Abstract
Background
The metrics used to assess quality of care and pay for performance are increasingly important. Medicare established the Physician Quality Reporting System (PQRS) that allowed physicians to report performance measures for many conditions including osteoporosis and rheumatoid arthritis (RA). We described the frequency and nature of physician-reported reasons why recommended care for individual osteoporosis and RA patients was not provided.
Methods
Using national data on Medicare fee-for-service beneficiaries (2007-2009), we identified healthcare providers reporting on quality of care for any of 3 osteoporosis or 3 RA measures. PQRS reason codes allowed physicians to submit explanations why recommended care was not given.
Results
In 2009, 1775 physicians reported on >= 1 osteoporosis PQRS measure, and 630 physicians reported on >= 1 RA measure. For patients for whom their physician reported via PQRS on lifetime DXA screening since age 60, 76% of older women had received such screening. Among patients with physician-diagnosed osteoporosis reported via PQRS, 82% received prescription osteoporosis medication in the preceding year. For RA medication use reported via PQRS, 89% of patients received a DMARD or a biologic. For the remaining 11-24% of osteoporosis and RA patients, their physicians reported medical, patient, system, or other reasons why care was considered but not provided.
Conclusions
A substantial fraction of Medicare enrollees who did not receive recommended osteoporosis or RA care had physician-documented reasons for why care was not provided. For Medicare and other health plans that implement penalties for apparent nonperformance (or delivery of suboptimal care), it will be important for allow physicians to provide reasons that care was considered medically inappropriate, refused, or otherwise not feasible.
Keywords: osteoporosis, rheumatoid arthritis, quality, Medicare, DMARD
Introduction
Controversies around the best methods to measure, benchmark, and reimburse for high quality care continue to spur debate at a national level[1, 2]. In 2004, the Centers for Medicare and Medicaid Services (CMS) implemented the Physician Quality Reporting Initiative, subsequently named the Physician Quality Reporting System (PQRS). This pilot program tested a system to allow healthcare providers to report on the quality of care they were delivering to patients enrolled in the Medicare program. PQRS was designed as “a first step toward linking Medicare health professionals’ payments to quality, which is consistent with Medicare’s ongoing transformation from passive payer to active purchaser of high-value healthcare.”[3]. Physicians who participated in the program could receive an annual incentive bonus equal to 2% of their annual revenues for all fees billed for their fee-for-service Medicare patients. This initially voluntary, pay for reporting program was intended to transition from an incentive-based program to improve quality to a mechanism by which physicians not meeting certain quality performance measures will receive reduced Medicare payments[4].
The number and breadth of PQRS measures have continued to grow over time. In 2007, quality measures relevant for musculoskeletal care were added in the form of 5 new quality measures for osteoporosis screening and treatment. The osteoporosis measures have changed over time, and new measures were added in 2008 for patients with rheumatoid arthritis (RA). While administrative databases from large health plans have long been used to assess quality of care in osteoporosis [5-9] and in RA [10-13], a unique feature of the PQRS program is that it allows providers to report care that was considered but not actually provided for one of several reasons, including those beyond the physicians’ control (e.g. cost of medication, patient refusal, non-adherence, etc.). This provides an important advantage over alternate reporting systems that do not allow the provider to document potentially legitimate reasons why quality indicator-recommend care was not provided.
To date, evaluation of the PQRS program has provided only limited results to inform the quality of care reported and delivered by participating healthcare providers. We therefore used national Medicare data to evaluate the physician-reported care provided for quality measures in osteoporosis and RA, with a particular focus on circumstances where the physician reported that they did NOT provide the recommended care for a reason beyond the physician’s control.
Methods
Data sources and patient eligibility
Study population and data source
After Institutional Review Board approval, we used the national Medicare data from CMS from 2007-2009 to identify enrollees in traditional fee-for-service Medicare with Part A and Part B coverage who were not enrolled in a Medicare Advantage plan. Analyses of medication use also required enrollment in a Part D prescription drug benefit plan. Characteristics of the 2009 source Medicare population in Supplemental Table 1 showed that patients eligible for this analysis had slightly lower income and were more likely to live in rural areas and to have a higher one year mortality compared to those with Medicare and non-part D drug plans or to those enrolled in Medicare Advantage. For the osteoporosis PQRS measures of interest, the Medicare random 5% sample data were available. For the RA measures, the full 100% Medicare population of RA patients was available. Physicians are identified in CMS data by unique physician identification numbers (UPIN) and national provider identifier (NPI) numbers. Patients were clustered within physician practices using UPIN and NPI numbers.
Identification and validation of claims-based PQRS measures for osteoporosis and RA
We used person-level Medicare claims that contained the relevant Healthcare Common Procedure Coding System (HCPCS) level II codes relevant for each PQRS measure. Some PQRS measures are appropriate on an ongoing, periodic basis (e.g. percentage of patients with an assessment and classification of RA disease activity within 12 months), some are relevant only once (e.g. any DXA or osteoporosis medication since the age of 60, tuberculosis testing prior to the first use of any biologic for RA), and some might never be relevant (e.g. counseling for glucocorticoid use among RA patients with documentation of a management plan, a measure irrelevant for patients not treated with glucocorticoids). We selected and evaluated the three osteoporosis and three RA PQRS measures, described in Table 1, based upon clinical interest and the expectation of the broadest applicability to a diverse osteoporosis and RA patient population.
