Abstract
Uromodulin (UMOD) genetic variants cause familial juvenile hyperuricemic nephropathy, characterized by hyperuricemia, decreased renal excretion of UMOD and uric acid; such findings suggest a role for UMOD in the regulation of plasma uric acid. We screened common variants across the UMOD locus in two populations, one from a community-based Chinese population, the other from California twins and siblings. Transcriptional activity of promoter variants was estimated in luciferase reporter plasmids transfected into HEK293 cells and mlMCD3 cells. By variance components in twin pairs, uric acid concentration and excretion were heritable traits. In the primary population from Beijing, we identified that carriers of haplotype GCC displayed higher plasma uric acid, and 3 UMOD promoter variants associated with plasma uric acid. UMOD promoter variants displayed reciprocal effects on urine uric acid excretion and plasma uric acid concentration, suggesting a primary effect on renal tubular handling of urate. These UMOD genetic marker-on-trait associations for uric acid were replicated in an independent American population sample. Site-directed mutagenesis at trait-associated UMOD promoter variants altered promoter activity in transfected luciferase reporter plasmids. These results suggest that UMOD promoter variants seem to initiate a cascade of transcriptional and biochemical changes influencing UMOD secretion, eventuating in elevation of plasma uric acid.
Keywords: Uromodulin, uric acid, Tamm-Horsfall protein, UMOD
INTRODUCTION
Circulating uric acid concentration constitutes a risk factor for both renal and cardiovascular disease(1) and unusual mutations at the uromodulin (UMOD) locus exhibit disturbances in uric acid metabolism. UMOD is an ~80–90 kDa glycoprotein, consisting of 640 amino acids including 48 cysteine residues, exclusively synthesized by the thick ascending limb (TAL) and early distal convoluted tubule in the kidney(2). UMOD is initially trafficked into the endoplasmic reticulum, then shuttled to the apical membrane of cells as a GPI-linked molecule, and finally released into the urine by proteolytic cleavage (3–5). Healthy individuals typically excrete as much as 20–70 mg of UMOD per day, rendering it the most abundant protein in human urine (6).
UMOD mutations have been identified in patients with familial juvenile hyperuricemia nephropathy (OMIM 162000), medullary cystic kidney disease 2 (OMIM 603860) and glomerulocystic kidney disease (OMIM 609886) (7–9), which are characterized by hyperuricemia and progressive kidney disease. Thus UMOD is a candidate regulator of renal uric acid excretion. In a recent genome-wide association study, common genetic variant rs4293393 in the UMOD gene promoter was associated with serum uric acid(10).
UMOD may contribute to regulation of sodium reabsorption through the TAL(11): decreased UMOD secretion might lead to a reduction of sodium reabsorption by the TAL (12), perhaps compensated through increased reabsorption of sodium by the proximal tubule. Since sodium reabsorption is coupled to increased urate reabsorption in the proximal tubule (13), plausible mechanisms linking UMOD to serum uric acid are apparent.
In this study, we typed common genetic variants spanning the UMOD locus, to establish associations between the UMOD gene and plasma uric acid in different populations, and also explored whether the effect of UMOD on plasma uric acid was mediated by regulation of renal uric acid or sodium excretion.
RESULTS
Primary studies in a community-based cohort of Beijing
Association of UMOD haplotypes and common variants with plasma uric acid
We genotyped 7 common variants (supplementary table 1 and 2) across the UMOD locus. We also used 7 common variants to construct haplotype blocks in Haploview (14) for all subjects in the Chinese population (Table 1). The analysis revealed 3 blocks across the UMOD genes, in which SNPs in UMOD promoter region, intron and 3’ downstream were all highly correlated within each block (Supplementary table 3). Common promoter haplotypes displayed associations with plasma uric acid levels: carriers of promter haplotype GCC showed higher plasma uric acid, while carriers of promoter haplotype ATT presented lower plasma uric acid levels (Table 2).
Table 1.
Characteristics of the Chinese community-based cohort in this study.
| Variables | Participants (n=1000) |
|---|---|
| Age (years) | 63.7±0.3 |
| Male (%) | 48.5% |
| BMI (kg/m2) | 25.3±0.1 |
| SBP (mmHg) | 137.8±0.6 |
| DBP (mmHg) | 81.0±0.3 |
| Plasma uric acid (µmol/L) | 265.7±2.6 |
| Plasma creatinine (µmol/L) | 138.33±0.6 |
| eGFR (mL/min/1.73 m2) | 70.8±0.5 |
| Cholesterol (mmol/L) | 5.3 (4.7–6.0) |
| Triglycerides (mmol/L) | 1.3 (1.0–1.8) |
| Urinary ACR (median [IQR]) | 2.6 (1.3–4.9) |
| Hypertension(%) | 46.3 |
| Anti-hypertension medication(%) |
81.6 |
Values shown are mean +/− SEM, or median (inter-quartile range [IQR]). Samples shown here were collected in 2008. eGFRs presented in this table were estimated from plasma creatinine using the equation developed from data on the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI)[34]. ACR: albumin to creatinine ratio in urine.
Table 2.
UMOD promoter common haplotypes and SNPs: Effects on plasma uric acid in the Chinese population (by univariate analyis of variance).
| HAP/ SNP |
Copy (N)/ Allele (N) |
pUA (umol/L) (mean+/−SEM) |
P value for |
FDR p | |||||
|---|---|---|---|---|---|---|---|---|---|
| model | gene | significant covariate |
|||||||
| Age | Gender | Waist | BMI | ||||||
| GCC | 0 (812) | 263.9+/−2.8 | <0.0001 | 0.017 | 0.006 | <0.0001 | <0.0001 | 0.013 | 0.023 |
| 1+2 (114) | 279.4+/−7.6 | ||||||||
| ATT | 0+1 (138) | 278.7+/−6.8 | <0.0001 | 0.021 | 0.006 | <0.0001 | <0.0001 | 0.012 | 0.021 |
| 2 (788) | 263.8+/−2.9 | ||||||||
| rs13333226 | |||||||||
| AA (791) | 263.4+/−2.9 | <0.0001 | 0.009 | 0.020 | <0.0001 | <0.0001 | >0.05 | 0.018 | |
| GA+AA(135) | 279.2+/−7.0 | ||||||||
| rs 6497476 | |||||||||
| TT (781) | 263.3+/−2.9 | <0.0001 | 0.005 | 0.027 | <0.0001 | <0.0001 | >0.05 | 0.020 | |
| CC+CT (114) | 281.2+/−7.7 | ||||||||
| Rs4293393 | |||||||||
| TT (792) 264.3+/−2.9 | <0.0001 | 0.032 | 0.042 | <0.0001 | <0.001 | >0.05 | 0.026 | ||
| CC+CT (137) | 279.3+/−7.1 | ||||||||
HAP: haplotype. pUA: plasma uric acid. The haplotypes located 5’ near gene region of UMOD, consisted of rs13333226 (A>G) → rs6497476 (T>C) → rs4293393 (T>C). Univariate analysis of variance was performed to test the association of haplotypes with plasma uric acid level. Age, gender, eGFR, BMI, height, weight, waist, SBP and DBP were selected as covariates. Covariates with significant p values (p<0.05) are listed in the Table.
We then tested the association of each of the 7 individual SNPs with plasma uric acid in the cohort. 3 promoter SNPs, rs4293393 C/T, rs6497476 C/T, and rs13333226 G/A, each associated with plasma uric acid. rs13333226 G allele carriers (n=135) have higher plasma uric acid than A/A homozygotes (n=791, 279.2±7.0 vs. 263.4±2.9 µmol/L, p=0.009); rs6497476 C allele carriers (n=114) have higher plasma uric acid than T/T homozygotes (n=781, 281.2±7.7 vs. 263.3±2.9 µmol/L, p=0.005); and finally rs4293393 C allele carriers (n=137) have higher plasma uric acid compared with T/T homozygotes (n=792) (279.3±7.1 vs. 264.3±2.9 µmol/L, p=0.032) (Table 2).
