a constant weight in a lyophilizer. Fecal fats were extracted from 100 mg of dried feces as described (21). The extract was completely evaporated and the fat was weighed and expressed as a percentage of the total fecal dry weight. Lipid classes were analyzed by TLC on a silica G plate (9). A standard mixture of oleic acid, monoolein, 1,2-diolein, 1,3-diolein, and triolein was included on each plate. Three to 4 different amounts of the standards were applied to each plate to generate a standard curve for quantitation. The plate was developed by a 2-stage, 1-dimensional method, developed in cupric acetate/phosphoric acid, and the individual lipid species quantitated as described (22,23). Only lipid species for which we were able to generate a standard curve were quantitated. The quantity of each lipid species was normalized to the fatty acid signal.
Statistical methods
Statistical analysis was performed with SigmaStat software (version 3.5, Systat Software). Values are expressed as the means ± SD. Pairwise comparisons of weight or fecal fat values were analyzed by Student’s t test. Two-way ANOVA was used to test for an interaction of dam or pup genotype on fecal fat values with α = 0.05. We made post hoc multiple comparisons using the Holm-Sidak method. Comparison of experimental results with expected results was done by chi-square with a Yates correction applied to the calculations for the matings of PLRP2−/+/CEL−/+ mice and by Fisher Exact Test for the PLRP2−/+/CEL−/− × PLRP2−/−/CEL−/+ matings. All tests were considered significant at P < 0.05.
Results
Expression of mRNA encoding CEL in mouse pups
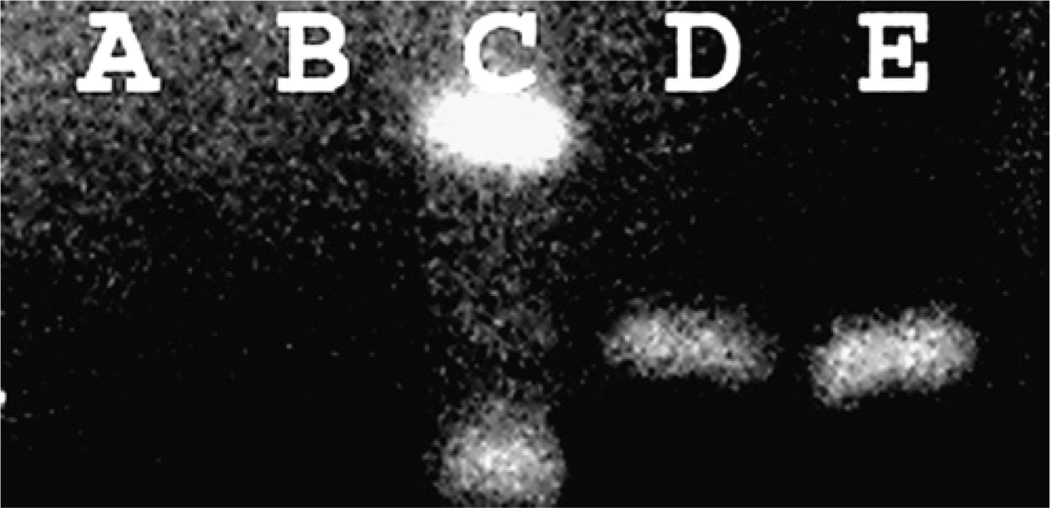
Because the expression of mRNA encoding CEL is temporally regulated during mouse development, we characterized our experimental model to determine whether 8-d-old wild-type mouse pups express CEL (24). mRNA encoding CEL was present in the pancreas at this age (Fig. 1).
FIGURE 1.
RT-PCR analysis of CEL expression in the pancreas from wild-type 8-d-old mice. Water control (A), RT reaction mixture without added RT control (B). Markers (C), wild-type pup 1 nursed by wild-type dam (D), wild-type pup 2 nursed by wild-type dam (E).
Fecal fat measurement
We found no subjective difference in the appearance or consistency of the stool collected from pups of the 4 feeding pairs. There was an interaction between dam and pup genotypes on the pups’ level of fecal fat (P < 0.001) (Table 1). The percent fecal fat was higher in CEL−/− pups when dams were CEL−/− (P = 0.001) rather than CEL+/+ (P = 0.001), but that of CEL+/+ pups was not affected by the genotype of the dam. In dams that were CEL−/−, the level of fecal fat in pups that were CEL−/− was greater than in those that were CEL+/+ (P = 0.001). In dams that were CEL+/+, there was no effect of the pups’ genotype.
