Abstract
Background
Whether human immunodeficiency virus (HIV) infection is a risk factor for heart failure (HF) is not clear. The presence of coronary heart disease and alcohol consumption in this population may confound this association.
Methods
To determine whether HIV infection is a risk factor for incident HF, we conducted a population-based, retrospective cohort study of HIV-infected and HIV-uninfected veterans enrolled in the Veterans Aging Cohort Study Virtual Cohort (VACS-VC) and the 1999 Large Health Study of Veteran Enrollees (LHS) from January 1, 2000, to July 31, 2007.
Results
There were 8486 participants (28.2% HIV-infected) enrolled in the VACS-VC who also participated in the 1999 LHS. During the median 7.3 years of follow-up, 286 incident HF events occurred. Age- and race/ethnicity–adjusted HF rates among HIV-infected and HIV-uninfected veterans were 7.12 (95% confidence interval [CI],6.90-7.34) and 4.82 (95% CI, 4.72-4.91) per 1000 person-years, respectively. Compared with HIV-uninfected veterans, those who were HIV infected had an increased risk ofHF (adjusted hazard ratio [HR], 1.81; 95% CI, 1.39-2.36). This association persisted among veterans who did not have a coronary heart disease event or a diagnosis related to alcohol abuse or dependence before the incident HF event (adjusted HR, 1.96; 95% CI, 1.29-2.98). Compared with HIV-uninfected veterans, those who were HIV infected with a baseline Human immunodeficiency virus 1 (HIV-1) RNA level of 500 or more copies/mL had a higher risk of HF (adjusted HR, 2.28; 95% CI, 1.57-3.32), while those with baseline and a recent HIV-1 RNA level less than 500 copies/mL did not (adjusted HR, 1.10; 95% CI, 0.64-1.89; P< .001 for comparison between high and low HIV-1 RNA groups).
Conclusions
Our data suggest that HIV infection is a risk factor for HF. Ongoing viral replication is associated with a higher risk of developing HF.
Human Immunodeficiency virus (HIV) infection as an independent risk factor for cardiovascular disease has been reported in some1,2 but not all studies.3,4 Whether HIV infection is an independent risk factor for heart failure (HF), particularly among patients without a prior coronary heart disease (CHD) event or significant alcohol use before developing HF, is not known.5 Prior reports2,6 suggest that class of antiretroviral drugs (particularly protease inhibitors), Human immunodeficiency virus 1 (HIV-1) RNA levels,7 and low CD4+ lymphocyte counts8 are each associated with an increased risk of cardiovascular disease events, an important risk factor for HF. Heavy alcohol consumption, which is more prevalent among HIV-infected people, is also an established risk factor for HF.9,10 In addition, zidovudine (azidothymidine) use has been associated11,12 with a specific dose-dependent skeletal myopathy attributed to mitochondrial toxicity and with cardiac dysfunction that resolves with discontinuation of the drug. In addition, HIV infection has been linked with dilated cardiomyopathy, myocarditis, and left ventricular dysfunction, the last even in the absence of clinically advanced HIV disease.13,14 However, not all studies have confirmed the association between HIV, antiretroviral therapy, and myocardial dysfunction.5 Other important risk factors for HF in the general population include increasing age, hypertension, diabetes mellitus, and obesity,15 factors that are being seen with increasing frequency among the HIV-infected population. With improved survival among individuals with HIV infection and possible effects of HIV infection and antiretroviral therapy on body habitus and metabolic factors, it is important to understand the associations of established and novel risk factors and define the role of HIV itself in the risk of HF. An understanding of risk factors is critical in designing targeted intervention strategies to reduce such risk and improve cardiovascular clinical outcomes in this population. The objective of this study, therefore, was to determine whether HIV infection was independently associated with an increased risk of incident HF. We performed additional analyses in patients without a prior CHD event or alcohol abuse or dependence diagnosis before developing HF and assessed the association between suppressed HIV-1 RNA viral loads and HF to further understand the impact of HIV itself on the risk of HF.
Methods
We conducted a population-based, retrospective cohort study combining 2 established data sources: the Veterans Aging Cohort Study Virtual Cohort (VACS-VC) and the 1999 Large Health Study of Veteran Enrollees (LHS). The VACS-VC is a cohort of individuals with or without HIV infection matched on age, sex, race/ethnicity, and clinical site who were identified from US Department of Veterans Affairs (VA) administrative data in the fiscal years 1998-2003. This cohort has been described and used for studies16-18 of clinical outcomes in the HIV-infected and HIV-uninfected veteran population. Briefly, the cohort consists of extensive clinical, laboratory, and pharmacy data from the Immunology Case Registry, the Pharmacy Benefits Management database, the Decision Support System database, and the National Patient Care Database. The 1999 LHS was a survey administered between June 1999 and January 2000 designed to assess the health status of veterans. The institutional review boards at the University of Pittsburgh, Yale University, and the West Haven VA Medical Center approved this study.
All participants in the VACS-VC/LHS were eligible for the present study. We excluded those with prevalent CHD, angina, or HF at baseline (n=3209). We also excluded patients who were diagnosed as having cancer (except nonmelanoma skin cancer [n=1716]) to increase the possibility of adequate length of survival to develop an HF event during the follow-up period and to remove any possible bias resulting from the use of some antineoplastic drugs that are cardiotoxic; women were excluded because of the small number (n=276).