The effects of age, gender, eGFR, BMI, height, weight, waist, SBP and DBP and UMOD gene (SNP) on plasma uric acid were tested by linear regression: a model combining age, gender, waist, eGFR, DBP and gene (SNP) predicted plasma uric acid (p<0.001); rs13333226 itself accounted for ~0.4% of plasma uric acid variance (p=0.015; table 3), while rs6497476 itself accounted for ~0.5% of serum uric acid variance (p=0.013; table 3), and rs4293393 itself accounted for ~0.3% of serum uric acid variance (p=0.037; table 3).
Table 3.
Regression: Fractional effects of UMOD common variants on plasma uric acid in the Chinese cohort.
| MLR model | ||||||
|---|---|---|---|---|---|---|
| Predictors: SNP (one SNP at a time), Gender GFR DBP, waist | ||||||
| Dependent variables: Plasma uric acid level | ||||||
| RefSNP | F | P | Adjusted R2 | |||
| rs13333226 | 86.005 | 3.72*10–74 | 0.316 | |||
| rs6497476 | 83.789 | 4.27*10–72 | 0.317 | |||
| rs4293393 | 86.183 | 3.34*10–74 | 0.318 | |||
| Model component (with all independent variables) p values | ||||||
| RefSNP | SNP | R2 for SNP | Gender | GFR | DBP | Waist |
| rs13333226 | 0.015 | 0.4% | <0.001 | <0.001 | 0.027 | <0.001 |
| rs6497476 | 0.013 | 0.5% | <0.001 | <0.001 | 0.036 | <0.001 |
| rs4293393 | 0.037 | 0.3% | <0.001 | <0.001 | 0.04 | <0.001 |
MLR: Multiple linear regression, by the stepwise regression method. Plasma uric acid was the dependent variable. Single SNP, age, gender, eGFR, BMI, height, weight, waist, SBP and DBP were selected as independent variables. A model combining UMOD SNP genotype, gender, DBP and waist predicted plasma uric acid level.
We employed estimation of the FDR (False Discovery Rate), in order to minimize false negative results while maximizing true positive results, using the Excel calculator of FDRs from p-values, at <http://www.rowett.ac.uk/~gwh/fdr.html>. All the FDR p values for SNPs and haplotypes are listed in Table 2. 3 promoter SNP effects were also confirmed by permutation (non-parametric) tests.
Association of UMOD variant with urinary uric acid excretion
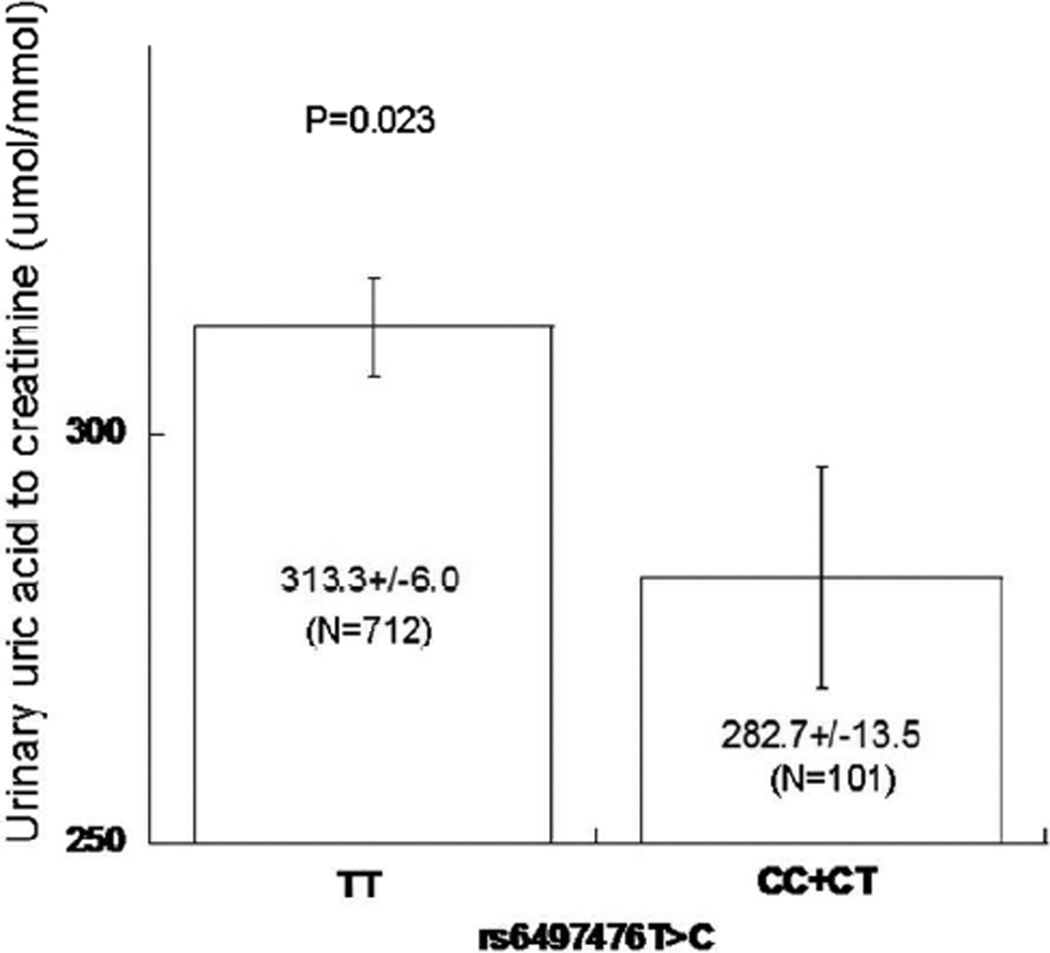
We also investigated the association of UMOD SNPs with urinary uric acid excretion. rs6497476 C allele carriers (n=101) have lower urinary uric acid to creatinine ratio than T/T homozygotes (n=712, 282.7±13.5 vs. 313.3±6.0 µmol/mmol, p=0.023) (Figure 1), consistent with previous results(15) that the C allele predicted higher plasma uric acid.
Figure 1. UMOD promoter common genetic variation: Effect on urine uric acid excretion in a community based cohort of the Chinese population.
Urine uric acid excretion was measured in spot (untimed) urine samples from year 2008. Promoter rs6497476 results indicated that C allele carriers had lower urinary uric acid-to-creatinine ratios than T/T homozygotes.