Table 1.
Description of Medicare PQRS Osteoporosis and Rheumatoid Arthritis Quality Measures Relevant in 2009
| PQRS Measure number and construct | Criteria used to Define Eligible Population | Care Recommended by CMS PQRS Measure | Relevant HCPCS II Codes |
|---|---|---|---|
|
| |||
| Measure #39: Screening or Therapy for Osteoporosis for Women Aged 65 Years and Older | Female patients aged 65 years and older | Had a central dual-energy X-ray absorptiometry (DXA) measurement ordered or performed at least once since age 60 or pharmacologic therapy prescribed within 12 months | 3095F – DXA results available, DXA not ordered |
| 3096F – DXA ordered | |||
| 4005F – prescription osteoporosis medication ordered | |||
|
| |||
| Measure #40: Osteoporosis: Management Following Fracture | Patients aged 50 years and older with fracture of the hip, spine or distal radius | Central dual-energy X-ray absorptiometry (DXA) measurement ordered or performed or pharmacologic therapy prescribed | 3095F – DXA results available, DXA not ordered |
| 3096F – DXA ordered | |||
| 4005F – prescription osteoporosis medication ordered | |||
|
| |||
| Measure #41: Osteoporosis: Pharmacologic Therapy | Patients aged 50 years and older with a diagnosis of osteoporosis | Pharmacologic therapy (other than minerals/vitamins) for osteoporosis prescribed | 4005F – prescription osteoporosis medication ordered |
|
| |||
| #108: Disease Modifying Anti-Rheumatic Drug Therapy in Rheumatoid Arthritis | Patients 18 years and older with a diagnosis of RA | Prescribed, dispensed, or administered a disease modifying anti-rheumatic drug (DMARD) or biologic (at least 1 prescription or infusion) | 4187 – DMARD prescribed |
|
| |||
| Measure #177: Rheumatoid Arthritis (RA): Periodic Assessment of Disease Activity | Patients 18 years and older with a diagnosis of RA | Assessment and classification of disease activity within 12 months | 3470F – RA disease activity assessed |
|
| |||
| Measure #178: Rheumatoid Arthritis (RA): Functional Status Assessment | Patients 18 years and older with a diagnosis of RA | Functional status assessment was performed at least once within 12 months | 1170F – RA functional status assessed |
DXA = dual x-ray energy absorptiometry; RA = rheumatoid arthritis; DMARD = disease modifying anti-rheumatic drug; HCPCS = Healthcare Common Procedure Coding System
The HCPCS II codes that allow for PQRS reporting are submitted to CMS at the time when a physician has a face-to-face encounter with a patient with a qualifying diagnosis (e.g. osteoporosis, RA). If a patient had the same PQRS measure reported more than once in each calendar year, the last claim in each calendar year that applied to the PQRS measure was used. Modifier codes to the HCPCS II codes allow the physician to designate whether the patient actually received the recommended care, or whether there were medical (care not indicated or contraindicated), patient-related (e.g. patient declined, economic, religious, non-adherence), system (e.g. resources to perform service not available, insurance coverage/ payer related limits), or other unspecified reasons why the care was considered but was not provided.
For two PQRS measures, we used Medicare prescription drug data and claims for administration of parenteral medications to assess the validity of PQRS reporting. Measure 41 indicates that a patient received at least one prescription for an oral, infusion, or injectable prescription osteoporosis medication [an oral or intravenous bisphosphonate, raloxifene, teriparatide, calcitonin, or systemic hormone therapy]. Measure 108 indicates that an RA patient received at least one oral, infused, or injected DMARD including biologics [hydroxychloroquine, methotrexate, leflunomide, sulfasalazine, gold, azathioprine, cyclosporin, cyclophosphomide, mycophenalate, minocycline, penacillamine, anti-tumor necrosis factor (TNF) therapy, rituximab, abatacept, anakinra]. To assess drug utilization in relation to the PQRS claim reported, we required that each patient have Part D coverage in the 12 months prior to and the 2 months following the PQRS claim date. The purpose of this 14 month window was to assess whether the patient had filled any osteoporosis or RA medication in the 12 months prior to the PQRS measure being reported, or 2 months into the future for new prescriptions.
For these two drug-related PQRS measures, we then evaluated PQRS performance for patients whose physicians reported the PQRS measure compared to that physicians’ patients meeting the same eligibility criteria described in Table 1 (i.e. had osteoporosis or RA physician encounters with the pre-specified diagnosis codes) yet did not have the PQRS measure reported. Eligible patients of physicians who never reported on any osteoporosis or RA PQRS measure were also examined in a similar fashion.