Definitions
Infection with HIV was defined as 1 or more inpatient and/or 2 or more outpatient International Classification of Diseases, Ninth Revision (ICD-9) codes for HIV infection confirmed by the participant's presence in the VA Immunology Case Registry. Hepatitis C virus infection was defined as a positive result of a hepatitis C virus antibody test or 1 or more inpatient and/or 2 or more outpatient ICD-9 codes for this diagnosis. These definitions have been used in multiple studies19-22 and have been found to correlate well with medical record reviews and/or laboratory test–based diagnoses. We used the presence of 1 or more inpatient and/or 2 or more outpatient ICD-9 codes to identify CHD (ICD-9 codes 410 and 411) events and HF (ICD-9 codes 425 and 428). The ICD-9 codes for various cardiovascular diagnoses are highly predictive of actual adjudicated clinical diagnoses. The positive predictive values of ICD-9 codes for HF, myocardial infarction, cerebrovascular disease, and diabetes mellitus are 94.3%, 81.9%, 89.4%, and 96.2%, respectively.23 We selected these codes on the basis of prior studies within the VA health care system24 and because these definitions had high agreement with the formal adjudication process within the Cardiovascular Health Study.3,25,26
Hypertension was also defined using ICD-9 codes (401,401.1, 401.9, 402, 402.1, 402.11, 402.9, 403, 404, 404.1, 404.9, 405, 405.1,405.11,405.19,405.9,405.91,405.99, and437.2). Diabetes and dyslipidemia were diagnosed according to our previously published20,21,25 algorithms using a combination of laboratory measurements, medication prescriptions, and ICD-9 codes. Self-reported height and weight were used to calculate body mass index (calculated as weight in kilograms divided by height in meters squared). Self-reported data on smoking were obtained from the LHS survey and categorized as history of current smoking, past smoking, or never smoking. History of cocaine abuse or dependence was defined using ICD-9 codes (304.20-304.23 and 305.6-305.63). History of alcohol abuse or dependence was defined using ICD-9 codes based on previous work in the VACS.27
Among the HIV-infected patients, we collected data on baseline CD4+ lymphocyte counts, HIV-1 RNA levels, and use of combination antiretroviral therapy (CART). Baseline CD4+ lymphocyte count and HIV-1 RNA level measurements were from 180 days before and up to 180 days after the time of enrollment in the LHS, and recent measurements were the CD4+ lymphocyte count and HIV-1 RNA level determined closest to the date of the incident HF event, death, or last follow-up observation. Baseline CART was defined as receipt of 3 or more antiretroviral agents for 30 or more days at time of enrollment into the study. Patients who had not received CART at time of enrollment through their last observation date or censor date were considered CART naive. Because CART data were available only through June 2005, the analyses involving CART-naive participants were truncated to 2005. Follow-up time was time to an HF event, death, or last known visit within the VA health care system during the study period. We confirmed deaths using the VA vitals status file, the Social Security Administration death master file, the Beneficiary Identification and Records Locator Subsystem, and the Veterans Health Administration's medical SAS inpatient data sets.
Statistical Methods
Descriptive statistics for all variables by HIV status were assessed using t test or its nonparametric counterpart for continuous variables and χ2 test or Fisher exact test for categorical variables. Cox proportional hazards models were used to estimate the hazard ratio (HR) and 95% confidence intervals (CIs) for incident HF associated with HIV infection vs noninfection after adjusting for confounders. The proportional hazards assumption was assessed using the Grambsch-Therneau method.28 Additional analyses were performed to determine whether the association between HIV infection and HF persisted after excluding patients who had a diagnosis of alcohol abuse or dependence before the development of the incident HF event during the follow-up period. Secondary analyses also examined the association between HIV status and HF stratified by HIV-1 RNA level, CD4+ lymphocyte count, and antiretroviral therapy status. To explore the impact of viral suppression, we compared HIV-uninfected participants with HIV-infected participants who had baseline and recent HIV-1 RNA levels less than 500 copies/mL, baseline but not recent HIV-1 RNA levels of less than 500 copies/mL, and those with baseline HIV-1 RNA levels of 500 or more copies/mL. Additional analysis included interaction terms between HIV status and individual comorbidities. None of these factors were found to be statistically significant and they are not presented here.
Results
Between January 1, 2000, and July 31, 2007, a total of 8486 patients met our criteria for inclusion, of whom 2391 (28.2%) were HIV infected and 6095 (71.8%) were HIV uninfected (Table 1). The median duration of follow-up was 7.3 years (range, 0.01-7.48 years) for the entire cohort. There were 286 incident HF events and 1096 deaths during the follow-up period. Among patients who did not develop HF or die (n = 7104), 87.1% completed follow-up to within 1 year of the end of the follow-up period.