TABLE 1.
Fecal fat levels in CEL+/+ and CEL−/− mouse pups nursed by CEL+/+ and CEL−/− dams1
| Genotype |
||
|---|---|---|
| Dam | Pups | Fecal fat2, % |
| CEL+/+ | CEL+/+ | 10.8 ± 4.1a |
| CEL+/+ | CEL−/− | 5.1 ± 2.8a |
| CEL−/− | CEL+/+ | 8.0 ± 3.6a |
| CEL−/− | CEL−/− | 34.8 ± 9.2b |
Values are means ± SD, n = 3–5. Effects of dam genotype, pup genotype, and their interaction were significant, P < 0.001. Within a genotype for the dam or pup, values without a common letter differ, P < 0.05.
Fecal fat = (weight of extracted fat/dry weight of feces) × 100.
Composition of fecal fats
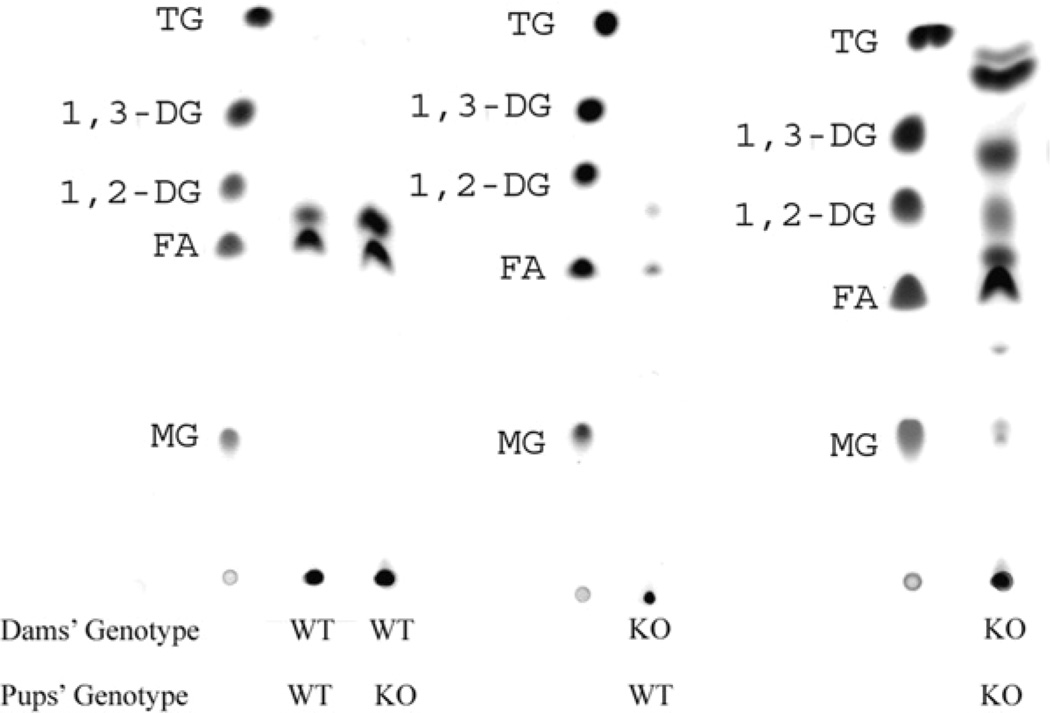
Representative TLC analyses for each of the genotype combinations are presented (Fig. 2). When 1 of the feeding dyad was wild type, only fatty acids, a lipid species migrating at the position of sterol and polar lipids, which remain at the origin, were present. No undigested or partially digested neutral lipids were present in any of these samples. In contrast, the fecal fat extract from the CEL-deficient pup and dam combination always had abundant diglycerides and triglycerides (Fig. 2; Table 2).
FIGURE 2.
TLC of fat in feces of CEL+/+ and CEL−/− mouse pups nursed by CEL+/+ and CEL−/− dams. Although samples from each mouse pup were analyzed by TLC, only a single, representative run is presented for each nursing pair. The standards for each plate are labeled. The anomalous migration of triglycerides in the CEL−/−/CEL−/− sample compared with the migration of the triglyceride standard is an artifact of sample amount in the experimental sample. The genotypes of the mother and the pups are given below each lane.