Statistical analysis
Descriptive statistics compared the characteristics of physicians reporting on any of the osteoporosis or RA PQRS measures to those of physicians who did not report. Physician characteristics, such as specialty, age, and rural/urban practice setting were obtained from the American Medical Association Masterfile and provided information even for doctors who did not report on any PQRS measure.
We performed a validity check on the legitimacy of the reasons given on PQRS claims as to why patients were not treated with osteoporosis or RA medications (PQRS measures 41 and 108). Alternating logistic regression [14] was used to account for the clustering of patients within physician practices and to identify factors associated with patients having reason(s) given on a PQRS claim as to why recommended care was not provided referent to patients with PQRS claims treated by those same physicians and for whom no reason codes were given (indicating that the recommended care was provided). Age and comorbidities were the key independent factors of interest and were selected based upon content knowledge regarding conditions for which a physician might deem that a patient might be contraindicated to receive a prescription osteoporosis or RA medication. Comorbidities included chronic kidney disease, hepatitis C, liver disease, chronic obstructive pulmonary disease (COPD), diabetes, HIV, malignancy, and heart failure. We also included in statistical models gender, race/ethnicity, dual eligible status, low income subsidy, geographic region of the U.S., and household income defined by census block group. All analyses were performed in SAS version 9.3 (SAS Institute, Cary, NC).
Results
PQRS reporting was examined to inform the quality of care provided in both RA and osteoporosis across the entire Medicare program. Using data from the random 5% sample, a total of 1,775 physicians reported on any of the 3 listed osteoporosis PQRS measures in 2009 (Table 2). This number represented a minority of physicians treating osteoporosis and fracture patients, as 373,780 additional physicians treating these kinds of patients did not report on an osteoporosis PQRS measure. The median age of the physicians reporting was 50 years, and 71% were male. The majority of physicians (91%) reported on DXA screening (PQRS Measure 39), few (8%) reported on post-fracture care (Measure 40), and 60% reported on prescription medication use for osteoporosis patients (Measure 41).
Table 2.
Characteristics of Physicians* Reporting at Least Once in 2009 for Any Osteoporosis or Rheumatoid Arthritis PQRS Measure
| PQRS Measure Group | Reported on at Least 1 Measure among any of the 3 OP or RA PQRS Measures | Did not Report on any of the 3 OP or RA PQRS Measures |
|---|---|---|
|
| ||
|
Osteoporosis Measures
| ||
| Physicians, n | 1,775 | 373,780 |
| Median age, years (IQR)* | 50 (44,56) | 52 (45,59) |
| Male sex* | 71.1% (981) | 80.1% (246,797) |
| Rural practice setting | 12.8% (176) | 12.6% (31,008) |
| Specialty** | ||
| Rheumatology | 13.3% (184) | N/A |
| Internal Medicine | 35.4% (488) | |
| Family Practice | 25.9% (437) | |
| PQRS Measure Reported | ||
| Measure 39 (DXA screening) | 91.1% (1,617) | |
| Measure 40 (post fracture care) | 7.9% (141) | |
| Measure 41 (OP medication use) | 60.6% (1,076) | |
|
| ||
|
Rheumatoid Arthritis Measures
| ||
| Physicians, n | 630 | 83,849 |
| Median age, years (IQR)* | 52 (47,60) | 53 (46, 60) |
| Male gender* | 75.5% (360) | 80.5% (50,630) |
| Rural practice setting | 5.8% (28) | 18.8% (11,811) |
| Specialty** | ||
| Rheumatology | 70.3% (336) | N/A |
| Internal Medicine | 18.2% (87) | |
| Family Practice | 3.5% (17) | |
| Measure reported on | ||
| Measure 108 (DMARD use) | 95.8% (604) | |
| Measure 177 (RA disease activity) | 29.0% (183) | |
| Measure 178 (functional status) | 34.0% (215) | |
N/A = not applicable; IQR = inter-quartile range; OP = osteoporosis; RA = rheumatoid arthritis; DXA = dual xray absorptiometry
using information available in the American Medical Association Masterfile
specialty proportions listed do not sum to 100% as only the top 3 specialties reporting were shown
For RA patients using the 100% CMS data, 630 physicians reported on PQRS measures in 2009; most were rheumatologists. The majority reported on DMARD use (Measure 108, 96%); approximately one-third reported on RA disease activity and/or functional status. Physicians reporting PQRS measures generally were similar in age to those not reporting PQRS measures, and a slightly greater proportion of women physicians reported. In 2009, 11.6% of all U.S. rheumatologists reported on DMARD use for their patients through the PQRS program, which was a higher proportion than in 2008 (6.4% of rheumatologists) [data not shown].
