Abstract
Rationale
Alcohol use is often implicated in initial lapses to smoking during quit smoking attempts. Mechanisms explaining this association are unknown but could include (a) learned associations between drinking and smoking or (b) direct pharmacologic effects of alcohol.
Objectives
In a 2 (Told Alcohol vs. Told Placebo) × 2 (0.4g/kg vs. 0.0 g/kg ethanol) between-subjects balanced-placebo design, we examined instruction and beverage condition effects on smokers’ ability to resist initiating smoking and whether these effects differed by sex.
Methods
Participants were 96 heavy alcohol drinkers, smoking 10–30 cigarettes per day. After 15 hours of smoking abstinence, participants consumed either an alcoholic or a non-alcoholic beverage and 35 minutes later completed a smoking lapse task.
Results
Overall, neither instructions nor beverage contents influenced behavior on the smoking lapse task. However, the instruction condition had different effects in men and women. Women, but not men, were more likely to smoke and reported expecting greater satisfaction from smoking when they were Told Alcohol compared to Told Placebo. The effects of instruction condition on smoking behavior were not mediated by self-reported expected satisfaction from smoking.
Conclusions
Women may be more likely to choose to smoke after drinking moderate amounts of alcohol because of their expectations rather than the pharmacological effects of the alcohol.
Keywords: Alcohol, smoking relapse, nicotine, balanced placebo design, craving, alcohol administration, pharmacologic effects, expectancy effects
Introduction
Cigarette smokers tend to consume more alcohol than nonsmokers (Anthony et al. 2000; Chiolero et al. 2006; Dawson 2000; Falk et al. 2006; Kahler et al. 2008). Greater alcohol use decreases the odds of smoking cessation in community samples (Augustson et al. 2008; Dawson 2000; Dollar et al. 2009; Hymowitz et al. 1997; Kahler et al. 2009; Osler et al. 1999; Sorlie et al. 1990) and in samples of smokers trying to quit (Humfleet et al. 1999; Leeman et al. 2008; Murray et al. 1995; Sherman et al. 1996; Smith et al. 1999). Approximately one quarter of initial smoking incidents following a quit attempt (lapses) occur in contexts involving alcohol use (Baer et al. 1988; Borland 1990; Shiffman 1982), and ecological assessment data indicate a temporal link between drinking and lapsing (Gwaltney et al. 2005; Shiffman et al. 2008; Shiffman et al. 1996). Among heavy drinkers, the risk of a smoking lapse occurring on days when light to moderate drinking occurs (1–3 drinks for women, 1–4 drinks for men) is more than 4 times greater than the risk of lapsing on a non-drinking day, and heavy drinking doubles the risk of lapse compared to moderate drinking (Kahler et al. 2010). However, the mechanisms accounting for such effects are not well understood.
A number of laboratory studies have examined the effects of alcohol compared to placebo on urge to smoke, smoking behavior, and smoking lapse behavior. These studies indicate that alcohol consumption increases urges to smoke (Burton et al. 1997; Kouri et al. 2004; Sayette et al. 2005) and increases smoking (Glautier et al. 1996; Griffiths et al. 1976; Henningfield et al. 1983; 1984; Mintz et al. 1985; Nil et al. 1984). Furthermore, a laboratory analogue study for smoking lapse found that a priming dose of alcohol (target BAC of 0.03 g/dl) compared to placebo, decreased time to initiating smoking when monetary reinforcement for delaying smoking was provided (McKee et al. 2006). However, these studies have not directly addressed the extent to which alcohol’s effects on smoking are due to its direct pharmacologic effects or to stimulus expectancy effects
Evidence does suggest that alcohol affects smoking, in part, through direct pharmacologic effects. For example, alcohol increases craving for stimulating properties of smoking in a dose dependent manner during both the ascending and descending limbs of the BAC curve (Epstein et al. 2007; King and Epstein, 2005). Furthermore, nicotine compared to placebo enhances alcohol’s subjective and stimulating effects and attenuates its sedating effects (Kouri et al. 2004; Perkins et al. 1995; Rose et al. 2002; see also Acheson et al. 2006). Finally, alcohol compared to placebo more strongly increases satisfaction with smoking when individuals smoke nicotine-containing cigarettes compared to denicotinized cigarettes (Rose et al. 2002; 2004).
Evidence also suggests that learned associations between alcohol consumption and smoking may contribute to the ability of alcohol to facilitate smoking lapses. Because smoking is more likely to occur when smokers are drinking compared to when they are not (Shiffman et al. 1994; Shiffman et al. 2002), drinking alcohol may activate expectations that smoking also will occur and that it will be especially reinforcing based on prior experiences of enhanced reward from smoking while drinking. Indeed, two studies found that providing placebo beverage with cues and verbal instructions that the beverage contains alcohol significantly increased craving for cigarettes and subjective effects of smoking (McKee et al. 2010; Sayette et al. 2005).
No studies to date have fully disaggregated pharmacologic vs. expectancy effects of alcohol on urge to smoke and smoking lapse. To do so requires a balanced placebo design (BPD) in which participants are assigned in a 2 × 2 design (a) to be given a beverage that contains either alcohol or placebo [beverage] and (b) to be told they are receiving either alcohol or placebo [instruction] (Marlatt and Rohsenow, 1980). Analyzing simultaneously the main effects of beverage condition and instruction condition allows for an unconfounded measure of each effect controlling for the other and could provide valuable information on the mechanisms through which alcohol consumption affects smoking.
The BPD also can be used to examine potential sex differences in pharmacologic vs. stimulus expectancy effects of alcohol on smoking. Although alcohol use is associated with smoking lapse and relapse in both men and women (Kahler et al. 2009; Kahler et al. 2010), the mechanisms through which alcohol affects smoking may differ by sex. In women, smoking is generally more dependent on non-pharmacologic factors than in men (Perkins 2009; Perkins et al. 2006), while alcohol (0.8 g/kg) increases smoking to a greater extent in men than women (King et al. 2009). Thus, expectancy effects of alcohol on smoking may be stronger in women compared to men, whereas pharmacologic effects may be stronger for men.