TABLE 2.
Lipid species in the feces of CEL-deficient mouse pups nursed by CEL-deficient dams
| Sample1 |
||||||
|---|---|---|---|---|---|---|
| Lipid species | 1 | 2 | 3 | 4 | 5 | Mean ± SD |
| Monoglyceride | 0.05 | 0.06 | 0.04 | 0.05 | 0.06 | 0.05 ± 0.01 |
| Fatty acid | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 1,2-Diglyceride | 0.60 | 0.63 | 1.38 | 0.70 | 0.51 | 0.76 ± 0.35 |
| 1,3-Diglyceride | 1.02 | 1.01 | 1.73 | 1.10 | 1.19 | 1.21 ± 0.30 |
| Triglyceride | 1.45 | 3.49 | 3.27 | 2.22 | 3.30 | 2.75 ± 0.88 |
The quantity of each lipid species was normalized to the excretion of fatty acids. Samples were obtained from 5 litters of 5–9 pups each.
Effect of fat maldigestion on weight gain of mouse pups
At 10 d of age, CEL-deficient pups raised by CEL-deficient dams weighed 5.97 ± 0.72 g (n = 15). Wild-type (n = 12) or heterozygous pups (n = 3) nursed by wild-type mothers weighed 5.45 ± 0.9 g (P = 0.092).
PLRP2/CEL-deficient mice
In an attempt to create mice carrying null alleles for the genes encoding PLRP2 and CEL, we mated mice with heterozygous null alleles for both genes (PLRP2+/−/CEL+/−). Ninety-three pups were born. Four died and 89 pups survived to weaning. None of the surviving pups had homozygous null alleles for both genes. Given the number of surviving pups, we would expect 5–6 double knockouts (P = 0.046). We also mated PLRP2−/−/CEL+/− × PLRP2+/−/CEL−/− mice. These matings produced 52 newborns. Twelve pups died and 40 pups survived to weaning. None of the surviving pups were double knockouts, although we expected to have 10 (P = 0.002).
Discussion
Digestive lipases show a temporal pattern of expression during development (6,8,24). The predominant digestive lipase in adults, PTL, is expressed at low levels in newborns even though they have relatively high consumption of dietary fats (17). These disparities lead to the hypothesis that other lipases must contribute to dietary fat digestion in the newborn. Previously, we showed that PLRP2 contributes to dietary fat digestion in newborns (9,25). Others have suggested that CEL in mother’s milk or in the pancreas also contributes, but the evidence has been mixed (10,13,18,19,26). In this article, we report studies to address the role of CEL in neonatal dietary fat digestion.
Our results demonstrate that CEL contributes to dietary triglyceride digestion whether it originates from mother’s milk or pancreatic secretions. However, only the absence of both mother’s milk and pancreatic CEL produces fat maldigestion. The absence of only mother’s milk CEL or only pancreatic CEL does not affect the efficiency of dietary fat absorption. Combined with our previous data on PLRP2-deficient mice, these results suggest that CEL partially compensates for PLRP2 deficiency and permits survival of the PLRP2-deficient pups. Our results do not address the question of whether CEL makes a substantial contribution to the digestion of other lipids besides triglycerides and if mother’s milk CEL is necessary for efficient digestion of these other lipids.
Even though CEL clearly contributes to dietary triglyceride digestion in the pups, the fat and resultant energy losses are not sufficient to affect weight gain. By comparison, in our other models of fat malabsorption in suckling mice, PLRP2-deficient pups weighed 16% less and procolipase-deficient pups weighed 35% less than wild-type pups at 10 d (5,9). The effect on weight gain and the higher fat content of the feces of PLRP2 pups (nearly 60% of the dry weight was fat) suggests that PLRP2 is the predominant triglyceride lipase in the newborn (9).
At first glance, our results in the procolipase-deficient mice might argue against the conclusion that the increased fecal fat losses account for the slower weight gain in the PLRP2-deficient mice. The fecal fat content in the feces from procolipase-deficient mice (25%) was lower than that in the CEL-deficient mice (35%); yet, the procolipase-deficient mice had the largest defect in weight gain of all the genotypes. The comparison of the procolipase-deficient mice with the other 2 genotypes is complicated, because the gene encoding procolipase includes enterostatin, a peptide with effects on appetite (27). The poorer weight gain in the procolipase-deficient pups may result from the combined effects of energy loss and the absence of enterostatin. Whatever the explanation, our results show that weight gain alone is not an adequate surrogate for defects in fat digestion or fat absorption.