Table 3 describes the patients and reason codes (if present) that physicians used to report whether osteoporosis and RA care was provided, and if not, to provide further explanation(s). For Measure 39 (DXA screening, 4,179 patients) 76% of physicians reported that the patient received a DXA at least once since the age of 60, or were prescribed an osteoporosis medication. The remaining 24% had one or more reason why DXA was not provided. For Measure 40 (osteoporosis management post fracture, 172 patients), only 37.8% of providers reported that the patient received recommended care. Reasons were given for the remaining patients, the most common of which was “other” i.e. “reason not otherwise categorized”. Among 2,351 patients with osteoporosis, their physicians reported that they provided prescription medication for 81.9%.; medical (e.g. comorbidities) and patient-related reasons (e.g. refusal) were less common explanations than non-specific ‘other’ reasons for not providing medication.
Table 3.
Provision of Care and Reasons Care was Not Provided According to Results Reported by Physicians via PQRS, Osteoporosis and Rheumatoid Arthritis Patients from PQRS in 2009*
| Osteoporosis PQRS Measures** | RA PQRS Measures** | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Measure 39 – DXA Screening | Measure 40 – Post Fracture Management | Measure 41 – Prescription OP Medication | Measure 108 – DMARD Use | Measure 177 – Disease Activity Measurement*** | Measure 178 – Functional Status Measurement | |
|
| ||||||
| Unique Patients, n | 4,179 | 172 | 2,351 | 26,408 | 6,563 | 8,362 |
|
| ||||||
| Reported as Providing Recommended Care | 76.0 (3,178) | 37.8 (65) | 81.9 (1,927) | 88.8 (23,458) | 85.9 (5,639) | 80.4 (6,721) |
|
| ||||||
| Physician-Provided Reason for Not Providing Recommended Care * | N/A | N/A | ||||
| Medical Reason | 6.4 (269) | 9.3 (16) | 3.2 (76) | 6.6 (1,740) | ||
| Patient reason (e.g. refusal) | 4.1 (172) | 2.9 (5) | 3.1 (72) | N/A | ||
| System reason (e.g. cost) | 1.6 (66) | 4.1 (7) | 0.1 (14) | N/A | ||
| Other reason not specified | 12.9 (540) | 45.9 (79) | 11.4 (269) | 5.0 (1,333) | ||
Data shown are % (n)
N/A = not applicable (i.e. reason codes not relevant for this measure)
these reasons are not mutually exclusive and therefore column percentages may sum to greater than 100%
these data represent only results for patients for whom the measure was reported on, as described in Table 2
using a validated instrument
For RA, physicians reported on DMARD use for 26,408 of their RA patients. A total of 88.8% of patients were described as having received recommended care. Medical reasons (e.g. patient in remission, medical contraindication) were the most common reason given for why care was not provided. A smaller group of RA patients had their RA disease activity or functional status measured. Among the 6,563 patients who had their RA disease activity measured and reported, 14.1% were measured using a non-standardized instrument (e.g. physician gestalt). For the remaining patients, 44.0% of patients were reported by their physicians as being in low disease activity, 32.0% as moderate disease activity, and 9.9% as having high disease activity.
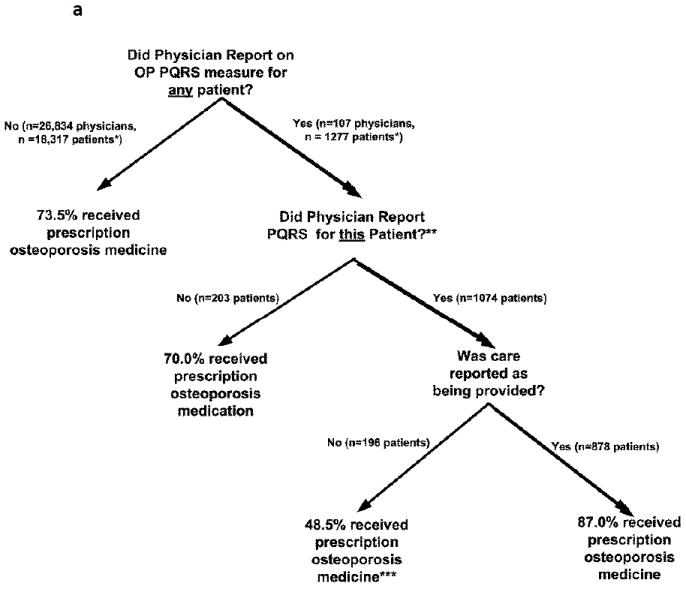
We validated prescription medication use for patients with osteoporosis (Measure 41) and DMARD/biologic use for RA patients (Measure 108) (Figure 1). The analysis was restricted to patients who had 12 months of part D Medicare coverage prior to and 2 months’ coverage after the relevant osteoporosis or RA physician visit. For patients whose physicians reported that they had prescribed an osteoporosis drug (the majority of all that physicians’ osteoporosis patients), confirmation using pharmacy or infusion codes was obtained for 87% in the previous 12 months or subsequent 2 months from the date of the PQRS claim (Figure 1a). Even for patients whose physicians reported that there was a reason for which care was not provided, 49% had evidence of a filled prescription or infusion for an osteoporosis medication. Overall, 77% of osteoporosis patients treated by physicians reporting on PQRS Measure 41 had evidence that they received a prescription medication. Overall, 74% of patients (across all nodes in the tree) received a prescription osteoporosis medication.