Aims and Hypotheses
The purpose of the present study was to investigate the extent to which the effects of alcohol on smoking initiation during a laboratory analogue task of smoking lapse are due to alcohol’s pharmacologic effects vs. expectancy effects (i.e., the belief that alcohol was administered). Specifically, we hypothesized that both receiving alcohol and expecting that one is drinking alcohol would independently increase risk of initiating smoking. We further hypothesized that expectancy effects of alcohol would be stronger for women than for men whereas the pharmacologic effects of alcohol would be stronger for men than for women. Finally, we hypothesized that both pharmacologic and expectancy effects of alcohol on initiating smoking would be mediated by increases in urge to smoke and expected satisfaction from smoking.
Method
Participants
The study was approved by the Brown University Institutional Review Board. Participants recruited from the community had to meet the following inclusion criteria: 21 to 65 years of age; smoking 10–30 cigarettes a day; a carbon monoxide (CO) level >10 ppm; current heavy drinking (≥ 5 drinks per occasion for men; ≥ 4 drinks for women) at least twice a month; report no history or intention to seek alcohol treatment. Exclusion criteria were: using other tobacco products or nicotine replacement therapy; plan to quit smoking in the next month; incapable of abstaining from alcohol for 24 hours without significant withdrawal symptoms; current affective disorder or psychotic symptoms; current pregnancy or nursing; illicit drug use on more than four occasions in the past four weeks; medical issues or medications contraindicated for alcohol consumption; weighing greater than 250 lbs; prior knowledge about study procedures or contact with study participants.
Potential participants were screened by telephone (N = 1246) before completing a baseline interview, at which they signed informed consent. Participants were informed that the study evaluated effects of alcohol on smoking behavior and that they would be randomly assigned to consume a beverage containing alcohol or a non-alcoholic beverage. Of the 162 potential participants screened, 57 were deemed ineligible at baseline, and 9 dropped out prior to the second session. Results are based on the 96 participants who completed the experimental session.
Design
Participants were randomized to conditions in a 2 × 2 factorial design crossing alcohol administration (beverage condition: Receive Alcohol [a moderate dose of 0.4g/kg, equivalent to about 2 drinks] vs. Receive Placebo) with instructional set (instruction condition: Told Alcohol vs. Told Placebo). Urn randomization (Wei, 1978) balanced conditions on sex, level of nicotine dependence, and drinks consumed per week: (a) Told Alcohol/Received Alcohol (n = 24), (b) Told Alcohol/Received Placebo (n = 25), (c) Told Placebo/Received Alcohol (n = 24), and (d) Told Placebo/Received Placebo (n = 23). Research assistants were blind to the alcohol content of the beverage.
Procedure
At baseline, participants completed a battery of interview and self-report assessments including demographics, diagnostic interview, and smoking and alcohol use questions. They then were scheduled for an experimental session, which was completed, on average, 14.2 (SD = 7.6) days later. Participants were instructed to refrain from drinking alcohol for 24 hours prior to both study sessions. They were instructed to abstain from smoking overnight before the session, not to eat any solid foods within 4 hours, and not to drink any liquids within 2 hours prior to the session. On arrival, compliance was confirmed with a CO reading of less than 50% of their baseline CO level (Odum et al. 2002; Tidey et al. 1999) and a zero breath alcohol concentration (BrAC) per an Alco-Sensor IV (Intoximeters, Inc., St Louis, MO., USA). Experimental sessions occurred in an 80 square-ft ventilated smoking room with a one-way mirror window.
At the start of the experimental session, participants completed measures of urge to smoke and expected satisfaction from smoking. The research assistant then opened a sealed envelope that specified the instruction condition. Participants in the “Told Alcohol” conditions were told, “You have been assigned to the condition to drink an alcoholic beverage. In this condition, we need to test how drinking alcohol will affect your mood, responses, and behavior. Participants in this condition will be compared to others who receive non-alcoholic beverages.” Participants in the “Told Placebo” condition were told, “You have been assigned to the condition to drink a non-alcoholic beverage. In this condition, we need to test how people operate in a normal state of consciousness while engaging in all other actions associated with drinking. Participants in this condition will be compared to others who receive alcohol. Therefore, we will still be taking all of the same measurements as of participants receiving alcohol, which include taking your breath alcohol level, CO, and heart rate levels.”
Alcohol administration began at 15:00. Following established BPD procedures (Rohsenow et al. 1981), beverages were prepared by the study coordinator who randomized participants to the experimental conditions but had no contact with study participants. For the “Told Alcohol” conditions, the research assistant brought in a tray sprayed with vodka and containing a chilled full bottle labeled as 80-proof vodka and a chilled full bottle of tonic water (each with the original labels), a bottle of lime juice, and three glasses already rubbed with a cotton ball soaked in vodka for olfactory cues. For participants in the “Told Alcohol/Receive Placebo” condition, the labeled vodka bottle contained only flat tonic water with a 5 ml vodka float for olfactory cues and as a taste mask. The research assistant prepared the drinks in view of the participant by measuring the assigned weight and sex-adjusted dose (0.4 g/kg; 90% of this dose for women) from the vodka bottle in a graduated cylinder and then mixing in 5 parts tonic water and 5 ml of lime juice from a dropper. For the “Told Placebo” conditions, the tray contained a chilled full bottle labeled as tonic water, a bottle of lime juice, and three glasses. For the “Told Placebo/Received Alcohol” condition, the bottle actually contained 5:1 ratio of tonic/vodka, and for the “Told Placebo/Received Placebo” condition, the bottle contained 5:1 ratio of fresh/flat tonic. The research assistant prepared drinks in view of the participant by measuring the assigned weight and sex-adjusted dose from the tonic bottle and then mixing in 5 ml of lime juice. In all conditions, the beverage was poured into glasses in three equal-sized portions. Participants had to consume each drink in 5 minutes over 15 total minutes. Research assistants were kept unaware of the alcohol content of the beverage by conducting BrAC assessments with the digital readout covered and stored in memory for later retrieval. Participants remained seated until the end of the smoking lapse task to minimize interoceptive cues of intoxication.
