Abstract
Bone tissue has the capacity to adapt to its functional environment such that its morphology is “optimized” for the mechanical demand. The adaptive nature of the skeleton poses an interesting set of biological questions (e.g., how does bone sense mechanical signals, what cells are the sensing system, what are the mechanical signals that drive the system, what receptors are responsible for transducing the mechanical signal, what are the molecular responses to the mechanical stimuli). Studies of the characteristics of the mechanical environment at the cellular level, the forces that bone cells recognize, and the integrated cellular responses are providing new information at an accelerating speed. This review first considers the mechanical factors that are generated by loading in the skeleton, including strain, stress and pressure. Mechanosensitive cells placed to recognize these forces in the skeleton, osteoblasts, osteoclasts, osteocytes and cells of the vasculature are reviewed. The identity of the mechanoreceptor(s) is approached, with consideration of ion channels, integrins, connexins, the lipid membrane including caveolar and noncaveolar lipid rafts and the possibility that altering cell shape at the membrane or cytoskeleton alters integral signaling protein associations. The distal intracellular signaling systems on-line after the mechanoreceptor is activated are reviewed, including those emanating from G-proteins (e.g., intracellular calcium shifts), MAPKs, and nitric oxide. The ability to harness mechanical signals to improve bone health through devices and exercise is broached. Increased appreciation of the importance of the mechanical environment in regulating and determining the structural efficacy of the skeleton makes this an exciting time for further exploration of this area.
Keywords: Mechanoreceptor, Skeleton, Ion channel, Integrin, Connexin, Lipid raft, MAPK, Nitric oxide
1. Introduction
The skeleton is an extremely complex tissue that is able to regulate its own mass and architecture to meet two critical and competing responsibilities: one structural and the other metabolic. While metabolic demands on the skeleton are managed largely through calciotropic hormones, the structural functions of bone are set first through genetic determinants, and then through the ability of the skeleton to adapt to its loading environment. Skeletal diseases, such as osteoporosis, can be defined by a failure of the adaptive response to maintain the structure needed to withstand daily loading. Understanding the molecular basis by which bone responds – or fails to respond – to mechanical stimuli should help identify new approaches for treatment of musculoskeletal diseases and injuries.
The success of the skeleton as a structure is ultimately a product of its mechanical properties—how stiff it is, how resilient to fatigue, and how effectively it withstands the extremes of physical activity. Bone tissue has the capacity to adapt to its functional environment such that its morphology is “optimized” for the mechanical demand. Wolff's law, proposed in 1892, was the first to suggest that the skeleton could respond to even subtle changes in functional demands. With modern understanding that it is the cells of the skeleton—the osteoblasts, osteocytes and osteoclasts – that orchestrate the remodeling process to buttress or erode the skeleton – scientists have concentrated on trying to characterize the range of biophysical signals generated by mechanical load, and to understand how these factors are translated into intracellular signals that regulate bone cell behavior. Thus, those cells responsible for bone remodeling must be able to both perceive and respond to the mechanical environment; in this way mechanical forces exert regulatory control over skeletal morphology, and ultimately, produce a skeleton that can withstand the rigors of daily use.
Bone cells are not unique in the ability to respond to mechanical signals; in fact most cells are mechanosensitive. Much progress has been made in understanding the mechanical response of cardiovascular tissue where cells function in a dynamic system of pulsatile blood flow that creates shear, tension and pressure waves in expandable tubes at a rate of more than 60 times per minute (1 Hz, or 1 cps). Endothelial cells are known to respond to shear stress and cardiac myocytes to strain with teleologically understandable alterations (Li et al., 1999a; Wung et al., 1999). As well, the response of cells of the kidney (Hirakata et al., 1997; Ingram et al., 1999) and lung (Kulik and Alvarado, 1993), to name but a few other organs, demonstrably respond to their mechanical environments. Indeed, as onecelled organisms are known to respond to pressure generated by the height of fluid in which they exist (Sukharev et al., 1997), it would seem logical that an ability to respond to the mechanical environment would be an adaptive evolutionary stress that might have affected the joining of cells into complex organisms during evolution.
That bone cells can respond to increases and/or decreases in input from the mechanical environment of the skeleton is well accepted by the scientists, physicians, surgeons and engineers within the musculoskeletal disciplines. Bone's sensitivity to mechanical demands in the “positive” direction is well illustrated by studies that show that the humerae in the “playing” arms of elite tennis players have 35% thicker cortices than the arm that simply throws the ball into the air, emphasizing the extent, and site specificity of the response (Jones et al., 1977; Haapasalo et al., 2000; Kontulainen et al., 2003). Exercise studies have shown that certain degrees of loading must be exceeded to elucidate changes (MacKelvie et al., 2003) and that the degree of the response is determined not only by the magnitude of the load (Rubin and Lanyon, 1985), but the rate (O'Connor et al., 1982), cycle number (Rubin and Lanyon, 1984a), and even the frequency of the load (Rubin and McLeod, 1994; Qin et al., 1998), emphasizing that “load” can be anabolic to bone, but that the relationship between loading and adaptation is complex, and that beyond deformation, such as fluid flow and streaming potentials, may help mediate the mechanical challenge into an adaptive response.
It is also important to emphasize that the adaptive process in bone also can put the bone at risk: un-weighting causes negatively balanced bone remodeling, resulting in site-specific osteopenia. One of the most striking effects of long-duration human exposure to space is a dramatic loss of bone tissue (Baldwin et al., 1996; Carmeliet et al., 2001), reaching as much as a 2% bone density loss in the hip per month, while the upper extremities, which experience less dramatic changes in loading input, show negligible changes (Lang et al., 2004). Disuse, such as occurs in paralysis or prolonged bed rest, also can lead to a rapid loss of bone, with rates of loss approaching that realized during long-duration spaceflight (Gross and Rubin, 1995). The site-specific loss that occurs with disuse, or the gains that occur in response to exercise challenges, indicate that mechanosensitivity due to physical loading is important in regulating bone density, in essence ensuring an “appropriate” level of bone mass that is adequate for the functional demands placed upon the skeleton (Globus et al., 1986; Vico et al., 2000; Carmeliet et al., 2001).
While it is clear that bone cells are acutely sensitive to their mechanical environment, and that the adaptive response can alter both mass and morphology of bone, what remains unclear is how the mechanical force is recognized by the bone cell population (or perhaps other cells within the musculoskeletal system), and transduced into a cellular signal that controls transcriptional activity to recruit bone producing cells (osteoblasts) or bone eroding cells (osteoclasts). While it is not possible to provide an exhaustive review of the burgeoning literature on mechanotransduction in cells, here we will attempt to give readers a measured perspective of the research in this area and consider possible organizing principles for mechanotransduction in cells of the skeleton.
2. What mechanical factors are generated by loading?
The loads which arise from functional activity generate deformation in the bone tissue, pressure in the intramedullary cavity and within the cortices, transient pressure waves, shear forces through cannaliculi, and even dynamic electric fields as interstitial fluid flows past charged bone crystals (see Fig. 1). Alterations in bone remodeling are sensitive to changes in magnitude (Rubin and Lanyon, 1984a, 1985), the number of loading cycles (Rubin and Lanyon, 1984a), distribution of the loading (Lanyon et al., 1982), and the rate of strain (Harrigan and Hamilton, 1993).
Fig. 1.
Mechanical force in the cellular environment. Skeletal loading generates deformation of the hard tissue with strain across the cell's substrate, pressure in the intramedullary cavity and within the cortices with transient pressure waves, shear forces through cannaliculi which cause drag over cells, and dynamic electric fields as interstitial fluid flows past charged bone crystals.
A number of in vivo animal models have been developed to study the effect of specific components of an applied mechanical load on bone tissue including the following examples: compression of the functionally isolated turkey ulna (Rubin and Lanyon, 1984a), bending of the rat and mouse ulna (Mosley et al., 1997) and tibia (Gross et al., 2002), and 4-point bending to rat tibia (Lee et al., 2003). Distinct parameters of the applied loads can be correlated to changes in bone morphology (Rubin and Lanyon, 1985; Rubin et al., 1996, 2001a; Srinivasan et al., 2002) as well as changes in gene and protein expression (Sun et al., 1995; Rawlinson et al., 1998; Lee et al., 2003; Judex et al., 2004)
At the level of small volumes of tissue, all loads and bending moments resolve into strain, or change in length of a material from its original length. During vigorous activities, peak strain magnitudes measured in the load-bearing regions of the skeleton of adult species, including horse, human, lizard, sheep, goat, etc., are all remarkably similar ranging from 2000 to 3500 microstrain (note 1000 microstrain = 0.1% change in length) (Rubin and Lanyon, 1984b). It seems reasonable that the cell population responsible for adjusting skeletal mass can respond at least to these strain levels, if not lesser ones. Indeed, very low strains may be critical to maintenance of skeletal structure and inhibition of disuse bone loss (Rubin et al., 2001a).
Strain levels in the hard component of bone (up to 0.3% strain, or 3-mm deformation over a 1-m bone) are markedly lower than that which occurs in ligaments (5% strain), tendon (20% strain), or cartilage (30% strain). But what exact strain levels are experienced by cells fixed on and within the hard tissue environment of bone is unclear, and certainly, at the level of a 10 εm long bone lining cell or osteoblast, the deformation could be on the order of angstroms. By virtue of their adherence to substrate and the architecture of the plasma membrane (and the intracellular compartment itself) cells can already be considered to have a complex and heterogeneous strain distribution and experience stiffness. If the cell is then stretched or compressed, different portions of the cell will be subject to differential strain patterns. Han and Weinbaum have suggested, for instance, that the processes of osteocytes have a higher stiffness compared to the cell body, allowing normal physiologic strain to generate upwards of 5000 microstrain (0.5%) in the cells themselves (Han et al., 2004), establishing, in essence, an endogenous amplification system. Further, it has been proposed that the osteocyte population, and their interconnectedness via gap junctions, represent a three-dimensional sensing system, and that amplification of strain signals could further occur through fluid flow in the cannalicular system.
As to shear, bone volume includes a significant fraction of unbound interstitial fluid surrounding all of the cells found in bone tissue. Motion of this fluid is driven through two distinct sources. The first source of interstitial fluid flow is the pressure differential of the circulatory system (Dillaman et al., 1991; Keanini et al., 1995) and the second source is the externally applied mechanical loading (Piekarski and Munro, 1977). When bone is loaded, fluid is forced out of regions of high compressive strains, returning when the load is removed. This results in bone cells being exposed to an oscillating fluid flow. Theoretical models have been advanced suggesting that information regarding mechanical loading is perceived by bone cells through loading-induced oscillatory fluid flow (Kufahl and Saha, 1990; Weinbaum et al., 1994; Cowin et al., 1995), including the wall shear stress in the marrow compartment (Swan et al., 2003). These theoretical models also predict that bone cells are exposed to fluid induced shear stress that is on the order of 0.8 to 3 Pa in vivo (Weinbaum et al., 1994) (a paschal is force per area, where 1 Pa = N/m2). Furthermore, it is certain that any flow of fluid (flow is characterized as dynes/cm2, or the amount of drag across an area) due to periodic loading of the tissue, as occurs with physical activity, results in a flow profile that is dynamic and oscillatory in nature. The current evidence supports a significant role for loading-induced dynamic flow in the regulation of resorption through marrow stromal cells.
Pressure is also known to generate responses from bone cells. Continuous hydrostatic pressure at physiologic levels decreased osteoclast formation in marrow cultures (Rubin et al., 1997), and increased production of PGE2 and decreased collagen synthesis from osteoblast-like cells (Ozawa et al., 1990). Application of intermittent compressive force to fetal mouse calvariae was shown to increase bone formation and decrease bone resorption (Klein-Nulend et al., 1987). Indeed, application of pressure may have different effects than strain: chondrocytes subjected to cyclical tension caused increased MMP-13 and decreased TIMP-1 gene expression while cyclical hydrostatic pressure increased TIMP-1 and decreased MMP-13 (Wong et al., 2003). These results support the idea that chondrocytes can distinguish the type of mechanical force, responding appropriately to hydrostatic pressure with slowed chondrocytic differentiation and to cyclical strain with hypertrophy. As well, the dynamic, or time varying nature of the signal, is perceived by cells. When pressure is delivered to chondrocytes in a cyclical rather than constant fashion, it produces an anabolic response (Mizuno, 2005). A requirement for dynamic mechanical signals may indeed be necessary for physiological effects in bone (Lanyon and Rubin, 1984).
In any case, since the complex loading environment of the skeleton results in a diverse range and types of mechanical forces in the bone tissue, it is difficult to separate their effects; ultimately, a cell cannot experience any force in the absence of others, since loading the skeleton generates all of these physical signals concurrently. And despite the apparent ability of, say, chondrocytes to respond differently to pressure and strain, it is not yet clear if variable mechanical factors truly elicit differential responses or whether these are differences elicited by force level or rate of force application. In an attempt to reduce these signals to recognizable elements, controversy has arisen as to which signals are osteoregulatory, or predominant, or important at all. For instance, some have suggested that constant shear cannot represent a physiological stimulus in bone, and dynamic shear should be studied (Jacobs et al., 1998). Others, finding that constant shear caused increased prostaglandin secretion from osteoblasts, while strain at less than 1% magnitude did not (Owan et al., 1997; Smalt et al., 1997a), have declared that small strains cannot be recognized by bone. Yet we have shown that physiologic levels of strain have been shown to induce changes in bone cells such as decreased RANKL and increased eNOS expression (Rubin et al., 2003).