Our inability to generate mice deficient in both CEL and PLRP2 suggests that they are critical for survival. Whether the decreased survival is related to severe defects in fat digestion and the resultant energy loss or if it is due to another effect was not addressed in this study. PLRP2 and CEL are expressed in tissues outside the gastrointestinal tract, where they may have unknown functions.
In summary, we have demonstrated that CEL plays an important role in dietary fat digestion. It does not matter whether the CEL arises from mother’s milk, the pancreas, or both. Although our data clearly show that fat digestion is incomplete in the absence of CEL, we cannot reliably quantitate the contribution of CEL to overall digestion. Clearly, CEL probably makes a smaller contribution than does PLRP2, because PLRP2-deficient pups have higher amounts of fecal fat and gain weight at a slower than normal rate, whereas CEL-deficient pups gain weight normally even when nursed by CEL-deficient dams (19). Even so, this information advances our understanding of the role different lipases play in dietary fat digestion. Some day, the temporal differences in lipase expression may be exploited for nutritional therapy.
Footnotes
Supported by NIH grant DK53100.
Author disclosures: R. Miller and M. E. Lowe, no conflicts of interest.
Abbreviations used: CEL, carboxyl ester lipase; PLRP2, pancreatic lipase related protein 2; PTL, pancreatic triglyceride lipase.
Literature Cited
- 1.Carriere F, Barrowman JA, Verger R, Laugier R. Secretion and contribution to lipolysis of gastric and pancreatic lipases during a test meal in humans. Gastroenterology. 1993;105:876–888. doi: 10.1016/0016-5085(93)90908-u. [DOI] [PubMed] [Google Scholar]
- 2.Figarella C, De Caro A, Leupold D, Poley JR. Congenital pancreatic lipase deficiency. J Pediatr. 1980;96:412–416. doi: 10.1016/s0022-3476(80)80683-4. [DOI] [PubMed] [Google Scholar]
- 3.Ghishan FK, Moran JR, Durie PR, Greene HL. Isolated congenital lipase-colipase deficiency. Gastroenterology. 1984;86:1580–1582. [PubMed] [Google Scholar]
- 4.Fredikzon A, Olivecrona T. Decrease of lipase and esterase activities in intestinal contents of newborn infants during test meals. Pediatr Res. 1978;43:247–257. doi: 10.1203/00006450-197805000-00004. [DOI] [PubMed] [Google Scholar]
- 5.D’Agostino D, Cordle RA, Kullman J, Erlanson-Albertsson C, Muglia LJ, Lowe ME. Decreased postnatal survival and altered body weight regulation in procolipase deficient mice. J Biol Chem. 2002;277:7170–7177. doi: 10.1074/jbc.M108328200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Payne RM, Sims HF, Jennens ML, Lowe ME. Rat pancreatic lipase and two related proteins: enzymatic properties and mRNA expression during development. Am J Physiol. 1994;266:G914–G921. doi: 10.1152/ajpgi.1994.266.5.G914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lowe ME. Pancreatic triglyceride lipase and colipase: insights into dietary fat digestion. Gastroenterology. 1994;107:1524–1536. doi: 10.1016/0016-5085(94)90559-2. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, Sanchez D, Figarella C, Lowe ME. Discoordinate expression of pancreatic lipase and two related proteins in the human fetal pancreas. Pediatr Res. 2000;47:184–188. doi: 10.1203/00006450-200002000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Lowe ME, Kaplan MH, Jackson-Grusby L, D’Agostino D, Grusby MJ. Decreased neonatal dietary fat absorption and T cell cytotoxicity in pancreatic lipase-related protein 2-deficient mice. J Biol Chem. 1998;273:31215–31221. doi: 10.1074/jbc.273.47.31215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamosh M. Lipid metabolism in pediatric nutrition. Pediatr Clin North Am. 1995;42:839–859. doi: 10.1016/s0031-3955(16)39020-4. [DOI] [PubMed] [Google Scholar]