Table 1. Demographic and Clinical Characteristics of HIV-Infected and HIV-Uninfected Patientsa.
| Characteristic | HIV Infected (n = 2391) | HIV Uninfected (n = 6095) | P Value | |
|---|---|---|---|---|
| Age, median (SD), y | 48.0 (9.5) | 48.0 (9.5) | .70 | |
| Race | ||||
| White | 42.2 | 38.0 | ] | .001 |
| African American | 38.9 | 40.6 | ||
| Hispanic | 9.6 | 9.8 | ||
| Other | 9.3 | 11.6 | ||
| Hepatitis C virus infection | 30.5 | 11.4 | <.001 | |
| BMI, mean (SD) | 25.1 (4.2) | 28.1 (5.3) | <.001 | |
| Dyslipidemia | 29.5 | 31.6 | .06 | |
| Hypertension | 18.7 | 28.8 | <.001 | |
| Diabetes mellitus | 16.7 | 24.8 | <.001 | |
| Smoking status | ||||
| Never | 19.6 | 24.5 | ] | <.001 |
| Current | 55.0 | 45.3 | ||
| Past | 25.4 | 30.2 | ||
| History of alcohol abuse or dependence diagnosis | 34.0 | 32.5 | .18 | |
| History of cocaine abuse or dependence diagnosis | 21.9 | 15.7 | <.001 | |
| CART with HIV-1 RNA level <500 copies/mLat baseline | 51.0 | … | … | |
| CART with HIV-1 RNA level <50 copies/mL at baseline | 26.8 | … | … | |
| HIV-1 RNA median (mean) [SD], log10 (n = 1588) | 2.82 (3.12) [1.26] | … | … | |
| CD4+ lymphocyte count, median (mean) [SD],/mm3 | 366.0 (412.8) [277.4] | … | ||
| (n = 1636) | … | |||
| Heart failure events, No. | 97 | 189 | ||
| Adjusted heart failure incidence rate, per 1000 | 7.12 (6.90-7.34) | 4.82 (4.72-4.91) | ||
| person-yearsb | ||||
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CART, combination antiretroviral therapy; ellipses, not applicable; HIV, human immunodeficiency virus; HIV-1, Human immunodeficiency virus 1.
Data are percentage unless otherwise specified.
Adjusted for age and race/ethnicity.
The median age was 48.0 years in both groups. Participants with HIV infection were more likely to have hepatitis C virus coinfection (30.5% vs 11.4%) and cocaine abuse or dependence (21.9% vs 15.7%) and higher reported rate of current smoking (55.0% vs 45.3%), but were less likely to have hypertension (18.7% vs 28.8%) or diabetes (16.7% vs 24.8%) (P<.001 for all comparisons). The proportion of subjects with dyslipidemia or a diagnosis of alcohol abuse or dependence was similar in both groups. Patients with HIV infection had a lower mean (SD) body mass index compared with those without HIV infection (25.1 [4.2] vs 28.1 [5.3]).
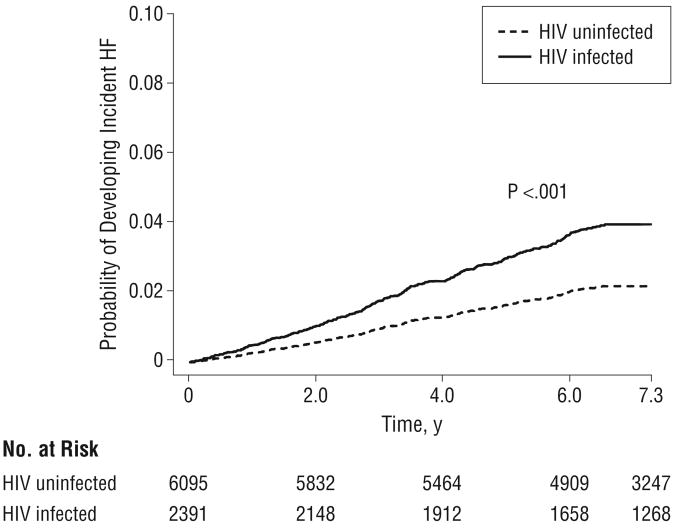
The age and race/ethnicity–adjusted rates of incident HF were 7.12 per 1000 person-years (95% CI, 6.90-7.34) for HIV-infected patients and 4.82 per 1000 person-years (95% CI, 4.72-4.91) for HIV-uninfected patients. Compared with HIV-uninfected patients, HIV-infected patients had a significantly increased risk of HF after adjusting for traditional risk factors (HR, 1.81; 95% CI, 1.39-2.36; Table 2 and Figure 1). Other factors significantly and positively associated with a risk of HF were increasing age, African American race, current smoking, body mass index greater than 30, hypertension, diabetes, and a diagnosis of alcohol abuse or dependence. Compared with patients without HIV infection, those with HIV infection who had baseline HIV-1 RNA levels of 500 or more copies/mL had a significantly higher risk of HF (adjusted HR, 2.28; 95% CI, 1.57-3.32). Those with HIV infection who had baseline and recent HIV-1 RNA levels less than 500 copies/mL, however, did not have an increased risk of HF (adjusted HR, 1.10; 95% CI, 0.64-1.89). The difference between the low HIV-1 RNA and high HIV-1 RNA group was statistically significant (P<.001) (Table 2).
Table 2. Association Between HIV Infection and Risk of Incident Heart Failurea.