Association of a UMOD common haplotype with urinary UMOD excretion
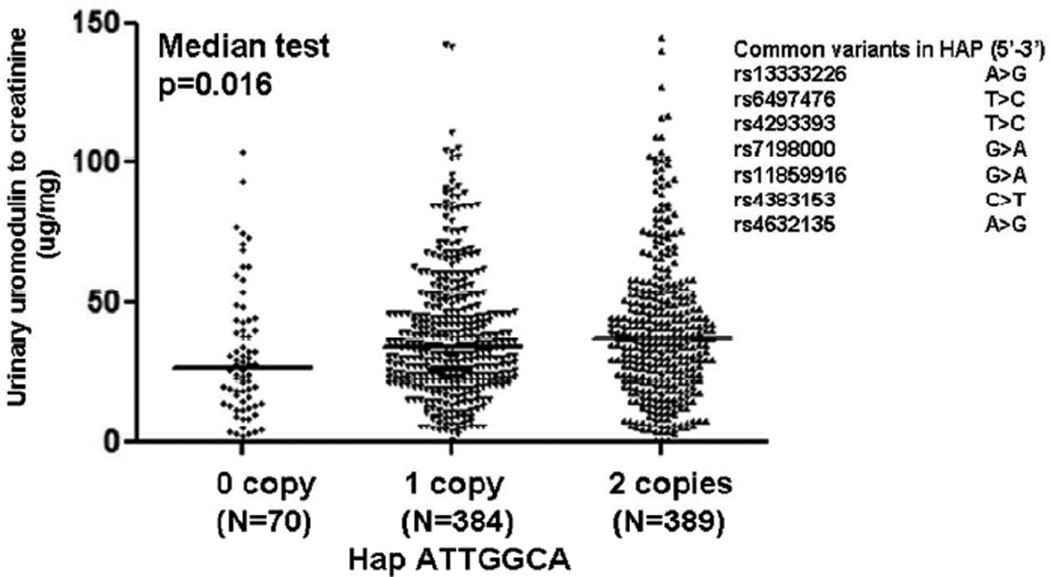
We then investigated whether the common haplotypes or SNPs associated with urinary UMOD excretion. Neither single SNPs nor promoter haplotypes was identified to be associated with UMOD excretion. Since a local region of high LD at the UMOD locus region was identified in the HapMap CHB (China-Han-Beijing) subjects (Supplementary Figure 1), we also inferred longer 7-SNP haplotypes across the UMOD locus. The most common haplotype, ATTGGCA (spanning rs13333226, rs6497476, rs4293393, rs7198000, rs11859916, rs4383153, rs4632135) associated urinary UMOD-creatinine ratio (Figure 2). Carriers of ATTGGCA displayed higher urinary UMOD excretion (p=0.016).
Figure 2. UMOD haplotype ATTGGCA: Effect on urinary UMOD excretion in a community-based cohort of Chinese population.
Haplotype homozygosities were confirmed by PLINK [URL: http://pngu.mgh.harvard.edu/purcell/plink/][14]. Urinary UMOD-to-creatinine ratio was presented as µg/mg. The most common haplotype ATTGGCA associated urinary UMOD-to-creatinine ratio.
Correlation between urinary UMOD, uric acid and sodium excretions
Urinary uric acid-to-creatinine ratio, urinary UMOD-to-creatinine ratio and urinary sodium-to-creatinine ratio were measured in spot urine in 898 cases. We found that plasma uric acid negatively correlated with urinary uric acid-to-creatinine ratio, as well as urinary UMOD-to-creatinine ratio. And we also found that the 3 biochemical traits, urinary uric acid-to-creatinine ratio, urinary UMOD-to-creatinine ratio and urinary sodium-to-creatinine ratio, positively correlated with each other (Table 4).
Table 4.
UMOD correlations (non-parametric, Spearman rank) with sodium and uric acid traits.
| uNa/Cr | uUA/Cr | uUMOD/Cr | pUA | |
|---|---|---|---|---|
| uNa/Cr (µmol/mmol) | ||||
| Correlation coefficient | 1.0 | 0.645 | 0.239 | −0.161 |
| Sig. (2-tailed) | 5.24*10−106 | 6.56*10−13 | 1.53*10−6 | |
| uUA/Cr (µmol/mmol) | ||||
| Correlation coefficient | 1.0 | 0.348 | −0.187 | |
| Sig. (2-tailed) | 1.66*10−26 | 2.11*10−8 | ||
| uUMOD/Cr (µg/mg) | ||||
| Correlation coefficient | 1.0 | −0.253 | ||
| Sig. (2-tailed) | 3.72*10−14 | |||
| pUA (µmol/L) | ||||
| Correlation coefficient | 1.0 | |||
| Sig. (2-tailed) |
Extension/replication studies in California twins and siblings
Of the 7 SNPs typed at/near UMOD in the Beijing cohort, 5 were either genotyped or imputed in the San Diego subjects; each of these 5 variants was significantly (p<0.05) associated with plasma uric acid: rs13333226 (p=0.0261), rs4293393 (p=0.0261), rs7198000 (p=0.0492), rs11859916 (p=0.0492), and rs4632135 (p=0.027). Of note, rs4293393 was previously associated with serum uric acid(10).
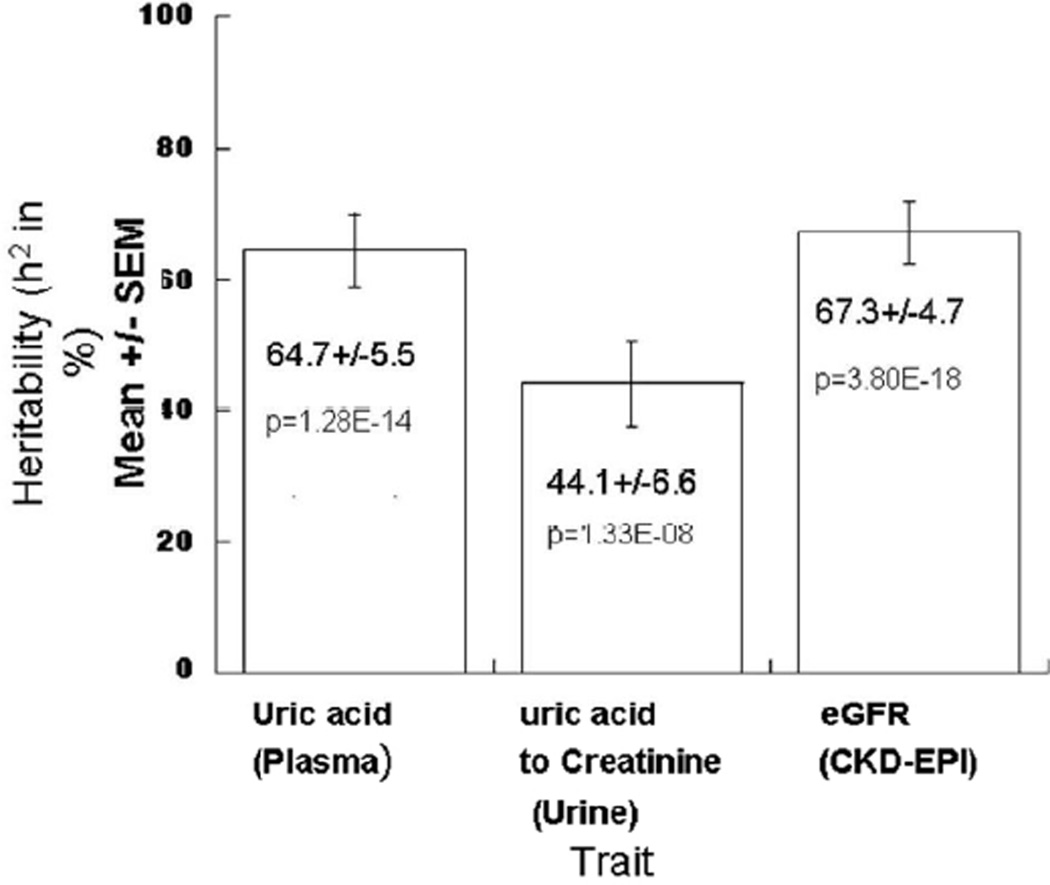
Heritability of plasma uric acid and uric acid excretion was also estimated in the twin cohort. H2 was substantial for both plasma uric acid (at 64.7+/−5.5%, p=1.28*10−14) and urinary uric acid excretion (uric acid to creatinine ratio, at 44.1+/−6.6%, p=1.33*10−8), suggesting that genetic factors play an important role in regulation of uric acid concentration, metabolism, and excretion (Figure 3).
Figure 3. Uric acid trait heritability in twin pairs.