- 11.Hernell O, Blackberg L, Lindberg T. Human milk enzymes with emphasis on the lipases. In: Lebenthal E, editor. Textbook of gastroenterology and nutrition in infancy. New York: Raven Press; 1989. pp. 209–221. [Google Scholar]
- 12.Fredrikzon B, Hernell O, Blackberg L, Olivecrona T. Bile salt-stimulated lipase in human milk: evidence of activity in vivo and of a role in the digestion of milk retinol esters. Pediatr Res. 1978;12:1048–1052. doi: 10.1203/00006450-197811000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Wang C-S, Martindale ME, King MM, Tang J. Bile-salt-activated lipase: effect on kitten growth rate. Am J Clin Nutr. 1989;49:457–463. doi: 10.1093/ajcn/49.3.457. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann AF, Mekhijan HS. Bile acids and the intestinal absorption of fat and electrolytes in health and disease. In: Nair PP, Kritchevsky D, editors. The bile acids. New York: Plenum Publishing; 1971. pp. 103–152. [Google Scholar]
- 15.Lindstrom MB, Sternby B, Borgstrom B. Concerted action of human carboxyl ester lipase and pancreatic lipase during lipid digestion in vitro: importance of the physiochemical state of the substrate. Biochim Biophys Acta. 1988;959:178–184. doi: 10.1016/0005-2760(88)90029-x. [DOI] [PubMed] [Google Scholar]
- 16.Morgan RGH, Borgstrom B. The mechanism of fat absorption in the bile fistula rat. Q J Exp Physiol Cogn Med Sci. 1969;54:228–243. doi: 10.1113/expphysiol.1969.sp002021. [DOI] [PubMed] [Google Scholar]
- 17.Fomon SJ, Ziegler EE, Thomas LN, Jensen RL, Filer LJ. Excretion of fat by normal full-term infants fed various milks and formulas. Am J Clin Nutr. 1970;23:1299–1313. doi: 10.1093/ajcn/23.10.1299. [DOI] [PubMed] [Google Scholar]
- 18.Howles P, Carter C, Hui D. Dietary free and esterified cholesterol absorption in cholesterol esterase (bile salt-stimulated lipase) gene-targeted mice. J Biol Chem. 1996;271:7196–7202. doi: 10.1074/jbc.271.12.7196. [DOI] [PubMed] [Google Scholar]
- 19.Weng W, Li L, van Bennekum AM, Potter SH, Harrison EH, Blaner WS, Breslow JL, Fisher EA. Intestinal absorption of dietary cholesteryl ester is decreased but retinyl ester absorption is normal in carboxyl ester lipase knockout mice. Biochemistry. 1999;38:4143–4149. doi: 10.1021/bi981679a. [DOI] [PubMed] [Google Scholar]
- 20.Sambrook J, Russell DW. Molecular cloning: a laboratory manual. 3rd ed. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 21.Schwarz M, Lund E, Setchell K, Kayden H, Zerwekh J, Bjorkhem I, Herz J, Russell D. Disruption of cholesterol 7alpha-hydroxylase gene in mice. J Biol Chem. 1996;271:18024–18031. doi: 10.1074/jbc.271.30.18024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bitman J, Wood DL. Quantitative densitometry in situ of lipids separated by thin layer chromatography. J Liq Chromatogr. 1981;4:1023–1034. [Google Scholar]
- 23.Bitman J, Wood DL, Ruth JM. Two-stage, one-dimensional thin layer chromatographic method for separation of lipid classes. J Liq Chromatogr. 1981;4:1007–1021. [Google Scholar]
- 24.Li X, Lindquist S, Lowe M, Noppa L, Hernell O. Bile salt-stimulated lipase and pancreatic lipase-related protein 2 are the dominating lipases in neonatal fat digestion in mice and rats. Pediatr Res. 2007;62:537–541. doi: 10.1203/PDR.0b013e3181559e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Agostino D, Lowe ME. Pancreatic lipase-related protein 2 is the major colipase-dependent pancreatic lipase in suckling mice. J Nutr. 2004;134:132–134. doi: 10.1093/jn/134.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howles PN, Stemmerman GN, Fenoglio-Preiser CM, Hui DY. Carboxyl ester lipase activity in milk prevents fat-derived intestinal injury in neonatal mice. Am J Physiol. 1999;277:G653–G661. doi: 10.1152/ajpgi.1999.277.3.G653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erlanson-Albertsson C, York D. Enterostatin: a peptide regulating fat intake. Obes Res. 1997;5:360–372. doi: 10.1002/j.1550-8528.1997.tb00565.x. [DOI] [PubMed] [Google Scholar]