Smoking Lapse Task
At 50 minutes from the start of drinking, participants were presented with a tray containing eight cigarettes of their preferred brand and an ashtray (McKee et al. 2006; McKee et al. 2011). Participants were instructed that they could commence smoking at any point over the next 50 minutes, but that for each 5 minutes they delayed smoking, they would earn $1 for a maximum of $10 during the delay period. They were further instructed that the session would end at 19:00 regardless of whether they chose to smoke. After choosing to smoke, participants completed brief self-report measures and then received a lighter. Participants were instructed to tell us when they wanted to smoke; the time at which this occurred was recorded as the primary dependent variable for this task (range 0–50 min), coded into 5-min intervals for analysis. Following the first cigarette (or at the end of the delay period if smoking was not initiated), all remaining cigarettes were available for purchase at $0.50 each from a $4.00 tab available to the participant. Participants smoked an average of 1.94 (SD = 0.96) cigarettes; amount smoked was not a focus of this study, was not predicted by experimental condition or sex × condition interactions and is not discussed further. Money earned during the 50-minute delay period and any of the remaining credit was paid at the end of the session.
Post Experimental Procedures
Participants remained in the laboratory until 19:00 and until their BrAC was below .04 g/dl, received a light meal and were allowed to watch movies and read. They were paid $115 for completing the sessions and an additional $14 that they could earn on the smoking lapse task and transported home by taxi. All participants in the deception conditions were fully debriefed regarding the deception following completion of the study. All participants were offered referrals for alcohol treatment and smoking cessation, with written resources available if interested.
Measures
Baseline
DSM-IV Axis I diagnoses were determined with the Structured Clinical Interview for DSM-IV Non-Patient Edition (SCID; First et al. 1995). Severity of nicotine dependence was assessed using the Fagerström Test for Nicotine Dependence (FTND; Heatherton et al. 1991). The Timeline Followback Interview (TLFB; Sobell et al. 1996), a well-validated daily calendar-assisted assessment of substance use, was used to assess past 60-day alcohol use.
Experimental sessions
BrAC was assessed at the beginning of the session and then at 26, 42, 60, 90, 120, and 160 minutes after drinking was initiated. Urge to smoke and expected satisfaction from smoking were assessed 42 minutes after initiating drinking. Urge to smoke was assessed with a single item 0–100 Visual Analogue Scale (VAS) and with the 10-item Brief Questionnaire of Smoking Urges (BQSU; Cox et al. 2001). BQSU items are rated on a 1=”strongly disagree” to 7=”strongly agree scale”, with higher scores indicating greater craving (α = .86). BQSU is a well-validated measure that includes items related to urge to smoke for positive reinforcement (Factor 1, the primary measure of interest) or negative reinforcement (Factor 2). Expected satisfaction from smoking was assessed with the respective 2-item subscale of the Cigarette Effects Scale (CES; Westman et al., 1992), a 10-item questionnaire (VAS scale, range 0–100) that has been used in studies of smoking lapse behavior (McKee et al., 2010); α = .66 at baseline and α = .87 prior to the smoking lapse task.
Credibility of the instructional set manipulation
At the end of the session, participants completed drink ratings, e.g., “drinks made me feel better”, on a scale from 0= “No effect at all” to 4= “a very strong effect” (Juliano et al. 2002; Rose et al. 2004 as adapted to alcohol-administration), estimated drink potency compared to usual drink (5-point scale, from 0 = “much weaker” to 4= “much stronger”) and content (estimated number of standard drinks consumed in half increments from 0 to 6), and indicated whether they consumed a drink with alcohol or a drink without alcohol. Finally, participants were asked on a separate page whether they felt deceived about anything during the experiment using open-ended responses sealed in an envelope for external review (Rohsenow and Bachorowski, 1984).
Data Analysis Plan
Baseline differences between experimental conditions were tested with two-way ANOVAs and chi-square tests. To test simultaneously the main effects of beverage condition and instruction condition on risk of initiating smoking during the delay period, we ran Cox proportional hazards survival analyses, which account for the fact that those not smoking by the end of the 50-min delay period were right-censored. The first step of the model included sex and FTND as predictors along with the dummy-coded main effects of Told Alcohol vs. Told Placebo and Received Alcohol vs. Received Placebo (i.e., both factors in the 2 × 2 BPD). In the second step, interactions between sex and experimental conditions were added. We next used linear regressions to test the effects of experimental conditions on VAS urge to smoke, BQSU Positive Reinforcement, and CES expected satisfaction. These models covaried sex, FTND, and the baseline value of the respective dependent variable and included dummy-codes for Told Alcohol and Told Placebo. Finally, we tested whether including measures of urge to smoke and expected satisfaction from smoking in the proportional hazards models predicting risk of initiating smoking significantly reduced (i.e., mediated) the effect of experimental conditions on smoking.
Results
Table 1 presents descriptive statistics for the sample broken down by sex. The experimental conditions did not differ significantly on any of these descriptive variables, all ps > .14, with the exception of marital status, likely reflecting a chance difference. Regarding sex differences, women were less likely than men to be employed and more likely to be married/cohabiting.
Table 1.