Heritability (h2) of plasma uric acid and uric acid excretion, as well as eGFR. h2, the fraction of trait variance accounted for by genetic variance (h2 = VG/VP) was established by study of variance components in California 173 twin pairs (346 individuals). Plasma uric acid is in µmol/L, while urinary uric acid to creatinine ratio is in µmol/mmol, and eGFR was calculated by the CKD-EPI algorithm (normalized to ml/min/1.73m2).
Function of UMOD promoter variants (common haplotypes and single SNPs)
We constructed luciferase reporter plasmids for the 2 common naturally occurring UMOD haplotypes (HAP A-T-T and HAP G-C-C), and then created 3 mutant haplotypes by site-directed mutagenesis at promoter positions rs13333226, rs6497476, rs4293393, from A→G or T→C, forming 3 pairs of haplotypes in which differences only occur at one position: rs13333226, rs6497476, or rs4293393.
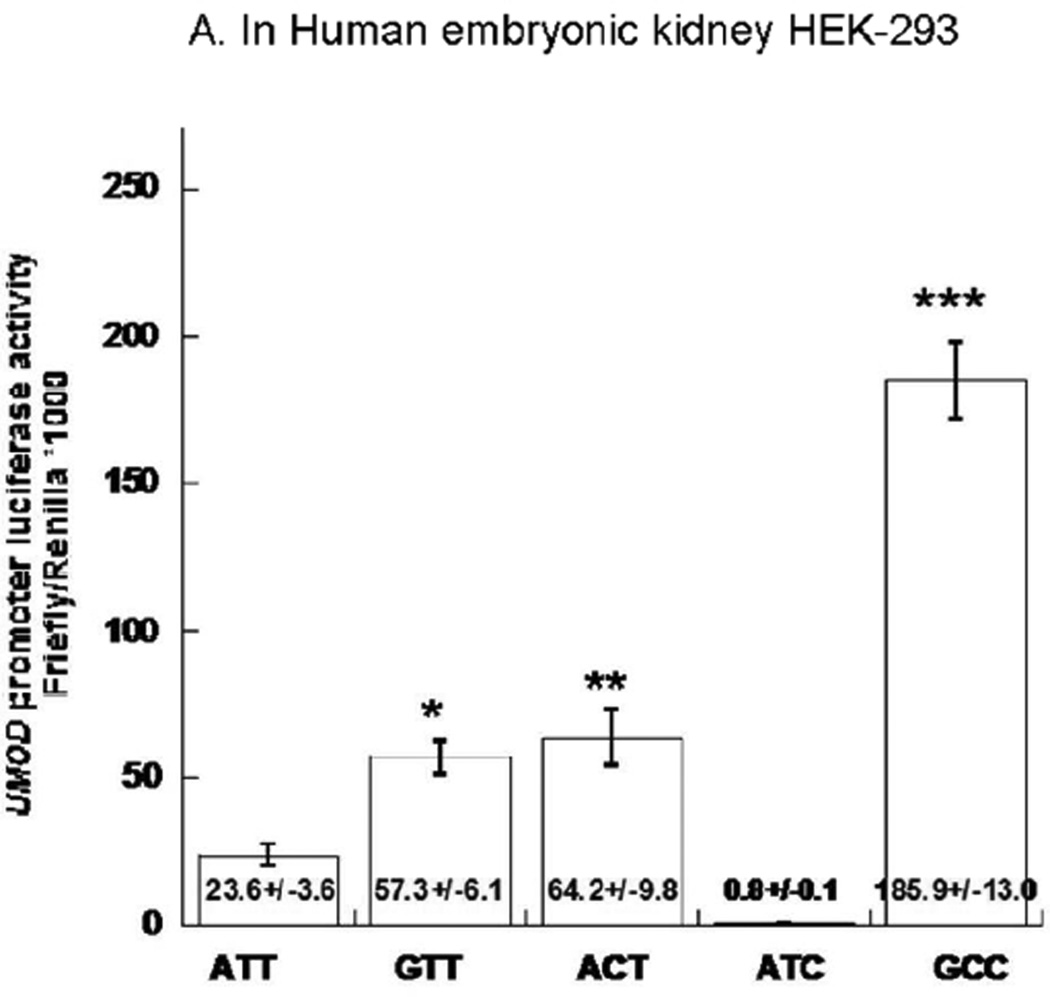
First we tested promoter strength by transfection of the 2 natural haplotypes into HEK-293 human kidney cells. Natural promoter haplotype A-T-T showed lower trascription of the reporter gene than haplotype G-C-C (Figure 4A). We then took advantage of mutant haplotypes to evaluate the transcriptional function of individual variants in transfected kidney cells. Higher gene trascription resulted from the G allele at rs13333226 and C allele at rs6497476 (Figure 4A), when tested upon balanced/matched haplotype backgrounds.
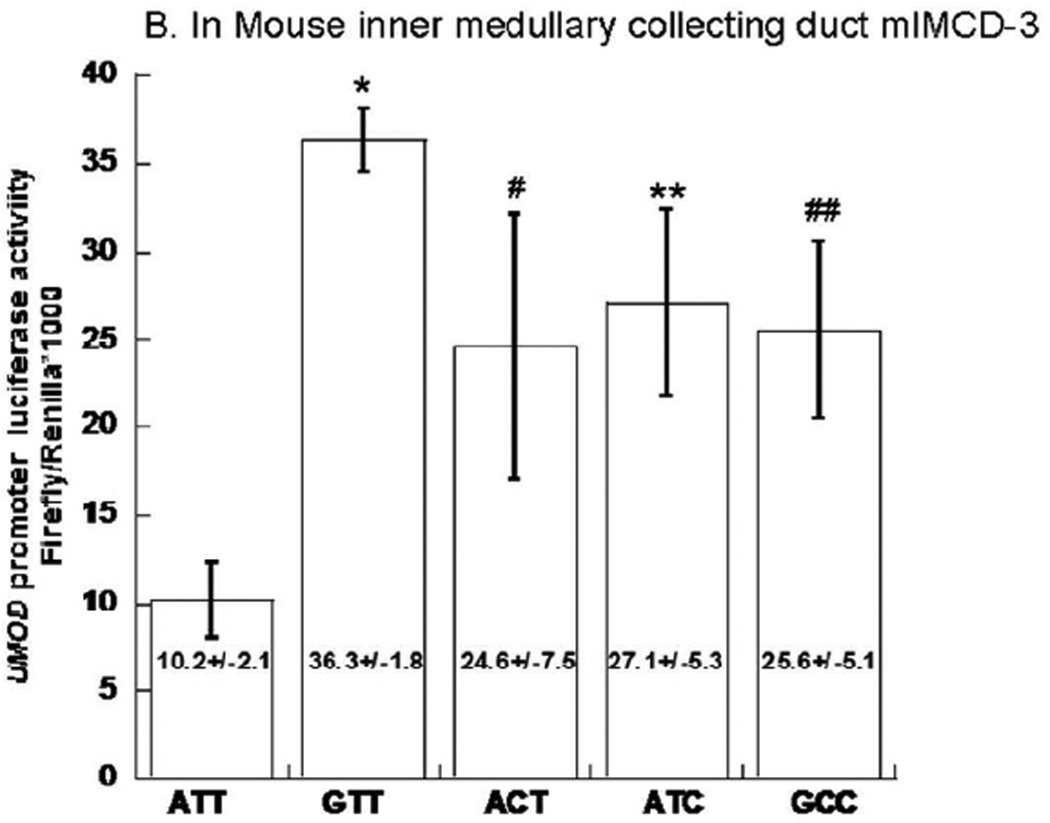
Figure 4. UMOD promoter haplotype/luciferase reporter functional studies.