| Characteristic | HR (95% CI) |
|---|---|
| HIV infection | 1.81 (1.39-2.36) |
| Age, per 10-y increase | 1.70 (1.50-1.93) |
| African American race vs non-African American | 1.32 (1.04-1.69) |
| race | |
| HCV infection | 1.00 (0.73-1.39) |
| BMI >30 vs <30b | 1.54 (1.18-2.01) |
| Dyslipidemia | 0.96 (0.74-1.23) |
| Hypertension | 1.95 (1.51-2.52) |
| Diabetes mellitus | 1.97 (1.53-2.53) |
| Current smoking | 1.31 (1.01-1.69) |
| History of alcohol abuse or dependence | 1.55 (1.16-2.08) |
| diagnosis | |
| History of cocaine abuse or dependence | 1.08 (0.76-1.54) |
| diagnosis | |
| Additional models for HIV infected vs | |
| HIV uninfectedb | |
| HIV-1 RNA | |
| HIV infected with baseline and recent HIV-1 | 1.10 (0.64-1.89)c |
| RNA levels <500 copies/mL | |
| HIV infected with baseline but no recent | 2.39 (1.25-4.58)c |
| HIV-1 RNA level <500 copies/mL | |
| HIV infected with baseline HIV-1 RNA level | 2.28 (1.57-3.32)c |
| ≥500 copies/mL | |
| CD4+ lymphocyte count | |
| <200/mm3 | 1.72 (1.23-2.40)d |
| ≥200/mm3 | 1.98 (1.09-3.61) |
| CART exposure | |
| CART naive | 2.22 (1.45-3.40)e |
| CART experienced | 1.60 (1.15-2.24) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CART, combination antiretroviral therapy; CI, confidence interval; HCV, hepatitis C virus; HIV, human mmunodeficiency virus; HIV-1, Human immunodeficiency virus 1; HR hazard ratio.
Multivariable Cox proportional hazards regression model of 234 events Patients with a diagnosis of coronary heart disease at study entry were excluded from analysis.
The HRs are adjusted for all variables listed.
P= 0.03 between patients with baseline and recent HIV-1 RNA levels less than 500 copies/mL and those with baseline but no recent HIV-1 RNA leve less than 500 copies/mL; P< 0.001 between patients with baseline and recent HIV-1 RNA levels less than 500 copies/mL and those with baseline HIV-1 RNA level of 500 or more copies/mL.
P= 0.65 between low CD4+ and high CD4+ groups.
P= 0.18 between CART-naive and CART-experienced groups
Figure 1.
Human immunodeficiency virus (HIV) status and the risk of heart failure (HF) for the total cohort.
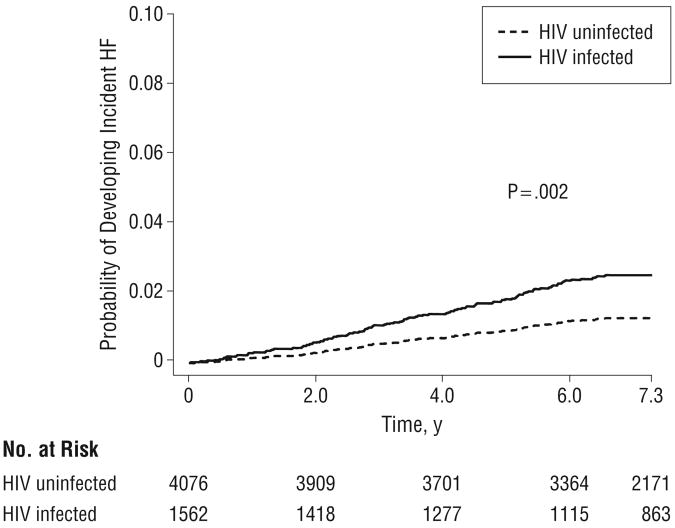
Although all participants in our study did not have CHD, HF, or angina at the time of enrollment, we conducted additional analyses excluding patients who developed CHD during the follow-up period prior to the diagnosis of HF, had a diagnosis of alcohol abuse or dependence, or both (Table 3 and Figure 2). Among patients without a CHD event prior to the diagnosis of HF, the association between HIV infection and HF persisted (HR, 1.92; 95% CI, 1.42-2.61). Similarly, among patients who did not have a diagnosis of alcohol abuse or dependence at baseline or during the follow-up period, HIV infection remained significantly associated with an increased risk of HF (HR, 1.94; 95% CI, 1.36-2.76). These results persisted when we further restricted the sample to patients who had neither a diagnosis of CHD during the follow-up period prior to the diagnosis of HF nor a diagnosis of alcohol abuse or dependence (HR, 1.96; 95% CI, 1.29-2.98).
Table 3.
Association Between HIV Infection and Risk of Incident Heart Failure Among Patients Without a CHD or Alcohol Abuse or Dependence Diagnosis Prior to the Incident Heart Failure Eventa
| HR (95% CI) | |||
|---|---|---|---|
|
|
|||
| Characteristic | Model 1b | Model 2c | Model 3d |
| HIV infection | 1.92 (1.42-2.61) | 1.94 (1.36-2.76) | 1.96 (1.29-2.98) |
| HCV infection | 0.99 (0.68-1.43) | 0.99 (0.59-1.66) | 1.12 (0.63-1.97) |
| Age | 1.50 (1.29-1.75) | 1.71 (1.47-1.99) | 1.49 (1.24-1.79) |
| African American race | 1.55 (1.16-2.06) | 1.34 (0.98-1.83) | 1.71 (1.18-2.48) |
| BMI >30 | 1.29 (0.94-1.78) | 1.91 (1.37-2.68) | 1.49 (0.99-2.20) |
| Dyslipidemia | 0.86 (0.64-1.17) | 1.00 (0.73-1.39) | 0.89 (0.61-1.31) |
| Hypertension | 2.14 (1.58-2.89) | 2.14 (1.52-3.02) | 2.51 (1.67-3.79) |
| Diabetes mellitus | 1.94 (1.45-2.61) | 1.96 (1.41-2.71) | 1.81 (1.23-2.68) |
| Current smoking | 1.41 (1.04-1.90) | 1.32 (0.95-1.83) | 1.35 (0.92-1.98) |
| History of alcohol abuse or dependence diagnosis | 1.53 (1.09-2.15) | ||
| History of cocaine abuse or dependence diagnosis | 0.98 (0.65-1.48) | 1.21 (0.49-3.02) | 0.87 (0.27-2.79) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CHD, coronary heart disease; CI, confidence interval; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HR, hazard ratio.