Demographics and substance use characteristics of the whole sample and broken down by sex
| Variable | Total N = 96 |
Women N = 42 |
Men N = 54 |
|---|---|---|---|
|
| |||
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Age | 38.6 (11.1) | 39.4 (10.3) | 37.9 (11.7) |
| Education (Years) | 13.2 (2.1) | 12.9 (2.1) | 13.5 (2.0) |
| FTND Score | 5.3 (2.1) | 5.2 (2.2) | 5.4 (2.1) |
| Number of cigarettes/day | 17.3 (6.0) | 17.0 (6.2) | 17.4 (5.8) |
| Percent drinking days | 49.9 (26.4) | 50.3 (27.3) | 49.6 (26.0) |
| Average drinks per drinking day | 6.0 (3.0) | 5.4 (2.6) | 6.5 (3.2) |
|
| |||
| n (%) | n (%) | n (%) | |
|
| |||
| Race | |||
| White | 62 (65.3) | 26 (61.9) | 36 (67.9) |
| African-American | 23 (24.2) | 9 (21.4) | 14 (26.4) |
| American Indian/Alaskan Native | 1 (1.1) | 1 (2.4) | 0 (0.0) |
| Asian | 2 (2.1) | 0 (0.0) | 2 (3.8) |
| Multiracial / Other | 7 (7.4) | 6 (14.3) | 1 (1.9) |
| Hispanic Ethnicity | 4 (4.2) | 0 (0.0) | 4 (7.4) |
| Marital Status ** | |||
| Married / Cohabiting | 30 (31.3) | 20 (47.6) | 10 (18.5) |
| Divorced/Separated/Never Married/ Widowed | 66 (68.8) | 22 (52.4) | 44 (81.5) |
| Employment * | |||
| Unemployed/Home-maker/Retired | 63 (66.3) | 33 (80.5) | 30 (55.6) |
| Employed | 26 (27.4) | 7 (17.1) | 19 (35.2) |
| Student | 6 (6.3) | 1 (2.4) | 5 (9.3) |
| % Very low family income (< 20,000) | 49 (51.0) | 23 (54.8) | 26 (48.1) |
| DSM-IV alcohol abuse (lifetime) | 63 (65.6) | 26 (61.9) | 37 (68.5) |
| DSM-IV alcohol dependence (lifetime) | 27 (28.1) | 8 (19.0) | 19 (35.2) |
| DSM-IV alcohol dependence (past 12 months) | 7 (7.3) | 1 (2.4) | 6 (11.1) |
Note: Percentages are based upon available data per group. FTND = Fagerström Test for Nicotine Dependence
p < .05 and
p < .01 for tests of the difference between men and women.
Credibility of the Instructional Set Manipulation
On the open-ended post-experiment credibility questions, one participant (4%) in the Told Alcohol/Received Placebo condition detected deception and reported receiving placebo. In the Told Placebo/Received Alcohol condition, four (16.7%) reported receiving alcohol not placebo. No participants in the other conditions reported being deceived. Analyses excluding the participants for whom the deception failed produced the same findings for all analyses, so all participants are retained in analyses presented. Means (SDs) for main effects of the experimental manipulations with corresponding effect sizes for drink ratings are presented in Table 2. There were significant, large effects for Told Alcohol vs. Told Placebo on estimated number of alcoholic drinks and drink potency and smaller significant effects on satisfaction and feeling better, but no significant effects of Received Alcohol vs. Received Placebo on any variables. Told × Received interactions were nonsignificant. Sex did not predict responses on the measures noted above or interact with condition to predict responses.
Table 2.
Means and effect sizes for the main effects of instruction condition and beverage condition on drink ratings and time to choosing to smoke
| Told | Received | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Placebo | Alcohol | Placebo | Alcohol | |||||||
|
|
||||||||||
| M | (SD) | M | (SD) | Effect size | M | (SD) | M | (SD) | Effect size | |
| Est. number of drinks | 0.75 | (1.47) | 2.17 | (1.03) | .25*** | 1.23 | (1.31) | 1.71 | (1.55) | .04 |
| Smell | 1.28 | (1.17) | 1.53 | (1.24) | .01 | 1.35 | (1.21) | 1.47 | (1.21) | .002 |
| Satisfaction | 1.15 | (1.23) | 1.69 | (1.31) | .05* | 1.35 | (1.38) | 1.50 | (1.22) | .004 |
| Made me feel better | .79 | (1.14) | 1.31 | (1.03) | .06* | .98 | (1.16) | 1.13 | (1.06) | .01 |
| Liking | 1.15 | (1.23) | 1.61 | (1.24) | .04 | 1.23 | (1.23) | 1.54 | (1.27) | .02 |
| Similar to usual | .53 | (.95) | .76 | (1.23) | .01 | .52 | (.95) | .77 | (1.24) | .01 |
| Taste | 1.28 | (1.33) | 1.67 | (1.44) | .02 | 1.29 | (1.38) | 1.67 | (1.39) | .02 |
| Potency | 1.33 | (.79) | 2.22 | (1.09) | .19*** | 1.63 | (1.06) | 1.96 | (1.02) | .03 |
| Time to smoke | 23.39 | (22.55) | 20.74 | (22.02) | .004 | 24.76 | (22.54) | 19.32 | (21.75) | .015 |
Note. Estimated number of standard drinks (rated on a scale from 0 to 6 drinks in half increments and converted to whole drinks in the table). Potency rated on a 5-point scale (0 = “much weaker” to 4 = “much stronger”). Other drink ratings completed on a 5-point scale (0 = “not at all” to 4 = “extremely”). Time to smoke indicates the mean number of minutes to choosing to smoke a cigarette during the 50-minute delay period with those not smoking set to the maximum of 50 minutes. Tests of the effect of sex on these variables were nonsignificant as were sex × condition interactions.
Effect size is indicated as partial η 2 of the main effects from two-way (Told × Received) analyses of variance.
p < .05;
p < .01;
p < .001.
Breath Alcohol Levels
In the Received Alcohol conditions, participants had mean (SD) BrACs of .046% (.01) at 26 minutes from the start of drinking and then .044% (.01) at 42 minutes, .041% (.01) at 60 minutes, .030% (.01) at 90 minutes, .021% (.01) at 120 minutes, and .010% (.01) at 160 minutes. The two alcohol groups did not differ.