Transfection results for human UMOD promoter haplotype/luciferase reporters are shown in human embryonic kidney (HEK-293) cells as well as in mouse inner medullary collecting duct (mlMCD-3). Results are expressed as the ratio of firefly/Renilla luciferase activity. As a negative control, the ratio of firefly/Renilla luciferase activities for the promoter-less luciferase reporter vector (empty vector pGL3-Basic) co-transfected with transfection efficiency control plasmid Renilla plasmid (pTK-RL) was 0.002±0.001 for HEK-293 cells and 8.44*10−5±1.84*10−6 for mlMCD-3. The absolute firefly luminescence for UMOD haplotype ATT was 22826±1739 RLU/sec in HEK293 cells; 235±28RLU/sec in mlMCD-3. 50 µl from each 500 µl cell lysate were used for luciferase assay. Each experiment was performed in triplicate, and such experiments were repeated at least three times. Statistical significance refers to the haplotype effects. Results are compared by ANOVA followed by post-hoc LSD.
We repeated the same procedure in mlMCD3 cells, and obtained similar results which showed that 3-SNP promoter haplotype G-C-C displayed increased transcription compared to haplotype A-T-T (Figure 4B). Individually, the rs13333226 G allele and rs6497476 C allele each elevated reporter gene activity. However the rs4293393 C vs T difference was only identified in mlMCD3 (Figure 4B).
DISCUSSION
Overview: UMOD and uric acid
Aberrant renal handling of uric acid excretion is a major contributor to serum uric acid elevation in adults with hyperuricemia (16). UMOD may be candidate protein to regulate uric acid, based on studies of medullary cystic kidney disease 2 (MCKD2) (17) and general cohorts (18). Our study indicated haplotypes across the whole gene associated with plasma uric acid as well as urinary UMOD excretion, while only variants in promoter region had effects on plasma uric acid in a community-based Asian population. Carriers of the rs13333226 G allele, rs6497476 C allele or rs4293393 C allele have higher plasma uric acid compared to rs13333226 A/A, rs6497476 T/T or rs4293393 T/T homozygotes, respectively. In an extension/replication study of European-ancestry individuals in San Diego, the same genetic variants across the UMOD locus also predicted plasma uric acid. In our study, the correlation between plasma uric acid and UMOD excretion, as well as urinary UMOD correlation with urinary uric acid/sodium excretion suggested that UMOD may play a role in renal tubular handling of uric acid. Studies with the isolated/transfected UMOD promoter showed that G allele at rs13333226 and C allele at rs6497476 changed the reporter gene activity compared with A alleles at rs13333226 and T allele at rs6497476. These results suggest a mechanistic chain of events whereby common UMOD promoter variants play a role in the determination of human plasma uric acid.
Plasma uric acid concentration is a complex biochemical trait with multiple contributory factors derived from both genes (19) and environment (20). In our replication study, high heritability (~64.7%) of plasma uric acid was identified in twins with Caucasian ancestry, while similarly high heritability (~70.5%) in Chinese twins was reported previously(21)
The setting of late penetrance of the ultimate disease trait (such as hyperuricemia), as well as likely genetic heterogeneity, the “intermediate phenotype” (22) strategy may be a useful approach in the search for disease predisposition loci. In accordance with this pathway concept, we pursued intermediate traits in this study. Excretion of UMOD, estimated by the urinary UMOD/creatinine ratio, was not only correlated with plasma uric acid but also influenced by UMOD haplotype ATTGGCA (based on 7 genotyped UMOD SNPs: rs13333226, rs6497476, rs4293393, rs7198000, rs11859916, rs4383153, rs4632135). Haplotype ATTGGCA predicted higher UMOD excretion (Figure 5), which suggested that ATTGGCA carriers might have more uric acid excretion and lower plasma uric acid. Although we did not identify such correlations, in another haplotype. GCCGGTA as compared with ATTGGCA, which allelic changes in the promoter region (G-C-C versus A-T-T), predicted higher plasma uric acid (Figures 2A/2B).
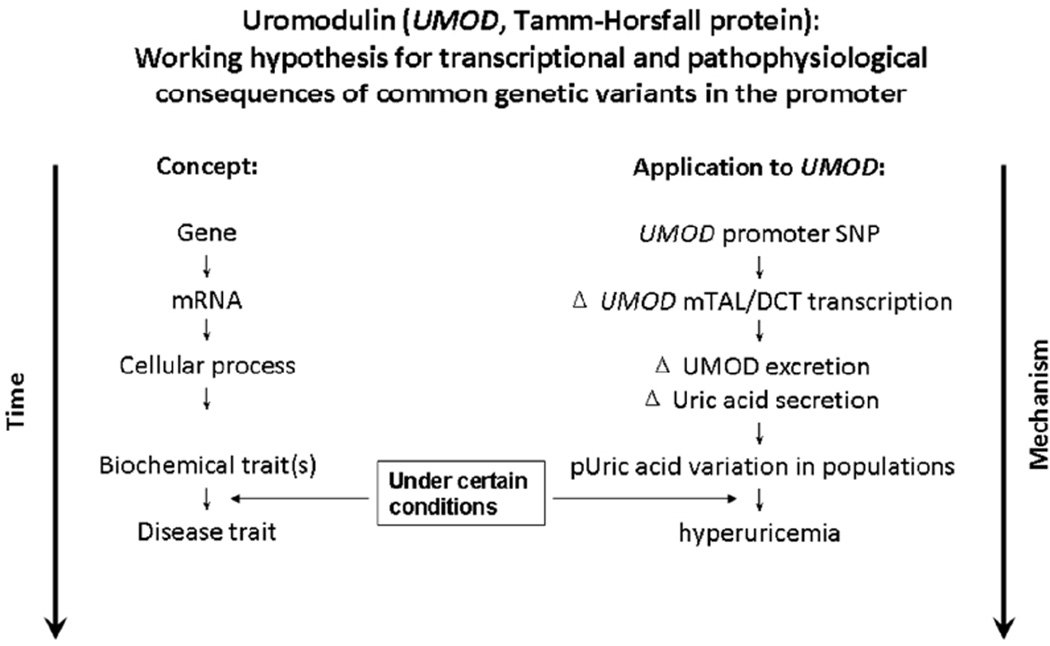
Figure 5.
Uromodulin (UMOD, Tamm-Horsfall protein): Working hypothesis for transcriptional and pathophysiological consequences of common genetic variants in the promoter.
UMOD in the kidney
UMOD is exclusively synthesized by the tubular cells of the TAL and early distal convoluted tubule in the kidney (23). It has been speculated that UMOD contributes to colloid osmotic pressure and retardation of sodium/potassium passage through the TAL, thus enhancing active transport in this segment(23). Recently, UMOD has proved to play a role in regulation of the renal outer medullary potassium channel, thus increasing salt re-absorption along the TAL (24). In THP−/− mice, urinary sodium/creatinine ratio and FENA/FEUA decreased substantially, without a significant difference in either FENA or FEUA, comparing knockout to wild-type (25). Based on these findings, it appeared that a decrease of UMOD excretion may result in diminished reabsorption of sodium in the TAL, thus triggering an increase of sodium reabsorption in the proximal tubule, with an accompanying increase of uric acid reabsorption. In this study we also found positive correlations among UMOD, uric acid and sodium excretions, indicating functional links among renal excretions of UMOD, uric acid and sodium in the population. Furthermore the correlations between plasma and urinary uric acid, as well as with urinary UMOD, support an influence of UMOD upon plasma uric acid.