So far, isolating a specific component of the physical milieu that regulates skeletal morphology has been difficult—no single parameter of the mechanical environment reliably predicts bone remodeling in all naturally observed or experimentally created conditions (Brown et al., 1990). Furthermore, in vitro systems are models only: cells plated in monolayer in a tissue culture vehicle don't experience force as they might within an organ. As well, the times studied in tissue culture are short compared to a remodeling cycle in bone. Thus, in vitro systems, while critical to understanding the molecules from whence the response is derived, can simply not replicate the temporal, spatial and contextual experience of a cell in the living skeleton.
The results obtained to date do not support abandoning any one physical signal as conclusively playing no role in bone adaptation. Indeed, in vitro application of forces to cells seems to stimulate many of the same signal transduction pathways, perhaps resulting in the same changes in gene expression (see Table 1). To the extent that there is similarity in the cellular response to diverse physical signals might suggest that a common molecular event such as a conformation change in a transducer protein is induced by all of these signals. There is evidence that the multiple mechanical factors induce the same classic distal signal transduction pathways. Perhaps an equally important question to ask is how the cell interprets the multitude of signals in the mechanical environment – indeed a veritable cacophony of noise – and sums up the incoming information to generate an appropriate adaptive response.
Table 1.
| Alteration | Shear | Strain |
|---|---|---|
| Proliferation | (Li et al., 2004) | (Kaspar et al., 2002; Boutahar et al., 2004) |
| Differentiation: | ||
| RUNX2, Osterix |
(Li et al., 2004) | Rubin J., unpub data |
| β-catenin | (Norvell et al., 2004) | |
| Remodeling: | ||
| RANKL | (Kim and Jacobs, 2005) | (Rubin et al., 2003) |
| Osteoprotegerin | Jacobs C, unpub data | Unaffected (Rubin et al., 2002c) |
| Cytokine | ||
| secretion: | ||
| PGE, NO | (Smalt et al., 1997a; Klein-Nulend et al., 1998; McAllister and Frangos, 1999) |
(Rubin et al., 2003) (Zaman et al., 1999) |
| Extracellular | ||
| matrix: | ||
| Osteopontin | (You et al., 2001) | (Toma et al., 1997; Ziambaras et al., 1998) |
| Collagenase-3 | (Yang et al., 2004) |
3. Mechanosensitive bone cells
Which cell in the bone compartment responds to skeletal loading? As discussed, there is ample evidence that most cells in the body are able to sense their mechanical environment. Indeed, there seems to be a generality in cell responses to mechanical force, at least at the current level of understanding. Thus, MAPK is activated by stretch and shear in cells in endothelium, smooth muscle and bone. It follows that osteoblasts, stromal cells and osteocytes, as well as chondrocytes or alveoloblasts, will have similar basic machinery for responding to physical signals and that differential responses arise out of site-specific and temporal-specific gene patterns associated with the target cell.
Mechanical responses of osteoprogenitor cells, including stromal cells, osteoblasts and osteocytes, have all been documented. It is often difficult to isolate the critical responding cell: for example, exposure to microgravity results in a decreased number of osteoblasts, but what cell senses and responds to the loss of gravity—the stromal cell, the differentiated osteoblast, or the distant, entombed osteocyte? Li et al. found that marrow stromal cells change their proliferation rate and gene expression patterns in response to mechanical stimulation (Li et al., 2004). With respect to osteoclast number: stromal cell expression of the osteoclastogenic facto, RANKL is sensitive to mechanical force (Rubin et al., 1999, 2002c), suggesting that the number of osteoclasts present is controlled through mechanical regulation sensed by stromal cells. However, the osteoclast itself has also been shown to respond to mechanical signals adding another layer of control by which mechanical force might limit bone resorption (Wiltink et al., 1995).
Other cells present in bone, such as endothelial and smooth muscle cells in the penetrating vasculature, might also contribute to the skeleton's adaptive response to loading. After all, endothelial cells respond to shear stress and tensile strain generated by increased heart rate during exercise, by producing nitric oxide (Davis et al., 2001; Boo and Jo, 2003). Nitric oxide is an important humoral factor to transduction of mechanical input in vascular cells (Ralston, 1997; Lane and Gross, 1999). Increased vascular release of nitric oxide might be important in regulating bone cell response: nitric oxide has pleiotropic effects in bone cells, and certainly potently decreases resorption through decreasing osteoclast formation and activity (Fan et al., 2004). But bone stromal cells also release nitric oxide as a result of mechanical input (Rubin et al., 2003) providing a secondary cell target for mechanical induction of this freely diffusible antiresorptive agent. Thus, targets of skeletal loading may include extraskeletal cells and systems.
The vast majority of cells in cortical bone are osteocytes, and due to their pervasive, three-dimensional distribution throughout both trabecular and cortical bone, these cells are, potentially, well placed to sense the magnitude and direction mechanical strain within the tissue. Although this cell is enclosed in calcified tissue it is connected to equally encased cells, as well as to the bone surface, through a network of cannaliculi through which these cells cast long cell processes. Osteocytes are indeed able to respond to strain in vivo as shown by increased glucose-6 phosphate dehydrogenase activity (Skerry et al., 1989), or earlier response of c-fos mRNA (Inaoka et al., 1995) after loading. As well, unloading causes osteopontin expression in osteocytes (Gross et al., 2005). Dentin matrix protein (DMP1), which is a secreted matrix protein expressed in late osteoblasts and osteocytes, has been shown to increase in osteocytes after tooth movement in the jaw (Gluhak-Heinrich et al., 2003).
If we consider that strain signals of even very low magnitude can stimulate an anabolic response in bone tissue (Rubin et al., 2001b), the osteocyte may be best placed to sense such a signal (Rubin et al., 2002a). A mathematical theory which takes this into account suggests that osteocytes stimulate osteoblast bone formation (Huiskes et al., 2000). This might occur through fluid flow through cannaliculi, as well as through deformation, both which have been shown to cause changes in osteocyte function (Klein-Nulend et al., 1998; Plotkin et al., 2005). It has also been suggested that, due to the modulus mismatch of the bone material and the lacunae, the osteocyte within the “lake” would be subject to strains as high as 30,000 microstrain, even though the bulk material was strained only to 3000 microstrain (Nicolella et al., in press). In other words, the microarchitecture of the bone tissue could serve to indirectly (fluid pressure through cannaliculi) or directly (strain amplification via the lacunae) amplify the strain signal. While some have suggested that osteocytes might be more sensitive to shear than, for instance, transient pressure (Klein-Nulend et al., 1995), there are divergent opinions: e.g., substrate strain prevents osteocyte apoptosis (Plotkin et al., 2005). Recently Han and coworkers have developed a 3D model for the osteocyte process and used large-deformation “elastica” theory to predict the deformed shape of the cell (Han et al., 2004). Because the model predicts a cell process that is very stiff, hard tissue strains will be amplified through the cell process, indeed into the magnitudes that have been studied in vitro in many systems. Whether the osteocyte is the major responder to mechanical strain in the skeleton is still to be determined.
4. Candidate mechanoreceptors
One of the “big” questions in mechanotransduction is the nature of the receptor(s) for mechanical force. Actual receptors for sensing pressure and movement have been described in single-celled organisms, and have parallels in mammalian sense organs. The first described mechanosensor was a stretch-activated channel that could respond directly to membrane perturbation (Guharay and Sachs, 1984). A channel of this type was purified from E. coli: the mechanosensitive channel large conductance (MscL) forms a nonselective ion channel (Sukharev et al., 1997) that responds to hyperosmotic tension.
A more complex version of an ion channel is found in C. elegans where a mechanosensory complex involves necessary parts from matrix, microtubules, and a linker protein that all encircle a central ion channel (Huang et al., 1995; Tavernarakis and Driscoll, 1997). The central ion channel is similar to the protein stomatin that is expressed in red blood cells and regulates membrane conductance; stomatin dysfunction in red blood cells of patients with hereditary stomatocytosis leads to a hemolytic anemia (Stewart, 2004). The C. elegans stomatinlike protein is part of a mechanosensory transduction channel that connects an inside-out channel to underlying microtubules. Interestingly this channel functions within a specific lipid-raft membrane microdomain, which may define underlying responsiveness to the mechanical environment (discussed below).
For non-sensory tissues, however, mechanoreceptors are less well understood. The ability of cells to read their biomechanical environment requires that their mechanoreceptors must either be in contact with the outside, through the cell membrane and its attachment to substrate, or that the mechanoreceptor be able to sense changes in a loading-induced physical intermediary such as fluid shear on the apical membrane. While there are examples of channels that are regulated by movement of mechanosensory bristles (Sukharev and Corey, 2004), or by tension waves (Morris, 1990), a unified model of the most proximal events that lead to intracellular signal transduction in non-sensory tissues does not yet exist. Theoretical considerations may be moving toward an architectural/spatial concept which integrates positional changes between signaling proteins, scaffolds, membrane domains and structural components of the cell. All can be perturbed by mechanical force. Whether each aspect is a mechanoreceptor in itself, or works in a holistic context combining many aspects of cell architecture and response, is the work of the coming years (see Fig. 2).
Fig. 2.
Mechanosensors activate intracellular signals. Multiple mechanosensors may be involved in receiving mechanical signals.
4.1. Channels
Alterations in ion channel activity in osteoblasts have been associated with bone cell activation, whether through alteration in conductance stimulated by PTH (Ferrier et al., 1986), or by stretch/strain (Duncan et al., 1992). Patch-clamp techniques have been used to show at least three classes of mechanosensitive ion channels in human osteoblasts (Davidson et al., 1990). Through this set of channels mechanical stimulus could induce membrane hyper and de/polarization or a complex multiphasic response. Indeed two stretch-activated membrane channels have been identified in bone cells (Duncan et al., 1996; Kizer et al., 1997). Cyclical strain has been shown to modulate the activity of certain channels—chronically strained osteoblasts had significantly larger increases in whole cell conductance when subjected to additional mechanical strain than unstrained controls (Duncan and Hruska, 1994). More recently, radial membrane strains of 800% were shown to be necessary to open half of the mechanosensitive channels in bone cells (Charras et al., 2004). Mechanosensitive channels have also been implicated in the response of bone cells to fluid shear stress (Ryder and Duncan, 2001). In addition to direct activation of intracellular signaling cascades, influx of a charged species such as calcium can also alter membrane potential and activate voltage sensitive channels that are not directly mechanosensitive. For example, the L-type voltage sensitive calcium channel has been implicated in mechanosensitivity in vivo (Li et al., 2002).
Mechanically activated channels have been studied in limb bone cultures: gadolinium chloride, which blocks some stretch/ shear-sensitive cation channels, was able to block load-related increases in PGI2 and nitric oxide (Rawlinson et al., 1996). In these experiments, osteoblast response to load was even more sensitive to nifedipine, which blocks calcium channels. Thus, many studies have demonstrated that mechanically sensitive ion channels exist in bone, but the exact identification of such a channel or channels remains elusive.
4.2. Integrins and integrin associated proteins
Membrane deformation and shear across the membrane, as well as pressure transients, are transmitted to the cytoskeleton and ultimately to the cell-matrix adhesion proteins that anchor the cell in place (Katsumi et al., 2004). Thus, the cell represents a load transmission network whereby surface forces will affect proteins that span or are associated with the plasma membrane including the cytoskeleton, linker proteins at sites of cell attachment, and membrane spanning integrin adhesion proteins. In this way mechanotransduction might be expected to be dependent on the mechanical integrity of this network with constituents of this network serving as molecular mechanotransducers.
Integrins are membrane spanning proteins that couple the cell to its extracellular environment. They are also known to initiate cytosolic signaling upon binding to extracellular ligands (outside-in signaling) as well having their binding affinity regulated intracellularly (inside-out signaling). Functional integrins are heterogeneous dimers made of α and β subunits. In osteoblasts the β1 subunit has the predominant functional role, dimerized with α subunits including α1 through α5 and αV (Zimmerman et al., 2000; Bennett et al., 2001). The β1 subunit has also been reported in osteocytes (Hughes et al., 1993) along with β3 and CD44. The ligands for this β1 containing class of integrins are diverse, including collagen I and III and fibronectin. The cytoplasmic tail of the β subunit has been identified as playing a critical role in integrin signaling (Liu et al., 2000). Although the details of integrin signaling are not entirely clear, it appears to be primarily via interaction with other structural proteins. Integrins have been implicated as mechanoreceptors in a wide range of cells including myocytes (Aikawa et al., 2002), fibroblasts (Tadokoro et al., 2003), endothelial cells (Shyy and Chien, 2002), chondrocytes (Millward-Sadler and Salter, 2004), and bone cells (Salter et al., 1997). Fluid flow has been shown to activate MAPK via β1 integrin and FAK dependent tyrosine phosphorylation in endothelial cells (Ishida et al., 1996). In osteoblasts, steady fluid flow has been shown to upregulate β1 expression (Kapur et al., 2003) and activate αvβ3 which co-localized with src (Weyts et al., 2002). However, blocking β3 with RGD containing peptides was not found to affect ERK activation (Weyts et al., 2002).