Figure 1. Receipt of Osteoporosis and RA Prescription Medications in 2009 according to PQRS Measure Reporting Status.
a
* all patients in this & subsequent branches had to have Medicare part A + B + part D coverage as described in the text
** overall, 77% of the 1,277 patients of these physicians received a prescription osteoporosis medication
*** care was reporting at a physician office visit as having not been provided. However, it may have been provided at a previous point in time
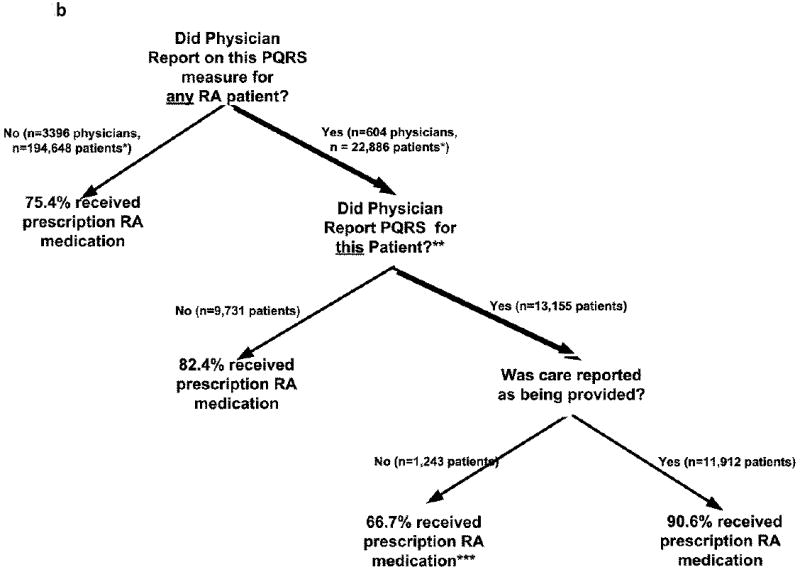
b
* all patients in this & subsequent branches had to have Medicare part A + B + part D coverage as described in the text
** overall, 86% of the 22,886 patients of these physicians received a prescription RA medication
*** care was reporting at a physician office visit as having not been provided. However, it may have been provided at a previous point in time.
In RA, the trends were similar (Figure 1b). Among the patients whose physicians reported to the PQRS program that they prescribed a DMARD or biologic in 2009 (Measure 108), evidence of a DMARD or biologic was found for 91% of them. This was higher than for patients of these same doctors when they did not report the PQRS measure (82%). Overall, 86% of patients of physicians who reported on measure 108 had evidence of a DMARD or a biologic. This proportion was 10% higher than the prevalence of DMARD use among patients of physicians not reporting Measure 108 (75.4%). Among all patients in this analysis (i.e. across all nodes in the tree) the overall prevalence of DMARD use was 76%. These results in 2009 were similar to the corresponding results in 2008 (data not shown).
Table 4 describes factors associated with the PQRS RA Measure 108 that physicians used to describe medical reasons for the patient not receiving a DMARD or biologic. As shown, older age, black race, and a number of comorbidities were significantly associated with physicians reporting a medical reason why the patient was not treated for their RA. In osteoporosis, all reason codes (medical, patient, system, and other) had to be combined given the distribution of the PQRS reason codes. Considering all factors listed in the rows or footnotes in Table 4, the only factors significantly associated with a physician reporting that a patient did not receive osteoporosis medications was the presence of chronic kidney disease (multivariable-adjusted odds ratio [OR] = 2.18, 95% CI 1.20 – 3.97) and female gender (OR = 0.54, 95% CI 0.32 – 0.89).
Table 4.
Factors Associated with a Physician-Reported Medical Reason for Which Care Was Not Provided among RA Patients whose Physicians reported on RA Measure 108*
| Adjusted** Odds Ratio (95% Confidence Interval) for PQRS Measure 108: Medical Reason why DMARD/Biologic Was Not Prescribed to RA Patient | |
|---|---|
| Age (5 year increments) | 1.08 (1.04 – 1.12) |
| Black race (referent to white) | 1.44 (1.14-1.84) |
| Comorbidities (each row referent to not having the comorbidity) | |
| Renal disease | 1.83 (1.45 - 2.29) |
| COPD | 1.19 (1.03 – 1.37) |
| Malignancy | 1.54 (1.27 – 1.86) |
| Liver disease | 3.06 (1.29-7.26) |
the dependent variable modeled was whether the patient was given a medical reason (on the PQRS claim) for why a non-biologic or biologic DMARD or biologic was prescribed (n = 645 patients) referent to patients for whom the physician reported to PQRS that a non-biologic or biologic DMARD was prescribed (n =11,945 patients). Within-practice clustering was adjusted for using alternating logistic regression [14].
adjusted for gender, other racial/ethnic groups (asian, Hispanic, and others), dual eligible status, urban/rural residence, low income subsidy, and socio-economic status (defined by census block group). Also adjusted for diabetes, hepatitis C, HIV disease, heart failure, and treating physician age and gender, none of which was significantly associated with the outcome.