Risk of Initiating Smoking
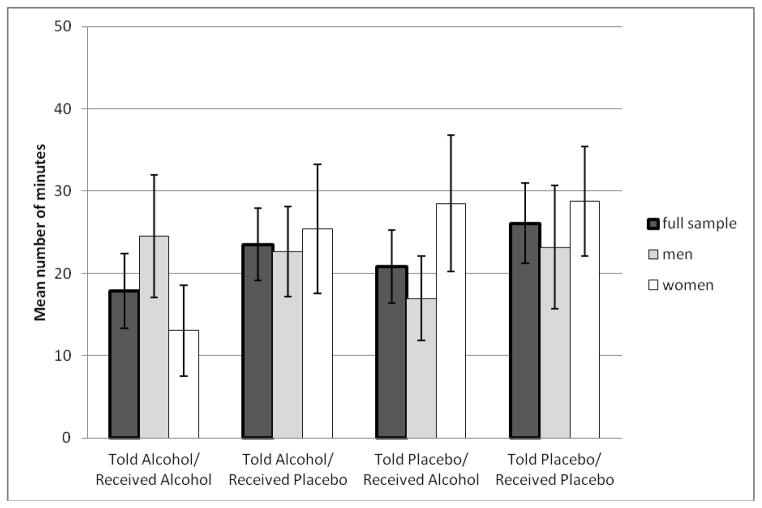
Mean number of minutes before initiating smoking during the 50-minute delay period is presented by Figure 1 broken down by the four cells of the BPD design and by sex and in Table 2 by the main effect of each experimental factor. Those who did not choose to smoke (n = 33; 34.4%) during the delay period were assigned a value of 50 when calculating means. Results of proportional hazards models predicting risk of initiating smoking are presented in Table 3. The main effects of instruction condition and beverage condition were nonsignificant, as was the main effect of sex. There was a robust effect of FTND, with greater tobacco dependence predicting greater risk of initiating smoking, supporting the validity of the lapse model. In the second step of the model, there was a significant interaction between sex and instruction condition. Women in the Told Alcohol condition smoked sooner than women in the Told Placebo condition (Hazard ratio [HR] = 2.87, 95% confidence interval [CI] = 1.20–6.90, p=.018); 77.3% of women in the Told Alcohol condition smoked compared to 50.0% of women in the Told Placebo condition. This effect was not apparent in men (HR = 0.70, 95% CI = 0.35–1.40, p=.31); 63.0% of men in Told Alcohol smoked compared to 70.4% of men in Told Placebo. In both sexes, the main effect of beverage condition and the instruction condition by beverage condition interaction were nonsignificant, ps > .45.
Figure 1.
Mean number of minutes to choosing to smoke a cigarette during the 50-minute delay period broken down by the four experimental BPD groups and sex with standard error bars shown.
Table 3.
Hierarchical Cox proportional-hazards regression models predicting risk of initiating smoking during the 50-minute delay period (N = 96)
| Variable | Hazard ratio | 95 % CI | p |
|---|---|---|---|
| Step 1 | |||
| FTND | 1.22 | 1.07–1.40 | .003 |
| Female | 1.02 | 0.61–1.67 | 0.94 |
| Received Alcohol vs. Placebo | 1.26 | 0.76–2.08 | 0.37 |
| Told Alcohol vs. Placebo | 1.21 | 0.73–2.01 | 0.46 |
| Step 2 | |||
| Received Alcohol × Female | 1.14 | 0.41–3.19 | 0.81 |
| Told Alcohol × Female | 3.87 | 1.30–11.53 | 0.015 |
Note. FTND = Fagerström Test for Nicotine Dependence. In this analysis, time to choosing to smoke was divided into 10 discrete 5-min segments representing the possible time periods in which participants could choose to smoke.
We conducted follow-up analyses adding employment and marital status to the models as covariates given sex differences on these variables. Neither predicted smoking initiation or interacted with experimental conditions, and their addition did not alter the significance of the sex × instruction condition interaction. Income also did not predict smoking initiation.
Urge to Smoke and Expected Satisfaction from Smoking
Prior to drinking, participants reported scored on the high end of each self-report measure of urge to smoke and expected satisfaction from smoking: VAS urge to smoke mean = 72.8 (21.5); BQSU Positive Reinforcement mean = 6.0 (1.1) out of a maximum of 7; VAS CES satisfaction mean = 79.0 (20.2). Paired t-tests indicated that VAS urge to smoke increased significantly from pre-drinking to the assessment immediately prior to the lapse analogue task (42 min post-drinking; mean increase = 6.8 (23.3), t(94) = 2.84, p = .006). Significant increases were not seen for BQSU Positive Reinforcement or the satisfaction scale of the CES, ps > .20. Multiple regression analyses controlling for sex, FTND, and the baseline value of the respective dependent variable indicated no significant main effects of instruction or beverage condition on urge or expected smoking satisfaction, ps > .15. However, the interaction between sex and instruction condition was significant in the model predicting the CES satisfaction scale (B = 18.9, SE = 7.1, sr2 = .040, p = .009) but not VAS urge to smoke or BQSU Positive Reinforcement (ps = .08 and .10, respectively). Women in the Told Alcohol condition reported greater expected satisfaction from smoking than women in the Told Placebo condition (B = 12.0, SE = 6.0, sr2 = .063, p = .055), and decreased expected satisfaction in men (B = −7.2, SE = 4.3, sr2 = .024, p = .10), with both effects approaching significance. Mediational analyses, however, indicated that CES satisfaction did not predict risk of initiating smoking, and its inclusion in the proportional hazards model did not alter the sex × instruction condition interaction.
Discussion
To our knowledge this is the first study to utilize the alcohol BPD methodology to examine experimentally the independent effects on smoking of (a) expecting that one is drinking alcohol and (b) actually drinking alcohol. Among women, alcohol stimulus expectancy effects on smoking initiation predominated over pharmacologic effects at moderate alcohol doses. Women told that their drink contained alcohol had almost three times greater risk of initiating smoking than women told that their drink did not contain alcohol. Although expected satisfaction from smoking was more strongly affected by alcohol stimulus expectancy effects in women compared to men, this effect did not account for the observed differences in risk of initiating smoking. Findings support the notion that women’s smoking is more dependent than men’s on nonpharmacologic factors such as context or cues (Perkins et al., 2006; Perkins, 2009) and suggest that beliefs associated with drinking alcohol, rather than the pharmacologic effects of a moderate alcohol dose, may increase smoking lapse risk for women who are heavy drinkers.