As mentioned above, a large number of integrin and focal adhesionassociated structural proteins have been identified and their function has only begun to be elucidated. Playing a central role is focal adhesion kinase (FAK). FAK is known be critical to integrin clustering as well being a potent signaling molecule with important kinase activity and several sites of tyrosine phosphorylation (Schaller, 1996; Schlaepfer et al., 1999). FAK is known to be involved in cell growth, survival and migration and is increasingly suspected to be involved in mechanotransduction in a number of cells (Lee et al., 2000; Gerthoffer and Gunst, 2001; Keller et al., 2001; Orr and Murphy-Ullrich, 2004) including bone cells (Wozniak et al., 2000; Rezzonico et al., 2003). When activated, FAK autophosphorylates tyrosine 397 which allows interaction with a number of src-family proteins and other molecules with SH2 domains (src homology 2, a specific sequence involved in binding to a phosphorylated tyrosine). Tyrosine phosphorylation, predominantly in FAK (and specifically at tyrosine 397), is known to occur in osteoblasts upon mechanical stimulation (Boutahar et al., 2004). Upon phosphorylation, FAK contributes to MAPK activation via interaction with c-src, Grb2, and the small GTPase Ras (Schlaepfer et al., 1997, 1998, 1999). This is significant since MAPK activation is also one of the effectors of oscillatory flow in bone cells (You et al., 2001) and this pathway has been observed in response to fluid flow in endothelial cells (Berk et al., 1995; Ishida et al., 1996; Li et al., 1997). It is also notable that FAK activation has been observed to cause intracellular calcium mobilization via activation of the γ1 isoform of PLC (the only SH2 containing isoform known). A similar pathway may be important in bone cells since fluid induced calcium mobilization has been shown to require PLC mediated IP3 release.
A large number of additional adhesion-associated linker proteins with both structural and biochemical roles are potential molecular mechanotransducers. For example, both talin and paxillin are unique in that they are known to bind to the focal adhesion targeting sequence of FAK (Schaller, 1996) and talin also binds directly to the integrin cytoplasmic β tail (Horwitz et al., 1986). Paxillin is also a substrate for FAK and src kinase activity and hence a potential signal initiator (Subauste et al., 2004). Upon tyrosine phosphorylation, paxillin is known to create binding sites for interaction with SH2 domain containing adaptor and signaling proteins. In contrast, talin appears primarily to regulate integrin activation via inside-out signaling. Both are strong candidates for further investigation: talin due to its direct interaction with actin and integrins, and paxillin due to its potential for direct downstream signal activation.
4.3. Connexins
Connexins are membrane spanning proteins that form regulated channels that allow the direct exchange of small molecules with adjacent cells resulting in intercellular communication between cells. Intercellular communication via gap junctions has been suggested to be central to the transmission of information about the mechanical environment of a given cell and ultimately allowing a sensing cell to elicit a change in behavior at an actor cell some distance removed from the mechanosensing event (Donahue, 2000; Yellowley et al., 2000). Interconnected cells have also been proposed to form a cellular network that can exhibit an enhanced sensitivity to biophysical stimuli than occurs in individual cells. This application might occur due to the larger area occupied by a cell network, and therefore larger net effect of the biophysical signal, than by individual cells. Indeed, when the communication of an ensemble of cells is interrupted, a reduced sensitivity has been observed in response to biophysical signals such as electric fields (Vander Molen et al., 2000), fluid flow (Saunders et al., 2001) or biochemical stimulation (Vander Molen et al., 1996). Interestingly, it has recently been suggested that in addition to regulating cell–cell communication, connexins can also form regulatory channels between the cell and its extracellular environment. These channels (known as connexin hemi-channels) have been identified at the tips of the dendritic processes and knockdown with connexin antisense inhibits the release of PGE2 in response to fluid shear, suggesting that perhaps they are being mechanically regulated (Cherian et al., 2005), although this mechanism does not appear to play a role in ATP release by osteoblasts in response to shear (Genetos et al., 2005). Finally, mechanical stimulation has been shown to increase expression of connexins in vitro and in vivo, suggesting that cells become better connected with their neighbors perhaps acting as a sort of positive feed-back loop (Alford et al., 2003).
4.4. Membrane structure
Cells possess a complex organizational structure that supports compartmentalization of signals within an equally complex plasma membrane. While aspects of the plasma membrane can still be represented by the Singer–Nicholson fluid mosaic concept of a neutral lipid bilayer, this sandwich has been found to contain several phases of lipid including gel, liquid-ordered and liquid-disordered states (Simons and Toomre, 2000). Lipid rafts are highly condensed assemblies of cholesterol and sphin-golipid. This organized structure creates a complex association between the inner and outer leaflets where transmembrane proteins are involved in stabilizing the coupling. As such, the lipid raft is a stiff base within a fluid – or disordered – lipid bilayer. Lipid rafts can be categorized into caveolar and noncaveolar forms (Anderson, 1998; Cho et al., 2003). Unlike noncaveolar rafts, caveolae contain caveolins as their structural and functional molecules (Anderson, 1998; Okamoto et al., 1998). Both forms of detergent-resistant organized membrane have been shown to be critical to the integration of mechanical information at the plasma membrane surface (Anderson, 1998).
The components of the lipid core (glycosphingolipid, sphingolipid and cholesterol) integrate the array of proteins that localize to the caveolar system. When membrane integrity is breached, for instance by leaching cholesterol (Park et al., 1998) or sequestering membrane cholesterol with filipin (Rizzo et al., 1998), signaling networks are disrupted. A large variety of signaling molecules have been found to be discretely concentrated in detergent-resistant membrane, including GTP-binding proteins, tyrosine kinases, calcium and lipid signaling molecules. With such an abundance of signaling effectors located in one place, caveolae are regulatory hot spots where cross talk and directionality rapidly, efficiently and specifically propagate signals to downstream targets (Rizzo et al., 1998).
Certain proteins are integral to membrane structure while creating docking positions for signaling complexes. Caveolins-1 (α and β), −2 and −3, named for their association with caveolae (as well as lipid rafts), each contain a 33-aa hydrophobic domain that anchors the protein to the membrane (Anderson, 1998). Caveolin-1 in particular associates with invaginated caveolae, and its role in shaping the local membrane morphology is confirmed by the loss of flask-like caveolae in caveolin-1 null animals (Drab et al., 2001; Kranenburg et al., 2001). The protein structure of caveolins allows both amino and carboxyl termini to remain in the cell cytoplasm and interact with proteins that kink around the central hydrophobic domain. Many pro teins found in caveolae contain caveolinbinding motifs that may represent a mechanism for membrane sequestration (Okamoto et al., 1998). The tendency of caveolin to form homooligomers that associate with lipid-modified signaling molecules suggests that caveolin serves as a scaffold protein (Couet et al., 1997). Indeed, “caveolin scaffolding domains” are postulated to function like other modular protein domains (e.g., SHC2, pleckstrin).
Caveolins-1 and −2 have been described in human fetal osteoblasts and in murine MC3T3-E1 cells along with multiple caveolar flasks in the membrane (Solomon et al., 2000a). Similar findings are corroborated in osteoblastic outgrowths from normal adult bone (Lofthouse et al., 2001). Bone cell caveolae are associated with important signaling molecules including G-proteins, Ras, nitric oxide synthase, and tyrosine kinases (Solomon et al., 2000b). These membrane domains, whether caveolae or non-caveolar lipid rafts, likely provide a microenvironment that enhances both efficiency and fidelity of signal transduction.
The organized membrane may have greater significance for mechanical response than for parsing signals arising from liganded receptors. Non-caveolar and caveolar organized membranes have been shown to be critical for mechanically induced signals. In the vascular endothelium for instance, increased flow causes the translocation of signaling molecules to caveolae; if caveolae are disassembled, both proximal and downstream signaling events, including activation of the MAPK pathway, are abrogated (Rizzo et al., 1998). Stretch activation of small GTPases in cardiac myocytes has, as well, been associated with caveolae: the stretch activation of the GEFs RhoA and Rac1 fails to occur when caveolae are disrupted by treatment with methyl-β-cyclodextrin (MβCD) (Kawamura et al., 2003). Disruption of membrane caveolae with MβCD abolishes the stretch-induced activation of ERK1/2 as well as the subsequent increase in protein synthesis in pulmonary vasculature (Zeidan et al., 2003).
Another concept that relates to membrane structure is the ability of strain or shear to induce conformational changes in intracellular proteins—separating linked proteins, or inducing protein structural changes. There is evidence that this can occur. Fibronectin lengthening due to tension exposes cryptic-binding sites, which should enable association of as yet unidentified molecules (Craig et al., 2001; Gao et al., 2002). Intracellular forces due to strain, shear, or drag can be related to exposure of critical-binding sites necessary for signal activation of structural molecules (see Fig. 3).
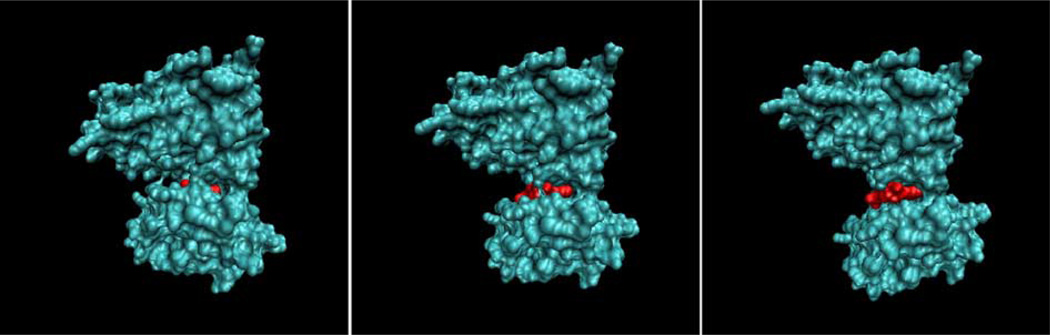
Fig. 3.
Computer simulation of Focal Adhesion Kinase (FAK). The figure shows three frames from a computer simulation of the change in conformation of the Focal Adhesion Kinase (FAK) molecule under load. Note that loading results in the exposure of an ATP-binding site (depicted in white). Courtesy of Ronald Kwon, C. Jacobs laboratory.
4.5. Cellular architecture and tensegrity
Perhaps all the proceeding concepts can be summed up as contributing to a greater whole — a concept that holds that the entire cell, by virtue of its intrinsic architecture, is poised to receive information regarding its mechanical environment. Mechanical stress alters cell shape and structure which is now known to affect changes in the internal cytoskeleton, which is in turn composed of a network of microfilaments and microtubules that link adhesion receptors to the cell nucleus. Rearrangement in proteins of the cell cytoskeleton can lead to adaptive responses. Cell spreading on the extracellular matrix has been associated with increased tyrosine phosphorylation of focal adhesion kinases (Bockholt and Burridge, 1993). Changes in cell sensing of its neighbors secondary to distortional changes from physical loading, might invoke mechanisms where changes in cell architecture activate classic signaling pathways.
Tensegrity, a concept elucidated by Ingber, may come close to tying together structural organization of the cell as it interacts with the physical environment. In the tensegrity model, the cell is hard wired through its cytoskeleton to cell surface receptors that physically couple the internal architecture to the extracellular matrix (Ingber, 1997). Thus, the cytoskeleton not only stabilizes cell shape, and generates tension via an actinomyosin filament sliding mechanism: there is a measurable isometric tension that generates an internal “pre-stress”. Thus, external mechanical loads are imposed on a pre-stressed structure (Wang et al., 2001). This can be compared, for instance, to the way our skeleton is held up against gravity through tensile linkages with surrounding muscle and tendon. Since the membrane attaches to substrate in a point fashion, through focal adhesions, the pull through the CSK is complex. When microtubules are assembled, exerting pushing forces as they grow, cells can become mobile if the assembly is at the leading edge. Forces generated by protein polymerization have been measured, and speculation that the interaction of the growing microtubule end with a specific attachment might modify the force (Dogterom and Yurke, 1997). If the force emanates from a focal adhesion point, one can imagine that the force generation will be differentially felt throughout the cell body, perhaps activating specific signaling systems. In any case, it appears that altering stress in one part of the cell can affect another part.
Tensegrity models are consistent with recognition of multiple types of mechanical input—from shear to strain and pressure. Indeed, they would predict that force could be nearly instantaneously transferred from one side of the cell to the other. It might also explain signal sorting, through macromolecules assembled along CSK at a focal adhesion, and molecules that are not assembled, but exactly nearby, might feel this stress.
4.6. Making sense of multiple putative mechanosensors
With the multiplicity of mechanical signals presented to the cell, it is likely that no one mechanosensor or receptor mechanism is responsible for all of the integrated cell responses to the mechanical environment. As will be described below, different cellular level mechanical stimulation has been shown to regulate a variety of intracellular signaling and processes, often with substantial overlap. This is likely to be true for the multiple putative mechanosensors. At the very least, these sensors are likely to interact with each other in integrating information which the cell receives via chemical and the mechanical signals.