Discussion
Overall, using nationally representative data from the U.S. Medicare fee for service program through 2009, we found that only 74% of osteoporosis patients and 76% of RA patients received recommended care in the form of a prescription medication to treat these conditions. These estimates were obtained from administrative data via methods that are commonly used to assess quality of care. However, the incremental data from physicians participating in the Medicare PQRS program suggested that for 11 to 24% of osteoporosis and rheumatoid arthritis patients of these physicians, care was considered yet was not provided because it was not indicated, was refused, or could not be provided for another reason. Our findings suggest that the osteoporosis and RA care gaps that have previously been identified on the basis of administrative claims data [10-12] may have overestimated the magnitude of the actual care gap.
Our overall estimate of DMARD use in 2009 for RA patients (76%) is comparable to, albeit somewhat higher than, the previously reported prevalence of DMARD use (67%) for older RA patients enrolled in Medicare Advantage in 2008[12]. It was also consistent with 2009 results from the Health Employer Data Information Set (HEDIS) where the prevalence of DMARD use for RA patients enrolled in HEDIS-reporting managed care plans was approximately 74% [15]. Numerous factors may account for these modest differences including better access to rheumatology care, specificity of the criteria used to identify RA patients within health plans, and differences in patient populations (e.g. Medicare Advantage versus traditional fee for service Medicare)[16-18]. We also provide physician-reported information on the reasons why care was considered but not provided. The most common reason why a DMARD was not provided was a medical reason (e.g. RA was in remission, medical contraindication). Even after controlling for these factors, and consistent with other reports [19], we found that African Americans with RA were more likely to have their physician provide a medical reason as to why they were not prescribed DMARDs or biologics.
Although national estimates of prescription medication use are not available for osteoporosis patients, HEDIS does collect information regarding DXA use. HEDIS is a widely used group of performance measures collected by the managed care industry, and these measures are developed and maintained by the National Committee for Quality Assurance (NCQA). The 2009 estimate of the lifetime receipt of DXA for women age 65 and older was approximately 70% [15], which is consistent with our results shown in Table 3 that showed that 76% of women had their physician report via PQRS they had received a DXA at least once since age 60. These estimates are substantially higher than estimates using up to 7 years of administrative data [5], which have shown much lower rates (e.g.33% or less) of DXA utilization among older women. Given that the optimal interval for repeat DXA testing is not clear but may span many years if the first DXA test is normal [20] even five or more years of administrative data may be insufficient to assess use of infrequently ordered tests such as DXA. This under-ascertainment likely is due to the time horizon for assessment, since administrative data does not cover patients across their life span. In the U.S., these data are often left or right-censored at age 65 when most patients transition from commercial insurance to Medicare. For that reason, systems such as PQRS that can query patients or physicians about their health care for tests ordered infrequently, such as screening DXA or colonoscopy, provide a valuable complement to administrative data sources.
A key strength of our analysis is the use of our national U.S. Medicare data. For osteoporosis, we used the 5% random sample, which allowed us to make inference to the entire Medicare traditional fee-for-service population of osteoporosis patients. For RA, we had access to 100% of the relevant data, therefore, no extrapolation or inferences were required. Additionally, and unlike most quality of care studies that only have access to diagnoses on claims used for billing purposes, the PQRS measures we examined provide greater certainty that the billing diagnoses accurately reflected the clinical conditions. For example, the RA disease activity PQRS measure (measure 177) would likely not have been reported by a physician for a patient who did not actually have RA. Thus, the patients and their associated diagnoses on which the PQRS measure was reported could reasonably be inferred to have greater validity given this ‘second level’ of diagnosis confirmation via PQRS. Of note, it is possible that comparator patients for whom PQRS measures were not reported were somewhat less likely to actually have osteoporosis or RA, despite validation studies that have generally shown high validity of administrative data to accurately identify the diseases of interest [21]. This misclassification of comparator patients could have modestly impacted the results in Figure 2 and presumably would have attenuated the differences in treatment rates observed between patients for whom PQRS measures were reported compared to those for whom they were not reported.
As a further limitation of our results, we recognize that there is more than one method to report PQRS measures to the Medicare program. Our analyses focused only on claim-based reporting, but reporting also may be done through a qualified registry. To our knowledge, there are no large osteoporosis registries in the U.S. that are qualified to perform PQRS reporting. However, a few arthritis registries collect information on RA patients. The Consortium of Rheumatology Researchers of North America (CORRONA), for example, provides the capability to report to PQRS; however, such reporting was not possible until 2010. In contrast, the American College of Rheumatology began PQRS reporting in June 2009 through the Rheumatology Clinical Registry (RCR), and reporting through this mechanism was not captured within our data. In 2009, for example, 210 rheumatologists reported on DMARD use (Measure 108) and on disease activity or functional status (Measures 177 or 178) through the RCR [Itara Barnes, personal communication]. The only other method of reporting, though a qualified electronic health record (EHR), began in 2010 and is limited to 20 measures focused on primary care and preventive services [22]. In 2012, only 1 of these EHR measures is relevant to osteoporosis (measure 39), and none relevant to RA.