Contrary to expectations, the pharmacologic effects of the 0.4g/kg dose of alcohol on initiating smoking were nonsignificant and were not significantly greater among men compared to women. McKee et al. (2006) found a similar alcohol dose (target BrAC of 0.03 g/dl) decreased time to initiate smoking on the same smoking lapse analogue task among 16 heavy drinking daily smokers. The discordance with our findings may be due to the shorter smoking deprivation period (3 hr) used by McKee et al., which resulted in a longer latency to smoking initiation in the placebo condition (~35 min). The drinkers in the present study were more than 10 years older on average than those in McKee et al., and alcohol effects on smoking may be more pronounced among less established smokers (Epstein et al. 2007). McKee et al. also did not use a BPD design, so pharmacologic effects of alcohol could not be fully separated from expectancy effects. Finally, McKee et al. used a within-subjects design (compared to our between-subjects design), which can increase statistical power. Although we were powered to detect medium effect sizes, smaller sample sizes when examining effects of condition within each sex had reduced power.
The lack of a significant pharmacologic effect of alcohol on smoking urge was surprising, particularly in light of alcohol’s established dose-dependent effects on tobacco craving (e.g., Burton and Tiffany 1997; Kouri et al. 2004; McKee et al. 2006; Sayette et al. 2005). A number of studies have used a similar dose of alcohol but among lighter smokers with less tobacco deprivation. Because urge to smoke after 15 hours of smoking abstinence was relatively high, it is possible that this ceiling effect overrode the hypothesized main effect of alcohol on smoking urge in the present study. Also, it is important to note that participants were established regular heavy drinkers, many of whom had a history of alcohol dependence, unlike in many studies. These participants reported little subjective effects of receiving a moderate dose of alcohol compared to placebo. Thus, in this sample of heavy drinkers, the amount of alcohol consumed may have been too low to affect urge to smoke. Examination of other moderators of alcohol effects on smoking beyond sex should be conducted in the present data and in other samples.
Limitations
Because deception is increasingly hard to maintain in the Told Placebo/Received Alcohol condition when doses of alcohol become high (e.g., Martin et al. 1993), we were not able to test higher doses of alcohol that would have more meaningful subjective effects in heavy drinkers. A dose-dependent effect of a higher dose of alcohol on initiating smoking should be explored in a within-subjects experiment. Also, in order to maintain deception we used a vodka-tonic beverage in the Told Alcohol condition (Keane at al., 1980; Marlatt et al., 1973), which was not similar to the beverages that participants usually drank. Providing participants’ their preferred beverage may increase the potency of the alcohol expectancy effect to elicit urges to smoke and smoking behavior but at the risk of decreasing the effectiveness of the deception.
We used overnight smoking abstinence to increase the odds that individuals would choose to smoke (McKee 2009). Although the biochemical verification used cannot guarantee that all participants refrained from smoking for at least 15 hours, the high levels of baseline urge to smoke strongly suggest considerable deprivation. Because many alcohol-involved lapses to smoking occur in contexts with relatively low levels of craving and negative affect (Kahler et al. 2010), future BPD studies should explore alcohol effects on craving and smoking initiation using a shorter deprivation period that results in lower baseline craving. Finally, all smokers denied intention to quit smoking in the next 30 days; studies with smokers who want to quit would be valuable.
Conclusions
Alcohol use is a known risk factor for lapses to smoking in those trying to quit. The present study suggests that during acute smoking abstinence, moderate alcohol consumption (approximately two drinks) may have limited pharmacologic effects on the ability to resist smoking among heavy drinkers. However, at least for women, whose smoking may be more cue or context-driven than men’s, the belief that one has consumed alcohol may facilitate smoking through associative processes. Further research is needed to understand mechanisms through which intoxicating levels of alcohol consumption may additionally reduce the ability to resist smoking, such as through increasing impulsivity and decreasing inhibitory control. Studies also should examine whether other individual difference variables (e.g., genetic factors, severity of alcohol dependence) and state-related factors (e.g., stress [McKee et al. 2011], negative affect [Perkins et al. 2010], or positive affect) may interact with alcohol administration to increase further the risk of lapsing to smoking after drinking.
Acknowledgments
source of funding: This study was funded by the National Institute on Alcohol Abuse and Alcoholism, grant R01AA016978 to Dr. Kahler and by a Senior Research Career Scientist award from the Department of Veterans Affairs to Dr. Rohsenow.
Footnotes
Conflicts of interest
The authors have no financial relationship with the study sponsor, and no conflicts of interest to disclose.