In our study, we systemically genotyped several common variants across the UMOD gene, and thereby identified additional SNPs potentially contributing to control of plasma uric acid in two populations of different ancestries. In our Asian population, promoter SNPs appeared to play especially important roles, including rs4293393, which was reported previously related to serum uric acid and reduced risk of kidney stone formation (10); additional trait-associated UMOD promoter SNPs upstream of rs4293393 were also identified. Although these additional variants were not previously reported to be associated with uric acid in earlier studies, they have been associated with sodium excretion (15), hypertension(15) or chronic kidney disease progression (10, 26,27). Such associations might begin to explain why serum uric acid, hypertension and chronic kidney disease are tightly linked together in many cohort studies (28). In the California subjects, SNPs across the UMOD locus (including the promoter) predicted uric acid. In the Third National Health and Nutrition Examination Survey (NHANES III), interactions between race (white, black, Hispanic and others) and abnormal levels of uric acid were observed(29). Previous GWAS studies found that UMOD-region tagging-SNP rs12917707 associated with CKD and eGFR (27), however the position of rs12917707 is far 5’ of UMOD, indeed >5 kbp upstream of the UMOD 5’-UTR. Thus we did not include tagging-SNP rs12917707 in our functional studies.
Functional genetic variation in the UMOD promoter
To investigate whether the promoter SNPs associated with plasma uric acid are functional, we constructed UMOD promoter/reporter plasmids, focusing on 3 common promoter variants: rsl3333226 → rs6497476 → rs4293393. We tested luciferase reporter acitvity in two cell lines: human embryonic kidney cell HEK-293 and mouse inner medullary collecting duct cell mlMCD-3. Since Hepatocyte Nuclear Factor 1b (HNF1b) is a transcription factor expressed in tubular epithelial cells as a regulator of UMOD transcription(30,31), we verified expression of UMOD and HNF1b mRNAs in renal/kidney cell lines at NCBI-GEO <http://www.ncbi.nlm.nih.gov/geo/> transcriptome database. Both UMOD and HNF1b were shown to be expressed in both human HEK-293 and mouse mlMCD3 cells.
In vivo chromatin immunoprecipitation experiments demonstrated that HNF1b binds to two sites close to the UMOD gene (at −1.1 and −0.58 kb with respect to the transcriptional start/cap site) in the mouse (32). Although SNP rs4293393 (from this study) is located at around −0.55 kb in the human UMOD promoter, it is unclear whether HNF1b binds to a similar site in the human UMOD promoter region.
In both cell lines we obtained similar results wherein 3-SNP promoter haplotype G-C-C displayed increased transcription compared to haplotype A-T-T. Individually, the rs13333226 G allele and rs6497476 C allele each elevated reporter gene activity, suggesting functional transcriptional roles for these variants in both cell lines. The difference between rs4293393 C vs T alleles was only identified in mlMCD3 cells.
We were initially surprised to note that UMOD promoter haplotypes and individual variants had opposite effects on transcription in cella (reporter gene activity in transfected cells) and urinary uromodulin excretion in vivo. Thus, there might be several explanations for this paradox.
First, although we constructed luciferase reporter plasmids by inserting a 1,794 bp promoter fragment of UMOD, this length might still not be sufficient to reflect the promoter regulation in vivo. However the luciferase activity difference between genotypes at same background indicated that all 3 SNPs were functional in the regulation of UMOD transcription. UMOD is a GPI-anchored cell membrane protein, whose biosynthesis and trafficking involve a complex secretory pathway: UMOD is cleaved, glycosylated and glypiated in the ER, and then N-glycan moieties are further trimmed in the Golgi apparatus, after which the mature moieties and GPI modification act as sorting signals, routing uromodulin to the apical membrane, from which it is released into the urine. Urinary uromodulin may thus be influenced by several steps: synthesis, glycosylation, trafficking and cleavage. Alternatively, negative feedback mechanisms can be proposed to explain the contrasting results in vivo and in cella: for example, after synthesis, accumulation of UMOD in the ER might delay glycosylation and thus UMOD expression on the apical cell membrane, or cleavage of the UMOD anchor for release into the urine.
Conclusions and perspectives
In summary, our data suggest that common genetic variation in the promoter region of UMOD confers a series of changes upon gene trascription and secretion of UMOD, renal tubular sodium/uric acid reabsorption, and finally serum uric acid concentration. Our observations are consistent with the “intermediate phenotype”(33) framework for complex traits as well as with the “common disease/common allele” hypothesis for frequent traits in the population (34), and suggest new molecular strategies for probing the pathophysiology, risk, and rational treatment of hyperuricemia (Figure 5).
METHODS
Subjects
Primary studies in a community-based cohort in an urban area of Beijing
Individuals were from a community-based cohort in an urban area of Beijing. Briefly, individuals aged >=40 years were evaluated for chronic kidney disease (CKD) and associated factors in 2004 (35). As a consequence of urban renewal in Beijing, part of the participants moved out of the area. Survival status and indicators of kidney damage were re-evaluated in 67.7% (n = 1563) of them in 2008 (36). We were able to ascertain, consent, and prepare genomic DNA from blood leukocytes obtained from 1000 of the original 1563 participants of 2008 in this study.
We were able to ascertain, consent, and prepare genomic DNA from 1000 of the original participants of 2008 in this study. The subset reported here (n=1000) did not differ from the complete cohort in age (p=0.603), gender (p=0.718), blood pressure (SBP p=0.35, DBP p=0.207), eGFR (p=0.308), or plasma uric acid (p=0.622). Characteristics of the cohort are listed in Table 1. eGFR was estimated with the equation developed from data based on Chinese patients with CKD (37) and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (38). Since eGFR caculated with these two equationa were highly correlated, we used the CKD-EPI equation to generate eGFR for both populations (Table 1).
Replication/extension studies: American twins and siblings
642 American individuals of white (European or Hispanic) ancestry, from 242 nuclear families ascertained at UCSD were subjected to genome-wide genotyping with the lllumina-610-Quad array, encompassing −590K SNP genotypes, after which an additional ~2 million Hap-Map-2 SNPs were imputed with MACH v. 1.0.16 (http://www.sph.umich.edu/csg/abecasis/MaCH/). This population included 24% Males, with age 40.6+/−0.8 years, SBP 123.0+/−0.6mmHg, DBP 75.6+/−0.4mmHg, plasma uric acid 414.3+/−0.1umol/L and eGFR 97.8+/−1.0 ml/min/1.73m2. SNP-on-phenotype effects with tested in MERLIN v1.1.2 (http://www.sph.umich.edu/csg/abecasis/merlin/) in order to explicitly account for family structure. For the plasma uric acid trait, the Q-Q plot of −log10 expected (theoretical uniform distribution) versus observed significance (p-values) across the genome did not show deviation across the range from −log10 = 0 to 5.5. Heritability (h2, or the fraction of trait variance accounted for by genetic variance) of uric acid traits was estimated by variance components in twin pairs, using SOLAR, available at <http://txbiomed.org/departments/genetics/genetics-detail?r=37>.
Genomics
Genomic DNA was isolated from whole blood using a modified salt extraction technique (39). Single nucleotide polymorphisms were obtained from public SNP databases (http://www.ncbi.nih.nlm.gov/SNP). Genomic DNA was typed using a matrix assisted laser desorption ionization time-of-flight mass spectrometry system(40), or by Taqman assay on an ABI PRISM Sequence Detection System 7500. Reproducibility of genotyping was verified with 50 blinded replicate samples. We selected 7 UMOD SNPs according to previously published research and the dbSNP database in NCBI. SNPs were selected for minor allele freq (>=5%), and to span functional domains in the gene, as well as LD blocks according to pertinent HapMap CHB results. SNPs evaluated in this study were in Hardy Weinberg equilibrium (p>0.05). Genotyping failing rates were less than 10%. In order to prove that the missing data were randomizely distributed, we compared clinical features between subgroups (whole population group, successfully genotyped group and genotype failed group) for each SNP and no differences were identified. Allele distribution of these SNPs were listed in supplementary table 1.