5. Mechanically activated intracellular signaling systems
Application of mechanical force to bone cells causes modulation of cell function—including changes in proliferation and function. Indeed, most aspects of cell behavior can be elucidated by a number of mechanical forces (Table 1). Thus, straining osteoprogenitor cells can cause them to proliferate (Zhuang et al., 1996) and to secrete extracellular matrix (Harter et al., 1995). Similarly, shear stress can induce beta-catenin signaling (Norvell et al., 2004) and secretion of osteopontin (You et al., 2001). To achieve these ends, it is quite clear that multiple classic signaling pathways are activated after force application.
Since the distal responses to mechanical factors are similar to those elicited by ligand-receptor pairing, and result in changes in gene expression, mechanotransduction must eventually end up utilizing similar intracellular signaling cascades. This has been demonstrated convincingly in endothelial cell response to shear (Chien et al., 1998) where shear induces the macrophage chemoattractant peptide-1 (MCP-1) through activation of MAPK pathways (Jalali et al., 1998) similarly to the actions of VEGF, which also stimulates MCP-1 through MAPK pathways activated after binding to the VEGF receptor.
In essence, mechanical forces have been shown to activate every type of signal transduction cascade, from increases in intracellular cAMP (Lavandero et al., 1993), IP3 and intracellular calcium (Dassouli et al., 1993; Li et al., 2004), guanine regulatory proteins (Gudi et al., 2003), and MAPK (Rubin et al., 2002c). As previously stated, this review does not aim to collect each and every report of each signal cascade stimulated by mechanical force in bone cells. Instead, we will examine several examples as models.
5.1. Signaling through G-proteins
Guanine regulatory proteins are some of the most proximal elements in many signaling cascades, thus the ability of mechanical force to generate intracellular increases in cAMP and MAPK, for instance, with distal gene regulation relevant to those signals, most certainly involves GTPase regulated proteins.
Cyclic AMP generated functions are ubiquitous. cAMP, IP3 and PKC activity were shown to increase in primary osteoblasts isolated from dentoalveolar bone after stretch (Carvalho et al., 1994). It is certain that when cAMP rises that Gα would be invoked to activate adenylate cyclase. Fluid flow regulates osteocyte gap junctions by causing PGE2 release from osteocytes (Cherian et al., 2005). Activation of the G-protein coupled PGE2 receptor causes increased intracellular cAMP and PKA activity, eventually resulting in connexin-43 expression. The initial response to flow may derive from activation of a non-cAMP pathway. Stretch also increases gap junctional communication in osteoblasts through changing connexin-43 function: cyclic stretch increased dye diffusion (Ziambaras et al., 1998). Whether this latter effect required PGE2 release is not known.
Several laboratories have shown that Ras and Rho GTPases are activated after mechanical force in vascular cells (Jalali et al., 1998; Li et al., 1999b). This activation may be even more extensive as recently shear was shown to have downstream effects that required Gα(q) and Gβγ to activate Ras itself (Gudi et al., 2003). While this specific activation has not been shown in bone cells, as we have stated, mechanically responsive systems appear to be somewhat universal with regard to cells and are likely to be true for osteoblasts and osteocytes.
5.2. Calcium signaling
Both strain and pressure result in mobilization of intracellular calcium (Wiltink et al., 1995). Numerous studies of both osteoblasts and osteocytes demonstrate that this is a consistent and robust response to both static and dynamic patterns of shear (Hung et al., 1995; Jacobs et al., 1998). Furthermore when this critical early second messenger is blocked during mechanical stimulation mechanically regulated changes in gene expression do not occur (Chen et al., 2000; You et al., 2001), although PGE2 release in response to fluid flow occurs independent of intracellular calcium signaling (Saunders et al., 2003). This rapid increase in intracellular calcium concentration appears to occur due to phospholipase C activity and IP3 signaling resulting in release of calcium from intracellular stores in many cases. Interestingly, calcium-induced calcium release has been suggested in the transduction of steady fluid flow (Chen et al., 2000) but was not found to occur in response to dynamic flow (You et al., 2001) suggesting that cells may be able to distinguish temporal patterns of mechanical stimulation. Stretch is also known to stimulate the IP3 pathway in cardiomyocytes (Dassouli et al., 1993), and is probably a fairly universal response in many tissues. Osteoblasts subjected to fluid shear increase the expression of c-fos and cyclooxygenase-2 (Chen et al., 2000). This response appears to be based on a rapid increase in intracellular calcium concentration thought to derive from phospholipase C via IP3 induction of calcium release from intracellular stores (Chen et al., 2000).
Calcium transients can also derive from opening of stretchactivated channels that are permeable to calcium. Stretching osteoclasts isolated from bone marrow of chickens, Wiltnik et al. reported a calcium-conducting ion channel in these bone resorbing cells that leads to cell membrane hyperpolarization (Wiltink et al., 1995). Since increased intracellular calcium concentration in osteoclasts induces a reduction in adhesive molecules and decreased resorptive activity, these authors speculate that stretch, through opening the channel, decreases osteoclastic activity through this pathway.
5.3. MAPK signaling
Mechanical force has been shown to activate mitogen-activated protein kinases in every cell type studied to date. It has long been known to occur in endothelial cells, where not only ERK1/2 kinase, but also p38, BMK-1 and JNK are activated (Yan et al., 1999). In bone cells many groups have shown activation of ERK1/2 in particular (You et al., 2001; Jessop et al., 2002; Weyts et al., 2002; Yang et al., 2004; Plotkin et al., 2005).
It has been demonstrated that mechanical activation of ERK1/2 is required for certain measurable responses to strain in bone stromal and osteoblast-like cells (Rubin et al., 2002c) (see Fig. 4). The strained bone cell downregulates its expression of RANKL, and upregulates expression of eNOS (Rubin et al., 2003). Although strain also activates c-jun kinase (JNK), JNK inhibition does not prevent strain effects on either RANKL or eNOS (Rubin et al., 2002c). Reduced display of RANKL by cells present in bone diminishes the local osteoclastogenic potential. As a result of increased eNOS expression, nitric oxide (NO) synthesis is enhanced.
Fig. 4.
Strain stimulation of MAPK is required for downstream gene regulation. The effect of strain to decrease RANKL (left graph) and increase eNOS (right graph) was complete blocked by the presence of an ERK1/2 kinase inhibitor (ERK-i). These results show that strain activation of ERK1/2 is necessary for the downstream effects in the bone stromal cell model. From (Rubin et al., 2003).
ERK1/2 activation by mechanical stress has been linked to other specific genes such as strain induction of collagenase-3 (Yang et al., 2004), as well as to proliferation (Boutahar et al., 2004). Both fosB and its spliced variant, deltafosB, which has been shown to stimulate increases in bone density in vivo, are induced by mechanical loading in mouse hind limb bone and by fluid shear stress in mouse calvarial osteoblasts (Inoue et al., 2004). The ERK1/2 dependent increase in the fos gene targets a CRE/AP-1 type element in the promoter that binds CREB.
The proximal events leading to ERK1/2 activation are the subject of much continued research. This may certainly involve integrins (Whedon, 1984) as well as multiple other effectors, including small GEFs (Jin et al., 2005) or changes in membrane structure (Boyd et al., 2003).
5.4. Nitric oxide signaling
Nitric oxide has pleiomorphic effects on bone cells (Ralston, 1997), and may have a role in mechanical signaling in bone. Nitric oxide is released shortly after shear stress from osteoblasts and osteocytes (Reich et al., 1990; Smalt et al., 1997b) likely due to activation of endothelial nitric oxide synthase (Klein et al., 2004) similarly to known effects of shear in vascular cells (Boo and Jo, 2003). The rapid activation of nitric oxide in endothelial cells requires, in part, an intact plasma membrane, including lipid rafts (Park et al., 1998) and cyto-skeleton (Knudsen and Frangos, 1997), and this is likely to be true for mechanical release of nitric oxide in bone cells. Endothelial nitric oxide synthase is the predominant nitric oxide synthase isoform in adult bone (Helfrich et al., 1997), and expression of this gene with subsequent increase in nitric oxide production is upregulated by strain in marrow stromal cells (Rubin et al., 2003). Downstream nitric oxide signaling can depend on activation of guanylate cyclase or on direct actions of the molecule to nitrosylate proteins as has been shown for NO action to decrease the RANKL/osteoprotegerin ratio in stromal cells (Fan et al., 2004). Nitric oxide has also been shown to be necessary for the response to in vivo loading in rodents, although whether the nitric oxide derives from bone or the vasculature in bone is not yet clear (Turner et al., 1996; Armour et al., 2001; Kunnel et al., 2004).
6. The therapeutic promise of mechanical signals
Considering the increase in bone mass that occurs with exercise (Jones et al., 1977), or the rapid bone loss that parallels disuse (Leblanc et al., 1990) and microgravity (Lang et al., 2004), it is clear that some specific mechanical components within the functional environment of the skeleton influence bone mass and morphology. By improving our understanding how loads are anabolic to bone, as well as identifying those specific components within the complex loading environment which are anabolic, mechanical signals themselves may represent the very essence of a non-pharmacologic means to inhibit osteoporosis, promote osseointegration, ensure mineralization following distraction of osteogenesis or accelerate the healing of fractures. Whether this is used to inhibit bone loss in the spine, ensure the healing of a fracture in the tibia, or promote bony ingrowth into a dental implant, mechanical signals represent a physiologically based “factor” which is intrinsic to the skeletal system and critical to achieving a balanced state of bone remodeling. While exercise, per se, has been used to some success in augmenting the skeleton, issues such as compliance, safety, and efficacy have kept osteoporosis interventions focused on pharmaceutical interventions. Recent evidence has suggested, however, that high-frequency, low-magnitude mechanical signals are anabolic to the skeleton (Rubin et al., 2001a), and considering that they are three orders of magnitude below those strains which generate damage in bone, and that they can enhance both bone quantity and quality (Rubin et al., 2004) suggest that they may represent a means of harnessing bone's sensitivity to mechanical signals and establish a non-pharmacological treatment for osteoporosis (see Fig. 5).
Fig. 5.
Mechanical input improves trabecular bone microarchitecture. Fluorescent photomicrographs of a transverse section of proximal femur of adult (8 y) sheep, comparing a control animal (left) to an animal subject to 20 min per day of 30 Hz (cycles per second) of a low-level (0.3 g) mechanical vibration for one year (Rubin et al., 2001a). The large increase in trabecular bone density results in enhanced bone quality (Rubin et al., 2002b), achieved with tissue strains three orders of magnitude below those which cause damage to the tissue. These data suggest that specific mechanical parameters may represent a non-pharmacologic basis for the treatment of osteoporosis (Ward et al., 2004).
In a first effort to demonstrate a proof of principle of this concept, sixty-two post-menopausal women were randomized into in a double-blind, placebo controlled pilot study to investigate if mechanical signals could modulate bone mass and morphology (Rubin et al., 2004). 32 women underwent mechanical loading for two 10-min periods per day, through floor mounted devices that produced a 0.2 g mechanical stimulus at 30 Hz while 32 women received placebo devices. Evaluating those in the highest quartile of compliance, placebo controls lost 2% in femoral neck density over the year, while the treatment group lost no BMD indicating a statistical relative benefit of treatment. In a parallel study, twenty children with cerebral palsy were randomized into active mechanical treatment (0.3 g, 90 Hz, ten min per day) and placebo control groups (Ward et al., 2004). After six months, the placebo controls lost 12% of volumetric trabecular bone mineral density, while those in the active treatment arm gained 6.3%.
These animal and human studies indicate that brief exposure to mechanical signals can benefit bone quantity and quality. Such a biomechanical intervention is self-targeting, endogenous to bone tissue, and auto-regulated, and provides insight towards a unique, non-pharmacological intervention for osteoporosis. In contrast to systemic, pharmaceutical intervention such as estrogens, PTH or bisphosphonates, the attributes of such mechanical prophylaxes are that they are native to the bone tissue, safe at low intensities, incorporate all aspects of the remodeling cycle, and the relative amplitude of the signal will subside as formation persists (self-regulating and self-targeting). However, the widespread use of mechanical – or other physical – stimuli in the treatment of skeletal disorders will undoubtedly be delayed until we achieve a better understanding of the mechanisms by which they act, regardless of the osteogenic potential of mechanical stimuli which clearly points to their potential as a unique intervention for disorders and injuries of the musculoskeletal system, including the cranial skeleton.
7. Outstanding questions
Bone is extremely sensitive to mechanical signals, thus, skeletal mass and morphology are determined in large part to its ability to adapt to the functional challenges to which it is subject. The adaptive nature of the skeleton poses an interesting set of biological questions (e.g., how does bone sense mechanical signals, what cells are the sensing system, what are the receptors for force, what are the mechanical signals that drive the system, what are the molecular responses to the mechanical stimuli). We have seen that the resident bone cell populations possess mechanoreceptors which sense the mechanical environment and that virtually every type of signaling cascade is subject to activation (or in the case of osteoclast recruitment, suppression) by mechanical forces. Certainly, it is clear that new mechanical signals in bone alter the transcriptional activity of bone (Judex et al., 2005), and that the resultant adaptation is an effective strategy for a “smart” material to accommodate new loading challenges. The growing amount of information in this area highlights critical questions that need to be addressed by investigators in mechanotransduction and the signaling world in general. First, do bone cells respond to all of the many physical signals generated during loading? If this is the case, how do bone cells sort this signal noise into recognizable cues resulting in a coordinated adaptive response? Secondly, what receptor is activated or what other molecular level events occur in the cell in response to these various physical signals? For example, is the membrane deformation generated during fluid shear across the apical surface of a cell comparable to the deformation generated in a substrate stretch experiment? And then, while significant overlap exists in the signals activated by different physical forces, in some cases the response to different biomechanical stimulation may be different. Furthermore, even should we find that a common pathway is activated by a variety of physical signals, are we confident that this indicates a single common response? And considering that bone is, under normal conditions, essentially always being loaded, how the bone can differentiate a “new” load, and thus stimulate adaptation, from a “typical” load, and thus retain homeostasis?