References
- Acheson A, Mahler SV, Chi H, de Wit H. Differential effects of nicotine on alcohol consumption in men and women. Psychopharmacology. 2006;186:54–63. doi: 10.1007/s00213-006-0338-y. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Echeagaray-Wagner F. Epidemiologic analysis of alcohol and tobacco use. Alcohol Res Health. 2000;24:201–8. [PMC free article] [PubMed] [Google Scholar]
- Augustson EM, Wanke KL, Rogers S, Bergen AW, Chatterjee N, Synder K, Albanes D, Taylor PR, Caporaso NE. Predictors of sustained smoking cessation: A prospective analysis of chronic smokers from the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study. Am J Public Health. 2008;98:549–555. doi: 10.2105/AJPH.2005.084137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer JS, Lichtenstein E. Classification and prediction of smoking relapse episodes: An exploration of individual differences. Journal of Consulting and Clinical Psychology. 1988;56:104–110. doi: 10.1037//0022-006x.56.1.104. [DOI] [PubMed] [Google Scholar]
- Borland R. Slip-ups and relapse in attempts to quit smoking. Addictive Behaviors. 1990;15:235–245. doi: 10.1016/0306-4603(90)90066-7. [DOI] [PubMed] [Google Scholar]
- Burton SM, Tiffany ST. The effect of alcohol consumption on craving to smoke. Addiction. 1997;92:15–26. [PubMed] [Google Scholar]
- Chiolero A, Wietlisbach V, Ruffieux C, Paccaud F, Cornuz J. Clustering of risk behaviors with cigarette consumption: A population-based survey. Prev Med. 2006;42:348–53. doi: 10.1016/j.ypmed.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Dawson DA. Drinking as a risk factor for sustained smoking. Drug Alcohol Depend. 2000;59:235–49. doi: 10.1016/s0376-8716(99)00130-1. [DOI] [PubMed] [Google Scholar]
- Dollar KM, Homish GG, Kozlowski LT, Leonard KE. Spousal and alcohol-related predictors of smoking cessation: a longitudinal study in a community sample of married couples. Am J Public Health. 2009;99:231–3. doi: 10.2105/AJPH.2008.140459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein AM, Sher TG, Young MA, King AC. Tobacco chippers show robust increases in smoking urge after alcohol consumption. Psychopharmacology (Berl) 2007;90:321–329. doi: 10.1007/s00213-006-0438-8. [DOI] [PubMed] [Google Scholar]
- Falk DE, Yi HY, Hiller-Sturmhofel S. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Res Health. 2006;29:162–71. [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- Glautier S, Clements K, White JA, Taylor C, Stolerman IP. Alcohol and the reward value of cigarette smoking. Behav Pharmacol. 1996;7:144–154. [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Liebson I. Facilitation of human tobacco self-administration by ethanol: a behavioral analysis. J Exp Anal Behav. 1976;25:279–92. doi: 10.1901/jeab.1976.25-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwaltney CJ, Shiffman S, Sayette MA. Situational correlates of abstinence self-efficacy. J Abnorm Psychol. 2005;114:649–60. doi: 10.1037/0021-843X.114.4.649. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerstrom test for nicotine dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Chait LD, Griffiths RR. Cigarette smoking and subjective response in alcoholics: effects of pentobarbital. Clin Pharmacol Ther. 1983;33:806–12. doi: 10.1038/clpt.1983.110. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Chait LD, Griffiths RR. Effects of ethanol on cigarette smoking by volunteers without histories of alcoholism. Psychopharmacology (Berl) 1984;82:1–5. doi: 10.1007/BF00426371. [DOI] [PubMed] [Google Scholar]
- Humfleet G, Munoz R, Sees K, Reus V, Hall S. History of alcohol or drug problems, current use of alcohol or marijuana, and success in quitting smoking. Addict Behav. 1999;24:149–54. doi: 10.1016/s0306-4603(98)00057-4. [DOI] [PubMed] [Google Scholar]
- Hymowitz N, Cummings KM, Hyland A, Lynn WR, Pechacek TF, Hartwell TD. Predictors of smoking cessation in a cohort of adult smokers followed for five years. Tob Control. 1997;6:S57–62. doi: 10.1136/tc.6.suppl_2.s57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano LM, Brandon TH. Effects of nicotine dose, instructional set, and outcome expectancies on the subjective effects of smoking in the presence of a stressor. J Abnorm Psychol. 2002;111:88–97. doi: 10.1037//0021-843x.111.1.88. [DOI] [PubMed] [Google Scholar]
- Kahler CW, Borland R, Hyland A, McKee SA, Thompson ME, Cummings KM. Alcohol consumption and quitting smoking in the International Tobacco Control (ITC) Four Country Survey. Drug Alcohol Depend. 2009a;100:214–220. doi: 10.1016/j.drugalcdep.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler CW, Spillane NS, Metrik J. Alcohol use and initial smoking lapses among heavy drinkers in smoking cessation treatment. Nicotine Tob Res. 2010;12:781–785. doi: 10.1093/ntr/ntq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler CW, Strong DR, Papandonatos GD, Colby SM, Clark MA, Boergers J, Niaura R, Abrams DB, Buka SL. Cigarette smoking and the lifetime alcohol involvement continuum. Drug Alcohol Depend. 2008;93:111–120. doi: 10.1016/j.drugalcdep.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane TM, Lisman SA, Kreutzer J. Alcoholic beverages and their placebos: An empirical evaluation of expectancies. Addict Beh. 1980;5:313–328. doi: 10.1016/0306-4603(80)90005-2. [DOI] [PubMed] [Google Scholar]
- King A, McNamara P, Conrad M, Cao D. Alcohol-induced increases in smoking behavior for nicotinized and denicotinized cigarettes in men and women. Psychopharmacology (Berl) 2009;207:107–17. doi: 10.1007/s00213-009-1638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Epstein AM. Alcohol dose-dependent increases in smoking urge in light smokers. Alcohol Clin Exp Res. 2005;29:547–52. doi: 10.1097/01.alc.0000158839.65251.fe. [DOI] [PubMed] [Google Scholar]
- Kouri EM, McCarthy EM, Faust AH, Lukas SE. Pretreatment with transdermal nicotine enhances some of ethanol’s acute effects in men. Drug Alcohol Depend. 2004;75:55–65. doi: 10.1016/j.drugalcdep.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Leeman RF, McKee SA, Toll BA, Krishnan-Sarin S, Cooney JL, Makuch RW, O’Malley SS. Risk factors for treatment failure in smokers: relationship to alcohol use and to lifetime history of an alcohol use disorder. Nicotine Tob Res. 2008;10:1793–809. doi: 10.1080/14622200802443742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt GA, Rohsenow DJ. Cognitive processes in alcohol use: Expectancy and the balanced placebo design. In: Mello NK, editor. Advances in Substance Abuse: Behavioral and Biological Research. JAI Press; Greenwich, CT: 1980. [Google Scholar]