Patients with a diagnosis of CHD at study entry were excluded from all models.
Patients without prior CHD; number of events, 207; number of participants, 8406.
Patients without prior alcohol abuse or dependence; number of events, 167; number of participants, 5689.
Patients without prior CHD or alcohol abuse or dependence; number of events, 117; number of participants, 5638.
Figure 2.
Human immunodeficiency virus (HIV) status and the risk of heart failure (HF) among patients without a coronary heart disease or alcohol abuse or dependence diagnosis prior to the incident HF event.
Comment
Our data suggest that HIV infection is associated with an increased risk of HF after adjusting for traditional CHD risk factors. This risk persisted even after restricting the sample to patients who did not have a diagnosis of CHD, HF, or angina at baseline or a diagnosis of CHD during the follow-up period prior to the diagnosis of HF, a diagnosis of alcohol abuse/dependence, or both. Ongoing viral replication (HIV-1 RNA level ≥500 copies/mL) was associated with a higher risk of developing HF. However, HIV-infected participants with baseline and recent HIV-1 RNA level less than 500 copies/mL did not have an increased risk of HF compared with HIV-uninfected participants.
To our knowledge, there are no definitive studies on the risk of HF that compared HIV-infected with HIV-uninfected people who are free of baseline CHD. Diastolic dysfunction has been reported in 48% to 50% of HIV-infected persons, but the proportion of this dysfunction attributable to preexisting CHD is not known.29-31 Myocarditis is present in up to half of patients with AIDS in autopsy studies,32,33 but overt antemortem HF or autopsy evidence of ventricular dysfunction is not present in all such cases. In addition, these smaller studies are from the pre-CART era and lack HIV-uninfected controls to determine a direct association with HIV infection. In contrast, one study5 of 91 HIV-infected participants did not find any significant evidence of right or left ventricular dysfunction as measured by radionuclide ventriculography. To our knowledge, our study is the largest to investigate the relationship between HF and HIV infection and the first to demonstrate such an association and provide evidence of HIV infection as an independent risk factor for HF.
Although the exact mechanism by which HIV infection is associated with HF is not well understood, several possible mechanisms exist, including direct effects of the HIV, comorbidities associated with HIV infection (eg, heavy alcohol consumption), antiretroviral therapy leading to an increased risk of CHD and subsequent HF, nutritional deficiencies, and immunologic damage to the myocardium. Our results suggest that HIV itself is playing an important and independent role. Even after excluding patients with a baseline history of CHD, HF, and angina, as well as a CHD event in the follow-up period prior to the diagnosis of HF and a history of alcohol abuse or dependence diagnosis, the risk of incident HF was still substantial among the HIV-infected cohort. Ongoing HIV replication appears to play an important role. Compared with HIV-uninfected participants, only participants with an HIV-1 RNA level greater than 500 copies/mL had a significantly increased risk of HF.
For participants who had baseline and recent HIV-1 RNA levels less than 500 copies/mL, there was no significantly increased risk of HF (Table 2). Antiretroviral therapy was associated with a slightly attenuated risk, although the difference between CART-naive and CART-experienced groups did not reach statistical significance. However, these results should be interpreted carefully given the small number of events in the stratified categories. Our findings that hypertension, obesity, alcohol abuse or dependence, and diabetes mellitus were associated with an increased risk of HF are consistent with reports34,35 from other established cohort studies of people without HIV infection and offer the possibility of interventions to reduce the risk of HF. In the current study, we were not able to assess the differential risk between controlled and uncontrolled hypertension or diabetes, but this is an attractive topic for further research.
Secondary infection of the myocardium in HIV-infected persons may also lead to myocarditis, myocardial dysfunction, and HF. Presence of cytomegalovirus, acid-fast bacilli, Toxoplasma gondii, Candida species, Histoplasma capsulatum, Cryptococcus neoformans, and Staphylococcus aureus in the myocardium of HIV-infected patients has been reported.11,33,36 A causal association is not clear in all instances, since in some cases, myocarditis is not associated with adjacent myocyte necrosis on histologic examination and evidence of disseminated disease is not present.
Other causes of heart muscle disease in HIV-infected persons include immunologic damage, nutritional deficiencies (eg, selenium, antioxidant vitamins, and carnitine), and antiretroviral therapy.11,13 Zidovudine has been associated with a specific dose-dependent skeletal myopathy attributed to mitochondrial toxicity and with cardiac dysfunction that resolves with discontinuation of the drug.11,12 However, in a study37 in HIV-infected children, zidovudine was not associated with worsening of cardiac function. We were unable to analyze the occurrence of HF associated with any specific antiretroviral drugs.
The role of traditional risk factors in the risk of HF needs to be emphasized. Increasing age, African American race, obesity, hypertension, diabetes, and alcohol use are established risk factors and were also significantly associated with a higher risk of HF in our study. Our data tend to suggest that these factors increase the risk of HF independent of their risk on clinically diagnosed CHD, evidenced by the fact that they remained significant even after people with prior CHD were excluded from the study. However, this finding should be interpreted with caution because we could not exclude subclinical CHD. Interventions to minimize the modifiable traditional risk factors, including glycemic and blood pressure control, weight reduction, and abstinence from alcohol, are prudent strategies that should be emphasized. The actual effect of such strategies on risk reduction among HIV-infected persons requires further study.