These questions arise because we do not yet understand with any confidence the biomechanical behavior of cell as structures. Cell mechanics has been an active area of research for decades, but we are still unable to determine the mechanical response of a specific cell in terms of its particular morphology or its connection with the extracellular environment. We are unable to determine, for example, what level of membrane deformation occurs or what level of force is transmitted across integrins or the cytoskeleton in the in vivo situation or in any but the simplest of in vitro cell loading systems. From a bioengineering perspective, these complexes, but clinically critical questions, are driving the field forward at an accelerating rate such that answers may soon be available. From a biological perspective, it highlights the increasing need for investigators to utilize consistent and well characterized cell loading approaches which can be mechanically understood and to be sensitive to the possibility that in some instances cells may actually respond to an unanticipated secondary physical signal. As with many emerging research areas at the interface of traditional disciplines, there is great potential and need for a deeper understanding of this fundamental process and an integrative approach is the most likely to succeed. These approaches should also represent a basis for understanding etiologies of musculoskeletal diseases such as osteoporosis, and should provide new insights in treatment strategies. In sum, an interdisciplinary approach, in this case combining endocrinology, bioengineering, cell biology and molecular biology, will be required to attain a clear understanding of how bone works.
Increased appreciation of the importance of the mechanical environment in regulating and determining the structural efficacy of the skeleton makes this an exciting time for investigations in this area. While much has been learned, the data, for the most part, suggests to us that we can now generate a series of questions upon which to concentrate. With these questions, we close this review.
Can cells sense multiple types of mechanical force, and, if so, is the response to each of these unique?
Which cells are critical for the skeleton's response to the mechanical environment?
Are temporal, directional and magnitude variations in mechanical force important for cell response?
Do all cells utilize the same molecular mechanotransducers to recognize force?
Does the origin/location of the cellular signal on the membrane or the cytoskeleton determine the distal event?
How shall these forces be harnessed to preserve skeletal structure during aging and disease?
Acknowledgements
NIH AR42360 (JR), VA Merit (JR), NIH AR43498 (CR), NASA E079/VIBE (CR), NIH AR45989 (CRJ), NASA NAG2-1601 (CRJ).
Abbreviations
- ERK
extracellular signal-regulated kinase
- MAPK
mitogen-activated protein kinase
- RANKL
receptor activator of NF-kappa-B ligand
- eNOS
endothelial nitric oxide synthase
- MMP-13
matrix metalloproteinase-13
- TIMP-1
tissue inhibitor of metalloproteinases-1
- FAK
focal adhesion kinase
- RUNX2
runt-related transcription factor 2
References
- Aikawa R, et al. Integrins play a critical role in mechanical stress-induced p38 MAPK activation. Hypertension. 2002;39:233–238. doi: 10.1161/hy0202.102699. [DOI] [PubMed] [Google Scholar]
- Alford AI, Jacobs CR, Donahue HJ. Oscillating fluid flow regulates gap junction communication in osteocytic MLO-Y4 cells by an ERK1/2 MAP kinase-dependent mechanism. Bone. 2003;33:64–70. doi: 10.1016/s8756-3282(03)00167-4. [DOI] [PubMed] [Google Scholar]
- Anderson RG. The caveolae membrane system. Annu. Rev. Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- Armour KE, et al. Defective bone formation and anabolic response to exogenous estrogen in mice with targeted disruption of endothelial nitric oxide synthase. Endocrinology. 2001;142:760–766. doi: 10.1210/endo.142.2.7977. [DOI] [PubMed] [Google Scholar]
- Baldwin KM, et al. Musculoskeletal adaptations to weightlessness and development of effective countermeasures. Med. Sci. Sports Exerc. 1996;28:1247–1253. doi: 10.1097/00005768-199610000-00007. [DOI] [PubMed] [Google Scholar]
- Bennett JH, Carter DH, Alavi AL, Beresford JN, Walsh S. Patterns of integrin expression in a human mandibular explant model of osteoblast differentiation. Arch. Oral. Biol. 2001;46:229–238. doi: 10.1016/s0003-9969(00)00114-x. [DOI] [PubMed] [Google Scholar]
- Berk BC, Corson MA, Peterson TE, Tseng H. Protein kinases as mediators of fluid shear stress stimulated signal transduction in endothelial cells: a hypothesis for calcium-dependent and calcium-independent events activated by flow. J. Biomech. 1995;28:1439–1450. doi: 10.1016/0021-9290(95)00092-5. [DOI] [PubMed] [Google Scholar]
- Bockholt SM, Burridge K. Cell spreading on extracellular matrix proteins induces tyrosine phosphorylation of tensin. J. Biol. Chem. 1993;268:14565–14567. [PubMed] [Google Scholar]
- Boo YC, Jo H. Flow-dependent regulation of endothelial nitric oxide synthase: role of protein kinases. Am. J. Physiol. Cell. Physiol. 2003;285:C499–C508. doi: 10.1152/ajpcell.00122.2003. [DOI] [PubMed] [Google Scholar]
- Boutahar N, Guignandon A, Vico L, Lafage-Proust MH. Mechanical strain on osteoblasts activates autophosphorylation of focal adhesion kinase and proline-rich tyrosine kinase 2 tyrosine sites involved in ERK activation. J. Biol. Chem. 2004;279:30588–30599. doi: 10.1074/jbc.M313244200. [DOI] [PubMed] [Google Scholar]
- Boyd NL, et al. Chronic shear induces caveolae formation and alters ERK and Akt responses in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H1113–H1122. doi: 10.1152/ajpheart.00302.2003. [DOI] [PubMed] [Google Scholar]
- Brown TD, Pedersen DR, Gray ML, Brand RA, Rubin CT. Toward an identification of mechanical parameters initiating periosteal remodeling: a combined experimental and analytic approach. J. Biomech. 1990;23:893–905. doi: 10.1016/0021-9290(90)90354-6. [DOI] [PubMed] [Google Scholar]
- Carmeliet G, Vico L, Bouillon R. Space flight: a challenge for normal bone homeostasis. Crit. Rev. Eukaryot. Gene Expr. 2001;11:131–144. [PubMed] [Google Scholar]
- Carvalho RS, Scott JE, Suga DM, Yen EH. Stimulation of signal transduction pathways in osteoblasts by mechanical strain potentiated by parathyroid hormone. J. Bone Miner. Res. 1994;9:999–1011. doi: 10.1002/jbmr.5650090707. [DOI] [PubMed] [Google Scholar]
- Charras GT, Williams BA, Sims SM, Horton MA. Estimating the sensitivity of mechanosensitive ion channels to membrane strain and tension. Biophys. J. 2004;87:2870–2884. doi: 10.1529/biophysj.104.040436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen NX, et al. Ca(2+) regulates fluid shear-induced cytoskeletal reorganization and gene expression in osteoblasts. Am. J. Physiol. Cell Physiol. 2000;278:C989–C997. doi: 10.1152/ajpcell.2000.278.5.C989. [DOI] [PubMed] [Google Scholar]
- Cherian PP, et al. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol. Biol. Cell. 2005 doi: 10.1091/mbc.E04-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien S, Li S, Shyy YJ. Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension. 1998;31:162–169. doi: 10.1161/01.hyp.31.1.162. [DOI] [PubMed] [Google Scholar]
- Cho KA, et al. Senescent phenotype can be reversed by reduction of caveolin status. J. Biol. Chem. 2003;278:27789–27795. doi: 10.1074/jbc.M208105200. [DOI] [PubMed] [Google Scholar]
- Couet J, Li S, Okamoto T, Ikezu T, Lisanti MP. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J. Biol. Chem. 1997;272:6525–6533. doi: 10.1074/jbc.272.10.6525. [DOI] [PubMed] [Google Scholar]
- Cowin SC, Weinbaum S, Zeng Y. A case for bone canaliculi as the anatomical site of strain generated potentials. J. Biomech. 1995;28:1281–1297. doi: 10.1016/0021-9290(95)00058-p. [DOI] [PubMed] [Google Scholar]
- Craig D, Krammer A, Schulten K, Vogel V. Comparison of the early stages of forced unfolding for fibronectin type III modules. Proc. Natl. Acad. Sci. U. S. A. 2001;98:5590–5595. doi: 10.1073/pnas.101582198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassouli A, Sulpice JC, Roux S, Crozatier B. Stretch-induced inositol trisphosphate and tetrakisphosphate production in rat cardiomyocytes. J. Mol. Cell. Cardiol. 1993;25:973–982. doi: 10.1006/jmcc.1993.1109. [DOI] [PubMed] [Google Scholar]
- Davidson RM, Tatakis DW, Auerbach AL. Multiple forms of mechanosensitive ion channels in osteoblast-like cells. Pflugers Arch. 1990;416:646–651. doi: 10.1007/BF00370609. [DOI] [PubMed] [Google Scholar]
- Davis ME, Cai H, Drummond GR, Harrison DG. Shear stress regulates endothelial nitric oxide synthase expression through c-Src by divergent signaling pathways. Circ. Res. 2001;89:1073–1080. doi: 10.1161/hh2301.100806. [DOI] [PubMed] [Google Scholar]
- Dillaman RM, Roer RD, Gay DM. Fluid movement in bone: theoretical and empirical. J. Biomech. 1991;24(Suppl. 1):163–177. doi: 10.1016/0021-9290(91)90386-2. [DOI] [PubMed] [Google Scholar]
- Dogterom M, Yurke B. Measurement of the force-velocity relation for growing microtubules. Science. 1997;278:856–860. doi: 10.1126/science.278.5339.856. [DOI] [PubMed] [Google Scholar]
- Donahue HJ. Gap junctions and biophysical regulation of bone cell differentiation. Bone. 2000;26:417–422. doi: 10.1016/S8756-3282(00)00245-3. [DOI] [PubMed] [Google Scholar]
- Drab M, et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- Duncan RL, Hruska KA. Chronic, intermittent loading alters mechanosensitive channel characteristics in osteoblast-like cells. Am. J. Physiol. 1994;267:F909–F916. doi: 10.1152/ajprenal.1994.267.6.F909. [DOI] [PubMed] [Google Scholar]
- Duncan RL, Hruska KA, Misler S. Parathyroid hormone activation of stretch-activated cation channels in osteosarcoma cells (UMR-106.01) FEBS Lett. 1992;307:219–223. doi: 10.1016/0014-5793(92)80771-8. [DOI] [PubMed] [Google Scholar]
- Duncan RL, Kizer N, Barry EL, Friedman PA, Hruska KA. Antisense oligodeoxynucleotide inhibition of a swelling-activated cation channel in osteoblast-like osteosarcoma cells. Proc. Natl. Acad. Sci. U. S. A. 1996;93:1864–1869. doi: 10.1073/pnas.93.5.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, et al. Nitric oxide regulates RANKL and OPG expression in bone stromal cells. Endocrinology. 2004;145:1–9. doi: 10.1210/en.2003-0726. [DOI] [PubMed] [Google Scholar]
- Ferrier J, Ward A, Kanehisa J, Heersche JN. Electrophysiological responses of osteoclasts to hormones. J. Cell. Physiol. 1986;128:23–26. doi: 10.1002/jcp.1041280105. [DOI] [PubMed] [Google Scholar]
- Gao M, Craig D, Vogel V, Schulten K. Identifying unfolding intermediates of FN-III(10) by steered molecular dynamics. J. Mol. Biol. 2002;323:939–950. doi: 10.1016/s0022-2836(02)01001-x. [DOI] [PubMed] [Google Scholar]
- Genetos DC, Geist DJ, Liu D, Donahue HJ, Duncan RL. Fluid shear-induced ATP secretion mediates prostaglandin release in MC3T3-E1 osteoblasts. J. Bone Miner. Res. 2005;20:41–49. doi: 10.1359/JBMR.041009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerthoffer WT, Gunst SJ. Invited review: focal adhesion and small heat shock proteins in the regulation of actin remodeling and contractility in smooth muscle. J. Appl. Physiol. 2001;91:963–972. doi: 10.1152/jappl.2001.91.2.963. [DOI] [PubMed] [Google Scholar]