- Martin CS, Sayette MA. Experimental design in alcohol administration research: limitations and alternatives in the manipulation of dosage-set. J Stud Alcohol. 1993;54:750–61. doi: 10.15288/jsa.1993.54.750. [DOI] [PubMed] [Google Scholar]
- McKee SA. Developing human laboratory models of smoking lapse behavior for medication screening. Addict Biol. 2009;14:99–107. doi: 10.1111/j.1369-1600.2008.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, Shi J. Alcohol expectancy increases positive responses to cigarettes in young, escalating smokers. Psychopharmacology (Berl) 2010;210:355–64. doi: 10.1007/s00213-010-1831-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Krishnan-Sarin S, Shi J, Mase T, O’Malley SS. Modeling the effect of alcohol on smoking lapse behavior. Psychopharmacology (Berl) 2006;189:201–10. doi: 10.1007/s00213-006-0551-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Sinha R, Weinberger AH, Sofuoglu M, Harrison EL, Lavery M, Wanzer J. Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. J Psychopharmacol. 2011;25:490–502. doi: 10.1177/0269881110376694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz J, Boyd G, Rose JE, Charuvastra VC, Jarvik ME. Alcohol increases cigarette smoking: A laboratory demonstration. Addictive Behaviors. 1985;10:203–207. doi: 10.1016/0306-4603(85)90001-2. [DOI] [PubMed] [Google Scholar]
- Murray RP, Istvan JA, Voelker HT, Rigdon MA, Wallace MD. Level of involvement with alcohol and success at smoking cessation in the lung health study. J Stud Alcohol. 1995;56:74–82. doi: 10.15288/jsa.1995.56.74. [DOI] [PubMed] [Google Scholar]
- Nil R, Buzzi R, Battig K. Effects of single doses of alcohol and caffeine on cigarette smoke puffing behavior. Pharmacol Biochem Behav. 1984;20:583–90. doi: 10.1016/0091-3057(84)90308-3. [DOI] [PubMed] [Google Scholar]
- Odum AL, Madden GJ, Bickel WK. Discounting of delayed health gains and losses by current, never- and ex-smokers of cigarettes. Nicotine Tob Res. 2002;4:295–303. doi: 10.1080/14622200210141257. [DOI] [PubMed] [Google Scholar]
- Osler M, Prescott E, Godtfredsen N, Hein HO, Schnohr P. Gender and determinants of smoking cessation: A longitudinal study. Prev Med. 1999;29:57–62. doi: 10.1006/pmed.1999.0510. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Acute responses to nicotine and smoking: implications for prevention and treatment of smoking in lower SES women. Drug Alcohol Depend. 2009;104(Suppl 1):S79–86. doi: 10.1016/j.drugalcdep.2008.10.022. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Doyle T, Ciccocioppo M, Conklin C, Sayette M, Caggiula A. Sex differences in the influence of nicotine dose instructions on the reinforcing and self-reported rewarding effects of smoking. Psychopharmacology (Berl) 2006;184:600–7. doi: 10.1007/s00213-005-0103-7. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Conklin CA, Sayette MA, Giedgowd GE. Acute negative affect relief from smoking depends on the affect situation and measure but not on nicotine. Biol Psychiatry. 2010;67:707–14. doi: 10.1016/j.biopsych.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Sexton JE, DiMarco A, Grobe JE, Scierka A, Stiller RL. Subjective and cardiovascular responses to nicotine combined with alcohol in male and female smokers. Psychopharmacology (Berl) 1995;119:205–12. doi: 10.1007/BF02246162. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Bachorowski JA. Effects of alcohol and expectancies on verbal aggression in men and women. J Abnorm Psychol. 1984;93:418–32. doi: 10.1037//0021-843x.93.4.418. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Marlatt GA. The balanced placebo design: methodological considerations. Addict Behav. 1981;6:107–22. doi: 10.1016/0306-4603(81)90003-4. [DOI] [PubMed] [Google Scholar]
- Rose JE, Brauer LH, Behm FM, Cramblett M, Calkins K, Lawhon D. Potentiation of nicotine reward by alcohol. Alcohol Clin Exp Res. 2002;26:1930–1. doi: 10.1097/01.ALC.0000040982.92057.52. [DOI] [PubMed] [Google Scholar]
- Rose JE, Brauer LH, Behm FM, Cramblett M, Calkins K, Lawhon D. Psychopharmacological interactions between nicotine and ethanol. Nicotine Tob Res. 2004;6:133–44. doi: 10.1080/14622200310001656957. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Martin CS, Wertz JM, Perrott MA, Peters AR. The effects of alcohol on cigarette craving in heavy smokers and tobacco chippers. Psychol Addict Behav. 2005;19:263–70. doi: 10.1037/0893-164X.19.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SE, Wang MM, Nguyen B. Predictors of success in a smoking cessation clinic. J Gen Intern Med. 1996;11:702–4. doi: 10.1007/BF02600163. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Relapse following smoking cessation: A situational analysis. Journal of Consulting and Clinical Psychology. 1982;50:71–86. doi: 10.1037//0022-006x.50.1.71. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Fischer LA, Paty JA, Gnys M, Hickcox M, Kassel JD. Drinking and smoking: A field study of their association. Annuals of Behavioral Medicine. 1994;16:203–209. [Google Scholar]
- Shiffman S, Gwaltney CJ. Does heightened affect make smoking cues more salient? J Abnorm Psychol. 2008;117:618–24. doi: 10.1037/0021-843X.117.3.618. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Gwaltney CJ, Balabanis MH, Liu KS, Paty JA, Kassel JD, Hickcox M, Gnys M. Immediate antecedents of cigarette smoking: an analysis from ecological momentary assessment. J Abnorm Psychol. 2002;111:531–45. doi: 10.1037//0021-843x.111.4.531. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcos M. First lapses to smoking: Within-subjects analysis of real-time reports. Journal of Consulting and Clinical Psychology. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Smith PM, Kraemer HC, Miller NH, Debusk RF, Taylor CB. In-hospital smoking cessation programs: Who responds, who doesn’t? Journal of Consulting and Clinical Psychology. 1999;67:19–27. doi: 10.1037//0022-006x.67.1.19. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline followback: A calendar method for assessing alcohol and drug use. Addiction Research Foundation; Toronto, Canada: 1996. [Google Scholar]
- Sorlie PD, Kannel WB. A description of cigarette smoking cessation and resumption in the Framingham Study. Prev Med. 1990;19:335–45. doi: 10.1016/0091-7435(90)90033-g. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Higgins ST, Bickel WK, Steingard S. Effects of response requirement and the availability of an alternative reinforcer on cigarette smoking by schizophrenics. Psychopharmacology (Berl) 1999;145:52–60. doi: 10.1007/s002130051031. [DOI] [PubMed] [Google Scholar]
- Westman EC, Levin ED, Rose JD. Smoking while wearing the nicotine patch; is smoking satisfying or harmful. Clin Res. 1992;40:871A. [Google Scholar]