The strengths of our study include large numbers, participants drawn from validated and well-established cohorts, a national rather than geographically limited sample, and availability of HIV-uninfected controls. Certain limitations need to be understood as well. The diagnosis of HF was based on ICD-9 codes; however, codes for various cardiovascular end points have been extensively used in previous publications3,24,25 and are considered reasonable alternatives to adjudicated clinical outcomes. Moreover, since HF is a chronic condition, our ability to capture outpatient clinical diagnoses in the present study is advantageous; if the initial diagnosis of HF were made at an outside hospital, the diagnosis could be captured in our study via routine follow-up outpatient care within the VA health care system. We did not assess the effect of specific antiretroviral drugs owing to the small number of events for individual drugs, nor did we assess the role of adherence to medication therapy and control of blood pressure or blood glucose levels in the risk of HF. Our study was limited to men and so may not be generalized to women. Other than men being the predominant population, the HIV infection epidemic in veterans is largely similar to that in nonveterans.20 Since we used administrative data to identify incident HF events, this study was not able to distinguish between HF associated with systolic vs diastolic dysfunction. Finally, while we excluded diagnosed CHD as a preceding event to HF, we did not exclude subclinical atherosclerosis as a cause of HF.
There are major clinical implications of our findings. If HF is a major cardiovascular consequence of HIV infection rather than atherosclerotic heart disease, different approaches to manage such consequences are warranted. Cardiovascular risk factor reduction and antiplatelet agents (eg, aspirin) are the mainstay in the management of atherosclerotic heart disease; however, these strategies, plus aggressive blood pressure control and the treatment of the HIV infection, may also be required to prevent development of HF in this population. The exact approach would depend on the mechanism and mediators of this risk; therefore, further studies are needed. Several risk factors identified are modifiable, and intervention studies may be designed to look at the effect of strict blood pressure control, tight glycemic control, and weight loss on the risk of HF. Additional studies are also warranted to fully understand the mechanism of HF in HIV-infected persons, especially to understand the nature of HF, eg, systolic vs diastolic dysfunction.
In conclusion, HIV infection is associated with an increased risk of HF after adjusting for traditional risk factors for HF. This association persisted even after exclusion of patients with a baseline history of CHD, HF, and angina, as well as a CHD event in the follow-up period prior to the diagnosis of HF and a history of alcohol abuse or dependence diagnosis. Ongoing viral replication is associated with a higher risk of HF. Further studies to fully characterize this association and to understand the underlying mechanisms are warranted.
Acknowledgments
Funding/Support: Funding for the Veterans Aging Cohort Study was provided by grant U10 AA 13566 from the National Institute on Alcohol Abuse and Alcoholism and Veterans Health Administration Public Health Strategic Health Core Group, grant HL095136-03 from the National Heart, Lung, and Blood Institute (Dr Freiberg), and grant AG024896 from the National Institute on Aging (Dr Oursler).
Footnotes
Author Contributions: Study concept and design: Butt, Leaf, Gibert, Oursler, Justice, and Freiberg. Acquisition of data: Leaf, Chang, Rimland, Kazis, Justice, and Freiberg. Analysis and interpretation of data: Butt, Chang, Kuller, Goetz, Leaf, Rimland, Gibert, Oursler, Rodriguez-Barradas, Lim, Gottlieb, Justice, and Freiberg. Drafting of the manuscript: Butt, Kuller, Leaf, and Freiberg. Critical revision of the manuscript for important intellectual content: Butt, Chang, Kuller, Goetz, Leaf, Rimland, Gibert, Oursler, Rodriguez-Barradas, Lim, Kazis, Gottlieb, Justice, and Freiberg. Statistical analysis: Chang and Rimland. Obtained funding: Oursler, Justice, and Freiberg. Administrative, technical, and material support: Goetz, Leaf, Rim-land, and Justice. Study supervision: Kuller, Lim, and Justice.
Financial Disclosure: None reported.
Publisher's Disclaimer: Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Additional Contributions: This material is the result of work supported with resources and the use of facilities at the VA Pittsburgh Healthcare System and the central data repositories maintained by the VA Information Resource center, including the National Patient Care Database, Decision Support System database, and the Pharmacy Benefits Management database.
Contributor Information
Dr Adeel A. Butt, Department of Medicine, University of Pittsburgh School of Medicine, and Veterans Affairs (VA) Pittsburgh Healthcare System, Pittsburgh, Pennsylvania.
Dr Chung-Chou Chang, Department of Medicine, University of Pittsburgh School of Medicine.
Dr Lewis Kuller, Department of Epidemiology, University of Pittsburgh Graduate School of Public Health, Pittsburgh.
Dr Matthew Bidwell Goetz, VA Greater Los Angeles Healthcare System and the David Geffen School of Medicine at the University of California, Los Angeles.
Dr David Leaf, VA Greater Los Angeles Healthcare System and the David Geffen School of Medicine at the University of California, Los Angeles.
Dr David Rimland, VA Medical Center and Emory University School of Medicine, Atlanta, Georgia.
Dr Cynthia L. Gibert, VA Medical Center and George Washington University, Washington, DC.
Dr Krisann K. Oursler, University of Maryland School of Medicine and VA Maryland Healthcare System, Baltimore.