- Globus RK, Bikle DD, Morey-Holton E. The temporal response of bone to unloading. Endocrinology. 1986;118:733–742. doi: 10.1210/endo-118-2-733. [DOI] [PubMed] [Google Scholar]
- Gluhak-Heinrich J, et al. Mechanical loading stimulates dentin matrix protein 1 (DMP1) expression in osteocytes in vivo. J. Bone Miner. Res. 2003;18:807–817. doi: 10.1359/jbmr.2003.18.5.807. [DOI] [PubMed] [Google Scholar]
- Gross TS, King KA, Rabaia NA, Pathare P, Srinivasan S. Upregulation of osteopontin by osteocytes deprived of mechanical loading or oxygen. J. Bone Miner. Res. 2005;20:250–256. doi: 10.1359/JBMR.041004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross TS, Rubin CT. Uniformity of resorptive bone loss induced by disuse. J. Orthop. Res. 1995;13:708–714. doi: 10.1002/jor.1100130510. [DOI] [PubMed] [Google Scholar]
- Gross TS, Srinivasan S, Liu CC, Clemens TL, Bain SD. Noninvasive loading of the murine tibia: an in vivo model for the study of mechanotransduction. J. Bone Miner. Res. 2002;17:493–501. doi: 10.1359/jbmr.2002.17.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudi S, et al. Rapid activation of Ras by fluid flow is mediated by Galpha(q) and Gbetagamma subunits of heterotrimeric G proteins in human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2003;23:994–1000. doi: 10.1161/01.ATV.0000073314.51987.84. [DOI] [PubMed] [Google Scholar]
- Guharay F, Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J. Physiol. 1984;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapasalo H, Kontulainen S, Sievanen H, Kannus P, Jarvinen M, Vuori I. Exercise-induced bone gain is due to enlargement in bone size without a change in volumetric bone density: a peripheral quantitative computed tomography study of the upper arms of male tennis players. Bone. 2000;27:351–357. doi: 10.1016/s8756-3282(00)00331-8. [DOI] [PubMed] [Google Scholar]
- Han Y, Cowin SC, Schaffler MB, Weinbaum S. Mechanotransduc-tion and strain amplification in osteocyte cell processes. Proc. Natl. Acad. Sci. U. S. A. 2004;101:16689–16694. doi: 10.1073/pnas.0407429101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrigan TP, Hamilton JJ. Bone strain sensation via transmembrane potential changes in surface osteoblasts: loading rate and microstructural implications. J. Biomech. 1993;26:183–200. doi: 10.1016/0021-9290(93)90048-j. [DOI] [PubMed] [Google Scholar]
- Harter L, Hruska K, Duncan R. Human osteoblast-like cells respond to mechanical strain with increased bone matrix protein production independent of hormonal regulation. Endocrinology. 1995;136:528–535. doi: 10.1210/endo.136.2.7530647. [DOI] [PubMed] [Google Scholar]
- Helfrich MH, Evans DE, Grabowski PS, Pollock JS, Ohshima H, Ralston SH. Expression of nitric oxide synthase isoforms in bone and bone cell cultures. J Bone Miner Res. 1997;12:1108–15. doi: 10.1359/jbmr.1997.12.7.1108. [published erratum appears in J Bone Miner Res 1997 Sep;12(9):1538] [DOI] [PubMed] [Google Scholar]
- Hirakata M, et al. Tyrosine kinase dependent expression of TGF-beta induced by stretch in mesangial cells. Kidney Int. 1997;51:1028–1036. doi: 10.1038/ki.1997.144. [DOI] [PubMed] [Google Scholar]
- Horwitz A, Duggan K, Buck C, Beckerle MC, Burridge K. Interaction of plasma membrane fibronectin receptor with talin—a transmembrane linkage. Nature. 1986;320:531–533. doi: 10.1038/320531a0. [DOI] [PubMed] [Google Scholar]
- Huang M, Gu G, Ferguson EL, Chalfie M. A stomatin-like protein necessary for mechanosensation in C. elegans . Nature. 1995;378:292–295. doi: 10.1038/378292a0. [DOI] [PubMed] [Google Scholar]
- Hughes DE, Salter DM, Dedhar S, Simpson R. Integrin expression in human bone. J. Bone Miner. Res. 1993;8:527–533. doi: 10.1002/jbmr.5650080503. [DOI] [PubMed] [Google Scholar]
- Huiskes R, Ruimerman R, van Lenthe GH, Janssen JD. Effects of mechanical forces on maintenance and adaptation of form in trabecular bone. Nature. 2000;405:704–706. doi: 10.1038/35015116. [DOI] [PubMed] [Google Scholar]
- Hung CT, Pollack SR, Reilly TM, Brighton CT. Real-time calcium response of cultured bone cells to fluid flow. Clin. Orthop. Relat. 1995:256–269. [PubMed] [Google Scholar]
- Inaoka T, et al. Sequential analysis of gene expression after an osteogenic stimulus: c-fos expression is induced in osteocytes. Biochem. Biophys. Res. Commun. 1995;217:264–270. doi: 10.1006/bbrc.1995.2773. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Tensegrity: the architectural basis of cellular mechan-otransduction. Annu. Rev. Physiol. 1997;59:575–599. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- Ingram AJ, Ly H, Thai K, Kang MJ, Scholey JW. Mesangial cell signaling cascades in response to mechanical strain and glucose. Kidney Int. 1999;56:1721–1728. doi: 10.1046/j.1523-1755.1999.00743.x. [DOI] [PubMed] [Google Scholar]
- Inoue D, Kido S, Matsumoto T. Transcriptional induction of fosB/ delta fosB gene by mechanical stress in osteoblasts. J. Biol. Chem. 2004;279:49795–49803. doi: 10.1074/jbc.M404096200. [DOI] [PubMed] [Google Scholar]
- Ishida T, Peterson TE, Kovach NL, Berk BC. MAP kinase activation by flow in endothelial cells. Role of beta 1 integrins and tyrosine kinases. Circ. Res. 1996;79:310–316. doi: 10.1161/01.res.79.2.310. [DOI] [PubMed] [Google Scholar]
- Jacobs CR, Yellowley CE, Davis BR, Zhou Z, Cimbala JM, Donahue HJ. Differential effect of steady versus oscillating flow on bone cells. J. Biomech. 1998;31:969–976. doi: 10.1016/s0021-9290(98)00114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalali S, et al. Shear stress activates p60src–Ras–MAPK signaling pathways in vascular endothelial cells. Arterioscler Thromb. Vasc. Biol. 1998;18:227–234. doi: 10.1161/01.atv.18.2.227. [DOI] [PubMed] [Google Scholar]
- Jessop HL, Rawlinson SC, Pitsillides AA, Lanyon LE. Mechanical strain and fluid movement both activate extracellular regulated kinase (ERK) in osteoblast-like cells but via different signaling pathways. Bone. 2002;31:186–194. doi: 10.1016/s8756-3282(02)00797-4. [DOI] [PubMed] [Google Scholar]
- Jin ZG, Wong C, Wu J, Berk BC. Flow shear stress stimulates Gab1 tyrosine phosphorylation to mediate protein kinase B and endothelial nitric-oxide synthase activation in endothelial cells. J. Biol. Chem. 2005;280:12305–12309. doi: 10.1074/jbc.M500294200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HH, Priest JD, Hayes WC, Tichenor CC, Nagel DA. Humeral hypertrophy in response to exercise. J. Bone Joint Surg. Am. 1977;59:204–208. [PubMed] [Google Scholar]
- Judex S, Garman R, Squire M, Donahue LR, Rubin C. Genetically based influences on the site-specific regulation of trabecular and cortical bone morphology. J. Bone Miner. Res. 2004;19:600–606. doi: 10.1359/JBMR.040101. [DOI] [PubMed] [Google Scholar]
- Judex S, et al. Mechanical modulation of molecular signals which regulate anabolic and catabolic activity in bone tissue. J. Cell. Biochem. 2005;94:982–994. doi: 10.1002/jcb.20363. [DOI] [PubMed] [Google Scholar]
- Kapur S, Baylink DJ, William Lau KH. Fluid flow shear stress stimulates human osteoblast proliferation and differentiation through multiple interacting and competing signal transduction pathways. Bone. 2003;32:241–251. doi: 10.1016/s8756-3282(02)00979-1. [DOI] [PubMed] [Google Scholar]
- Kaspar D, Seidl W, Neidlinger-Wilke C, Beck A, Claes L, Ignatius A. Proliferation of human-derived osteoblast-like cells depends on the cycle number and frequency of uniaxial strain. J. Biomech. 2002;35:873–880. doi: 10.1016/s0021-9290(02)00058-1. [DOI] [PubMed] [Google Scholar]
- Katsumi A, Orr AW, Tzima E, Schwartz MA. Integrins in mechanotransduction. J. Biol. Chem. 2004;279:12001–12004. doi: 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- Kawamura S, Miyamoto S, Brown JH. Initiation and transduction of stretch-induced RhoA and Rac1 activation through caveolae: cytoskeletal regulation of ERK translocation. J. Biol. Chem. 2003;278:31111–31117. doi: 10.1074/jbc.M300725200. [DOI] [PubMed] [Google Scholar]
- Keanini RG, Roer RD, Dillaman RM. A theoretical model of circulatory interstitial fluid flow and species transport within porous cortical bone. J. Biomech. 1995;28:901–914. doi: 10.1016/0021-9290(94)00157-y. [DOI] [PubMed] [Google Scholar]
- Keller RS, et al. Disruption of integrin function in the murine myocardium leads to perinatal lethality, fibrosis, and abnormal cardiac performance. Am. J. Pathol. 2001;158:1079–1090. doi: 10.1016/S0002-9440(10)64055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Jacobs CR. Oscillatory fluid flow induced shear stress suppresses osteoclastogenesis. Trans. Ortho. Res. Soc. 2005;30:912. [Google Scholar]
- Kizer N, Guo XL, Hruska K. Reconstitution of stretch-activated cation channels by expression of the alpha-subunit of the epithelial sodium channel cloned from osteoblasts. Proc. Natl. Acad. Sci. U. S. A. 1997;94:1013–1018. doi: 10.1073/pnas.94.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Nulend J, Veldhuijzen JP, de Jong M, Burger EH. Increased bone formation and decreased bone resorption in fetal mouse calvaria as a result of intermittent compressive force in vitro. Bone Miner. 1987;2:441–448. [PubMed] [Google Scholar]
- Klein-Nulend J, et al. Sensitivity of osteocytes to biomechanical stress in vitro. FASEB J. 1995;9:441–445. doi: 10.1096/fasebj.9.5.7896017. [DOI] [PubMed] [Google Scholar]
- Klein-Nulend J, et al. Nitric oxide response to shear stress by human bone cell cultures is endothelial nitric oxide synthase dependent. Biochem. Biophys. Res. Commun. 1998;250:108–114. doi: 10.1006/bbrc.1998.9270. [DOI] [PubMed] [Google Scholar]
- Klein RF, et al. Regulation of bone mass in mice by the lipoxygenase gene Alox15. Science. 2004;303:229–232. doi: 10.1126/science.1090985. [DOI] [PubMed] [Google Scholar]
- Knudsen HL, Frangos JA. Role of cytoskeleton in shear stress-induced endothelial nitric oxide production. Am. J. Physiol. 1997;273:H347–H355. doi: 10.1152/ajpheart.1997.273.1.H347. [DOI] [PubMed] [Google Scholar]
- Kontulainen S, Sievanen H, Kannus P, Pasanen M, Vuori I. Effect of long-term impact-loading on mass, size, and estimated strength of humerus and radius of female racquet-sports players: a peripheral quantitative computed tomography study between young and old starters and controls. J. Bone Miner. Res. 2003;18:352–359. doi: 10.1359/jbmr.2003.18.2.352. [DOI] [PubMed] [Google Scholar]
- Kranenburg O, Verlaan I, Moolenaar WH. Regulating c-Ras function. Cholesterol depletion affects caveolin association, GTP loading, and signaling. Curr. Biol. 2001;11:1880–1884. doi: 10.1016/s0960-9822(01)00582-6. [DOI] [PubMed] [Google Scholar]
- Kufahl RH, Saha S. A theoretical model for stress-generated fluid flow in the canaliculi-lacunae network in bone tissue. J. Biomech. 1990;23:171–180. doi: 10.1016/0021-9290(90)90350-c. [DOI] [PubMed] [Google Scholar]
- Kulik TJ, Alvarado SP. Effect of stretch on growth and collagen synthesis in cultured rat and lamb pulmonary arterial smooth muscle cells. J. Cell. Physiol. 1993;157:615–624. doi: 10.1002/jcp.1041570322. [DOI] [PubMed] [Google Scholar]
- Kunnel JG, Igarashi K, Gilbert JL, Stern PH. Bone anabolic responses to mechanical load in vitro involve COX-2 and constitutive NOS. Connect Tissue Res. 2004;45:40–49. doi: 10.1080/03008200490278133. [DOI] [PubMed] [Google Scholar]
- Lane P, Gross SS. Cell signaling by nitric oxide. Semin. Nephrol. 1999;19:215–229. [PubMed] [Google Scholar]
- Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J. Bone Miner. Res. 2004;19:1006–1012. doi: 10.1359/JBMR.040307. [DOI] [PubMed] [Google Scholar]
- Lanyon LE, Rubin CT. Static vs. dynamic loads as an influence on bone remodelling. J. Biomech. 1984;17:897–905. doi: 10.1016/0021-9290(84)90003-4. [DOI] [PubMed] [Google Scholar]
- Lanyon LE, Goodship AE, Pye CJ, MacFie JH. Mechanically adaptive bone remodelling. J. Biomech. 1982;15:141–154. doi: 10.1016/0021-9290(82)90246-9. [DOI] [PubMed] [Google Scholar]
- Lavandero S, et al. Changes in cyclic AMP dependent protein kinase and active stiffness in the rat volume overload model of heart hypertrophy. Cardiovasc. Res. 1993;27:1634–1638. doi: 10.1093/cvr/27.9.1634. [DOI] [PubMed] [Google Scholar]
- Leblanc AD, Schneider VS, Evans HJ, Engelbretson DA, Krebs JM. Bone mineral loss and recovery after 17 weeks of bed rest. J. Bone Miner. Res. 1990;5:843–850. doi: 10.1002/jbmr.5650050807. [DOI] [PubMed] [Google Scholar]
- Lee HS, Millward-Sadler SJ, Wright MO, Nuki G, Salter DM. Integrin and mechanosensitive ion channel-dependent tyrosine phosphory-lation of focal adhesion proteins and beta-catenin in human articular chondrocytes after mechanical stimulation. J. Bone Miner. Res. 2000;15:1501–1509. doi: 10.1359/jbmr.2000.15.8.1501. [DOI] [PubMed] [Google Scholar]
- Lee K, Jessop H, Suswillo R, Zaman G, Lanyon L. Endocrinology: bone adaptation requires oestrogen receptor-alpha. Nature. 2003;424:389. doi: 10.1038/424389a. [DOI] [PubMed] [Google Scholar]
- Li S, et al. Fluid shear stress activation of focal adhesion kinase. Linking to mitogen-activated protein kinases. J. Biol. Chem. 1997;272:30455–30462. doi: 10.1074/jbc.272.48.30455. [DOI] [PubMed] [Google Scholar]
- Li C, Hu Y, Mayr M, Xy Q. Cyclic strain stress-induced MAP kinase phosphatase 1 expression in vascular smooth muscle cells is regulated by Ras/Rac-MAPK pathways. J. Biol. Chem. 1999a;274:25273–25380. doi: 10.1074/jbc.274.36.25273. [DOI] [PubMed] [Google Scholar]
- Li S, et al. Distinct roles for the small GTPases Cdc42 and Rho in endothelial responses to shear stress. J. Clin. Invest. 1999b;103:1141–1150. doi: 10.1172/JCI5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Duncan RL, Burr DB, Turner CH. L-type calcium channels mediate mechanically induced bone formation in vivo. J. Bone Miner. Res. 2002;17:1795–1800. doi: 10.1359/jbmr.2002.17.10.1795. [DOI] [PubMed] [Google Scholar]
- Li YJ, et al. Oscillatory fluid flow affects human marrow stromal cell proliferation and differentiation. J. Orthop. Res. 2004;22:1283–1289. doi: 10.1016/j.orthres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Liu S, Calderwood DA, Ginsberg MH. Integrin cytoplasmic domain-binding proteins. J. Cell. Sci. 2000;113(20):3563–3571. doi: 10.1242/jcs.113.20.3563. [DOI] [PubMed] [Google Scholar]
- Lofthouse RA, Davis JR, Frondoza CG, Jinnah RH, Hungerford DS, Hare JM. Identification of caveolae and detection of caveolin in normal human osteoblasts. J. Bone Joint Surg. Br. 2001;83:124–129. doi: 10.1302/0301-620x.83b1.10604. [DOI] [PubMed] [Google Scholar]
- MacKelvie KJ, Khan KM, Petit MA, Janssen PA, McKay HA. A school-based exercise intervention elicits substantial bone health benefits: a 2-year randomized controlled trial in girls. Pediatrics. 2003;112:e447. doi: 10.1542/peds.112.6.e447. [DOI] [PubMed] [Google Scholar]
- McAllister TN, Frangos JA. Steady and transient fluid shear stress stimulate NO release in osteoblasts through distinct biochemical pathways. J. Bone Miner. Res. 1999;14:930–936. doi: 10.1359/jbmr.1999.14.6.930. [DOI] [PubMed] [Google Scholar]
- Millward-Sadler SJ, Salter DM. Integrin-dependent signal cascades in chondrocyte mechanotransduction. Ann. Biomed. Eng. 2004;32:435–446. doi: 10.1023/b:abme.0000017538.72511.48. [DOI] [PubMed] [Google Scholar]
- Mizuno S. A novel method for assessing effects of hydrostatic fluid pressure on intracellular calcium: a study with bovine articular chondrocytes. Am. J. Physiol. Cell. Physiol. 2005;288:C329–C337. doi: 10.1152/ajpcell.00131.2004. [DOI] [PubMed] [Google Scholar]
- Morris CE. Mechanosensitive ion channels. J. Membr. Biol. 1990;113:93–107. doi: 10.1007/BF01872883. [DOI] [PubMed] [Google Scholar]
- Mosley JR, March BM, Lynch J, Lanyon LE. Strain magnitude related changes in whole bone architecture in growing rats. Bone. 1997;20:191–198. doi: 10.1016/s8756-3282(96)00385-7. [DOI] [PubMed] [Google Scholar]
- Nicolella DP, Moravits DE, Gale AM, Bonewald LF, Lankford J. Osteocyte lacunae tissue strain in cortical bone. J Biomech. in press doi: 10.1016/j.jbiomech.2005.04.032. (2005 Jun 30; Electronic publication ahead of print; PMID: 15993413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norvell SM, Alvarez M, Bidwell JP, Pavalko FM. Fluid shear stress induces beta-catenin signaling in osteoblasts. Calcif. Tissue Int. 2004;75:396–404. doi: 10.1007/s00223-004-0213-y. [DOI] [PubMed] [Google Scholar]
- O'Connor JA, Lanyon LE, MacFie H. The influence of strain rate on adaptive bone remodelling. J. Biomech. 1982;15:767–781. doi: 10.1016/0021-9290(82)90092-6. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J. Biol. Chem. 1998;273:5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- Orr AW, Murphy-Ullrich JE. Regulation of endothelial cell function BY FAK and PYK2. Front Biosci. 2004;9:1254–1266. doi: 10.2741/1239. [DOI] [PubMed] [Google Scholar]
- Owan I, et al. Mechanotransduction in bone: osteoblasts are more responsive to fluid forces than mechanical strain. Am. J. Physiol. 1997;273:C810–C815. doi: 10.1152/ajpcell.1997.273.3.C810. [DOI] [PubMed] [Google Scholar]
- Ozawa H, et al. Effect of a continuously applied compressive pressure on mouse osteoblast-like cells (MC3T3-E1) in vitro. J. Cell. Physiol. 1990;142:177–185. doi: 10.1002/jcp.1041420122. [DOI] [PubMed] [Google Scholar]
- Park H, et al. Plasma membrane cholesterol is a key molecule in shear stress-dependent activation of extracellular signal-regulated kinase. J. Biol. Chem. 1998;273:32304–32311. doi: 10.1074/jbc.273.48.32304. [DOI] [PubMed] [Google Scholar]
- Piekarski K, Munro M. Transport mechanism operating between blood supply and osteocytes in long bones. Nature. 1977;269:80–82. doi: 10.1038/269080a0. [DOI] [PubMed] [Google Scholar]
- Plotkin LI, Mathov I, Aguirre JI, Parfitt AM, Manolagas SC, Bellido T. Mechanical stimulation prevents osteocyte apoptosis: requirement of Integrins, Src kinases and ERKs. Am. J. Physiol., Cell Physiol. 2005 doi: 10.1152/ajpcell.00278.2004. [DOI] [PubMed] [Google Scholar]
- Qin YX, Rubin CT, McLeod KJ. Nonlinear dependence of loading intensity and cycle number in the maintenance of bone mass and morphology. J. Orthop. Res. 1998;16:482–489. doi: 10.1002/jor.1100160414. [DOI] [PubMed] [Google Scholar]
- Ralston SH. The Michael Mason Prize Essay 1997. Nitric oxide and bone: what a gas! Br. J. Rheumatol. 1997;36:831–838. doi: 10.1093/rheumatology/36.8.831. [DOI] [PubMed] [Google Scholar]
- Rawlinson SC, Pitsillides AA, Lanyon LE. Involvement of different ion channels in osteoblasts' and osteocytes' early responses to mechanical strain. Bone. 1996;19:609–614. doi: 10.1016/s8756-3282(96)00260-8. [DOI] [PubMed] [Google Scholar]
- Rawlinson SC, Zaman G, Mosley JR, Pitsillides AA, Lanyon LE. Heme oxygenase isozymes in bone: induction of HO-1 mRNA following physiological levels of mechanical loading in vivo. Bone. 1998;23:433–436. doi: 10.1016/s8756-3282(98)00125-2. [DOI] [PubMed] [Google Scholar]
- Reich KM, Gay CV, Frangos JA. Fluid shear stress as a mediator of osteoblast cyclic adenosine monophosphate production. J. Cell. Physiol. 1990;143:100–104. doi: 10.1002/jcp.1041430113. [DOI] [PubMed] [Google Scholar]
- Rezzonico R, et al. Focal adhesion kinase pp125FAK interacts with the large conductance calcium-activated hSlo potassium channel in human osteoblasts: potential role in mechanotransduction. J. Bone Miner. Res. 2003;18:1863–1871. doi: 10.1359/jbmr.2003.18.10.1863. [DOI] [PubMed] [Google Scholar]
- Rizzo V, Sung A, Oh P, Schnitzer JE. Rapid mechanotransduction in situ at the luminal cell surface of vascular endothelium and its caveolae. J. Biol. Chem. 1998;273:26323–26329. doi: 10.1074/jbc.273.41.26323. [DOI] [PubMed] [Google Scholar]
- Rubin C, Lanyon LE. Regulation of bone formation by applied dynamic loads. J. Bone Jt. Surg. 1984a;66A:397–402. [PubMed] [Google Scholar]
- Rubin CT, Lanyon LE. Dynamic strain similarity in vertebrates: an alternative to allometric limb bone scaling. J. Theor. Biol. 1984b;107:321–327. doi: 10.1016/s0022-5193(84)80031-4. [DOI] [PubMed] [Google Scholar]
- Rubin C, Lanyon LE. Regulation of bone mass by mechanical strain magnitude. Calcif. Tissue Int. 1985;37:411–417. doi: 10.1007/BF02553711. [DOI] [PubMed] [Google Scholar]
- Rubin CT, McLeod KJ. Promotion of bony ingrowth by frequency-specific, low-amplitude mechanical strain. Clin. Orthop. Relat. Res. 1994;298:165–174. [PubMed] [Google Scholar]
- Rubin C, Gross T, Qin YX, Fritton S, Guilak F, McLeod K. Differentiation of the bone-tissue remodeling response to axial and torsional loading in the turkey ulna. J. Bone Jt. Surg., Am. 1996;78:1523–1533. doi: 10.2106/00004623-199610000-00010. [DOI] [PubMed] [Google Scholar]
- Rubin C, Turner AS, Bain S, Mallinckrodt C, McLeod K. Ana-bolism. Low mechanical signals strengthen long bones. Nature. 2001a;412:603–604. doi: 10.1038/35088122. [DOI] [PubMed] [Google Scholar]
- Rubin C, Xu G, Judex S. The anabolic activity of bone tissue, suppressed by disuse, is normalized by brief exposure to extremely low-magnitude mechanical stimuli. Faseb. J. 2001b;15:2225–2229. doi: 10.1096/fj.01-0166com. [DOI] [PubMed] [Google Scholar]
- Rubin C, Judex S, Hadjiargyrou M. Skeletal adaptation to mechanical stimuli in the absence of formation or resorption of bone. J. Musculoskelet. Neuronal Interact. 2002a;2:264–267. [PubMed] [Google Scholar]
- Rubin C, et al. Quantity and quality of trabecular bone in the femur are enhanced by a strongly anabolic, noninvasive mechanical intervention. J. Bone Miner. Res. 2002b;17:349–357. doi: 10.1359/jbmr.2002.17.2.349. [DOI] [PubMed] [Google Scholar]
- Rubin J, Biskobing D, Fan X, Rubin C, McLeod K, Taylor WR. Pressure regulates osteoclast formation and MCSF expression in marrow culture. J. Cell. Physiol. 1997;170:81–87. doi: 10.1002/(SICI)1097-4652(199701)170:1<81::AID-JCP9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Rubin J, Fan X, Biskobing D, Taylor W, Rubin C. Osteoclastogen-esis is repressed by mechanical strain in an in vitro model. J. Orthop. Res. 1999;17:639–645. doi: 10.1002/jor.1100170504. [DOI] [PubMed] [Google Scholar]
- Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, McLeod K. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J. Bone Miner. Res. 2004;19:343–351. doi: 10.1359/JBMR.0301251. [DOI] [PubMed] [Google Scholar]
- Rubin J, Murphy T, Fan X, Goldschmidt M, Taylor W. Mechanical strain inhibits RANKL expression through activation of ERK1/2 in bone marrow stromal cells. J. Bone Miner. Res. 2002c;17:1452–1460. doi: 10.1359/jbmr.2002.17.8.1452. [DOI] [PubMed] [Google Scholar]
- Rubin J, Murphy TC, Zhu L, Roy E, Nanes MS, Fan X. Mechanical strain differentially regulates endothelial nitric-oxide synthase and receptor activator of nuclear kappa B ligand expression via ERK1/2 MAPK. J. Biol. Chem. 2003;278:34018–34025. doi: 10.1074/jbc.M302822200. [DOI] [PubMed] [Google Scholar]