Physician practices voluntarily report on PQRS measures, and these practices and the patients that they treat may be different than those choosing not to report. For example, most RA patients for whom Measure 177 (RA disease activity measured using a validated instrument) was reported were described as having had disease activity measured using a validated instrument. In as much as a minority of physicians are typically observed to be using standardized disease activity measures in routine clinical practice [23, 24], it is likely that only a select group of physicians chose to report on this measure. Other factors may affect the decision to report to PQRS. For example, reporting for many PQRS measures is greatly facilitated by having an electronic medical record (EMR). Additionally, the Medicare bonus paid for reporting (but not for actual performance) is slowly being phased out; thus, the financial incentives for participation may be changing over time. The incentive payment that was initially 2% has now decreased to 0.5% in 2012. This bonus eventually will eventually become a penalty (e.g. a withheld amount of 2.0% based upon 2014 reporting).
Additionally, we recognize that the physician reported reasons why care was not provided are not specific; physicians reported these reasons in broad groups (e.g. medical, patient, system, other). We did find significant associations with a number of comorbidities that would seem reasonable medical contraindications to receipt of some DMARDs and biologics, supporting the face validity of these care exclusions. At a minimum, it is reasonable to assume that the care was considered during the office visit and not overlooked given that the PQRS reason codes were submitted at the time of the physician visit. However, the reasons why care was not provided might not be considered legitimate by all providers or health care plans. For example, data from a German study found that 13-19% of patients did not receive a DMARD within a 12 month period [25]. Although 21-31% of these patients were in remission and presumably did not need such treatment, others were not in remission and were only on prednisone as monotherapy (4-8%). Ideally, care exclusions for reasons such as high comorbidity burdens should conform to evidence-based recommendations, and quality reporting systems like PQRS should allow physicians to provide more precise reasons for such exclusions and avoid use of a non-specific ‘other’ category.
Conclusion
These results suggest that the substantial care gaps previously identified in published osteoporosis and RA studies using administrative data may have been somewhat overestimated because of care that was not provided for a presumed valid reason. Allowing physicians the opportunity to provide reasons why they considered but did not provide care appears useful to inform measurement in quality of care and should be an integral part of quality reporting systems in the future.
Supplementary Material
Significance & Innovation.
Quality of care and pay for performance are becoming increasingly important in evaluating and compensating physicians and health systems
The national Medicare Physician Quality Reporting System (PQRS) was established to allow physicians to describe care that was provided and care that was considered but not provided.
A systematic evaluation of results from PQRS related to 6 quality measures for osteoporosis and rheumatoid arthritis patients was performed to examine the frequency and reasons why recommended musculoskeletal care was not given.
Some apparent gaps in quality of care for these two conditions were described by physicians as having been considered but not provided for various reasons. The implications of this finding suggest that care gaps traditionally measured using health plan data may be somewhat overestimated when examined in light of additional information provided by physicians.
Acknowledgments
Dr. Curtis receives support from the NIH (AR 053351) and AHRQ (R01 HS018517). Dr. Saag receives salary support from the National Institutes of Health (AR052361) and the Agency for Healthcare Research and Quality (U18 HS016956).
This research was supported by AHRQ (R01 HS018517) and a contract between UAB and Amgen, Inc.
Only the authors from UAB had access to the Medicare data used. The analysis, presentation and interpretation of the results were solely the responsibility of the authors.
Footnotes
Conflicts of Interest:
JRC: Amgen (consulting and research)
KGS: Merck, Lilly, Amgen (consulting and research)
ED: Amgen (research)
Others: none
Bibliography
- 1. [November 8, 2011]; https://www.cms.gov/qualitymeasures/
- 2. [November 8, 2011]; http://www.qualityforum.org/Show_Content.aspx?id=119.
- 3.Medicare program; hospital inpatient value-based purchasing program. Final rule. Fed Regist. 2011;76(88):26490–26547. [PubMed] [Google Scholar]
- 4. [November 8, 2011]; http://www.ama-assn.org/ama1/pub/upload/mm/368/cms_pqri_chart.pdf.