Dr Maria C. Rodriguez-Barradas, Michael E. DeBakey VA Medical Center and Baylor College of Medicine, Houston, Texas.
Dr Joseph Lim, Yale University School of Medicine and Public Health, New Haven, Connecticut.
Dr Lewis E. Kazis, Center for the Assessment of Pharmaceutical Practices, Department of Health Policy and Management, Boston University School of Public Health, Boston, Massachusetts.
Dr Stephen Gottlieb, University of Maryland School of Medicine and Baltimore VA Medical Center, Baltimore.
Dr Amy C. Justice, Yale University School of Medicine and Public Health, New Haven, Connecticut, and VA Connecticut Healthcare System, West Haven.
Dr Matthew S. Freiberg, Department of Medicine, University of Pittsburgh School of Medicine.
References
- 1.Vittecoq D, Escaut L, Chironi G, et al. Coronary heart disease in HIV-infected patients in the highly active antiretroviral treatment era. AIDS. 2003 Apr;17(suppl 1):S70–S76. doi: 10.1097/00002030-200304001-00010. [DOI] [PubMed] [Google Scholar]
- 2.Friis-Møller N, Reiss P, Sabin CA, et al. DAD Study Group. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356(17):1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 3.Bozzette SA, Ake CF, Tam HK, Chang SW, Louis TA. Cardiovascular and cerebrovascular events in patients treated forhuman immunodeficiency virus infection. N Engl J Med. 2003;348(8):702–710. doi: 10.1056/NEJMoa022048. [DOI] [PubMed] [Google Scholar]
- 4.Currier JS, Taylor A, Boyd F, et al. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr. 2003;33(4):506–512. doi: 10.1097/00126334-200308010-00012. [DOI] [PubMed] [Google Scholar]
- 5.Kristoffersen US, Lebech AM, Gerstoft J, et al. Right and left cardiac function in HIV-infected patients investigated using radionuclide ventriculography and brain natriuretic peptide: a 5-year follow-up study. HIV Med. 2008;9(3):180–186. doi: 10.1111/j.1468-1293.2007.00531.x. [DOI] [PubMed] [Google Scholar]
- 6.Barbaro G, Di Lorenzo G, Cirelli A, et al. An open-label, prospective, observational study of the incidence of coronary artery disease in patients with HIV infection receiving highly active antiretroviral therapy. Clin Ther. 2003;25(9):2405–2418. doi: 10.1016/s0149-2918(03)80283-7. [DOI] [PubMed] [Google Scholar]
- 7.El-Sadr WM, Lundgren JD, Neaton JD, et al. Strategies for Management of Antiretroviral Therapy (SMART) Study Group. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355(22):2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 8.Lichtenstein KA, Armon C, Buchacz K, et al. HIV Outpatient Study (HOPS) Investigators. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin Infect Dis. 2010;51(4):435–447. doi: 10.1086/655144. [DOI] [PubMed] [Google Scholar]
- 9.Laonigro I, Correale M, Di Biase M, Altomare E. Alcohol abuse and heart failure. Eur J Heart Fail. 2009;11(5):453–462. doi: 10.1093/eurjhf/hfp037. [DOI] [PubMed] [Google Scholar]
- 10.Kloner RA, Rezkalla SH. To drink or not to drink? that is the question. Circulation. 2007;116(11):1306–1317. doi: 10.1161/CIRCULATIONAHA.106.678375. [DOI] [PubMed] [Google Scholar]
- 11.Currie PF, Boon NA. Immunopathogenesis of HIV-related heart muscle disease current perspectives. AIDS. 2003 Apr;17(suppl 1):S21–S28. doi: 10.1097/00002030-200304001-00004. [DOI] [PubMed] [Google Scholar]
- 12.Dalakas MC, Illa I, Pezeshkpour GH, Laukaitis JP, Cohen B, Griffin JL. Mitochondrial myopathy caused by long-term zidovudine therapy. N Engl J Med. 322(16):1990. 1098–1105. doi: 10.1056/NEJM199004193221602. [DOI] [PubMed] [Google Scholar]
- 13.Khunnawat C, Mukerji S, Havlichek D, Jr, Touma R, Abela GS. Cardiovascular manifestations in human immunodeficiency virus–infected patients. Am J Cardiol. 2008;102(5):635–642. doi: 10.1016/j.amjcard.2008.04.035. [DOI] [PubMed] [Google Scholar]
- 14.McDonald CL, Kaltman JR. Cardiovascular disease in adultand pediatric HIV/AIDS. J Am Coll Cardiol. 2009;54(13):1185–1188. doi: 10.1016/j.jacc.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bahrami H, Bluemke DA, Kronmal R, et al. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;51(18):1775–1783. doi: 10.1016/j.jacc.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 16.Fultz SL, Skanderson M, Mole LA, et al. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care. 2006;44(8)(suppl 2):S25–S30. doi: 10.1097/01.mlr.0000223670.00890.74. [DOI] [PubMed] [Google Scholar]
- 17.Butt AA, Fultz SL, Kwoh CK, Kelley D, Skanderson M, Justice AC. Risk of diabetes in HIV infected veterans pre- and post-HAART and the role of HCV coinfection. Hepatology. 2004;40(1):115–119. doi: 10.1002/hep.20289. [DOI] [PubMed] [Google Scholar]
- 18.McGinnis KA, Fultz SL, Skanderson M, Conigliaro J, Bryant K, Justice AC. Hepatocellular carcinoma and non-Hodgkin's lymphoma: the roles of HIV, hepatitis C infection, and alcohol abuse. J Clin Oncol. 2006;24(31):5005–5009. doi: 10.1200/JCO.2006.05.7984. [DOI] [PubMed] [Google Scholar]