UMOD measurement
Measurement of UMOD in urine was achieved by ELISA according to the Lau protocol[37]. 96-well microtiter plates were coated with 100 µl of 0.1mol/ml polyclonal mouse anti-human THP antibody (Biomedical Technologies Inc BT-590) overnight at 4°C. Plates were washed and treated with 200 µl blocking buffer (3% BSA in PBS) for 1 hour at room temperature. Urine samples and standards (Biomedical Technologies) were diluted in TEA buffer and added to wells in duplicate. After two hours incubation at 37°C, wells were washed and anti-UMOD antibody was added. After another one-hour incubation at 37°C, wells were again washed and goat anti-rabbit IgG horseradish peroxidase (CEDARLANE CL1032AP) was added. After 0.5 hour incubation at 37°C, wells are washed and color was developed by the addition of TMB substrate solution and incubation at room temperature for 15 minutes. The reaction is stopped by adding 2N H2S04, followed by reading immediately at OD490 and OD570. Urinary UMOD concentration was determined by interpolation on the standard curve.
Plasmid construction for luciferase reported assays
Human UMOD promoter/reporter plasmids were constructed as described (41). We isolated genomic DNA from human blood, from which we excised 1,794 bp of UMOD promoter region by PCR, containing common polymorphic sites for insertion into the upstream region polylinker of the firefly luciferase reporter plasmid pGL3-Basic (Promega). Synthetic replacements were made by site-directed mutagenesis (QuikChange; Stratagene) to produce the common haplotypes showed in Table 2. All inserts were sequence-verified before use.
HEK-293 (human embryonic kidney) cells and mlMCD3 (mouse inner medullary collecting duct) cells were both used to test transcriptional activity of transfected UMOD promoter/luciferase reporter plasmids. Cells were transfected (at ~50–60% confluence) with 1 µg of pGL3-Basic haplotype/firefly luciferase reporter plasmid and 10 ng of Renilla luciferase plasmid pRL-TK (Promega) as an internal control per well, by the liposome method (Superfect; Qiagen). The firefly and Renilla luciferase activities in the cell lysates were measured 48 hours after transfection, and results were expressed as the ratio of firefly/Renilla luciferase activities (“Stop & Glo®”; Promega). Each experiment was repeated a minimum of three times.
Statistical analyses
Typing 7 common variants (also 3 promoter common variants) across the UMOD locus allowed the inference of haplotypes from diploid genotypes in PLINK (version 3.0) <http://pngu.mgh.harvard.edu/purcell/plink/]> (42). Haplotype distribution was listed in supplementary table 3.
Statistical Package for the Social Sciences (SPSS v13.0; Chicago, IL) was used routinely. Baseline characteristics were reported as mean ±SEM or median (inter-quartile range [IQR]) for continuous variables. One-way ANOVA (using Bonferroni comparison tests) was performed in SPSS to test in vitro reporter activity assays. Genotypes and haplotypes were grouped by the number of copies of the particular allele or haplotype (0, 1, 2 copies). Mode of inheritance emerged from visual inspection of the marker-on-trait plots–scatterplots or bar-graphs -- to determine whether two of the three diploid genotypes (Major/Major, Major/Minor, or Minor/Minor) displayed the same trait clustering. In this study, we thus pooled the minor allele homozygotes with heterozygotes, in a minor-allele-dominant model for single SNPs or Haplotypes.
Univariate ANOVA analyses and multiple linear regression in SPSS were computed to evaluate the effects of genotype or haplotype on uric acid. Age, gender, eGFR, BMI, height, weight, waist, SBP or DBP were added as covariates in univariate ANOVA analysis, and as independent variables in multiple linear regression.
In consideration of multiple testing of the effects, we employed estimation of the FDR (False Discovery Rate), in order to minimize false negative results while maximizing true positive results, using the Excel calculator of FDRs from p-values, at <http://www.rowett.ac.uk/~gwh/fdr.html>.
Permutation tests was performed with “Resampling Stats Excel add-in version 3.2” (http://www.resample.com/content/software/excel/index.shtml) to confirm genotype effect on plasma uric acid.
Supplementary Material
Supplementary figure 1. Haplotype blocks at the UMOD locus for Chinese (CHB) ancestry.
Acknowledgments
Support: A grant of the Chinese 985 project (NCET-10-0186/BMU 20110156)
Footnotes
Disclosure
All authors participated in the work, and have seen the final results; none has conflict of interest. The work was supported by A grant of the Chinese 985 project (NCET-10-0186/BMU 20110156). The results presented in this paper have not been published previously in whole or part.
References
- 1.Feig DI, Mazzali M, Kang DH, Nakagawa T, et al. Serum uric acid: a risk factor and a target for treatment? J Am Soc Nephrol. 2006;17:S69–S73. doi: 10.1681/ASN.2005121331. [DOI] [PubMed] [Google Scholar]
- 2.Pennica D, Kohr WJ, Kuang WJ, Glaister D, et al. Identification of human uromodulin as the Tamm-Horsfall urinary glycoprotein. Science. 1987;236:83–88. doi: 10.1126/science.3453112. [DOI] [PubMed] [Google Scholar]
- 3.Kreft B, Jabs WJ, Laskay T, Klinger M, et al. Polarized expression of Tamm-Horsfall protein by renal tubular epithelial cells activates human granulocytes. Infect Immun. 2002;70:2650–2656. doi: 10.1128/IAI.70.5.2650-2656.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rindler MJ, Naik SS, Li N, Hoops TC, et al. Uromodulin (Tamm-Horsfall glycoprotein/uromucoid) is a phosphatidylinositol-linked membrane protein. J Biol Chem. 1990;265:20784–20789. [PubMed] [Google Scholar]
- 5.Serafini-Cessi F, Malagolini N, Hoops TC, Rindler MJ. Biosynthesis and oligosaccharide processing of human Tamm-Horsfall glycoprotein permanently expressed in HeLa cells. Biochem Biophys Res Commun. 1993;194:784–790. doi: 10.1006/bbrc.1993.1890. [DOI] [PubMed] [Google Scholar]
- 6.Kumar S, Muchmore A. Tamm-Horsfall protein--uromodulin (1950–1990) Kidney Int. 1990;37:1395–1401. doi: 10.1038/ki.1990.128. [DOI] [PubMed] [Google Scholar]
- 7.Hart TC, Gorry MC, Hart PS, Woodard AS, et al. Mutations of the UMOD gene are responsible for medullary cystic kidney disease 2 and familial juvenile hyperuricaemic nephropathy. J Med Genet. 2002;39:882–892. doi: 10.1136/jmg.39.12.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rampoldi L, Caridi G, Santon D, Boaretto F, et al. Allelism of MCKD, FJHN and GCKD caused by impairment of uromodulin export dynamics. Hum Mol Genet. 2003;12:3369–3384. doi: 10.1093/hmg/ddg353. [DOI] [PubMed] [Google Scholar]
- 9.Turner JJ, Stacey JM, Harding B, Kotanko P, et al. UROMODULIN mutations cause familial juvenile hyperuricemic nephropathy. J Clin Endocrinol Metab. 2003;88:1398–1401. doi: 10.1210/jc.2002-021973. [DOI] [PubMed] [Google Scholar]
- 10.Gudbjartsson DF, Holm H, Indridason OS, Thorleifsson G, et al. Association of variants at UMOD with chronic kidney disease kidney stones-role of age and comorbid diseases. PLoS Genet. 6:e1001039. doi: 10.1371/journal.pgen.1001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vyletal P, Bleyer AJ, Kmoch S. Uromodulin biology pathophysiology--an update. Kidney Blood Press Res. 33:456–475. doi: 10.1159/000321013. [DOI] [PubMed] [Google Scholar]