- 5.Curtis JR, Carbone L, Cheng H, Hayes B, Laster A, Matthews R, Saag KG, Sepanski R, Tanner SB, Delzell E. Longitudinal trends in use of bone mass measurement among older americans, 1999-2005. J Bone Miner Res. 2008;23(7):1061–1067. doi: 10.1359/JBMR.080232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curtis JR, Westfall AO, Allison JJ, Becker A, Casebeer L, Freeman A, Spettell CM, Weissman NW, Wilke S, Saag KG. Longitudinal patterns in the prevention of osteoporosis in glucocorticoid-treated patients. Arthritis Rheum. 2005;52(8):2485–2494. doi: 10.1002/art.21194. [DOI] [PubMed] [Google Scholar]
- 7.Saag KG, Gehlbach SH, Curtis JR, Youket TE, Worley K, Lange JL. Trends in prevention of glucocorticoid-induced osteoporosis. J Rheumatol. 2006;33(8):1651–1657. [PubMed] [Google Scholar]
- 8.Mudano A, Allison J, Hill J, Rothermal T, Saag K. Variations in Glucocorticoid Induced Osteoporosis Prevention in a Managed Care Cohort. J Rheumatol. 2001;28(6):1298–1305. [PubMed] [Google Scholar]
- 9.Mudano A, Casebeer L, Patino F, Allison J, Weissman N, Kiefe C, Person S, Gilbert D, Saag K. Racial disparities in osteoporosis prevention in a managed care population. South Med J. 2003;96(5):445–451. doi: 10.1097/01.SMJ.0000053918.93363.B0. [DOI] [PubMed] [Google Scholar]
- 10.MacLean CH, Louie R, Leake B, McCaffrey DF, Paulus HE, Brook RH, Shekelle PG. Quality of care for patients with rheumatoid arthritis. JAMA. 2000;284(8):984–992. doi: 10.1001/jama.284.8.984. [DOI] [PubMed] [Google Scholar]
- 11.Schmajuk G, Schneeweiss S, Katz JN, Weinblatt ME, Setoguchi S, Avorn J, Levin R, Solomon DH. Treatment of older adult patients diagnosed with rheumatoid arthritis: improved but not optimal. Arthritis Rheum. 2007;57(6):928–934. doi: 10.1002/art.22890. [DOI] [PubMed] [Google Scholar]
- 12.Schmajuk G, Trivedi AN, Solomon DH, Yelin E, Trupin L, Chakravarty EF, Yazdany J. Receipt of disease-modifying antirheumatic drugs among patients with rheumatoid arthritis in medicare managed care plans. JAMA. 2011;305(5):480–486. doi: 10.1001/jama.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curtis JR, Arora T, Narongroeknawin P, Taylor A, Bingham CO, 3, Cush J, Saag KG, Safford M, Delzell E. The delivery of evidence-based preventive care for older Americans with arthritis. Arthritis Res Ther. 2010;12(4):R144. doi: 10.1186/ar3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carey V, Zeger S, Diggle P. Modelling multivariate binary data with alternating logistic regressions. Biometrika. 1993;80(3):517–526. [Google Scholar]
- 15. [February 10th, 2012]; http://www.ncqa.org/tabid/784/Default.aspx.
- 16.Risk Selection and Risk Adjustment in Medicare. Annual Report to Congress. 1996;Chapter 15:255–279. [Google Scholar]
- 17.Batata A. The effect of HMOs on fee-for-service health care expenditures: evidence from Medicare revisited. J Health Econ. 2004;23(5):951–963. doi: 10.1016/j.jhealeco.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 18. [November 8, 2011]; http://www.medpac.gov/documents/MedPAC_Payment_Basics_11_MA.pdf.
- 19.Solomon DH, Ayanian JZ, Yelin E, Shaykevich T, Brookhart MA, Katz JN. Use of disease-modifying medications for rheumatoid arthritis by race and ethnicity in the National Ambulatory Medical Care Survey. Arthritis Care Res (Hoboken) 2012;64(2):184–189. doi: 10.1002/acr.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gourlay ML, Fine JP, Preisser JS, May RC, Li C, Lui LY, Ransohoff DF, Cauley JA, Ensrud KE. Study of Osteoporotic Fractures Research G: Bone-density testing interval and transition to osteoporosis in older women. N Engl J Med. 2012;366(3):225–233. doi: 10.1056/NEJMoa1107142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SY, Servi A, Polinski JM, Mogun H, Weinblatt ME, Katz JN, Solomon DH. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res Ther. 2011;13(1):R32. doi: 10.1186/ar3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. [November 8, 2011]; https://www.cms.gov/PQRS/20_AlternativeReportingMechanisms.asp.
- 23.Pincus T, Segurado OG. Most visits of most patients with rheumatoid arthritis to most rheumatologists do not include a formal quantitative joint count. Ann Rheum Dis. 2006;65(6):820–822. doi: 10.1136/ard.2005.044230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cush JJ. Trends in rheumatology practice: a survey of US rheumatologists. Presented at: 72nd annual meeting of ACR and 43rd annual meeting of the ARHP; October 25-29, 2008; San Francisco, Ca. Abstract 1573. [Google Scholar]
- 25.Ziegler S, Huscher D, Karberg K, Krause A, Wassenberg S, Zink A. Trends in treatment and outcomes of rheumatoid arthritis in Germany 1997-2007: results from the National Database of the German Collaborative Arthritis Centres. Ann Rheum Dis. 2010;69(10):1803–1808. doi: 10.1136/ard.2009.122101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