- 19.Butt AA, Khan UA, McGinnis KA, Skanderson M, Kent Kwoh C. Co-morbid medical and psychiatric illness and substance abuse in HCV-infected and uninfected veterans. J Viral Hepat. 2007;14(12):890–896. doi: 10.1111/j.1365-2893.2007.00885.x. [DOI] [PubMed] [Google Scholar]
- 20.Butt AA, McGinnis KA, Rodriguez-Barradas MC, et al. Veterans Aging Cohort Study. HIV infection and the risk of diabetes mellitus. AIDS. 2009;23(10):1227–1234. doi: 10.1097/QAD.0b013e32832bd7af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butt AA, Wang X, Moore CM. Effect of hepatitis C virus and its treatment on survival. Hepatology. 2009;50(2):387–392. doi: 10.1002/hep.23000. [DOI] [PubMed] [Google Scholar]
- 22.Butt AA, McGinnis KA, Skanderson M, Justice AC. Hepatitis C treatment completion rates in routine clinical care. Liver Int. 2010;30(2):240–250. doi: 10.1111/j.1478-3231.2009.02156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee DS, Donovan L, Austin PC, et al. Comparison of coding of heart failure and comorbidities in administrative and clinical data for use in outcomes research. Med Care. 2005;43(2):182–188. doi: 10.1097/00005650-200502000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Petersen LA, Wright S, Normand SL, Daley J. Positive predictive value of the diagnosis of acute myocardial infarction in an administrative database. J Gen Intern Med. 1999;14(9):555–558. doi: 10.1046/j.1525-1497.1999.10198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butt AA, Xiaoqiang W, Budoff M, Leaf D, Kuller LH, Justice AC. Hepatitis C virus infection and the risk of coronary disease. Clin Infect Dis. 2009;49(2):225–232. doi: 10.1086/599371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events: the Cardiovascular Health Study. Ann Epidemiol. 1995;5(4):278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 27.Kraemer KL, McGinnis KA, Skanderson M, et al. Alcohol problems and health care services use in human immunodeficiency virus (HIV)–infected and HIV–uninfected veterans. Med Care. 2006;44(8)(suppl 2):S44–S51. doi: 10.1097/01.mlr.0000223703.91275.78. [DOI] [PubMed] [Google Scholar]
- 28.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–526. doi: 10.1093/biomet/81.3.515. [DOI] [Google Scholar]
- 29.Reinsch N, Neuhaus K, Esser S, et al. German Competence Network for Heart Failure; German Competence Network for HIV AIDS. Prevalence of cardiac dia-stolic dysfunction in HIV-infected patients: results of the HIV-HEART study. HIV Clin Trials. 2010;11(3):156–162. doi: 10.1310/hct1103-156. [DOI] [PubMed] [Google Scholar]
- 30.Hsue PY, Hunt PW, Ho JE, et al. Impact of HIV infection on diastolic function and left ventricular mass. Circ Heart Fail. 2010;3(1):132–139. doi: 10.1161/CIRCHEARTFAILURE.109.854943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisher SD, Easley KA, Orav EJ, et al. Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted HIV Infection (P2C2 HIV) Study Group. Mild dilated cardiomyopathy and increased left ventricular mass predict mortality: the prospective P2C2 HIV Multicenter Study. Am Heart J. 2005;150(3):439–447. doi: 10.1016/j.ahj.2005.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reilly JM, Cunnion RE, Anderson DW, et al. Frequency of myocarditis, left ventricular dysfunction and ventricular tachycardia in the acquired immune deficiency syndrome. Am J Cardiol. 1988;62(10, pt 1):789–793. doi: 10.1016/0002-9149(88)91223-4. [DOI] [PubMed] [Google Scholar]
- 33.Anderson DW, Virmani R, Reilly JM, et al. Prevalent myocarditis at necropsy in the acquired immunodeficiency syndrome. J Am Coll Cardiol. 1988;11(4):792–799. doi: 10.1016/0735-1097(88)90213-6. [DOI] [PubMed] [Google Scholar]
- 34.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Levy D. Left ventricular dilatation and the risk of congestive heart failure in people without myocardial infarction. N Engl J Med. 1997;336(19):1350–1355. doi: 10.1056/NEJM199705083361903. [DOI] [PubMed] [Google Scholar]
- 35.Ingelsson E, Sundström J, Arnlöv J, Zethelius B, Lind L. Insulin resistance and risk of congestive heart failure. JAMA. 2005;294(3):334–341. doi: 10.1001/jama.294.3.334. [DOI] [PubMed] [Google Scholar]
- 36.Wu TC, Pizzorno MC, Hayward GS, et al. In situ detection of human cytomega-lovirus immediate-early gene transcripts within cardiac myocytes of patients with HIV-associated cardiomyopathy. AIDS. 1992;6(8):777–785. doi: 10.1097/00002030-199208000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Lipshultz SE, Orav EJ, Sanders SP, Hale AR, McIntosh K, Colan SD. Cardiac structure and function in children with human immunodeficiency virus infection treated with zidovudine. N Engl J Med. 1992;327(18):1260–1265. doi: 10.1056/NEJM199210293271802. [DOI] [PubMed] [Google Scholar]