- 12.Hoyer JR, Seiler MW. Pathophysiology of Tamm-Horsfall protein. Kidney Int. 1979;16:279–289. doi: 10.1038/ki.1979.130. [DOI] [PubMed] [Google Scholar]
- 13.Lhotta K. Uromodulin chronic kidney disease. Kidney Blood Press Res. 33:393–398. doi: 10.1159/000320681. [DOI] [PubMed] [Google Scholar]
- 14.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 15.Padmanabhan S, Melander O, Johnson T, Di Blasio AM, et al. Genome-wide association study of blood pressure extremes identifies variant near UMOD associated with hypertension. PLoS Genet. 6 doi: 10.1371/journal.pgen.1001177. e1001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terkeltaub R, Bushinsky DA, Becker MA. Recent developments in our understanding of the renal basis of hyperuricemia and the development of novel antihyperuricemic therapeutics. Arthritis Res Ther. 2006;8(Suppl 1):S4. doi: 10.1186/ar1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tinschert S, Ruf N, Bernascone I, Sacherer K, et al. Functional consequences of a novel uromodulin mutation in a family with familial juvenile hyperuricaemic nephropathy. Nephrol Dial Transplant. 2004;19:3150–3154. doi: 10.1093/ndt/gfh524. [DOI] [PubMed] [Google Scholar]
- 18.Lens XM, Banet JF, Outeda P, Barrio-Lucia V. A novel pattern of mutation in uromodulin disorders: autosomal dominant medullary cystic kidney disease type 2, familial juvenile hyperuricemic nephropathy, and autosomal dominant glomerulocystic kidney disease. Am J Kidney Dis. 2005;46:52–57. doi: 10.1053/j.ajkd.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Yang Q, Kottgen A, Dehghan A, Smith AV, et al. Multiple genetic loci influence serum urate levels and their relationship with gout and cardiovascular disease risk factors. Circ Cardiovasc Genet. 3:523–530. doi: 10.1161/CIRCGENETICS.109.934455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiecek A, Kokot F. Does industrial environment influence the prevalence of arterial hypertension, plasma cholesterol and uric acid concentration and activity of the renin-aldosterone system? Przegl Lek. 1996;53:356–359. [PubMed] [Google Scholar]
- 21.Duan HP, Pang ZC, Zhang DF, Wang SJ, et al. Heritability of serum uric acid in adult twins. Zhonghua Liu Xing Bing Xue Za Zhi. 31:384–388. [PubMed] [Google Scholar]
- 22.Lillie EO, O'Connor DT. Early phenotypic changes in hypertension: a role for the autonomic nervous system and heredity. Hypertension. 2006;47:331–333. doi: 10.1161/01.HYP.0000203980.44717.aa. [DOI] [PubMed] [Google Scholar]
- 23.Malagolini N, Cavallone D, Serafini-Cessi F. Intracellular transport, cell-surface exposure and release of recombinant Tamm-Horsfall glycoprotein. Kidney Int. 1997;52:1340–1350. doi: 10.1038/ki.1997.459. [DOI] [PubMed] [Google Scholar]
- 24.Renigunta A, Renigunta V, Saritas T, Decher N, et al. Tamm-Horsfall glycoprotein interacts with renal outer medullary potassium channel ROMK2 and regulates its function. J Biol Chem. 286:2224–2235. doi: 10.1074/jbc.M110.149880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gersch M, Mutig K, Bachmann S, Kumar S, et al. Is salt-wasting the long awaited answer to the hyperuricaemia seen in uromodulin storage diseases? Nephrol Dial Transplant. 2006;21:2028–2029. doi: 10.1093/ndt/gfk081. [DOI] [PubMed] [Google Scholar]
- 26.Ahluwalia TS, Lindholm E, Groop L, Melander O. Uromodulin gene variant is associated with type 2 diabetic nephropathy. J Hypertens. 29:1731–1734. doi: 10.1097/HJH.0b013e328349de25. [DOI] [PubMed] [Google Scholar]
- 27.Kottgen A, Glazer NL, Dehghan A, Hwang SJ, et al. Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet. 2009;41:712–717. doi: 10.1038/ng.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Townsend RR, Anderson AH, Chen J, Gadebegku CA, et al. Metabolic syndrome, components, and cardiovascular disease prevalence in chronic kidney disease: findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Nephrol. 33:477–484. doi: 10.1159/000327618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foley RN, Wang C, Ishani A, Collins AJ. NHANES III: influence of race on GFR thresholds and detection of metabolic abnormalities. J Am Soc Nephrol. 2007;18:2575–2582. doi: 10.1681/ASN.2006121411. [DOI] [PubMed] [Google Scholar]
- 30.Lazzaro D, De Simone V, De Magistris L, Lehtonen E, et al. LFB1 and LFB3 homeoproteins are sequentially expressed during kidney development. Development. 1992;114:469–479. doi: 10.1242/dev.114.2.469. [DOI] [PubMed] [Google Scholar]
- 31.Pontoglio M, Barra J, Hadchouel M, Doyen A, et al. Hepatocyte nuclear factor 1 inactivation results in hepatic dysfunction, phenylketonuria, and renal Fanconi syndrome. Cell. 1996;84:575–585. doi: 10.1016/s0092-8674(00)81033-8. [DOI] [PubMed] [Google Scholar]
- 32.Gresh L, Fischer E, Reimann A, Tanguy M, et al. A transcriptional network in polycystic kidney disease. EMBO J. 2004;23:1657–1668. doi: 10.1038/sj.emboj.7600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, Rao F, Rodriguez-Flores JL, Mahata M, et al. Naturally occurring human genetic variation in the 3'-untranslated region of the secretory protein chromogranin A is associated with autonomic blood pressure regulation and hypertension in a sex-dependent fashion. J Am Coll Cardiol. 2008;52:1468–1481. doi: 10.1016/j.jacc.2008.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reich DE, Lander ES. On the allelic spectrum of human disease. Trends Genet. 2001;17:502–510. doi: 10.1016/s0168-9525(01)02410-6. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Zuo L, Xu G, Wang F, et al. Community-based screening for chronic kidney disease among populations older than 40 years in Beijing. Nephrol Dial Transplant. 2007;22:1093–1099. doi: 10.1093/ndt/gfl763. [DOI] [PubMed] [Google Scholar]
- 36.Wang F, Zhang L, Zuo L, Liu L, et al. Mortality and renal function decline among a community-based Chinese population with normal or mildly impaired renal function. Nephrol Dial Transplant. 26:2847–2852. doi: 10.1093/ndt/gfq816. [DOI] [PubMed] [Google Scholar]
- 37.Ma YC, Zuo L, Chen JH, Luo Q, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2937–2944. doi: 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
- 38.Levey AS, Stevens LA, Schmid CH, Zhang YL, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buetow KH, Edmonson M, MacDonald R, Clifford R, et al. High-throughput development and characterization of a genomewide collection of gene-based single nucleotide polymorphism markers by chip-based matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Proc Natl Acad Sci U S A. 2001;98:581–584. doi: 10.1073/pnas.021506298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y, Wen G, Rao F, Zhang K, et al. Human dopamine beta-hydroxylase (DBH) regulatory polymorphism that influences enzymatic activity, autonomic function, blood pressure. J Hypertens. 28:76–86. doi: 10.1097/HJH.0b013e328332bc87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Purcell S, Neale B, Todd-Brown K, Thomas L, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1. Haplotype blocks at the UMOD locus for Chinese (CHB) ancestry.