- Ryder KD, Duncan RL. Parathyroid hormone enhances fluid shear-induced [Ca2+]i signaling in osteoblastic cells through activation of mechanosensitive and voltage-sensitive Ca2+ channels. J. Bone Miner. Res. 2001;16:240–248. doi: 10.1359/jbmr.2001.16.2.240. [DOI] [PubMed] [Google Scholar]
- Salter DM, Robb JE, Wright MO. Electrophysiological responses of human bone cells to mechanical stimulation: evidence for specific integrin function in mechanotransduction. J. Bone Miner. Res. 1997;12:1133–1141. doi: 10.1359/jbmr.1997.12.7.1133. [DOI] [PubMed] [Google Scholar]
- Saunders MM, et al. Gap junctions and fluid flow response in MC3T3-E1 cells. Am. J. Physiol,. Cell Physiol. 2001;281:C1917–C1925. doi: 10.1152/ajpcell.2001.281.6.C1917. [DOI] [PubMed] [Google Scholar]
- Saunders MM, et al. Fluid flow-induced prostaglandin E2 response of osteoblastic ROS 17/2.8 cells is gap junction-mediated and independent of cytosolic calcium. Bone. 2003;32:350–356. doi: 10.1016/s8756-3282(03)00025-5. [DOI] [PubMed] [Google Scholar]
- Schaller MD. The focal adhesion kinase. J. Endocrinol. 1996;150:1–7. doi: 10.1677/joe.0.1500001. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Broome MA, Hunter T. Fibronectin-stimulated signaling from a focal adhesion kinase-c-Src complex: involvement of the Grb2, p130cas, and Nck adaptor proteins. Mol. Cell. Biol. 1997;17:1702–1713. doi: 10.1128/mcb.17.3.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer DD, Jones KC, Hunter T. Multiple Grb2-mediated integrin-stimulated signaling pathways to ERK2/mitogen-activated protein kinase: summation of both c-Src- and focal adhesion kinase-initiated tyrosine phosphorylation events. Mol. Cell. Biol. 1998;18:2571–2585. doi: 10.1128/mcb.18.5.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Prog. Biophys. Mol. Biol. 1999;71:435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- Shyy JY, Chien S. Role of integrins in endothelial mechanosensing of shear stress. Circ. Res. 2002;91:769–775. doi: 10.1161/01.res.0000038487.19924.18. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell. Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Skerry TM, Bitensky L, Chayen J, Lanyon LE. Early strain-related changes in enzyme activity in osteocytes following bone loading in vivo. J. Bone Miner. Res. 1989;4:783–788. doi: 10.1002/jbmr.5650040519. [DOI] [PubMed] [Google Scholar]
- Smalt R, Mitchell F, Howard R, Chambers T. Induction of NO and prostaglandin E2 in osteoblasts by wall-shear stress but not mechanical strain. Am. J. Phys. 1997a;273:E751–E758. doi: 10.1152/ajpendo.1997.273.4.E751. [DOI] [PubMed] [Google Scholar]
- Smalt R, Mitchell FT, Howard RL, Chambers TJ. Mechano-transduction in bone cells: induction of nitric oxide and prostaglandin synthesis by fluid shear stress, but not by mechanical strain. Adv. Exp. Med. Biol. 1997b;433:311–314. doi: 10.1007/978-1-4899-1810-9_66. [DOI] [PubMed] [Google Scholar]
- Solomon KR, Adolphson LD, Wank DA, McHugh KP, Hauschka PV. Caveolae in human and murine osteoblasts. J. Bone Miner. Res. 2000a;15:2391–2401. doi: 10.1359/jbmr.2000.15.12.2391. [DOI] [PubMed] [Google Scholar]
- Solomon KR, Danciu TE, Adolphson LD, Hecht LE, Hauschka PV. Caveolin-enriched membrane signaling complexes in human and murine osteoblasts. J. Bone Miner. Res. 2000b;15:2380–2390. doi: 10.1359/jbmr.2000.15.12.2380. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Weimer DA, Agans SC, Bain SD, Gross TS. Low-magnitude mechanical loading becomes osteogenic when rest is inserted between each load cycle. J. Bone Miner. Res. 2002;17:1613–1620. doi: 10.1359/jbmr.2002.17.9.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart GW. Hemolytic disease due to membrane ion channel disorders. Curr. Opin. Hematol. 2004;11:244–250. doi: 10.1097/01.moh.0000132240.20671.33. [DOI] [PubMed] [Google Scholar]
- Subauste MC, Pertz O, Adamson ED, Turner CE, Junger S, Hahn KM. Vinculin modulation of paxillin-FAK interactions regulates ERK to control survival and motility. J. Cell. Biol. 2004;165:371–381. doi: 10.1083/jcb.200308011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukharev S, Corey DP. Mechanosensitive channels: multiplicity of families and gating paradigms. Sci STKE. 2004 doi: 10.1126/stke.2192004re4. 2004 re4 (online publ) [DOI] [PubMed] [Google Scholar]
- Sukharev SI, Blount P, Martinac B, Kung C. Mechanosensitive channels of Escherichia coli: the MscL gene, protein, and activities. Annu. Rev. Physiol. 1997;59:633–657. doi: 10.1146/annurev.physiol.59.1.633. [DOI] [PubMed] [Google Scholar]
- Sun YQ, McLeod KJ, Rubin CT. Mechanically induced periosteal bone formation is paralleled by the upregulation of collagen type one mRNA in osteocytes as measured by in situ reverse transcript-polymerase chain reaction. Calcif. Tissue Int. 1995;57:456–462. doi: 10.1007/BF00301950. [DOI] [PubMed] [Google Scholar]
- Swan CC, Lakes RS, Brand RA, Stewart KJ. Micromechanically based poroelastic modeling of fluid flow in Haversian bone. J. Biomech. Eng. 2003;125:25–37. doi: 10.1115/1.1535191. [DOI] [PubMed] [Google Scholar]
- Tadokoro S, et al. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 2003;302:103–106. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- Tavernarakis N, Driscoll M. Molecular modeling of mechanotransduc-tion in the nematode Caenorhabditis elegans . Annu. Rev. Physiol. 1997;59:659–689. doi: 10.1146/annurev.physiol.59.1.659. [DOI] [PubMed] [Google Scholar]
- Toma CD, Ashkar S, Gray ML, Schaffer JL, Gerstenfeld LC. Signal transduction of mechanical stimuli is dependent on microfilament integrity: identification of osteopontin as a mechanically induced gene in osteoblasts. J. Bone Miner. Res. 1997;12:1626–1636. doi: 10.1359/jbmr.1997.12.10.1626. [DOI] [PubMed] [Google Scholar]
- Turner CH, Takano Y, Owan I, Murrell GA. Nitric oxide inhibitor L-NAME suppresses mechanically induced bone formation in rats. Am. J. Physiol. 1996;270:E634–E639. doi: 10.1152/ajpendo.1996.270.4.E634. [DOI] [PubMed] [Google Scholar]
- Vander Molen MA, Donahue HJ, Rubin CT, McLeod KJ. Osteoblastic networks with deficient coupling: differential effects of magnetic and electric field exposure. Bone. 2000;27:227–231. doi: 10.1016/s8756-3282(00)00315-x. [DOI] [PubMed] [Google Scholar]
- Vander Molen MA, Rubin CT, McLeod KJ, McCauley LK, Donahue HJ. Gap junctional intercellular communication contributes to hormonal responsiveness in osteoblastic networks. J. Biol. Chem. 1996;271:12165–12171. doi: 10.1074/jbc.271.21.12165. [DOI] [PubMed] [Google Scholar]
- Vico L, et al. Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet. 2000;355:1607–1611. doi: 10.1016/s0140-6736(00)02217-0. [DOI] [PubMed] [Google Scholar]
- Wang N, et al. Mechanical behavior in living cells consistent with the tensegrity model. Proc. Natl. Acad. Sci. U. S. A. 2001;98:7765–7770. doi: 10.1073/pnas.141199598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward K, Alsop C, Caulton J, Rubin C, Adams J, Mughal Z. Low magnitude mechanical loading is osteogenic in children with disabling conditions. J. Bone Miner. Res. 2004;19:360–369. doi: 10.1359/JBMR.040129. [DOI] [PubMed] [Google Scholar]
- Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J. Biomech. 1994;27:339–360. doi: 10.1016/0021-9290(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Weyts FA, Li YS, van Leeuwen J, Weinans H, Chien S. ERK activation and alpha v beta 3 integrin signaling through Shc recruitment in response to mechanical stimulation in human osteoblasts. J. Cell. Biochem. 2002;87:85–92. doi: 10.1002/jcb.10278. [DOI] [PubMed] [Google Scholar]
- Whedon G. Disuse osteoporosis: physiologic aspects. Calcif. Tissue Int. 1984;36:S146–S150. doi: 10.1007/BF02406148. [DOI] [PubMed] [Google Scholar]
- Wiltink A, Nijweide PJ, Scheenen WJ, Ypey DL, Van Duijn B. Cell membrane stretch in osteoclasts triggers a self-reinforcing Ca2+ entry pathway. Pflugers Arch. 1995;429:663–671. doi: 10.1007/BF00373987. [DOI] [PubMed] [Google Scholar]
- Wong M, Siegrist M, Goodwin K. Cyclic tensile strain and cyclic hydrostatic pressure differentially regulate expression of hypertrophic markers in primary chondrocytes. Bone. 2003;33:685–693. doi: 10.1016/s8756-3282(03)00242-4. [DOI] [PubMed] [Google Scholar]
- Wozniak M, Fausto A, Carron CP, Meyer DM, Hruska KA. Mechanically strained cells of the osteoblast lineage organize their extracellular matrix through unique sites of alphavbeta3-integrin expression. J. Bone Miner. Res. 2000;15:1731–1745. doi: 10.1359/jbmr.2000.15.9.1731. [DOI] [PubMed] [Google Scholar]
- Wung BS, Cheng JJ, Chao YJ, Hsieh HJ, Wang DL. Modulation of Ras/Raf/extracellular signal-regulated kinase pathway by reactive oxygen species is involved in cyclic strain-induced early growth response-1 gene expression in endothelial cells. Circ. Res. 1999;84:804–812. doi: 10.1161/01.res.84.7.804. [DOI] [PubMed] [Google Scholar]
- Yan C, Takahashi M, Okuda M, Lee JD, Berk BC. Fluid shear stress stimulates big mitogen-activated protein kinase 1 (BMK1) activity in endothelial cells. Dependence on tyrosine kinases and intracellular calcium. J. Biol. Chem. 1999;274:143–150. doi: 10.1074/jbc.274.1.143. [DOI] [PubMed] [Google Scholar]
- Yang CM, Chien CS, Yao CC, Hsiao LD, Huang YC, Wu CB. Mechanical strain induces collagenase-3 (MMP-13) expression in MC3T3-E1 osteoblastic cells. J. Biol. Chem. 2004;279:22158–22165. doi: 10.1074/jbc.M401343200. [DOI] [PubMed] [Google Scholar]
- Yellowley CE, Li Z, Zhou Z, Jacobs CR, Donahue HJ. Functional gap junctions between osteocytic and osteoblastic cells. J. Bone Miner. Res. 2000;15:209–217. doi: 10.1359/jbmr.2000.15.2.209. [DOI] [PubMed] [Google Scholar]
- You J, et al. Osteopontin gene regulation by oscillatory fluid flow via intracellular calcium mobilization and activation of mitogen-activated protein kinase in MC3T3-E1 osteoblasts. J. Biol. Chem. 2001;276:13365–13371. doi: 10.1074/jbc.M009846200. [DOI] [PubMed] [Google Scholar]
- Zaman G, et al. Mechanical strain stimulates nitric oxide production by rapid activation of endothelial nitric oxide synthase in osteocytes. J. Bone Miner. Res. 1999;14:1123–1131. doi: 10.1359/jbmr.1999.14.7.1123. [DOI] [PubMed] [Google Scholar]
- Zeidan A, Broman J, Hellstrand P, Sward K. Cholesterol dependence of vascular ERK1/2 activation and growth in response to stretch: role of endothelin-1. Arterioscle. Thromb. Vasc. Biol. 2003;23:1528–1534. doi: 10.1161/01.ATV.0000090129.75275.C2. [DOI] [PubMed] [Google Scholar]
- Zhuang H, Wang W, Tahernia AD, Levitz CL, Luchetti WT, Brighton CT. Mechanical strain-induced proliferation of osteoblastic cells parallels increased TGF-beta 1 mRNA. Biochem. Biophys. Res. Commun. 1996;229:449–453. doi: 10.1006/bbrc.1996.1824. [DOI] [PubMed] [Google Scholar]
- Ziambaras K, Lecanda F, Steinberg TH, Civitelli R. Cyclic stretch enhances gap junctional communication between osteoblastic cells. J. Bone Miner. Res. 1998;13:218–228. doi: 10.1359/jbmr.1998.13.2.218. [DOI] [PubMed] [Google Scholar]
- Zimmerman D, Jin F, Leboy P, Hardy S, Damsky C. Impaired bone formation in transgenic mice resulting from altered integrin function in osteoblasts. Dev. Biol. 2000;220:2–15. doi: 10.1006/dbio.2000.9633. [DOI] [PubMed] [Google Scholar]